Assessing the Genetic Diversity of Daylily Germplasm Using SSR Markers: Implications for Daylily Breeding
Abstract
:1. Introduction
2. Results
2.1. SSR Primer Informativeness
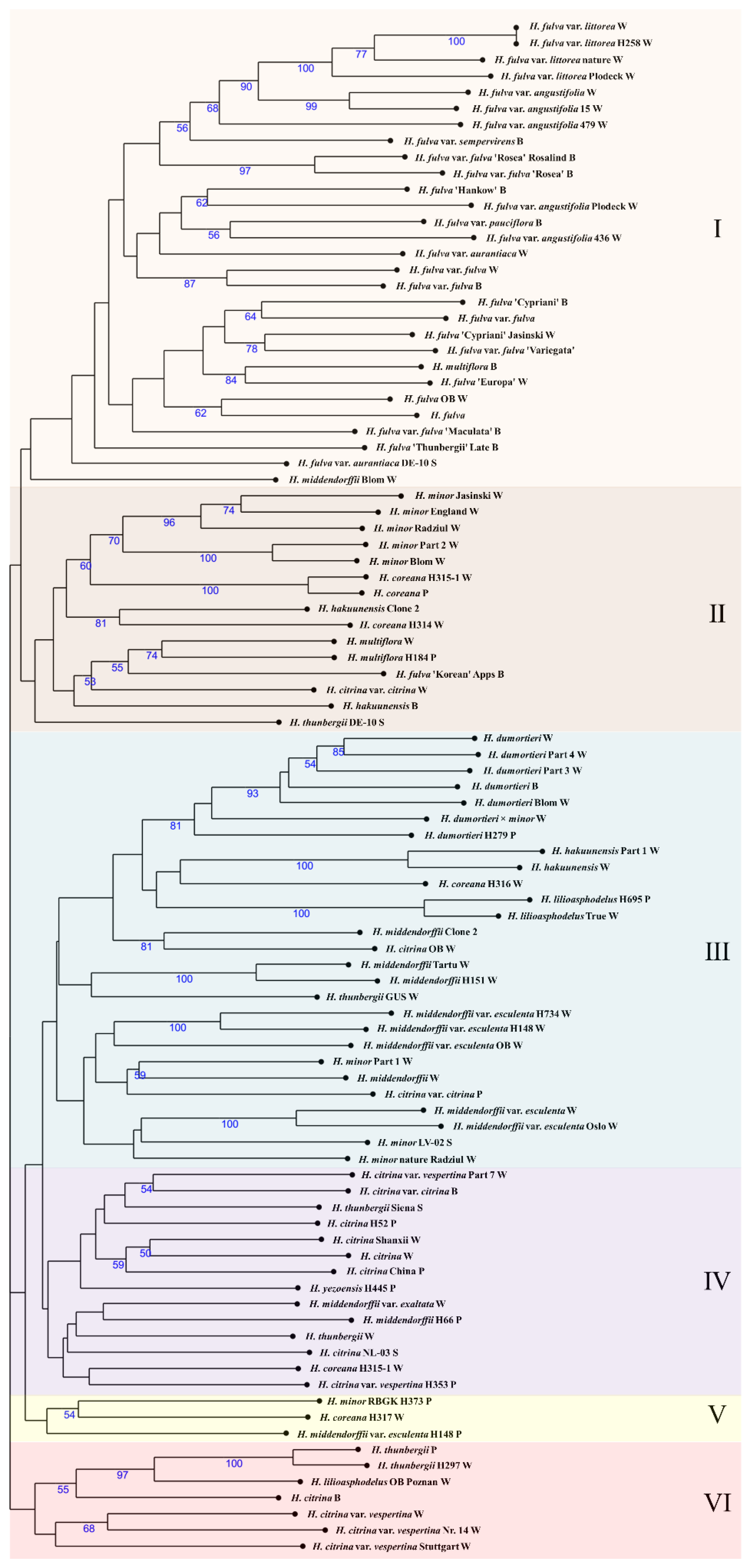
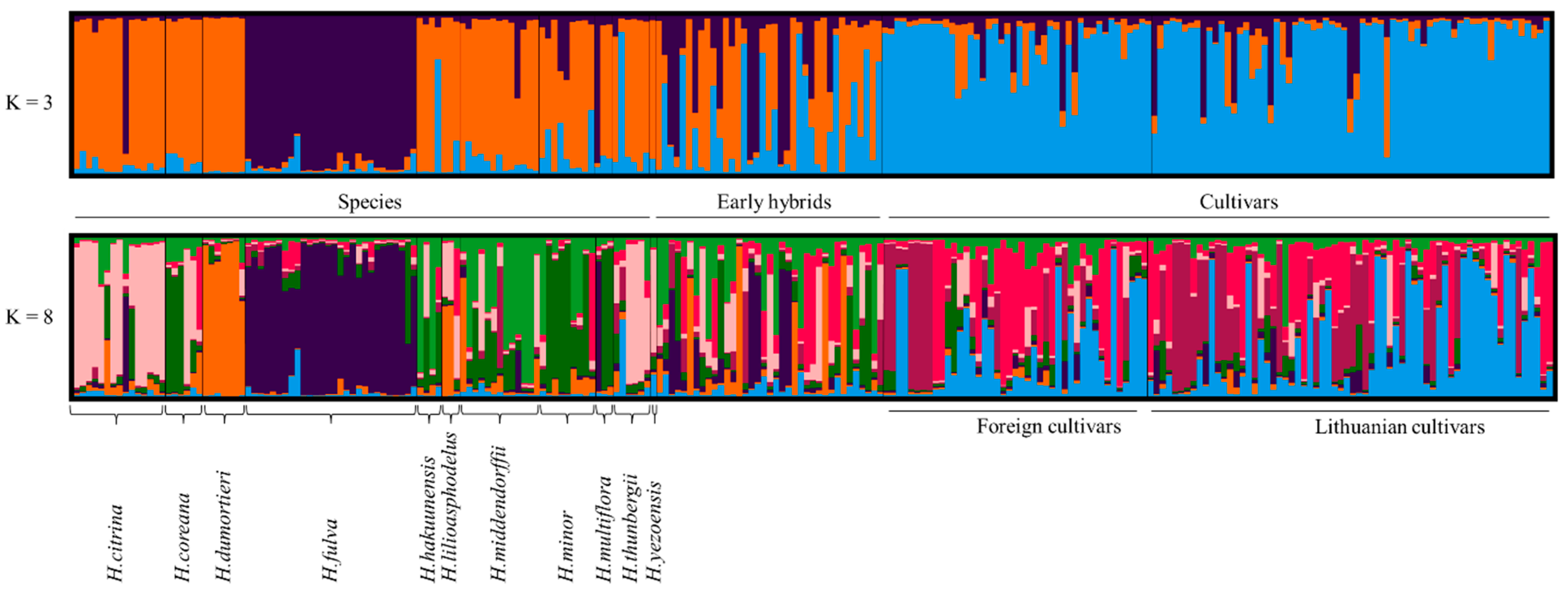
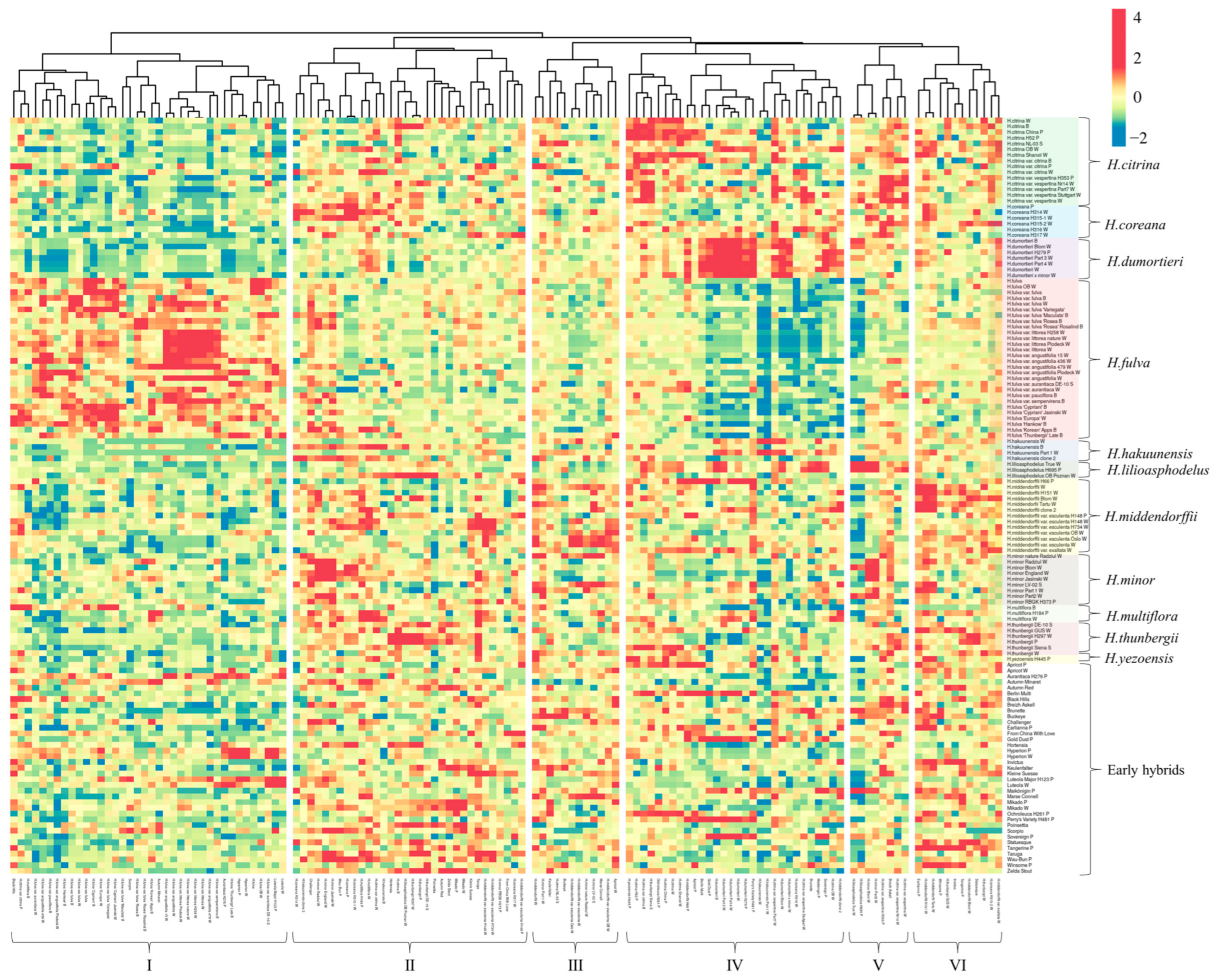
2.2. Genetic Relationships and Diversity of Daylily
2.3. Putative Origin of Early Hybrids
2.3.1. Diversity of Daylily Species in Early Hybrids
2.3.2. H. fulva Clones in Early Daylily Breeding Programs
2.3.3. Triploid H. fulva as Putative Origin to Both Diploid and Tetraploid Modern Daylily Cultivars
3. Discussion
3.1. Population Structure, Genetic Diversity and Relationships of Daylilies
3.2. Daylily Species Classification and Implication for Modern Breeding Programs
3.3. Ploidy in Breeding Programs
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Gao, Y.; Gao, S.; Yuan, L.; Wang, X.; Zhang, Q. Genetic characteristics of circadian flowering rhythm in Hemerocallis. Sci. Hortic. 2019, 250, 19–26. [Google Scholar] [CrossRef]
- Rodriguez-Enriquez, M.J.; Grant-Downton, R.T. A new day dawning: Hemerocallis (daylily) as a future model organism. AoB Plants 2013, 5, pls055. [Google Scholar] [CrossRef] [PubMed]
- Valder, P. The Garden Plants of China; Timber Press: Portland, OR, USA, 1999. [Google Scholar]
- Mlček, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Hao, Z.; Liang, L.; Liu, H.; Yan, Y.; Zhang, Y. Exploring the Extraction Methods of Phenolic Compounds in Daylily (Hemerocallis citrina Baroni) and Its Antioxidant Activity. Molecules 2022, 27, 2964. [Google Scholar] [CrossRef]
- Ma, T.; Sun, Y.; Wang, L.; Wang, J.; Wu, B.; Yan, T.; Jia, Y. An Investigation of the Anti-Depressive Properties of Phenylpropanoids and Flavonoids in Hemerocallis citrina Baroni. Molecules 2022, 27, 5809. [Google Scholar] [CrossRef] [PubMed]
- Manole, S. Proprietăţile biologice a speciilor şi soiurilor din genul Hemerocallis introduse în Republica Moldova. In Horticultură, Viticultură şi vinificaţie, Silvicultură şi grădini publice, Protecţia plantelor. Hortic. Mod. Realiz. Şi Perspect. 2018, 47, 478–482. [Google Scholar]
- Chung, M.G.; Kang, S.S. Morphometric analysis of the genus Hemerocallis L.(Liliaceae) in Korea. J. Plant Res. 1994, 107, 165–175. [Google Scholar] [CrossRef]
- Stout, A.B. Hemerocallis citrina. Addisonia 1930, 15, 3–4. [Google Scholar]
- Hu, S.Y. The species of Hemerocallis. Am. Hortic. Mag. 1968, 47, 86. [Google Scholar]
- Tomkins, J.; Wood, T.; Barnes, L.; Westman, A.; Wing, R.A. Evaluation of genetic variation in daylily (Hemerocallis spp.) using AFLP markers. Theor. Appl. Genet. 2001, 102, 489–496. [Google Scholar] [CrossRef]
- Stout, A.B. Sterility and fertility in species of Hemerocallis. Torreya 1921, 21, 57–62. [Google Scholar]
- Sochor, M.; Jemelková, M.; Doležalová, I. Phenotyping and SSR markers as a tool for identification of duplicates in lettuce germplasm. Czech J. Genet. Plant Breed. 2019, 55, 110–119. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Update of the Xylella spp. host plant database. EFSA J. Eur. Food Saf. Auth. 2018, 16, e05408. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, L.; Bi, X.; Lei, J. Compatibility of interspecific hybridization between Hemerocallis liloasphodelus and daylily cultivars. Sci. Hortic. 2017, 220, 267–274. [Google Scholar] [CrossRef]
- Swarup, S.; Cargill, E.J.; Crosby, K.; Flagel, L.; Kniskern, J.; Glenn, K.C. Genetic diversity is indispensable for plant breeding to improve crops. Crop Sci. 2021, 61, 839–852. [Google Scholar] [CrossRef]
- Mascher, M.; Schreiber, M.; Scholz, U.; Graner, A.; Reif, J.C.; Stein, N. Genebank genomics bridges the gap between the conservation of crop diversity and plant breeding. Nat. Genet. 2019, 51, 1076–1081. [Google Scholar] [CrossRef]
- Zhu, S.; Niu, E.; Shi, A.; Mou, B. Genetic diversity analysis of olive germplasm (Olea europaea L.) with genotyping-by-sequencing technology. Front. Genet. 2019, 10, 755. [Google Scholar] [CrossRef]
- Delfini, J.; Moda-Cirino, V.; dos Santos Neto, J.; Ruas, P.M.; Sant’Ana, G.C.; Gepts, P.; Gonçalves, L.S.A. Population structure, genetic diversity and genomic selection signatures among a Brazilian common bean germplasm. Sci. Rep. 2021, 11, 2964. [Google Scholar] [CrossRef] [PubMed]
- Mažeikienė, I.; Šikšnianienė, J.B.; Baniulis, D.; Gelvonauskienė, D.; Frercks, B.; Starkus, A.; Žebrauskienė, A.; Stanys, V. SSR analysis based on molecular characterisation of apple germplasm in Lithuania. Zemdirbyste-Agriculture 2019, 106, 159–166. [Google Scholar] [CrossRef]
- Chen, C.; Okie, W.R. Genetic relationship and parentages of historical peaches revealed by microsatellite markers. Tree Genet. Genomes 2021, 17, 35. [Google Scholar] [CrossRef]
- Emami-Tabatabaei, S.S.; Small, E.; Assadi, M.; Dehshiri, M.M.; Mehregan, I. Genetic variation among Iranian Medicago polymorpha L. populations based on SSR markers. Genet. Resour. Crop Evol. 2021, 68, 1411–1424. [Google Scholar] [CrossRef]
- Hamm, T.P.; Boggess, S.L.; Kandel, J.S.; Staton, M.E.; Huff, M.L.; Hadziabdic, D.; Shoemaker, D.; Adamczyk, J.J., Jr.; Nowicki, M.; Trigiano, R.N. Development and Characterization of 20 Genomic SSR Markers for Ornamental Cultivars of Weigela. Plants 2022, 11, 1444. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, Y.; Zhu, G.; Xu, Y.; Tan, J.; Liu, J. Diversity, classification, and EST-SSR-based association analysis of Caladium ornamental traits. Physiol. Plant. 2023, 175, e13841. [Google Scholar] [CrossRef]
- Song, L.; Wang, R.; Yang, X.; Zhang, A.; Liu, D. Molecular Markers and Their Applications in Marker-Assisted Selection (MAS) in Bread Wheat (Triticum aestivum L.). Agriculture 2023, 13, 642. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Poczai, P.; Etminan, A.; Jadidi, O.; Kianersi, F.; Shooshtari, L. An analysis of genetic variability and population structure in wheat germplasm using microsatellite and gene-based markers. Plants 2022, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Ben Romdhane, M.; Riahi, L.; Yazidi, R.; Mliki, A.; Zoghlami, N. Cross transferability of barley nuclear SSRs to pearl millet genome provides new molecular tools for genetic analyses and marker assisted selection. Open Agric. 2022, 7, 668–678. [Google Scholar] [CrossRef]
- Masuda, K.; Setoguchi, H.; Nagasawa, K.; Ishihara, M.; Sakaguchi, S. Development and Characterization of EST-SSR Markers for Amur Daylily, Hemerocallis middendorffii Trautv. & CA Mey.(Asphodelaceae). Acta Phytotaxon. Geobot. 2021, 72, 73–78. [Google Scholar] [CrossRef]
- Masuda, K.; Setoguchi, H.; Nagasawa, K.; Ishihara, M.I.; Sawa, K.; Horie, K.; Tsuboi, H.; Fukumoto, S.; Tango, T.; Sakaguchi, S. Rear-edge daylily populations show legacies of habitat fragmentation due to the Holocene climate warming. J. Biogeogr. 2023, 50, 551–563. [Google Scholar] [CrossRef]
- Li, S.; Ji, F.; Hou, F.; Cui, H.; Shi, Q.; Xing, G.; Weng, Y.; Kang, X. Characterization of Hemerocallis citrina transcriptome and development of EST-SSR markers for evaluation of genetic diversity and population structure of Hemerocallis collection. Front. Plant Sci. 2020, 11, 686. [Google Scholar] [CrossRef]
- Cao, D.M.; Zhang, C.; Zhang, X.C.; Kang, L.F.; Duan, J.J.; Ma, X.L.; Yan, G.J.; Wang, Y.S. Genetic diversity of wild daylily in Taihang Mountain areas based on ISSR markers. Acta Hortic. 2013, 977, 299–306. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, Y.K.; Liu, J.J.; Fu, M.; Zhang, Q.X. Investigation on resources of Hemerocallis in North China. Acta Hortic. 2017, 1185, 65–72. [Google Scholar] [CrossRef]
- Stanys, V.; Frercks, B.; Šikšnianienė, B.; Stepulaitienė, I.; Gelvonauskienė, D.; Stanienė, G.; Bobinas, C. Identification of sweet cherry (Prunus avium L.) cultivars using AFLP and SSR markers. Zemdirbyste-Agriculture 2012, 99, 437–444. [Google Scholar]
- Fu, Y.B. Understanding crop genetic diversity under modern plant breeding. Theor. Appl. Genet. 2015, 128, 2131–2142. [Google Scholar] [CrossRef]
- Gavin-Smyth, N.; Kramer, A.T.; Urbina-Casanova, R.; Vitt, P.; Fant, J.B. Genetic rescue reduces mate limitation in a threatened, clonal, and self-incompatible plant species. Restor. Ecol. 2021, 29, e13458. [Google Scholar] [CrossRef]
- Islam, M.R.; Zhang, Y.; Li, Z.Z.; Liu, H.; Chen, J.M.; Yang, X.Y. Genetic diversity, population structure, and historical gene flow of Nelumbo lutea in USA using microsatellite markers. Aquat. Bot. 2020, 160, 103162. [Google Scholar] [CrossRef]
- Gouda, A.C.; Warburton, M.L.; Djedatin, G.L.; Kpeki, S.B.; Wambugu, P.W.; Gnikoua, K.; Ndjiondjop, M.N. Development and validation of diagnostic SNP markers for quality control genotyping in a collection of four rice (Oryza) species. Sci. Rep. 2021, 11, 18617. [Google Scholar] [CrossRef]
- Lv, Y.; Gao, Y.; Cui, Y.; Wang, Y.; Fan, Z.; Guan, C.; Zhang, Q. Interfamily wide hybridization between daylily (Hemerocallis, Xanthorrhoeaceae) and lycoris (Lycoris, Amaryllidaceae). Plant Breed. 2022, 141, 820–827. [Google Scholar] [CrossRef]
- Polezhaeva, M.A.; Iunusova, D.R.; Tikhonova, N.A.; Polezhaev, A.N.; Koldaeva, M.N. Genetic Identification of Closely Related Endangered Rhododendron Species from East Asia. Russ. J. Genet. 2022, 58, 116–121. [Google Scholar] [CrossRef]
- Mohammed, I.; Rehman, S.I.; Mir, A.A.; Siddique, M.; Dar, M.S.; Shah, M.D.; Massodi, N.H.; Padder, B.A. Population genetics of Narcissus species reveals high diversity and multiple introductions into Kashmir. Agric. Res. 2020, 9, 536–542. [Google Scholar] [CrossRef]
- Hinge, V.R.; Shaikh, I.M.; Chavhan, R.L.; Deshmukh, A.S.; Shelake, R.M.; Ghuge, S.A.; Dethe, A.M.; Suprasanna, P.; Kadam, U.S. Assessment of genetic diversity and volatile content of commercially grown banana (Musa spp.) cultivars. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Miyake, T.; Yahara, T. Isolation of polymorphic microsatellite loci in Hemerocallis fulva and Hemerocallis citrina (Hemerocallidaceae). Mol. Ecol. Notes 2006, 6, 909–911. [Google Scholar] [CrossRef]
- Upadhyay, A.; Kadam, U.S.; Chacko, P.M.; Aher, L.; Karibasappa, G.S. Microsatellite analysis to differentiate clones of Thompson seedless grapevine. Indian J. Hortic. 2010, 67, 260–263. [Google Scholar]
- Wakui, K.; Mari, K.; Ran, M.; Kenji, K.; Junzo, F. RAPD Analysis to Evaluate the Genetic Variation and Relationships in Japanese Hemerocallis spp. J. Agric. Sci. Tokyo Univ. Agric. 2013, 57, 293–299. Available online: https://agriknowledge.affrc.go.jp/RN/2010851521.pdf (accessed on 10 January 2023).
- Upadhyay, A.; Kadam, U.S.; Chacko, P.; Karibasappa, G.S. Microsatellite and RAPD analysis of grape (Vitis spp.) accessions and identification of duplicates/misnomers in germplasm collection. Indian J. Hortic. 2010, 67, 8–15. [Google Scholar]
- Erhardt, W. Hemerocallis: Daylilies; Timber Press Inc.: Portland, OR, USA, 1992; pp. 31–35. [Google Scholar]
- Choi, J.S.; Huh, H.W.; Lee, S.A.; Huh, M.K. Genetic and phylogenetic relationships of genus Hemerocallis in Korea using ISSR. J. Life Sci. 2008, 18, 753–758. [Google Scholar] [CrossRef]
- Chung, M.G.; Noguchi, J. Geographic spatial autocorrelation of morphological characters of the Hemerocallis middendorffii complex (Liliaceae). Ann. Bot. Fenn. Finn. Zool. Bot. Publ. Board 1998, 35, 183–189. Available online: https://www.jstor.org/stable/23726560 (accessed on 19 February 2023).
- Hirota, S.K.; Yasumoto, A.A.; Nitta, K.; Tagane, M.; Miki, N.; Suyama, Y.; Yahara, T. Evolutionary history of Hemerocallis in Japan inferred from chloroplast and nuclear phylogenies and levels of interspecific gene flow. Mol. Phylogenetics Evol. 2021, 164, 107264. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yahara, T.; Yasumoto, A.; Hotta, M. Bimodal distribution of flowering time in a natural hybrid population of daylily (Hemerocallis fulva) and nightlily (Hemerocallis citrina). J. Plant Res. 2006, 119, 63–68. [Google Scholar] [CrossRef]
- Nitta, K.; Yasumoto, A.A.; Yahara, T. Variation of flower opening and closing times in F1 and F2 hybrids of daylily (Hemerocallis fulva; Hemerocallidaceae) and nightlily (H. citrina). Am. J. Bot. 2010, 97, 261–267. [Google Scholar] [CrossRef]
- Ramos, M.; Carvalho, R.; Soares da Silva, E.; Ramos, A.P.; Talhinhas, P. Pathological and Epidemiological Characterization of First Outbreak of Daylily Rust in Europe and Evaluation of Puccinia hemerocallidis Resistance in Hemerocallis Cultivars. Plants 2020, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Misiukevičius, E.; Stanys, V. Induction and analysis of polyploids in daylily (Hemerocallis L.) plants. Zemdirbyste-Agriculture 2022, 109, 373–382. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, D.; Kang, L.; Duan, J.; Ma, X.; Yan, G.; Wang, Y. Ploidy variation and karyotype analysis in Hemerocallis spp. (Xanthorrhoeaceae) and implications on daylily breeding, N. Z. J. Crop Hortic. Sci. 2014, 42, 183–193. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, D.M.; Zhang, X.C.; Kang, L.F.; Duan, J.J.; Ma, X.L.; Yan, G.J.; Wang, Y.S. Ploidy variation in Hemerocallis spp. and the implications on daylily breeding. Acta Hortic. 2013, 977, 197–203. [Google Scholar] [CrossRef]
- Gao, Y.; Qi, S. A study on the compatibility between different ploidy cultivars of Hemerocallis and the genetic relationships among their hybrids. Acta Hortic. 2012, 937, 547–554. [Google Scholar] [CrossRef]
- Stanys, V.; Baniulis, D.; Morkūnaitė-Haimi, Š.; Šikšnianienė, J.B.; Frercks, B.; Gelvonauskienė, D.; Stepulaitienė, I.; Stanienė, G.; Šikšnianas, T. Characterising the genetic diversity of Lithuanian sweet cherry (Prunus avium L.) cultivars using SSR markers. Sci. Hortic. 2012, 142, 136–142. [Google Scholar] [CrossRef]
- Roldán-Ruiz, I.; Dendauw, J.; Van Bockstaele, E.; Depicker, A.; De Loose, M.A.F.L.P. AFLP markers reveal high polymorphic rates in ryegrasses (Lolium spp.). Mol. Breed. 2000, 6, 125–134. [Google Scholar] [CrossRef]
- XLSTAT. Statistical software for Excel. 2007. Available online: https://www.xlstat.com (accessed on 19 February 2023).
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. 2006. Available online: https://darwin.cirad.fr/ (accessed on 19 February 2023).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A program for identifying clustering modes and packaging population structure inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. Clustvis: A Web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
| Locus | No. of Alleles | Observed Size Range, bp | H0 a | PIC b |
|---|---|---|---|---|
| SAU00006 | 14 | 225–255 | 0.53 | 0.17 |
| SAU00029 | 16 | 225–266 | 0.43 | 0.14 |
| SAU00042 | 24 | 182–219 | 0.67 | 0.13 |
| SAU00052 | 11 | 93–118 | 0.66 | 0.16 |
| SAU00096 | 25 | 161–223 | 0.78 | 0.13 |
| SAU00097 | 26 | 94–130 | 0.95 | 0.17 |
| SAU00150 | 19 | 272–299 | 0.69 | 0.14 |
| SAU00176 | 20 | 215–268 | 0.56 | 0.13 |
| SAU00008 | n.a. c | n.a. | n.a. | n.a. |
| SAU00048 | n.a. | n.a. | n.a. | n.a. |
| Mean | 19 | _ | 0.66 | 0.15 |
| Locus | H0 | PIC | H0 | PIC | H0 | PIC | H0 | PIC |
|---|---|---|---|---|---|---|---|---|
| Species | Early hybrids | Foreign cultivars | Lithuanian cultivars | |||||
| Investigated genotypes | 95 | 37 | 44 | 65 | ||||
| SAU00006 | 0.495 | 0.225 | 0.703 | 0.227 | 0.523 | 0.238 | 0.439 | 0.221 |
| SAU00029 | 0.453 | 0.175 | 0.487 | 0.252 | 0.341 | 0.212 | 0.386 | 0.167 |
| SAU00042 | 0.463 | 0.114 | 0.838 | 0.170 | 0.796 | 0.214 | 0.807 | 0.224 |
| SAU00052 | 0.568 | 0.167 | 0.757 | 0.241 | 0.727 | 0.232 | 0.649 | 0.198 |
| SAU00096 | 0.779 | 0.144 | 0.649 | 0.175 | 0.796 | 0.189 | 0.825 | 0.186 |
| SAU00097 | 0.916 | 0.163 | 1.000 | 0.187 | 0.977 | 0.217 | 0.947 | 0.211 |
| SAU00150 | 0.537 | 0.135 | 0.730 | 0.163 | 0.750 | 0.201 | 0.842 | 0.188 |
| SAU00176 | 0.453 | 0.146 | 0.432 | 0.177 | 0.523 | 0.218 | 0.807 | 0.222 |
| Mean | 0.583 | 0.159 | 0.700 | 0.199 | 0.679 | 0.215 | 0.713 | 0.202 |
| Source of Variation | df | SS | MS | Est. Var. | % |
|---|---|---|---|---|---|
| 10 species: H. citrina, H. coreana, H. dumortieri, H. fulva, H. hakuunensis, H. lilioasphodelus, H. middendorffii, H. minor, H. multiflora and H. thunbergii | |||||
| Among species | 9 | 228.245 | 25.361 | 1.885 | 18% |
| Within populations and species | 84 | 741.372 | 8.826 | 8.826 | 82% |
| Among individuals | 93 | 969.617 | 10.711 | 100% | |
| Four groups, combining species, early hybrids, foreign and Lithuanian cultivars | |||||
| Among groups | 3 | 125.613 | 41.871 | 0.540 | 5% |
| Within groups | 237 | 2571.764 | 10.851 | 10.851 | 95% |
| Among individuals | 240 | 2697.378 | 11.391 | 100% | |
| Two groups, combining cultivars of Lithuanian and foreign cultivars | |||||
| Among groups | 1 | 16.722 | 16.722 | 0.105 | 1% |
| Within groups | 107 | 1198.104 | 11.197 | 11.197 | 99% |
| Among individuals | 108 | 1214.826 | 11.303 | 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misiukevičius, E.; Frercks, B.; Šikšnianienė, J.B.; Kącki, Z.; Gębala, M.; Akulytė, P.; Trilikauskaitė, E.; Stanys, V. Assessing the Genetic Diversity of Daylily Germplasm Using SSR Markers: Implications for Daylily Breeding. Plants 2023, 12, 1752. https://doi.org/10.3390/plants12091752
Misiukevičius E, Frercks B, Šikšnianienė JB, Kącki Z, Gębala M, Akulytė P, Trilikauskaitė E, Stanys V. Assessing the Genetic Diversity of Daylily Germplasm Using SSR Markers: Implications for Daylily Breeding. Plants. 2023; 12(9):1752. https://doi.org/10.3390/plants12091752
Chicago/Turabian StyleMisiukevičius, Edvinas, Birutė Frercks, Jūratė Bronė Šikšnianienė, Zygmunt Kącki, Małgorzata Gębala, Paulina Akulytė, Emilija Trilikauskaitė, and Vidmantas Stanys. 2023. "Assessing the Genetic Diversity of Daylily Germplasm Using SSR Markers: Implications for Daylily Breeding" Plants 12, no. 9: 1752. https://doi.org/10.3390/plants12091752
APA StyleMisiukevičius, E., Frercks, B., Šikšnianienė, J. B., Kącki, Z., Gębala, M., Akulytė, P., Trilikauskaitė, E., & Stanys, V. (2023). Assessing the Genetic Diversity of Daylily Germplasm Using SSR Markers: Implications for Daylily Breeding. Plants, 12(9), 1752. https://doi.org/10.3390/plants12091752






