Efficacy of Biological Control Agents and Resistance Inducer for Control of Mal Secco Disease
Abstract
:1. Introduction
2. Results
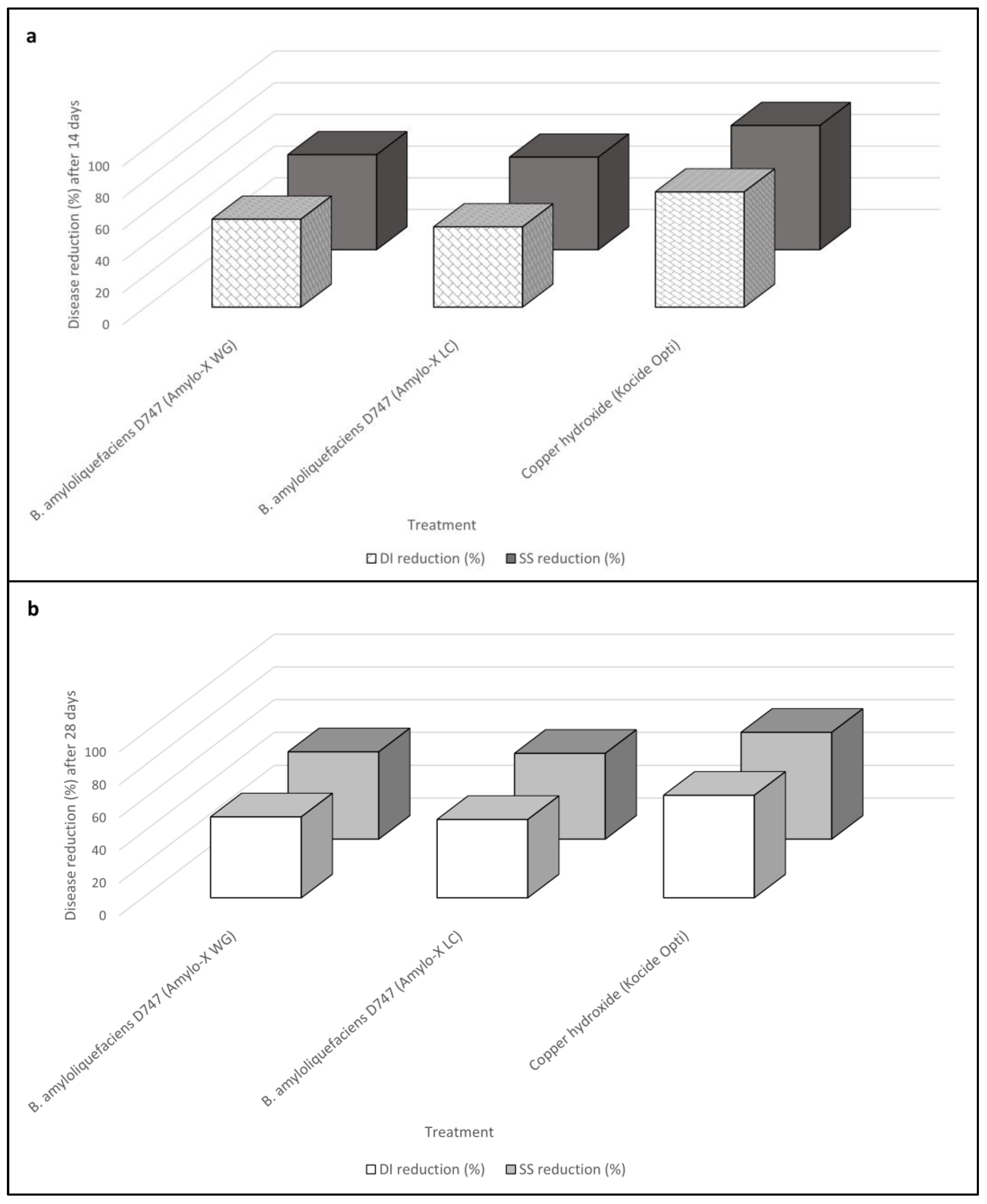
2.1. In Vivo Comparative Evaluation of Bacillus amyloliquefaciens Formulations and Mode of Application (Experiment I)
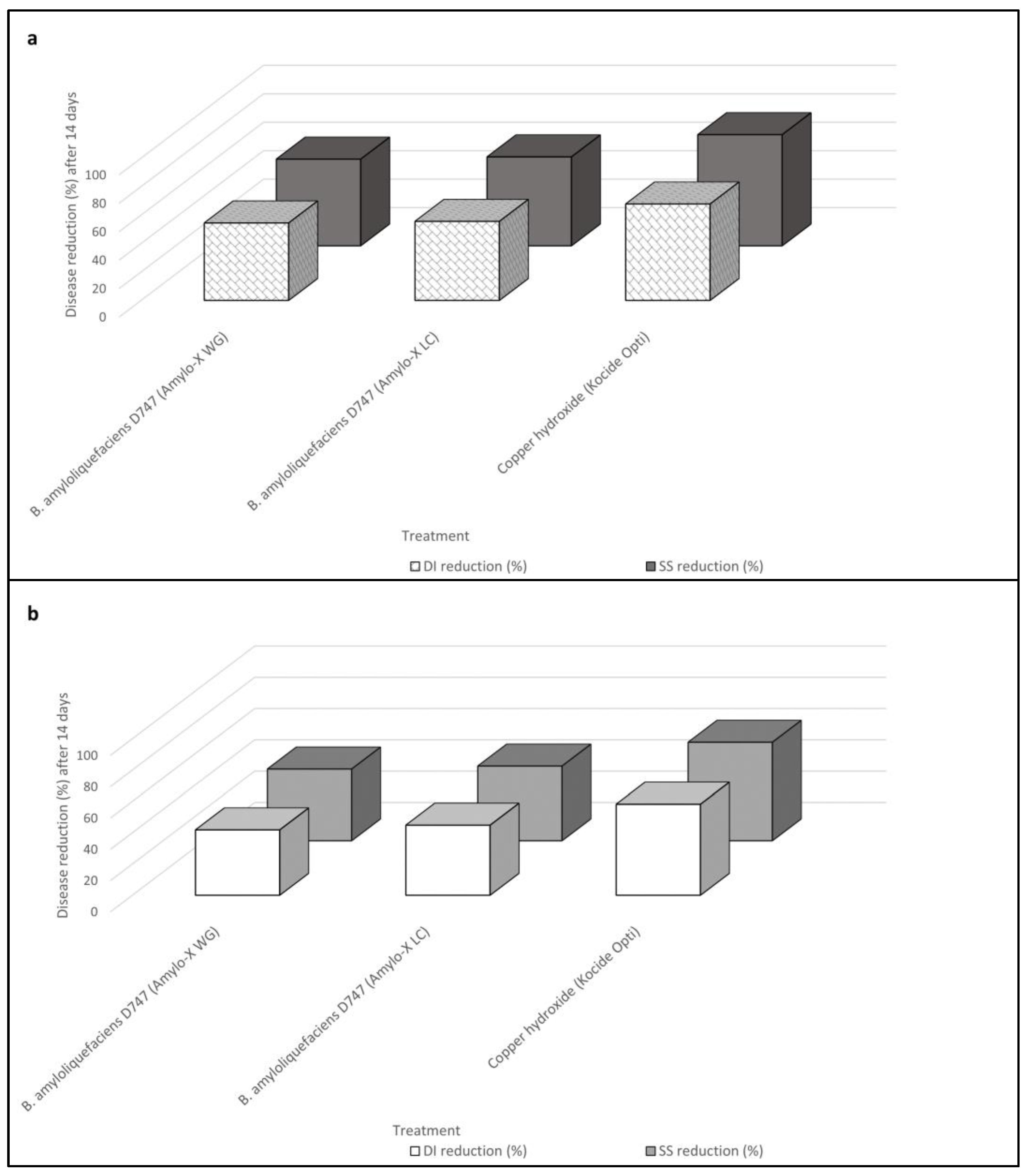
2.2. In Vivo Comparative Evaluation of Bacillus amyloliquefaciens Formulations and Mode of Application (Experiment II)
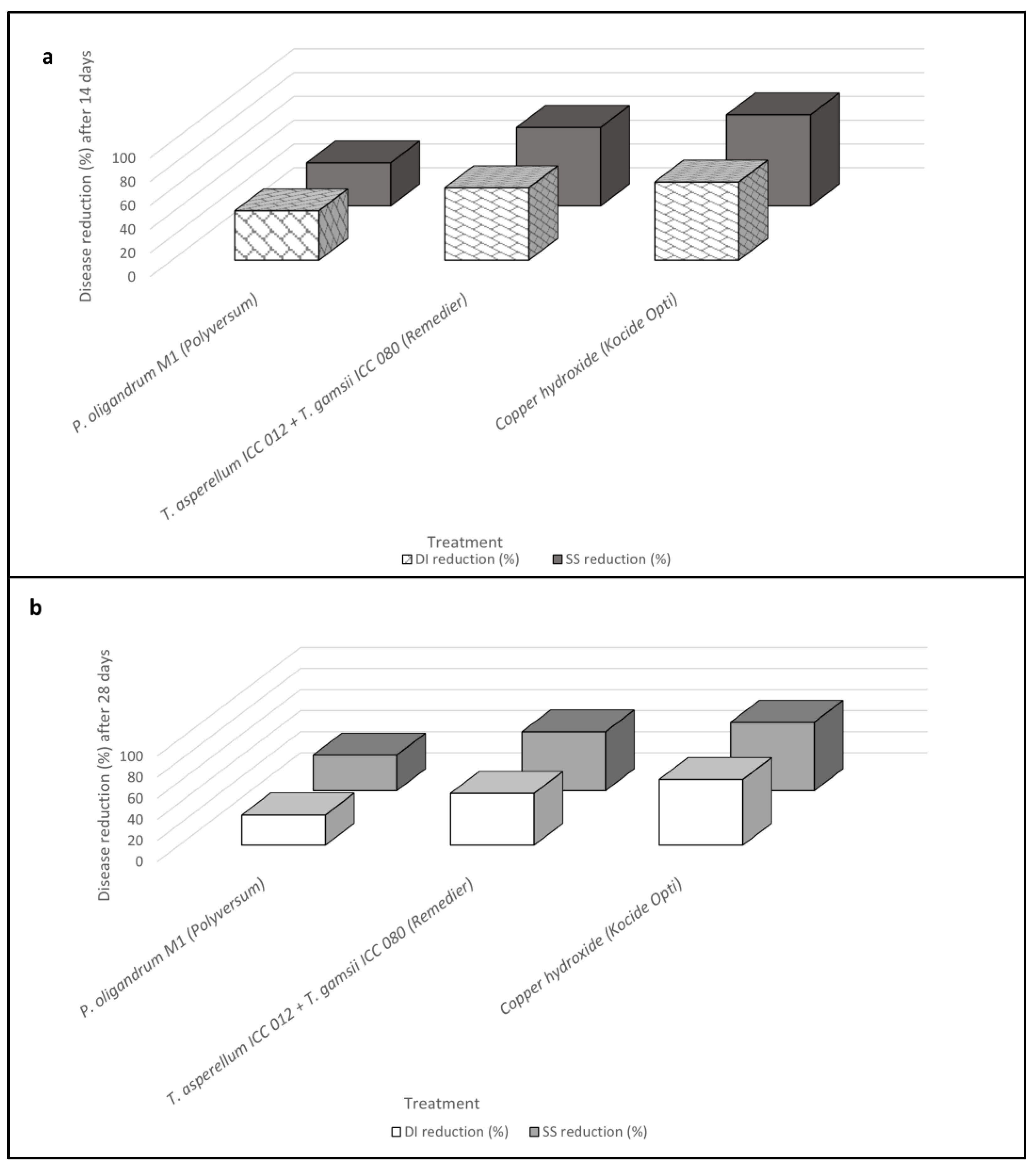
2.3. In Vivo Biocontrol Activity of Trichoderma spp. and Pythium oligandrum Formulates (Experiment III)
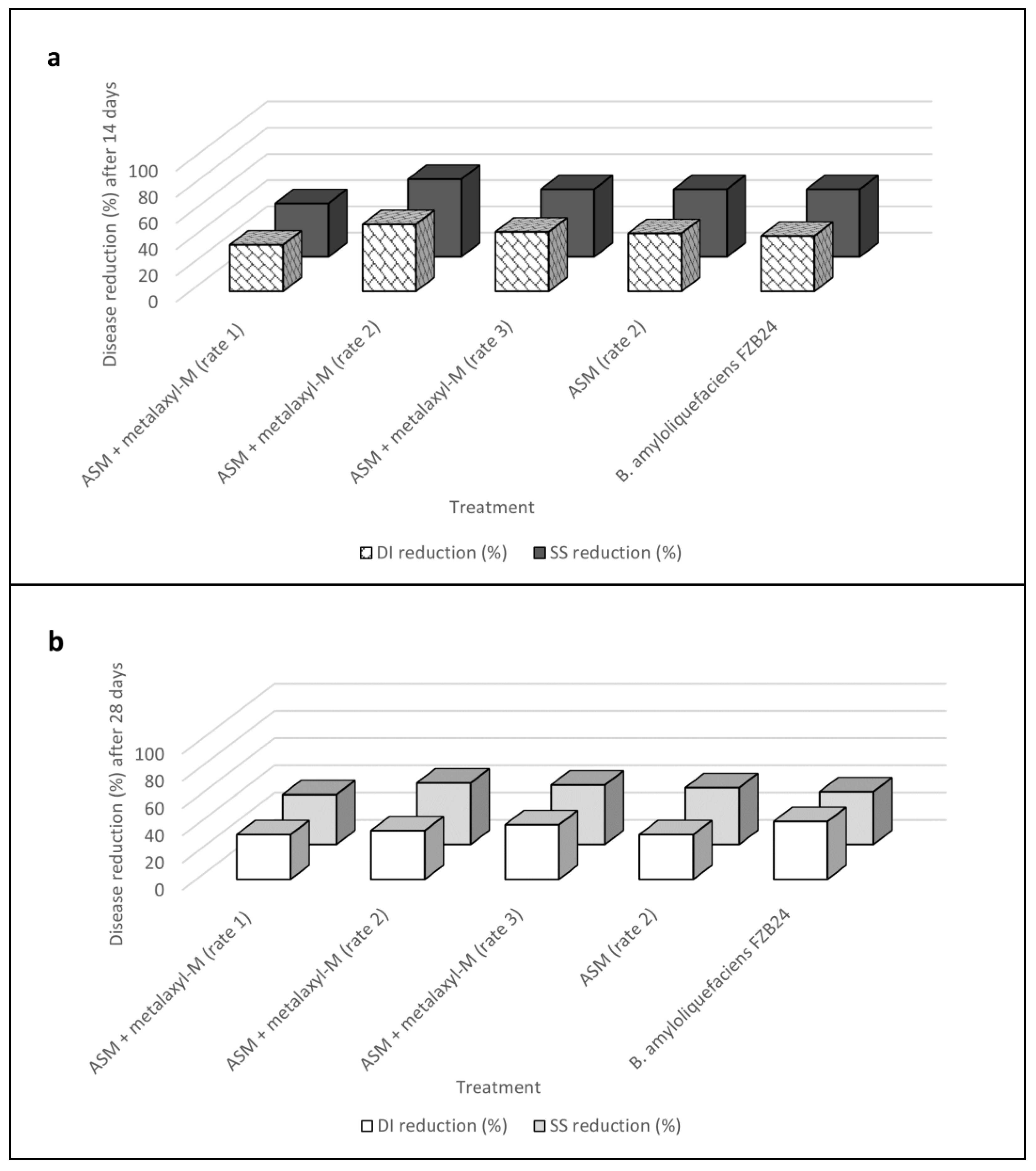
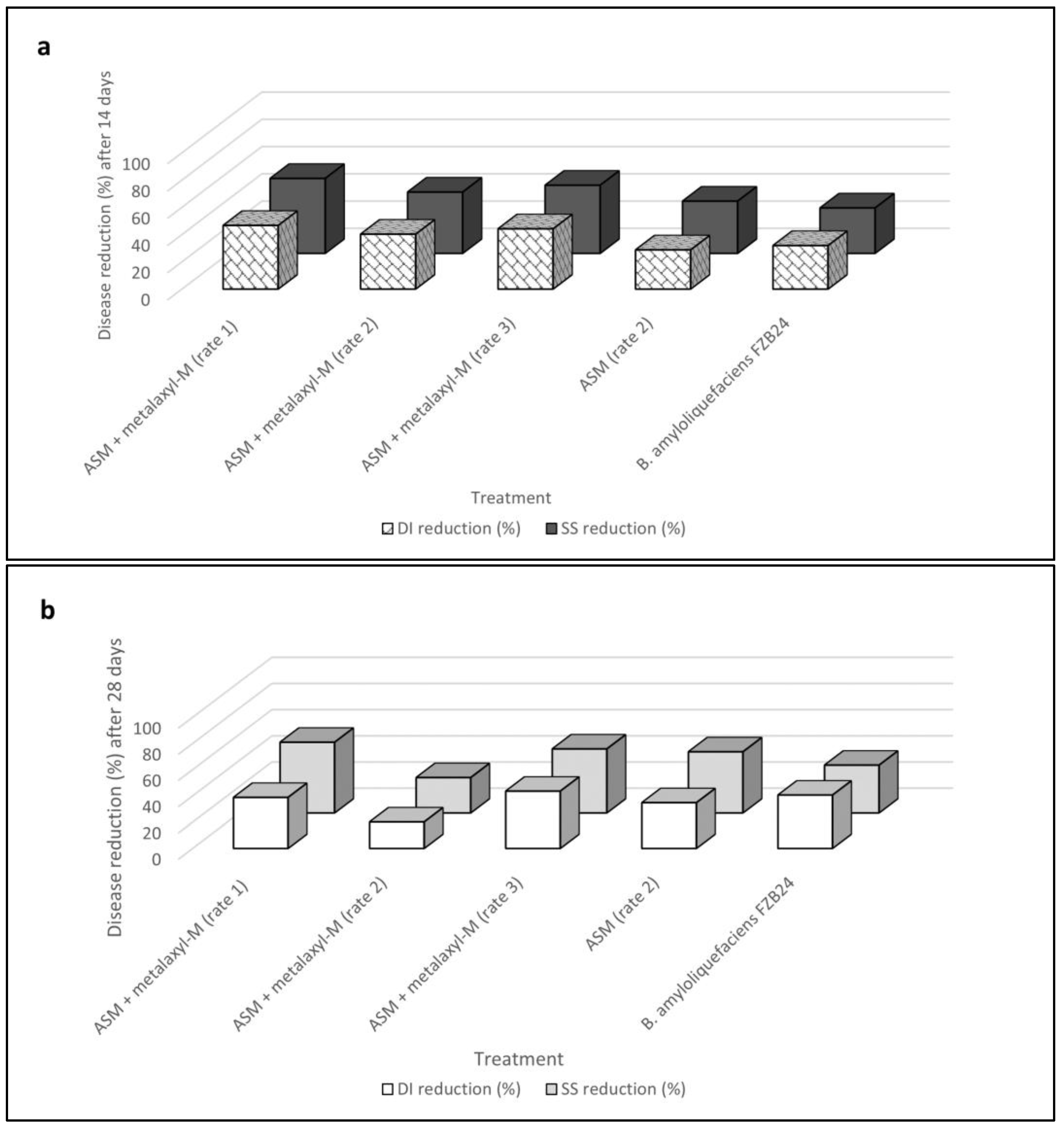
2.4. Effects of Acibenzolar-S-Methyl in Reducing Mal Secco Disease (Experiment IV)
3. Discussion
4. Materials and Methods
4.1. Collection and Spore Preparation of Plenodomus tracheiphilus Isolates
4.2. Plant Material and Growth Conditions
4.3. In Vivo Comparative Evaluation of Bacillus amyloliquefaciens Formulations and Mode of Application (Experiments I, II)
4.4. In Vivo Biocontrol Activity of Trichoderma spp. and Pythium oligandrum Formulates (Experiment III)
4.5. Disease-Controlling Effect of Acibenzolar-S-Methyl (Experiment IV)
4.6. Disease Assessment
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- EPPO. European and Mediterranean Plant Protection Organization. 2021. Available online: https://gd.eppo.int/ (accessed on 26 December 2022).
- Catara, A.; Cutuli, G. Osservazioni sulla suscettibilità di alcune Rutacee alle infezioni epigee di Phoma tracheiphila. Ann. Ist. Sper. Agrumic. 1972, 5, 29–49. [Google Scholar]
- Zuker, W.V.; Catara, A. Osservazioni al microscopio elettronico a scansione sulla penetrazione fogliare di Phoma tracheiphila. Inf. Fitopatol. 1985, 4, 33–35. [Google Scholar]
- Butera, N.; Cupperi, L.; Zucker, W.Z.; Catara, A.; Grasso, S. Observations of localization of Phoma tracheiphila in canopy infections. In Integrated Pest Control in Citrus Groves; Cavalloro, A.R., Martino, E.D., Eds.; Paper presented at: Expert Meeting (Italy); CRC Press: Boca Raton, FL, USA, 1985; pp. 257–266. [Google Scholar]
- Migheli, Q.; Cacciola, S.O.; Balmas, V.; Pane, A.; Ezra, D.; Magnano di San Lio, G. Mal secco disease caused by Phoma tracheiphila: A potential threat to lemon production worldwide. Plant Dis. 2009, 93, 852–867. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2018/1981 of the European Parliament and of the Council of 13 December 2018 Renewing the Approval of the Active Substances Copper Compounds, as Candidates for Substitution, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Off. J. Eur. Union 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R1981&from=EN (accessed on 26 December 2021).
- Lima, G.; Ippolito, A.; Nigro, F.; Salerno, M. Tentativi di lotta biologica contro il mal secco degli agrumi (Phoma tracheiphila) a mezzo di batteri endofiti. La Dif. Delle Piante 1994, 17, 43–49. [Google Scholar]
- Coco, V.; Grimaldi, V.; Licciardello, G.; Cirvilleri, G.; Grasso, S.; Catara, A. Inhibition of Phoma tracheiphila by pseudomonads in citrus seedlings. In Proceedings of the 8th International Citrus Congress, Agadir, Morocco. Int. Soc. Citric. 2004, 2, 729–732. [Google Scholar]
- Grasso, F.M.; Coco, V.; Grimaldi, V.; Catara, V. Contenimento delle infezioni Phoma tracheiphila in piante di limone mediante trattamenti con Pseudomonas spp. e Acibenzolar-S-methyl. Giornate Fitopatol. 2008, 191–195. [Google Scholar]
- Kalai-Grami, L.; Ben Slimane, I.; Mnari-Hattab, M.; Rezgui, S.; Aouani, M.A.; Hajlaoui, M.R.; Limam, F. Protective effect of Bacillus amyloliquefaciens against infections of Citrus aurantium seedlings by Phoma tracheiphila. World J. Microbiol. Biotechnol. 2014, 30, 529–538. [Google Scholar] [CrossRef]
- Aiello, D.; Leonardi, G.R.; Di Pietro, C.; Vitale, A.; Polizzi, G. A New Strategy to Improve Management of Citrus Mal Secco Disease Using Bioformulates Based on Bacillus amyloliquefaciens Strains. Plants 2022, 11, 446. [Google Scholar] [CrossRef]
- Fraver, D.R. Commercialization and Implementation of Biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Bělonožníková, K.; Hýsková, V.; Vašková, M.; Křížek, T.; Čokrtová, K.; Vaněk, T.; Halířová, L.; Chudý, M.; Žufić, A.; Ryšlavá, H. Seed Protection of Solanum lycopersicum with Pythium oligandrum against Alternaria brassicicola and Verticillium albo-atrum. Microorganisms 2022, 10, 1348. [Google Scholar] [CrossRef] [PubMed]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of Inducible Defense-related Proteins in Infected Plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Görlach, J.; Volrath, S.; Knauf-Beiter, G.; Hengy, G.; Beckhove, U.; Kogel, H.H.; Oostendorp, M.; Staub, T.; Ward, E.; Kessmann, H.; et al. Benzothiadiazole, a novel class of inducers of systemic acquired resistance, activates gene expression and disease resistance in wheat. Plant Cell 1996, 8, 629–643. [Google Scholar]
- Oostendorp, M.; Kunz, W.; Dietrich, B.; Staub, T. Induced Disease Resistance in Plants by Chemicals. Eur. J. Plant Pathol. 2001, 107, 19–28. [Google Scholar] [CrossRef]
- Spadaro, D.; Garibaldi, A.; Gullino, M.L. Control of Penicillium expansum and Botrytis cinerea on apple combining a biocontrol agent with hot water dipping and acibenzolar-S-methyl, baking soda, or ethanol application. Postharvest Biol. Technol. 2004, 33, 141–151. [Google Scholar] [CrossRef]
- Panebianco, S.; Vitale, A.; Platania, C.; Restuccia, C.; Polizzi, G.; Cirvilleri, G. Postharvest efficacy of resistance inducers for the control of green mold on important Sicilian citrus varieties. J. Plant. Dis. 2014, 121, 177–183. [Google Scholar] [CrossRef]
- Fragalà, F.; Castello, I.; Puglisi, I.; Padoan, E.; Baglieri, A.; Montoneri, E.; Vitale, A. New insights into municipal biowaste derived products as promoters of seed germination and potential antifungal compounds for sustainable agriculture. Chem. Biol. Technol. Agric. 2022, 9, 69. [Google Scholar] [CrossRef]
- Chen, K.; Tian, Z.; He, H.; Long, C.; Jiang, F. Bacillus species as potential biocontrol agents against citrus diseases. Biol. Control 2020, 151, 104419. [Google Scholar] [CrossRef]
- Munir, S.; Li, Y.; He, P.; Huang, M.; He, P.; He, P.; Cui, W.; Wu, Y.; He, Y. Core endophyte communities of different citrus varieties from citrus growing regions in China. Sci. Rep. 2020, 10, 3648. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, N.; Bélanger, R.R.; Rey, P.; Tirilly, Y. Oligandrin, the elicitin-like protein produced by the mycoparasite Pythium oligandrum, induces systemic resistance to Fusarium crown and root in tomato plants. Plant Physiol. Biochem. 2001, 39, 681–696. [Google Scholar] [CrossRef]
- Picard, K.; Ponchet, M.; Blein, J.P.; Rey, P.; Tirilly, Y.; Benhamou, N. Oligandrin, a proteinaceous molecule produced by the mycoparasite, Pythium oligandrum, induces resistance to Phytophthora parasitica infection in tomato plants. Plant Physiol. 2000, 124, 379–395. [Google Scholar] [CrossRef]
- McQuilken, M.P.; Whipps, J.M.; Cooke, R.C. Use of oospore formulations of Pythium oligandrum for biological control of Pythium damping-off in cress. J. Phytopathol. 1992, 135, 125–134. [Google Scholar] [CrossRef]
- Mohamed, N.; Lherminier, J.; Farmer, M.J.; Fromentin, J.; Béno, N.; Houot, V.; Milat, M.L.; Blein, J.P. Defense Responses in Grapevine Leaves Against Botrytis cinerea Induced by Application of a Pythium oligandrum Strain or Its Elicitin, Oligandrin, to Roots. Phytopathology 2007, 97, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Amira, M.B.; Lopez, D.; Mohamed, A.T.; Khouaja, A.; Chaar, H.; Fumanal, B.; Gousset-Dupont, A.; Bonhomme, L.; Label, P.; Goupil, P.; et al. Beneficial effect of Trichoderma harzianum strain Ths97 in biocontrolling Fusarium solani causal agent of root rot disease in olive trees. Biol. Control 2017, 110, 70–78. [Google Scholar] [CrossRef]
- Sanchez, A.D.; Ousset, M.J.; Sosa, M.C. Biological control of Phytophthora collar rot of pear using regional Trichoderma strains with multiple mechanisms. Biol. Control 2019, 135, 124–134. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Herrmann-Andrade, A.M.; Calabrese, C.D.; Bello, F.; Vázquez, D.; Musumeci, M.A. Effectiveness of Trichoderma strains isolated from the rhizosphere of citrus tree to control Alternaria alternata, Colletotrichum gloeosporioides and Penicillium digitatum A21 resistant to pyrimethanil in post-harvest oranges (Citrus sinensis L. (Osbeck)). J. Appl. Microbiol. 2020, 129, 712–727. [Google Scholar] [CrossRef]
- Díaz-Gutiérrez, C.; Arroyave, C.; Llugany, M.; Poschenrieder, C.; Martos, S.; Peláez, C. Trichoderma asperellum as a preventive and curative agent to control Fusarium wilt in Stevia rebaudiana, Biol. Control 2021, 155, 1049–9644. [Google Scholar]
- Leonardi, O.; Sesto, F.; Polizzi, G.; Di Silvestro, I.; Astuto, A. Antagonismo in vitro di microrganismi presenti nei terreni agrumetati nei confronti di Phoma tracheiphila. Tec. Agric. 1990, 42, 61–67. [Google Scholar]
- Gentile, A.; Deng, Z.; La Malfa, S.; Distefano, G.; Domina, F.; Vitale, A.; Polizzi, G.; Lorito, M.; Tribulato, E. Enhanced resistance to Phoma tracheiphila and Botrytis cinerea in transgenic lemon plants expressing a Trichoderma harzianum chitinase gene. Plant Breed. 2007, 126, 146–151. [Google Scholar] [CrossRef]
- Russo, M.; La Malfa, S.; Abbate, C.; Gentile, A.; Cirvilleri, G.; Catara, A. Transgenic endochitinase troyer citrange shows higher chitinase and glucanase activity and reduced colonization by fungi. J. Plant Pathol. 2009, 91, 84–86. [Google Scholar]
- Markovich, N.A.; Kononova, G.L. Lytic Enzymes of Trichoderma and Their Role in Plant Defense from Fungal Diseases: A Review. Appl. Biochem. Microbiol. 2003, 39, 341–351. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; de Masi, L.; de Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Bazghaleh, N.; Prashar, P.; Woo, S.; Vanderberg, A. Effects of lentil genotype on the colonization of beneficial Trichoderma species and biocontrol of Aphanomyces root rot. Microorganisms 2020, 8, 1290. [Google Scholar] [CrossRef]
- Ministero della Salute. Banca Dati dei Prodotti Fitosanitari. Available online: www.fitosanitari.salute.gov.it (accessed on 20 January 2023).
- Choudhary, A.K.; Singh, N.; Singh, D. Evaluation of the bioformulation of potent native strains of Trichoderma spp. against the foot rot/gummosis of Kinnow mandarin. Egypt. J. Biol. Pest Control 2021, 31, 90. [Google Scholar] [CrossRef]
- Panebianco, S.; Vitale, P.; Polizzi, G.; Scala, F.; Cirvilleri, G. Enhanced control of postharvest citrus fruit decay by means of the combined use of compatible biocontrol agents. Biol. Control 2015, 84, 19–27. [Google Scholar] [CrossRef]
- Muccilli, V.; Vitale, A.; Sheng, L.; Gentile, A.; Cardullo, N.; Tringali, C.; Oliveri, C.; La Rosa, R.; Di Guardo, M.; La Malfa, S.; et al. Substantial equivalence of a transgenic lemon fruit showing postharvest fungal pathogens resistance. J. Agric. Food Chem. 2020, 68, 3806–3816. [Google Scholar] [CrossRef] [PubMed]
- Resende, M.L.V.; Nojosa, G.B.A.; Cavalcanti, L.S.; Aguilar, M.A.G.; Silva, L.H.C.P.; Perez, J.O.; Andrade, G.C.G.; Carvalho, G.A.; Castro, R.M. Induction of resistance in cocoa against Crinipellis perniciosa and Verticillium dahliae by acibenzolar- S-methyl (ASM). Plant Pathol. 2002, 51, 621–628. [Google Scholar] [CrossRef]
- Barross, F.; Sagata, E.; César, L.; Ferreira, C.; Cezar, J. Induction of resistance in plants against phytopathogens. Biosci. J. 2010, 26, 231–239. [Google Scholar]
- Marucchini, C.; Zadra, C. Stereoselective degradation of metalaxyl and metalaxyl-M in soil and sunflower plants. Chirality 2002, 14, 32–38. [Google Scholar] [CrossRef] [PubMed]
- OEPP/EPPO Phoma tracheiphila. Bull. OEPP/EPPO Bull. 2005, 35, 307–311. [CrossRef]
- Luisi, N.; De Cicco, V.; Cutuli, G.; Salerno, M. Analisi della patogenicità di Phoma tracheiphila (Petri) Kanc. et Ghik. su alcune specie e cultivar di Agrumi. Phytopathol. Mediterr. 1979, 18, 162–165. [Google Scholar]
| Factor(s) | Disease Incidence y | Disease Severity y | |||
|---|---|---|---|---|---|
| df | F | p Value | F | p Value | |
| Treatment | 3 | 132.622 | >0.0000 | 120.403 | >0.0000 |
| Time | 1 | 13.215 | 0.001317 | 145.042 | >0.0000 |
| Treatment × time | 3 | 0.395 | 0.757782 ns | 3.903 | 0.021058 ns |
| After 14 Days | After 28 Days | |||||
|---|---|---|---|---|---|---|
| Treatments | DI (%) y | DI (va) y | SS (0-to-4) y | DI (%) y | DI (va) y | SS (0-to-4) y |
| Untreated control | 72.50 ± 3.13 a | 58.50 | 1.63 ± 0.08 a | 77.75 ± 3.43 a | 62.10 | 2.73 ± 0.13 a |
| Bacillus amyloliquefaciens strain D747 (Amylo-X® WG) | 32.25 ± 1.75 b | 34.57 | 0.65 ± 0.06 b | 39.25 ± 3.08 b | 38.75 | 1.28 ± 0.10 b |
| Bacillus amyloliquefaciens strain D747 (Amylo-X® LC) | 35.75 ± 2.31 b | 36.68 | 0.68 ± 0.10 b | 40.10 ± 1.10 b | 39.52 | 1.30 ± 0.08 b |
| Copper hydroxide (Kocide Opti®) | 19.75 ± 1.43 c | 26.34 | 0.35 ± 0.03 c | 29.00 ± 1.13 c | 32.57 | 0.95 ± 0.03 c |
| Factor(s) | Disease Incidence y | Disease Severity y | |||
|---|---|---|---|---|---|
| df | F | p Value | F | p Value | |
| Treatment | 3 | 98.599 | >0.0000 | 74.577 | >0.0000 |
| Time | 1 | 28.786 | 0.000017 | 121.467 | >0.0000 |
| Treatment × time | 3 | 0.258 | 0.854763 ns | 1.273 | 0.306233 ns |
| After 14 Days | After 28 Days | |||||
|---|---|---|---|---|---|---|
| Treatments | DI (%) y | DI (va) y | SS (0-to-4) y | DI (%) y | DI (va) y | SS (0-to-4) y |
| Untreated control | 67.50 ± 2.74 a | 55.30 | 1.60 ± 0.08 a | 74.75 ± 3.99 a | 60.04 | 2.63 ± 0.20 a |
| Bacillus amyloliquefaciens strain D747 (Amylo-X® WG) | 30.75 ± 1.53 b | 33.65 | 0.63 ± 0.07 b | 43.50 ± 1.71 b | 41.26 | 1.43 ± 0.08 b |
| Bacillus amyloliquefaciens strain D747 (Amylo-X® LC) | 30.00 ± 1.51 b | 33.19 | 0.60 ± 0.04 b | 41.25 ± 2.86 b | 39.94 | 1.38 ± 0.08 b |
| Copper hydroxide (Kocide Opti®) | 21.75 ± 2.46 c | 27.67 | 0.35 ± 0.06 c | 31.25 ± 1.90 c | 33.95 | 0.98 ± 0.06 c |
| Factor(s) | Disease Incidence y | Disease Severity y | |||
|---|---|---|---|---|---|
| df | F | p Value | F | p Value | |
| Treatment | 3 | 58.254 | >0.0000 | 59.9153 | >0.0000 |
| Time | 1 | 10.275 | 0.003790 | 73.4746 | >0.0000 |
| Treatment × time | 3 | 0.619 | 0.60933 ns | 1.5559 | 0.225941 ns |
| After 14 Days | After 28 Days | |||||
|---|---|---|---|---|---|---|
| Treatments | DI (%) y | DI (va) y | SS (0-to-4) y | DI (%) y | DI (va) y | SS (0-to-4) y |
| Untreated control | 51.75 ± 2.34 a | 46.01 | 1.18 ± 0.06 a | 55.25 ± 2.57 a | 48.03 | 1.93 ± 0.07 a |
| Pythium oligandrum M1 (Polyversum®) | 30.25 ± 3.53 b | 33.21 | 0.75 ± 0.15 b | 39.50 ± 2.84 b | 38.90 | 1.28 ± 0.10 b |
| Trichoderma asperellum ICC 012 + T. gamsii ICC 080 (Remedier®) | 20.25 ± 2.15 c | 26.64 | 0.40 ± 0.04 c | 28.25 ± 2.66 c | 32.02 | 0.85 ± 0.08 c |
| Copper hydroxide (Kocide Opti®) | 17.75 ± 2.12 c | 24.79 | 0.28 ± 0.04 c | 21.00 ± 0.76 c | 27.26 | 0.68 ± 0.02 c |
| 14 Days | 28 Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor(s) | DI y | SS y | DI y | SS y | |||||
| df | F | p Value | F | p Value | F | p Value | F | p Value | |
| Treatment | 5 | 28.791 | >0.0000 | 27.428 | >0.0000 | 13.919 | >0.0000 | 17.114 | >0.0000 |
| Trial | 1 | 93.793 | >0.0000 | 154.793 | >0.0000 | 14.124 | >0.0000 | 56.860 | >0.0000 |
| Trt × trial | 5 | 4.003 | 0.0088 | 6.903 | 0.0004 | 0.912 | 0.4896 | 1.974 | 0.1191 |
| 14 Days | 28 Days | |||||
|---|---|---|---|---|---|---|
| Treatments | DI (%) y | DI (va) y | SS (0-to-4) y | DI (%) y | DI (va) y | SS (0-to-4) y |
| Untreated control | 64.0 ± 1.2 a | 53.1 | 1.3 ± 0.06 a | 69.0 ± 4.5 a | 56.3 | 2.0 ± 0.1 a |
| Acibenzolar-S-methyl + metalaxyl-M (rate 1) | 41.3 ± 4.2 b | 40.0 | 0.8 ± 0.09 b | 46.3 ± 4.4 b | 42.9 | 1.3 ± 0.1 b |
| Acibenzolar-S-methyl + metalaxyl-M (rate 2) | 31.3 ± 4.2 b | 33.9 | 0.5 ± 0.03 b | 44.3 ± 5.7 b | 41.7 | 1.1 ± 0.1 b |
| Acibenzolar-S-methyl + metalaxyl-M (rate 3) | 35.0 ± 1.0 b | 36.3 | 0.6 ± 0.03 b | 41.3 ± 1.4 b | 40.0 | 1.1 ± 0.1 b |
| Acibenzolar-S-methyl | 35.7 ± 1.7 b | 36.7 | 0.6 ± 0.09 b | 46.3 ± 4.4 b | 42.9 | 1.2 ± 0.2 b |
| Bacillus amyloliquefaciens strain FZB24 | 37.0 ± 1.0 b | 37.5 | 0.6 ± 0.03 b | 39.7 ± 1.7 b | 39.0 | 1.2 ± 0.1 b |
| 14 Days | 28 Days | |||||
|---|---|---|---|---|---|---|
| Treatments | DI (%) y | DI (va) y | SS (0-to-4) y | DI (%) y | DI (va) y | SS (0-to-4) y |
| Untreated control | 40.7 ± 1.4 a | 39.6 | 0.6 ± 0.05 a | 56.7 ± 2.3 a | 48.8 | 1.4 ± 0.09 a |
| Acibenzolar-S-methyl + metalaxyl-M (rate 1) | 21.7 ± 1.8 b | 27.7 | 0.3 ± 0.03 b | 34.7 ± 2.6 b | 36.0 | 0.6 ± 0.09 b |
| Acibenzolar-S-methyl + metalaxyl-M (rate 2) | 24.3 ± 2.0 b | 29.5 | 0.3 ± 0.03 b | 45.3 ± 1.7 b | 42.3 | 1.0 ± 0.06 b |
| Acibenzolar-S-methyl + metalaxyl-M (rate 3) | 22.7 ± 1.7 b | 28.4 | 0.3 ± 0.00 b | 32.0 ± 5.9 b | 34.2 | 0.7 ± 0.1 b |
| Acibenzolar-S-methyl | 29.0 ± 1.1 b | 32.6 | 0.4 ± 0.03 b | 37.0 ± 3.2 b | 37.4 | 0.7 ± 0.1 b |
| Bacillus amyloliquefaciens strain FZB24 | 27.7 ± 2.9 b | 31.7 | 0.4 ± 0.06 b | 33.7 ± 1.4 b | 35.5 | 0.9 ± 0.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, G.R.; Polizzi, G.; Vitale, A.; Aiello, D. Efficacy of Biological Control Agents and Resistance Inducer for Control of Mal Secco Disease. Plants 2023, 12, 1735. https://doi.org/10.3390/plants12091735
Leonardi GR, Polizzi G, Vitale A, Aiello D. Efficacy of Biological Control Agents and Resistance Inducer for Control of Mal Secco Disease. Plants. 2023; 12(9):1735. https://doi.org/10.3390/plants12091735
Chicago/Turabian StyleLeonardi, Giuseppa Rosaria, Giancarlo Polizzi, Alessandro Vitale, and Dalia Aiello. 2023. "Efficacy of Biological Control Agents and Resistance Inducer for Control of Mal Secco Disease" Plants 12, no. 9: 1735. https://doi.org/10.3390/plants12091735
APA StyleLeonardi, G. R., Polizzi, G., Vitale, A., & Aiello, D. (2023). Efficacy of Biological Control Agents and Resistance Inducer for Control of Mal Secco Disease. Plants, 12(9), 1735. https://doi.org/10.3390/plants12091735







