Comparative Coexpression Analysis of Indole Synthase and Tryptophan Synthase A Reveals the Independent Production of Auxin via the Cytosolic Free Indole
Abstract
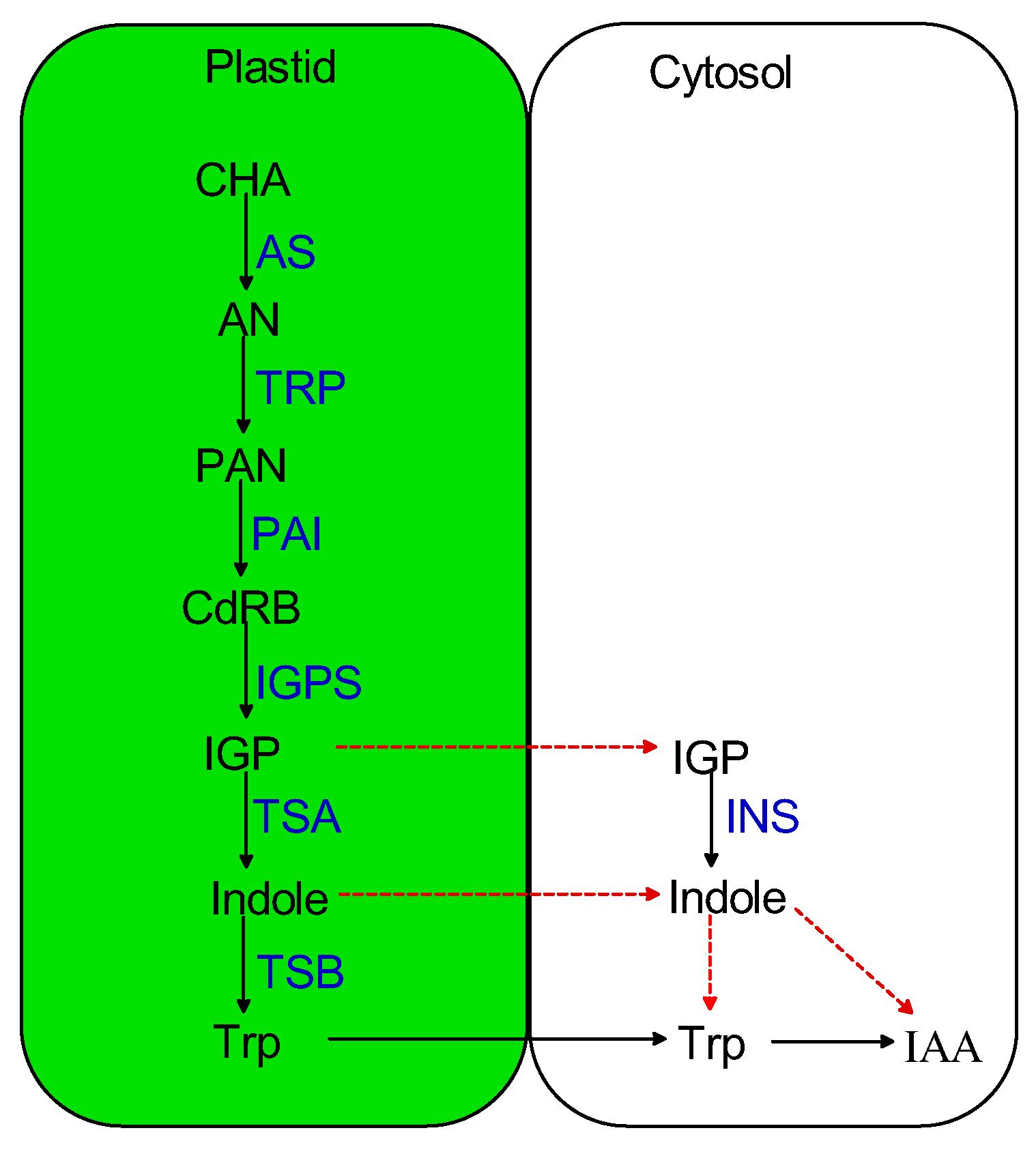
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Tryptophan Synthase A but Not Indole Synthase Is Coexpressed with Tryptophan Synthase B Genes
3.2. Indole Synthase Is not Coexpressed with the Upstream Tryptophan Synthase Genes in the Chorismic Acid Pathway
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y. Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu. Rev. Plant Biol. 2018, 69, 417–435. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, Q.; Tan, C.; Song, J.; Zhang, T.; Men, S. Biosynthesis-and transport-mediated dynamic auxin distribution during seed development controls seed size in Arabidopsis. Plant J. 2023, 113, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Ai, G.; Huang, R.; Zhang, D.; Li, M.; Li, G.; Li, W.; Ahiakpa, J.K.; Wang, Y.; Hong, Z.; Zhang, J. SlGH3. 15, a member of the GH3 gene family, regulates lateral root development and gravitropism response by modulating auxin homeostasis in tomato. Plant Sci. 2023, 330, 111638. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Normanly, J. Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harb. Perspect. Boil. 2010, 2, a001594. [Google Scholar] [CrossRef] [PubMed]
- Tivendale, N.D.; Ross, J.J.; Cohen, J.D. The shifting paradigms of auxin biosynthesis. Trends Plant Sci. 2014, 19, 44–51. [Google Scholar] [CrossRef]
- Chandler, J.W. Local auxin production: A small contribution to a big field. Bioessays 2009, 31, 60–70. [Google Scholar] [CrossRef]
- Abu-Zaitoon, Y.M.; Al Tawaha, A.R.; Alnaimat, S.M.; Al-Rawashdeh, I.M.; Abu-Zaiton, A.H.; Khalifat, A.H. Investigation of the potential role of aldehyde oxidase in indole-3-acetic acid synthesis of developing rice grains. Plant Cell Biotechnol. Mol. Biol. 2019, 20, 6–13. [Google Scholar]
- Gao, Y.; Dai, X.; Aoi, Y.; Takebayashi, Y.; Yang, L.; Guo, X.; Zeng, Q.; Yu, H.; Kasahara, H.; Zhao, Y. Two homologous INDOLE-3-ACETAMIDE (IAM) HYDROLASE genes are required for the auxin effects of IAM Arabidopsis. J. Genet. Genom. 2020, 47, 157–165. [Google Scholar] [CrossRef]
- Ortiz-García, P.; González Ortega-Villaizán, A.; Onejeme, F.C.; Müller, M.; Pollmann, S. Do Opposites Attract? Auxin-Abscisic Acid Crosstalk: New Perspectives. Int. J. Mol. Sci. 2023, 24, 3090. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Tanaka, K.; Sakai, T.; Sugawara, S.; Kawaide, H.; Natsume, M.; Kasahara, H. The main auxin biosynthesis pathway in Arabidopsis. Proc. Nat. Acad. Sci. USA 2011, 108, 18512–18517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y. Auxin biosynthesis. The Arabidopsis Book/American Society of Plant Biologists. Arab. Book 2014, 12, e0173. [Google Scholar] [CrossRef] [PubMed]
- Radwanski, E.R.; Last, R.L. Tryptophan biosynthesis and metabolism: Biochemical and molecular genetics. Plant Cell 1995, 7, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.H.; Dudareva, N. Aromatic amino acids: A complex network ripe for future exploration. Trends Plant Sci. 2020, 25, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Powell, A.F.; Mirzaei, M.; Wang, L.J.; Movahed, N.; Miller, J.K.; Jander, G. Indole-3-glycerolphosphate synthase, a branchpoint for the biosynthesis of tryptophan, indole, and benzoxazinoids in maize. Plant J. 2021, 106, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Shao, X.; Li, J. Indole-3-glycerol phosphate, a branchpoint of indole-3-acetic acid biosynthesis from the tryptophan biosynthetic pathway in Arabidopsis thaliana. Plant J. 2000, 24, 327–334. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, B.; Ouyang, J.; Li, J.; Wang, Y. Arabidopsis indole synthase, a homolog of tryptophan synthase alpha, is an enzyme involved in the trp-independent indole-containing metabolite biosynthesis. J. Integr. Plant Biol. 2008, 50, 1070–1077. [Google Scholar] [CrossRef]
- Wang, B.; Chu, J.; Yu, T.; Xu, Q.; Sun, X.; Yuan, J.; Li, J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 4821–4826. [Google Scholar] [CrossRef]
- Jin, Z.; Kim, J.H.; Park, S.U.; Kim, S.U. Cloning and characterization of indole synthase (INS) and a putative tryptophan synthase α-subunit (TSA) genes from Polygonum tinctorium. Plant Cell Rep. 2016, 35, 2449–2459. [Google Scholar] [CrossRef]
- Kriechbaumer, V.; Weigang, L.; Fießelmann, A.; Letzel, T.; Frey, M.; Gierl, A.; Glawischnig, E. Characterisation of the tryptophan synthase alpha subunit in maize. BMC Plant Boil. 2008, 8, 44. [Google Scholar] [CrossRef]
- Tillmann, M.; Tang, Q.; Cohen, J.D. Protocol: Analytical methods for visualizing the indolic precursor network leading to auxin biosynthesis. Plant Methods 2021, 17, 63. [Google Scholar] [CrossRef]
- Tillmann, M.; Tang, Q.; Gardner, G.; Cohen, J.D. Complexity of the auxin biosynthetic network in Arabidopsis hypocotyls is revealed by multiple stable-labeled precursors. Phytochemistry 2022, 200, 113219. [Google Scholar] [CrossRef] [PubMed]
- Pieck, M.; Yuan, Y.; Godfrey, J.; Fisher, C.; Zolj, S.; Vaughan, D.; Thomas, N.; Wu, C.; Ramos, J.; Lee, N.; et al. Auxin and tryptophan homeostasis are facilitated by the ISS1/VAS1 aromatic aminotransferase in Arabidopsis. Genetics 2015, 201, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zaitoon, Y.M.; Abu-Zaiton, A.; Al Tawaha, A.; Fandi, K.G.; Almomani, F.A.; Siddhartha, P. Evidence from co-expression analysis for the involvement of amidase and INS in the tryptophan-independent pathway of IAA synthesis in Arabidopsis. Appl. Biochem. Biotechnol. 2022, 194, 211–216. [Google Scholar] [CrossRef]
- Heather, M. Nonhebel. Tryptophan-Independent Indole-3-Acetic Acid Synthesis: Critical Evaluation of the Evidence. Plant Physiol. 2015, 169, 1001–1005. [Google Scholar] [CrossRef]
- Gupta, C.; Pereira, A. Recent advances in gene function prediction using context-specific coexpression networks in plants. F1000Research 2019, 8, F1000 Faculty Rev–153. [Google Scholar] [CrossRef]
- Zhang, B.; Horvath, S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005, 4, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Nakai, Y.; Shimizu, K. Ranking differentially expressed genes from Affymetrix gene expression data: Methods with reproducibility, sensitivity, and specificity. Algorithms Mol. Biol. 2009, 4, 7. [Google Scholar] [CrossRef]
- Movahedi, S.; Van de Peer, Y.; Vandepoele, K. Comparative network analysis reveals that tissue specificity and gene function are important factors influencing the mode of expression evolution in Arabidopsis and rice. Plant Physiol. 2011, 156, 1316–1330. [Google Scholar] [CrossRef]
- Ruprecht, C.; Proost, S.; Hernandez-Coronado, M.; Ortiz-Ramirez, C.; Lang, D.; Rensing, S.A.; Mutwil, M. Phylogenomic analysis of gene co-expression networks reveals the evolution of functional modules. Plant J. 2017, 90, 447–465. [Google Scholar] [CrossRef]
- Ghosh, A.; Arabia, S.; Shah, M.N.A.; Sami, A.A.; Islam, T. Identification and expression profiling of proline metabolizing genes in Arabidopsis thaliana and Oryza sativa to reveal their stress-specific transcript alteration. Physiol. Mol. Biol. Plants 2021, 27, 1469–1485. [Google Scholar]
- Obayashi, T.; Aoki, Y.; Tadaka, S.; Kagaya, Y.; Kinoshita, K. ATTED-II in 2018: A plant coexpression database based on investigation of the statistical property of the mutual rank index. Plant Cell Physiol. 2018, 59, e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ambaru, B.; Thakkar, P.; Marcotte, E.M.; Rhee, S.Y. Rational association of genes with traits using a genome-scale gene network for Arabidopsis thaliana. Nat. Biotechnol. 2010, 28, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Last, R.L.; Bissinger, P.H.; Mahoney, D.J.; Radwanski, E.R.; Fink, G.R. Tryptophan mutants in Arabidopsis: The consequences of duplicated tryptophan synthase beta genes. Plant Cell 1991, 3, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Lingling, L.; Xie, J.; Coulter, J.A.; Luo, Z. Synthesis and regulation of auxin and abscisic acid in maize. Plant Signal. Behav. 2021, 16, 1891756. [Google Scholar] [CrossRef]
- Pruitt Kim, D.; Robert, L.L. Expression Patterns of Duplicate Tryptophan Synthase (beta) Genes in Arabidopsis thaliana. Plant Physiol. 1993, 102, 1019–1026. [Google Scholar] [CrossRef]
- Yin, R.; Frey, M.; Gierl, A.; Glawischnig, E. Plants contain two distinct classes of functional tryptophan synthase beta proteins. Phytochemistry 2010, 71, 1667–1672. [Google Scholar] [CrossRef]
- Xie, G.; Forst, C.; Bonner, C.; Jensen, R.A. Significance of two distinct types of tryptophan synthase beta chain in Bacteria, Archaea and higher plants. Genome Biol. 2001, 3, 1–14. [Google Scholar] [CrossRef]
- Ahmad, S.; Veyrat, N.; Gordon-Weeks, R.; Zhang, Y.; Martin, J.; Smart, L.; Glauser, G.; Erb, M.; Flors, V.; Frey, M.; et al. Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol. 2011, 157, 317–327. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Fink, G.R. Two anthranilate synthase genes in Arabidopsis: Defense-related regulation of the tryptophan pathway. Plant Cell 1992, 4, 721–733. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Hoyt, J.M.; Hamilton, A.A.; Alonso, J.M. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 2005, 17, 2230–2242. [Google Scholar] [CrossRef] [PubMed]
- Less, H.; Galili, G. Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses. Plant Physiol. 2008, 147, 316–330. [Google Scholar] [CrossRef]
- Fattorini, L.; Veloccia, A.; Della Rovere, F.; D’Angeli, S.; Falasca, G.; Altamura, M.M. Indole-3-butyric acid promotes adventitious rooting in Arabidopsis thaliana thin cell layers by conversion into indole-3-acetic acid and stimulation of anthranilate synthase activity. BMC Plant Biol. 2017, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, K.K.; Last, R.L.; Fink, G.R.; Keith, B. Suppressors of trp1 fluorescence identify a new Arabidopsis gene, TRP4, encoding the anthranilate synthase beta subunit. Plant Cell 1993, 5, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. Arab. Book 2010, 8, e0132. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, S.; Zhu, L.; Last, R.L. Isolation of cDNAs encoding the tryptophan pathway enzyme indole-3-glycerol phosphate synthase from Arabidopsis thaliana. Plant Physiol. 1995, 108, 877. [Google Scholar] [CrossRef]
| Gene | AGI Locus | Gene | AGI Locus |
|---|---|---|---|
| ASA1 | At5g05730 | PAI3 | At1g29410 |
| ASA2 | At2g29690 | IGPS | At2g04400; At5g48220 |
| ASA3 | At3g55870 | TSA | At3g54640 |
| ASB1 | At1g25220 | TSB1 | At5g54810 |
| ASB2 | At5g57890 | TSB2 | At4g27070 |
| ASB (putative) | At1g24807 At1g24909 At1g25083 At1g25155 | TSB3 | At5g38530 |
| TRP1 | At5g17990; At1g70570 | TSB4 | At5g28237 |
| PAI1 | At1g07780 | INS | At4g02610 |
| PAI2 | At5g05590 |
| Gene | AGI Locus | TSA At3g54640 | Platform |
|---|---|---|---|
| TSB1 | At5g54810 | Ath-u.2 2 | |
| 10.8 | Ath-r.5 | ||
| TSB2 | At4g27070 | 6.4 | Ath-u.2 |
| 6.8 | Ath-r.5 | ||
| TSB3 | At5g38530 | 2.1 1 | Ath-u.2 |
| 1.6 1 | Ath-r.5 | ||
| 2.1 1 | Ath-m.9 | ||
| 2.7 1 | Abiotic | ||
| 3.6 | Hormone |
| Gene | Ath-u.2 | Ath-r.5 | Ath-m.9 | Tissue | Stress | Hormone | Biotic | Light |
|---|---|---|---|---|---|---|---|---|
| ASA1 | 12.9 | 11.6 | 10.5 | 5.0 | 4.4 | 4.3 | 5.3 | 4.8 |
| ASB1 | 7.9 | 8.2 | - | - | - | - | - | - |
| TRP1 | 12.9 | 11.8 | 10.3 | 3.8 | 5 | 5.2 | 5.2 | 4.4 |
| IGPS1 | 17.3 | 13.8 | 16.0 | 4.0 | 5.7 | 5.7 | 5.6 | - |
| Gene | AGI Locus | Average LS to Query Genes | New Rank |
|---|---|---|---|
| IGPS1 | At2g04400 | 8.06 | 6 |
| ASA1 | At5g05730 | 7.48 | 10 |
| TRP1 | At5g17990 | 7.32 | 13 |
| ASB1 | At1g25220 | 5.4 | 30 |
| TSA1 | At3g54640 | 12.73 | 1 |
| TSB1 | At5g54810 | 7.52 | 8 |
| TSB2 | AT4G27070 | 5.03 | 33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Zaitoon, Y.M.; Al-Ramamneh, E.A.-D.M.; Al Tawaha, A.R.; Alnaimat, S.M.; Almomani, F.A. Comparative Coexpression Analysis of Indole Synthase and Tryptophan Synthase A Reveals the Independent Production of Auxin via the Cytosolic Free Indole. Plants 2023, 12, 1687. https://doi.org/10.3390/plants12081687
Abu-Zaitoon YM, Al-Ramamneh EA-DM, Al Tawaha AR, Alnaimat SM, Almomani FA. Comparative Coexpression Analysis of Indole Synthase and Tryptophan Synthase A Reveals the Independent Production of Auxin via the Cytosolic Free Indole. Plants. 2023; 12(8):1687. https://doi.org/10.3390/plants12081687
Chicago/Turabian StyleAbu-Zaitoon, Yousef M., Ezz Al-Dein Muhammed Al-Ramamneh, Abdel Rahman Al Tawaha, Sulaiman M. Alnaimat, and Fouad A. Almomani. 2023. "Comparative Coexpression Analysis of Indole Synthase and Tryptophan Synthase A Reveals the Independent Production of Auxin via the Cytosolic Free Indole" Plants 12, no. 8: 1687. https://doi.org/10.3390/plants12081687






