Identification of Cumin (Cuminum cyminum) MicroRNAs through Deep Sequencing and Their Impact on Plant Secondary Metabolism
Abstract
:1. Introduction
2. Results
2.1. Sequence Analysis of C. cyminum Small RNAs
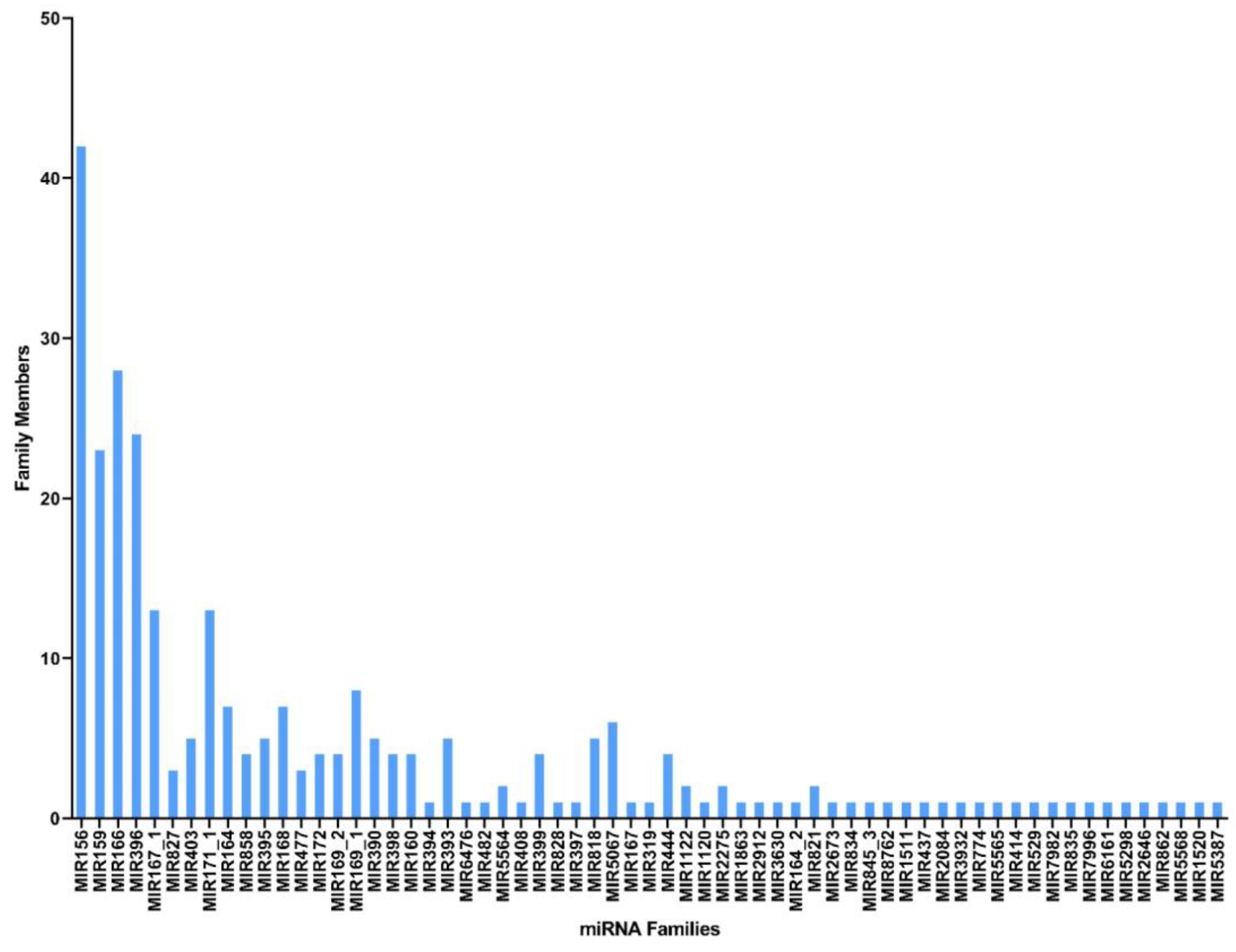
2.2. Identification of Conserved and Novel miRNAs in C. cyminum
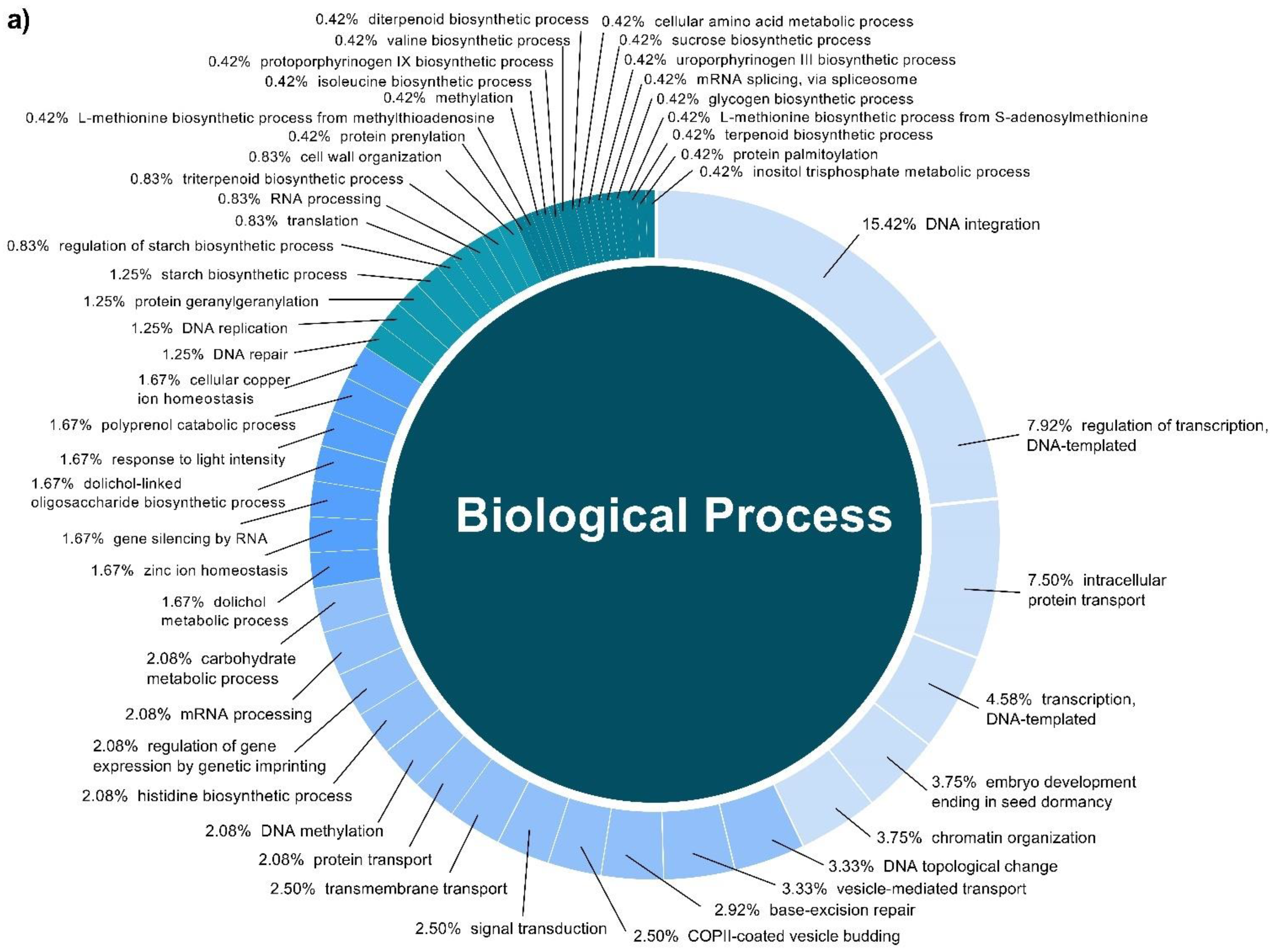
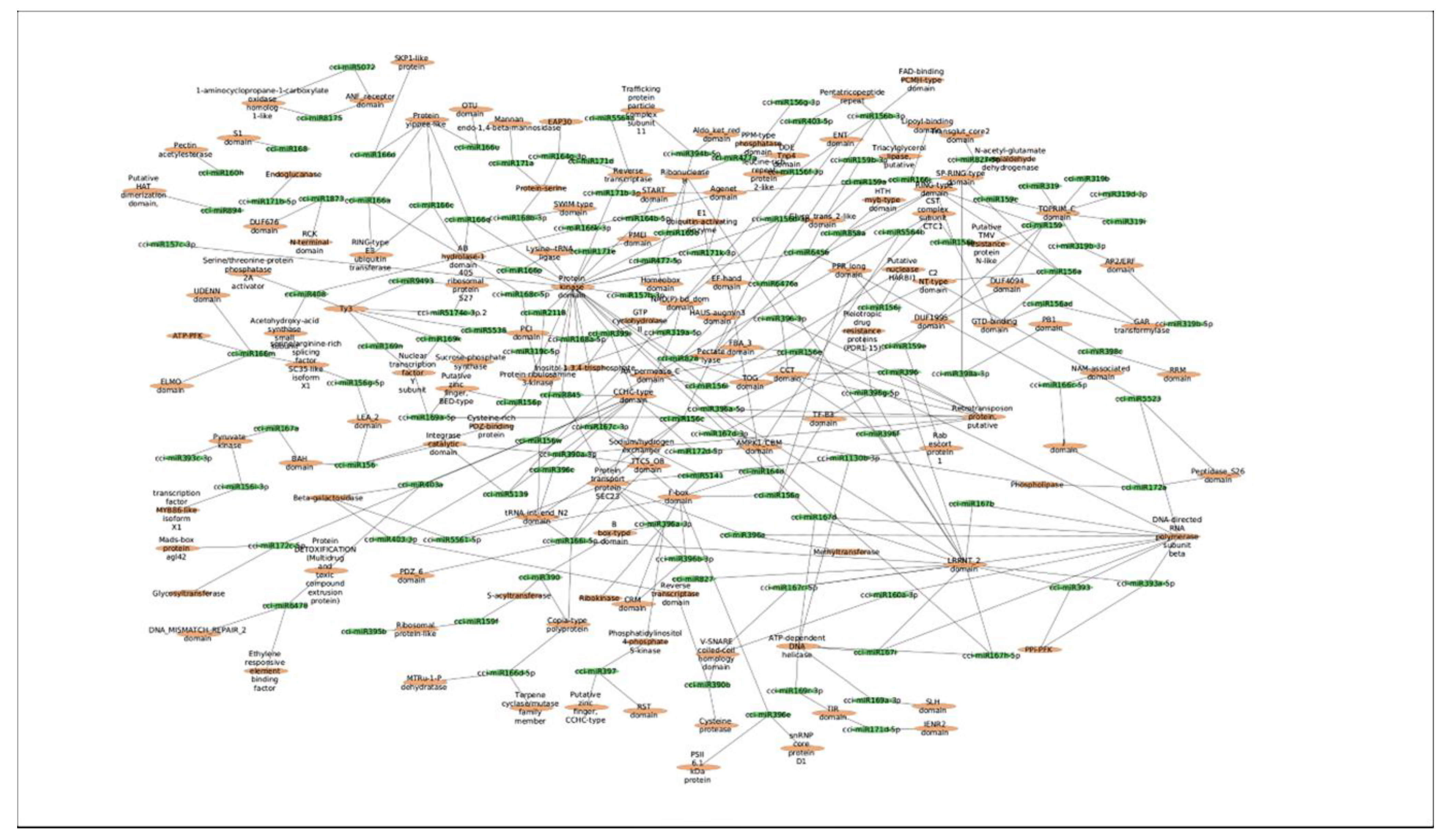
2.3. Target Prediction of Conserved and Novel C. cyminum miRNAs and Their Functional Analysis
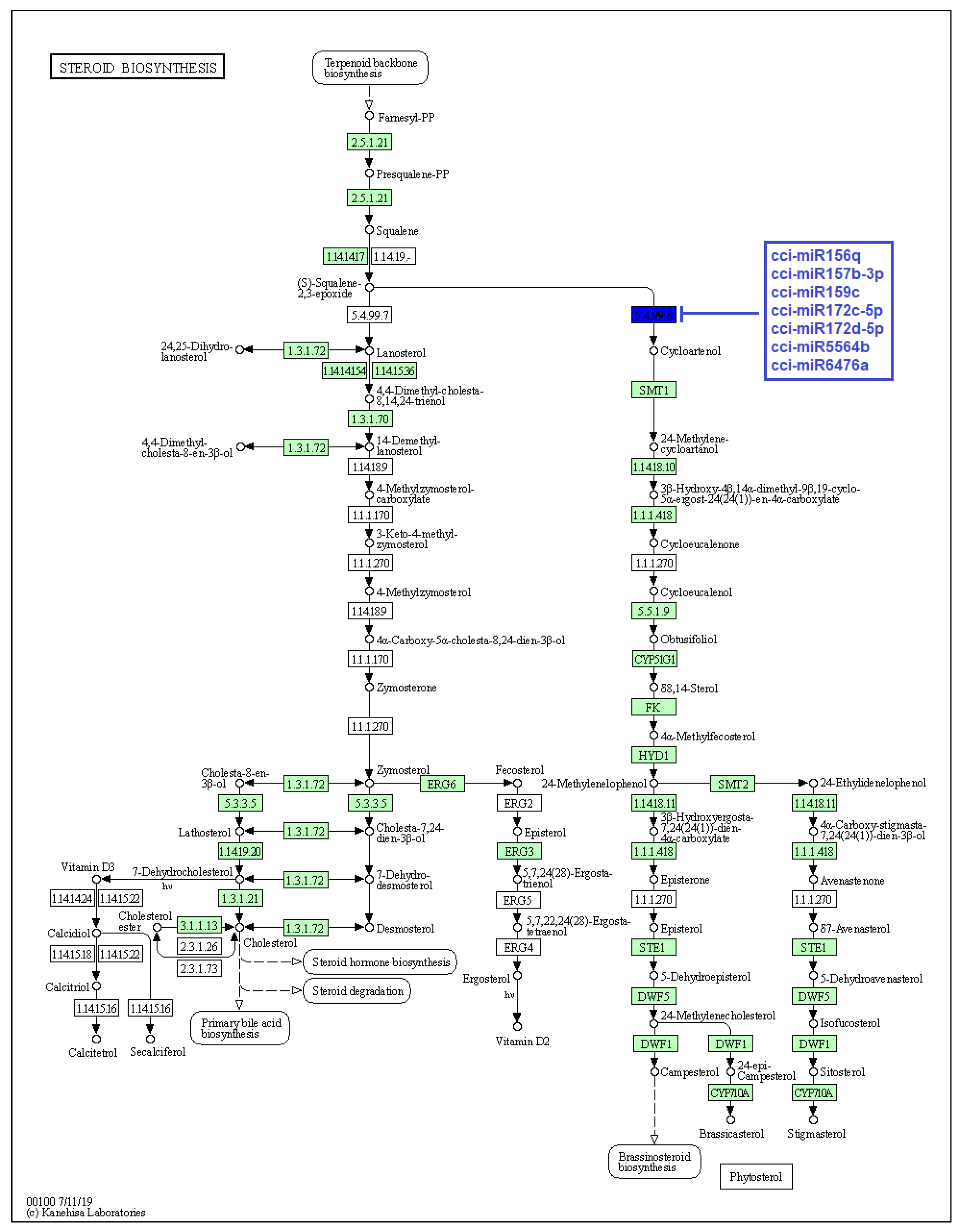
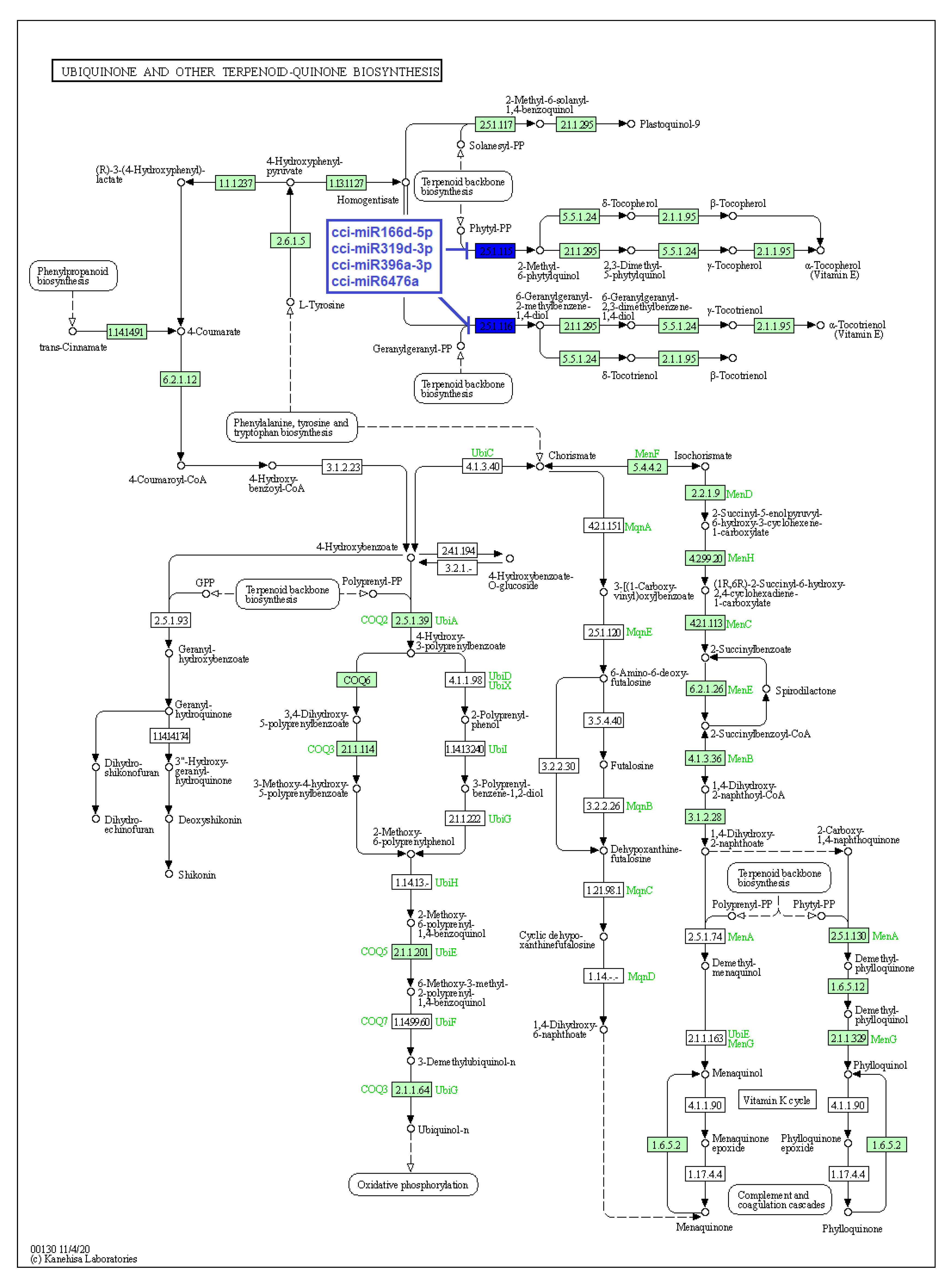
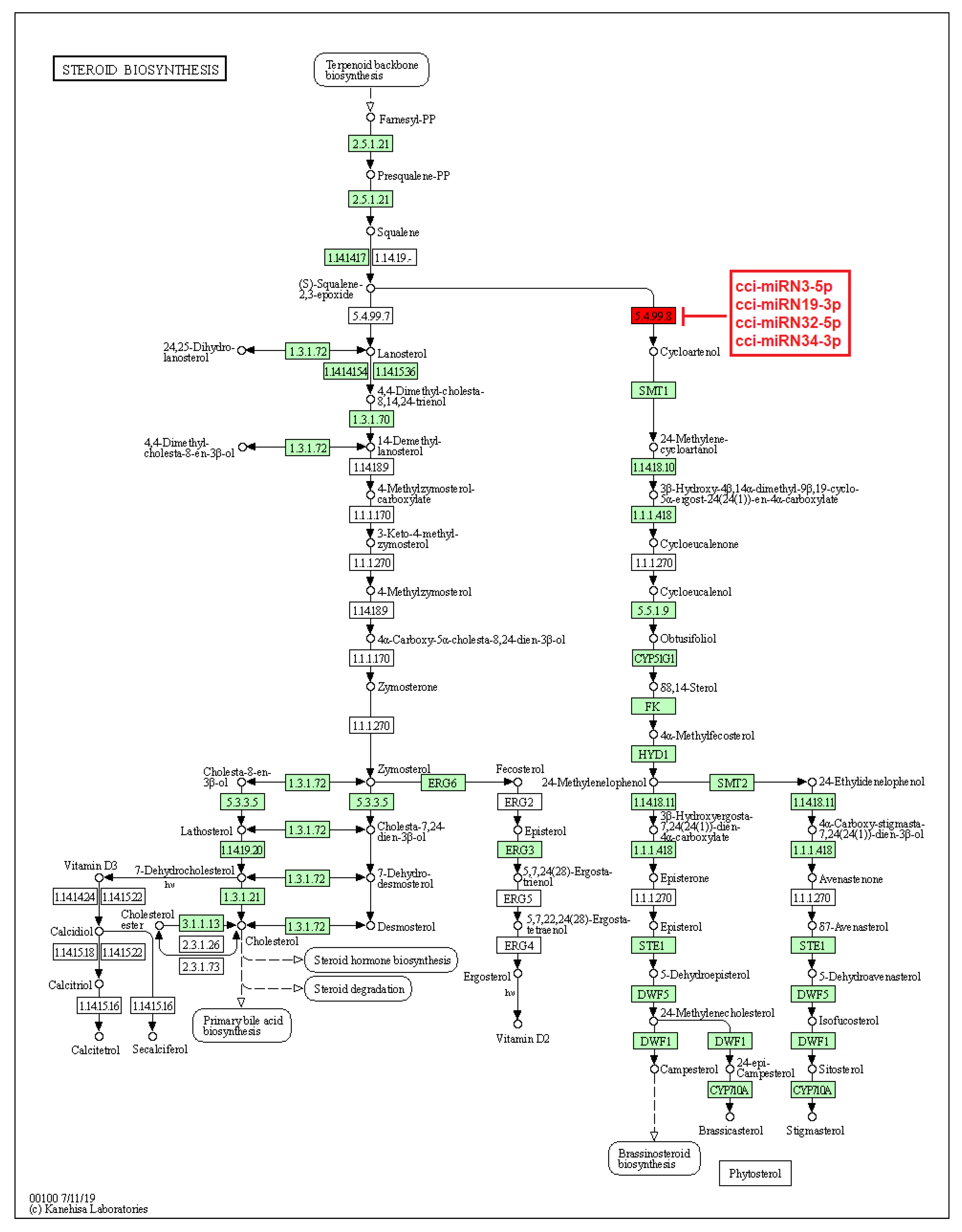
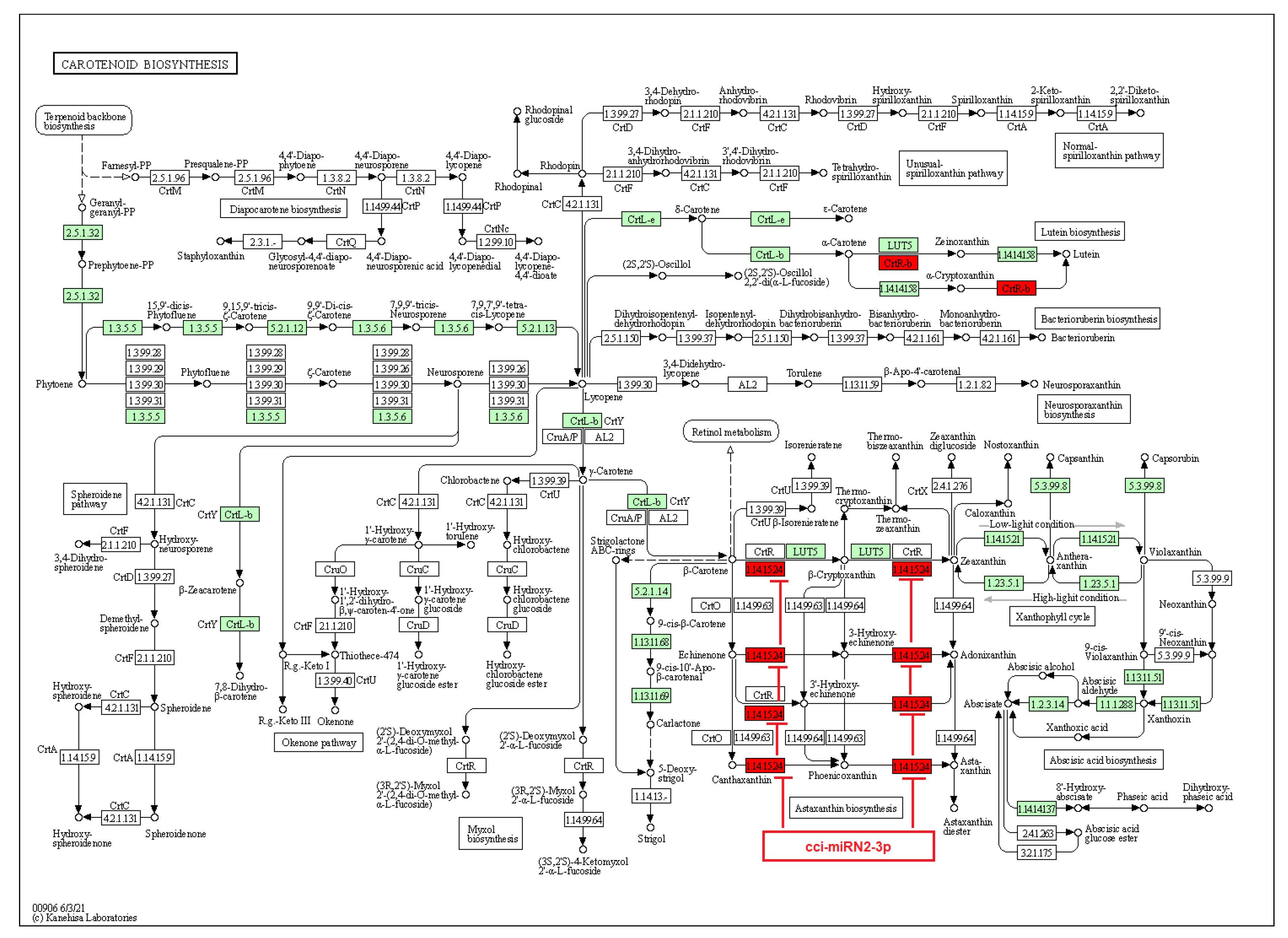
2.4. Identification of C. cyminum miRNA Targets Involved in Plant Secondary Metabolite Biosynthesis
2.5. Experimental Validation of C. cyminum miRNAs by qPCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials, RNA Extraction and Quality Control
4.2. Small RNA Library Construction and Sequencing
4.3. Small RNA Sequencing Data Analysis
4.4. Prediction of C. cyminum miRNA Targets, Their Functional Annotation, and Pathway Analysis
4.5. Extraction of Small RNA and Experimental Validation of C. cyminum miRNAs by qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Yadav, S.S.; Kumar, S.; Narashiman, B. A review on traditional uses, phytochemistry, pharmacology, and clinical research of dietary spice Cuminum cyminum L. Phyther. Res. 2021, 35, 5007–5030. [Google Scholar] [CrossRef] [PubMed]
- Minooeianhaghighi, M.H.; Sepehrian, L.; Shokri, H. Effets antifongiques des huiles essentielles de Lavandula binaludensis et Cuminum cyminum contre Candida albicans isolés de patientes atteintes de candidose vulvo-vaginale récidivante. J. Mycol. Med. 2017, 27, 65–71. [Google Scholar] [CrossRef]
- Balaram, P.K.; Vidhya, N.; Rojamathi, K.; Revathi Satheesh, E.; Sowmiya, P.; Sabarish, T.; Mahalingam, P. Antibacterial and Phytochemical Analysis of Cuminum Cyminum (Cumin) and Illicium Verum (Star Anise) against Clinical Pathogens. WJPR 2021, 10, 938–947. [Google Scholar]
- Archangia, A.; Bahram, H.; Mohammadi-Nejad, G. Association between seed yield-related traits and cDNA-AFLP markers in cumin (Cuminum cyminum) under drought and irrigation regimes. Ind. Crop. Prod. 2019, 133, 276–283. [Google Scholar] [CrossRef]
- Chaudhry, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Sumrra, S.H. Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–178. ISBN 9780081026595. [Google Scholar]
- Keerthiga, N.; Anitha, R.; Rajeshkumar, S.; Lakshmi, T.; Keerthiga, N.; Rajeshkumar, S. Antioxidant Activity of Cumin Oil Mediated Silver Nanoparticles. Pharmacogn. J. 2019, 11, 787–789. [Google Scholar] [CrossRef]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. LWT—Food Science and Technology Application of chitosan nanoparticles containing Cuminum cyminum oil as a delivery system for shelf life extension of Agaricus bisporus. LWT Food Sci. Technol. 2019, 106, 218–228. [Google Scholar] [CrossRef]
- Dinparvar, S.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Aydogdu, M.; Aktas, D. A nanotechnology-based new approach in the treatment of breast cancer: Biosynthesized silver nanoparticles using Cuminum cyminum L. seed extract. J. Photochem. Photobiol. B Biol. 2020, 208, 111902. [Google Scholar] [CrossRef] [PubMed]
- Armenta-Medina, A.; Gillmor, C.S. An introduction to methods for discovery and functional analysis of MicroRNAs in plants. Methods Mol. Biol. 2019, 1932, 1–14. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, L.; Xia, Y.; Wang, F.; Zhu, C. Emerging Roles of microRNAs in Plant Heavy Metal Tolerance and Homeostasis. J. Agric. Food Chem. 2020, 68, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, Y.; Cao, X.; Qi, Y. MicroRNAs and Their Regulatory Roles in Plant-Environment Interactions. Annu. Rev. Plant Biol. 2019, 70, 489–525. [Google Scholar] [CrossRef]
- Griffiths-Jones, S. MiRBase: MicroRNA sequences and annotation. Curr. Protoc. Bioinforma. 2010, 29, 1291–12910. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Quispe, C.; Saikat, A.S.M.; Jain, D.; Habib, A.; Janmeda, P.; Islam, M.T.; Radha; Daştan, S.D.; Kumar, M.; et al. Biosynthesis of Secondary Metabolites Based on the Regulation of MicroRNAs. Biomed Res. Int. 2022, 2022, 1–20. [Google Scholar] [CrossRef]
- Que, F.; Hou, X.L.; Wang, G.L.; Xu, Z.S.; Tan, G.F.; Li, T.; Wang, Y.H.; Khadr, A.; Xiong, A.S. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. 2019, 6, 69. [Google Scholar] [CrossRef]
- Barozai, M.Y.K.; Kakar, S.U.R.; Sarangzai, A.M. Profiling the carrot (Daucus carota L.) MicroRNAs and their targets. Pak. J. Bot. 2013, 45, 353–358. [Google Scholar]
- Srivastava, S.; Sanchita; Singh, R.; Srivastava, G.; Sharma, A. Comparative Study of Withanolide Biosynthesis-Related miRNAs in Root and Leaf Tissues of Withania somnifera. Appl. Biochem. Biotechnol. 2018, 185, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhong, Y.; Yu, F.; Xu, M. Deep sequencing identifies miRNAs and their target genes involved in the biosynthesis of terpenoids in Cinnamomum camphora. Ind. Crops Prod. 2020, 145, 111853. [Google Scholar] [CrossRef]
- Yin, D.D.; Li, S.S.; Shu, Q.Y.; Gu, Z.Y.; Wu, Q.; Feng, C.Y.; Xu, W.Z.; Wang, L.S. Identification of microRNAs and long non-coding RNAs involved in fatty acid biosynthesis in tree peony seeds. Gene 2018, 666, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-García, C.; Ahmed, S.S.S.J.; Ramalingam, S.; Selvaraj, D.; Srivastava, A.; Paul, S.; Sharma, A. Identification of microRNAs from medicinal plant Murraya Koenigii by high-throughput sequencing and their functional implications in secondary metabolite biosynthesis. Plants 2022, 11, 46. [Google Scholar] [CrossRef]
- Zhang, B.H.; Pan, X.P.; Cox, S.B.; Cobb, G.P.; Anderson, T.A. Evidence that miRNAs are different from other RNAs. Cell. Mol. Life Sci. 2006, 63, 246–254. [Google Scholar] [CrossRef]
- Vargas-Hernández, M.; Vázquez-Marrufo, G.; Aguilar-Ruiz, C.A.; González-Márquez, M.A.; Rocha, O.; Cerna-Pantoja, D.; Cruz-Hernández, A. MicroRNAs Associated with secondary metabolites production. In Plant Physiological Aspects of Phenolic Compounds; Soto-Hernández, M., García-Mateos, R., Palma-Tenango, M., Eds.; IntechOpen: London, UK, 2019; ISBN 978-1-78984-034-6. [Google Scholar]
- Liu, H.; Yu, H.; Tang, G.; Huang, T. Small but powerful: Function of microRNAs in plant development. Plant Cell Rep. 2018, 37, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: Traditional uses, chemical constituents, and nutraceutical effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Bettaieb, I.; Bourgou, S.; Wannes, W.A.; Hamrouni, I.; Limam, F.; Marzouk, B. Essential oils, phenolics, and antioxidant activities of different parts of cumin (Cuminum cyminum L.). J. Agric. Food Chem. 2010, 58, 10410–10418. [Google Scholar] [CrossRef]
- Rana, A.; Kumar, V.; Mirza, A.; Panghal, A. Efficacy of cumin (Cuminum cyminum L.) as a bionutrient and its management. Ann. Biol. 2018, 34, 218–222. [Google Scholar]
- Rogowska, A.; Szakiel, A. Enhancement of phytosterol and triterpenoid production in plant hairy root cultures—Simultaneous stimulation or competition? Plants 2021, 10, 2028. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Wang, C.; Chen, H.; Liu, Y.; Wang, Y.; Gao, W. Comprehensive Identification and Profiling of miRNAs Involved in Terpenoid Synthesis of Gleditsia sinensis Lam. Forests 2022, 13, 108. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, Q.; Mo, C.; Bai, L.; Tu, D.; Ma, X. Cloning and characterization of squalene synthase and cycloartenol synthase from Siraitia grosvenorii. Acta Pharm. Sin. B 2017, 7, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jeena, G.S.; Joshi, A.; Shukla, R.K. Bm-miR172c-5p Regulates Lignin Biosynthesis and Secondary Xylem Thickness by Altering the Ferulate 5 Hydroxylase Gene in Bacopa monnieri. Plant Cell Physiol. 2021, 62, 894–912. [Google Scholar] [CrossRef]
- Venkatesh, T.V.; Karunanandaa, B.; Free, D.L.; Rottnek, J.M.; Baszis, S.R.; Valentin, H.E. Identification and characterization of an Arabidopsis homogentisate phytyltransferase paralog. Planta 2006, 223, 1134–1144. [Google Scholar] [CrossRef]
- Zeng, W.; Sun, Z.; Lai, Z.; Yang, S.; Chen, H.; Yang, X.; Tao, J.; Tang, X. Determination of the MiRNAs related to bean pyralid larvae resistance in soybean using small RNA and transcriptome sequencing. Int. J. Mol. Sci. 2019, 20, 2966. [Google Scholar] [CrossRef] [PubMed]
- Kawaide, H.; Hayashi, K.I.; Kawanabe, R.; Sakigi, Y.; Matsuo, A.; Natsume, M.; Nozaki, H. Identification of the single amino acid involved in quenching the ent-kauranyl cation by a water molecule in ent-kaurene synthase of Physcomitrella patens. FEBS J. 2011, 278, 123–133. [Google Scholar] [CrossRef]
- Reid, B.; Meirong, J.; Reuben, J.P. A pair of threonines mark ent-kaurene synthases for phytohormone biosynthesis. Phytochemistry 2021, 184, 112672. [Google Scholar] [CrossRef]
- Aach, H.; Bode, H.; Robinson, D.G.; Graebe, J.E. ent-Kaurene synthase is located in proplastids of meristematic shoot tissues. Planta 1997, 202, 211–219. [Google Scholar] [CrossRef]
- Barozai, M.Y.K.; Baloch, I.A.; Din, M. Computational identification of microRNAs and their targets in two species of evergreen spruce tree (Picea). World Acad. Sci. Eng. Technol. 2011, 5, 1290–1295. [Google Scholar]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef] [PubMed]
- Stephen, N.M.; Gayathri, R.; Niranjana, R.; Prasad, Y.; Das, A.K.; Baskaran, V.; Ganesan, P. Carotenoids: Types, sources, ans biosynthesis. In Plant Secondary Metabolites; Mohammed Wasim, S., Vasudha, B., Kamlesh, P., Eds.; Apple Academic Press: New York, NY, USA, 2017; Volume 2, pp. 103–132. ISBN 9781771883566. [Google Scholar]
- Kumari, S.; Goyal, A.; Garg, M. Phytochemistry and Pharmacological Update on Tetraterpenoids. Nat. Prod. J. 2020, 11, 617–628. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Long, T.; Wang, S.; Yang, J. Regulation Mechanism of Plant Pigments Biosynthesis: Anthocyanins, Carotenoids, and Betalains. Metabolites 2022, 12, 871. [Google Scholar] [CrossRef]
- Du, H.; Liao, X.; Gao, Z.; Li, Y.; Lei, Y.; Chen, W.; Chen, L.; Fan, X.; Zhang, K.; Chen, S.; et al. Effects of methanol on carotenoids as well as biomass and fatty acid biosynthesis in Schizochytrium limacinum B4D1. Appl. Environ. Microbiol. 2019, 85, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Guo, J.; Chen, Q.; Wang, B.; He, X.; Zhuge, Q.; Wang, P. Different color regulation mechanism in willow barks determined using integrated metabolomics and transcriptomics analyses. BMC Plant Biol. 2022, 22, 1–15. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, N.; Wang, Q.; Fu, Y.; Wang, F.; Su, Y.; Xue, B.; Zhou, L.; Liao, H. The Effect of Low Temperature Stress on the Leaves and MicroRNA Expression of Potato Seedlings. Front. Ecol. Evol. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, J.; Hu, H.; Xue, J.; Yang, J.; Xu, J. Integrated Four Comparative-Omics Reveals the Mechanism of the Terpenoid Biosynthesis in Two Different Overwintering Cryptomeria fortunei Phenotypes. Front. Plant Sci. 2021, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pegler, J.L.; Oultram, J.M.J.; Grof, C.P.L.; Eamens, A.L. Profiling the abiotic stress responsive microRNA landscape of arabidopsis thaliana. Plants 2019, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Zhou, Z.; Zhao, L. Identification and expression analysis of miRNAs in germination and seedling growth of Tibetan hulless barley. Genomics 2021, 113, 3735–3749. [Google Scholar] [CrossRef] [PubMed]
- Qibin, L.; Jiang, W. MIREAP: MicroRNA Discovery By Deep Sequencing (Software, Version 0.2) 2008, Sourceforge. Available online: https://sourceforge.net/projects/mireap/ (accessed on 1 June 2019).





| Category | Total Reads | Unique Reads |
|---|---|---|
| Total Reads | 17,058,681 | 10,956,054 |
| Trimmed Reads | 12,917,048 | 2,126,409 |
| % reads aligned to ncRNA | 21.16% | |
| Reads aligned to ncRNA (rRNA, snoRNA, snRNA, tRNA) | 7,342,240 | 582,748 |
| Reads aligned to mirBase | 935,185 | 866,348 |
| Known miRNA | 353 | |
| Reads utilized for novel miRNA | 1,057,102 | 232,425 |
| Novel miRNA | 39 | |
| Putative reads | 3,582,521 | 866,078 |
| miRNA Family | Name | Sequence (5′–3′) | Length (nt) | Reference miRNA | No. of Mismatches | Read Counts | E Value |
|---|---|---|---|---|---|---|---|
| MIR156 | cci-miR156d-3p | GCTCTCTATGCTTCTGTCATCA | 22 | stu-miR156d-3p | 0 | 213,619 | 0.00000009 |
| cci-miR156 | TTGACAGAAGATAGAGAGCAC | 21 | bgy-miR156 | 0 | 155,965 | 0.0000004 | |
| cci-miR156a | TGACAGAAGAGAGTGAGCACA | 21 | bna-miR156a | 0 | 14,927 | 0.0000004 | |
| MIR159 | cci-miR159a | TTTGGATTGAAGGGAGCTCTA | 21 | ath-miR159a | 0 | 177,734 | 0.0000004 |
| cci-miR319b | TTGGACTGAAGGGAGCTCCCT | 21 | ath-miR319b | 0 | 5257 | 0.0000003 | |
| cci-miR319d-3p | CTTGGACTGAAGGGAGCTCCC | 21 | ppt-miR319d-3p | 0 | 346 | 0.0000003 | |
| MIR160 | cci-miR160h | TGCCTGGCTCCCTGCATGCCA | 21 | ptc-miR160h | 0 | 149 | 0.0000003 |
| cci-miR160a-3p | GCGTATGAGGAGCCAAGCATA | 21 | gma-miR160a-3p | 0 | 7 | 0.0000002 | |
| cci-miR160d | TGCCTGGCTCCCTGAATGCCA | 21 | cpa-miR160d | 0 | 1 | 0.0000002 | |
| MIR164 | cci-miR164b-5p | TGGAGAAGCAGGGCACGTGCA | 21 | ath-miR164b-5p | 0 | 1596 | 0.0000003 |
| cci-miR164d | TGGAGAAGCAGGGCACATGCT | 21 | mtr-miR164d | 0 | 116 | 0.0000002 | |
| cci-miR164g-3p | CACGTGCTCCCCTTCTCCA | 19 | zma-miR164g-3p | 0 | 36 | 0.000002 | |
| MIR164_2 | cci-miR164b | TGGAGAAGCAGGGCACTT | 18 | far-miR164b | 0 | 3 | 0.000009 |
| MIR166 | cci-miR166c | TCGGACCAGGCTTCATTCCTC | 21 | mtr-miR166c | 0 | 140,263 | 0.0000004 |
| cci-miR166u | TCTCGGACCAGGCTTCATT | 19 | gma-miR166u | 0 | 2404 | 0.000005 | |
| cci-miR166c-5p | GGAATGTTGTCTGGCTCGAGG | 21 | gma-miR166c-5p | 0 | 1176 | 0.0000004 | |
| MIR167 | cci-miR167h | TTGAAGCTGCCAGCATGA | 18 | gma-miR167h | 0 | 9 | 0.000009 |
| MIR167_1 | cci-miR167d | TGAAGCTGCCAGCATGATCTGG | 22 | ath-miR167d | 0 | 21,213 | 0.00000009 |
| cci-miR167c-5p | TGAAGCTGCCAGCATGATCTGC | 22 | tae-miR167c-5p | 0 | 2262 | 0.00000008 | |
| cci-miR167d | TGAAGCTGCCAGCATGATCTGA | 22 | cpa-miR167d | 0 | 1244 | 0.00000007 | |
| MIR168 | cci-miR168c-5p | TCGCTTGGTGCAGGTCGGGAC | 21 | bra-miR168c-5p | 0 | 694 | 0.0000004 |
| cci-miR168 | TCCCGCCTTGCATCAATTGAAT | 22 | aau-miR168 | 1 | 13 | 0.000009 | |
| cci-miR168a-5p | TCGCTTGGTGCAGATCGGGAC | 21 | osa-miR168a-5p | 0 | 13 | 0.0000002 | |
| MIR169_1 | cci-miR169k | TAGCCAAGGATGACTTGCCTGC | 22 | bna-miR169k | 0 | 232 | 0.00000008 |
| cci-miR169n | TAGCCAAGAATGACTTGCCT | 20 | osa-miR169n | 0 | 52 | 0.0000006 | |
| cci-miR169h | TAGCCAAGGATGACTTGCCTG | 21 | ath-miR169h | 0 | 3 | 0.0000002 | |
| MIR169_2 | cci-miR169a-5p | CAGCCAAGGATGACTTGCCGA | 21 | ath-miR169a-5p | 0 | 91 | 0.0000001 |
| cci-miR169r-3p | GGCAAGTTGTCTTTGGCTACA | 21 | zma-miR169r-3p | 1 | 45 | 0.00004 | |
| cci-miR169a-3p | GGCAAGTTGTCTTTGGCTAC | 20 | ath-miR169a-3p | 1 | 11 | 0.0001 | |
| MIR171_1 | cci-miR171b-3p | TTGAGCCGTGCCAATATCACG | 21 | ath-miR171b-3p | 0 | 879 | 0.0000003 |
| cci-miR171d-5p | TTGGCCGGGCTCACTCAGA | 19 | osa-miR171d-5p | 1 | 470 | 0.0007 | |
| cci-miR171d | TGATTGAGCCGTGCCAATATC | 21 | cpa-miR171d | 0 | 172 | 0.0000003 | |
| MIR172 | cci-miR172c-5p | GTAGCATCATCAAGATTCACA | 21 | mtr-miR172c-5p | 0 | 217 | 0.0000004 |
| cci-miR172d-5p | AGCACCATCAAGATTCACA | 19 | zma-miR172d-5p | 0 | 174 | 0.000002 | |
| cci-miR172a | AGAATCTTGATGATGCTGCAT | 21 | ath-miR172a | 0 | 38 | 0.0000002 | |
| MIR319 | cci-miR319i | TTGGGCTGAAGGGAGCTCCC | 20 | ptc-miR319i | 0 | 7 | 0.0000006 |
| MIR390 | cci-miR390b | AAGCTCAGGAGGGATAGCGCC | 21 | ppt-miR390b | 0 | 196 | 0.0000003 |
| cci-miR390a-3p | CGCTATCTATCCTGAGTTTCA | 21 | ath-miR390a-3p | 1 | 63 | 0.00005 | |
| cci-miR390 | AAGCACAGGATGGATAGCG | 19 | pta-miR390 | 1 | 14 | 0.0006 | |
| MIR393 | cci-miR393a-5p | TCCAAAGGGATCGCATTGATCC | 22 | ath-miR393a-5p | 0 | 66 | 0.00000007 |
| cci-miR393 | TCCAAAGGGATCGCATTGAT | 20 | ghr-miR393 | 0 | 23 | 0.0000006 | |
| cci-miR393c-3p | ATCATGCTATCCCTTTGGATT | 21 | gma-miR393c-3p | 0 | 19 | 0.0000003 | |
| MIR3932 | cci-miR3932a | TCGTCGTCATCACAAAGTT | 19 | ath-miR3932a | 1 | 1 | 0.0006 |
| MIR394 | cci-miR394b-5p | TTGGCATTCTGTCCACCTCC | 20 | ath-miR394b-5p | 0 | 119 | 0.0000006 |
| MIR395 | cci-miR395b | CTGAAGTGTTTGGGGGAACTCC | 22 | sly-miR395b | 0 | 821 | 0.00000004 |
| cci-miR395o | GAGTTCCTCCAAACACTT | 18 | osa-miR395o | 0 | 3 | 0.000009 | |
| cci-miR395a | CTGAAGTGTTCGGGGGAACTC | 21 | ath-miR395a | 1 | 2 | 0.00004 | |
| MIR396 | cci-miR396f | TTCCACGGCTTTCTTGAACTG | 21 | ptc-miR396f | 0 | 41,200 | 0.0000004 |
| cci-miR396 | TTCCACAGCTTTCTTGAACTT | 21 | pta-miR396 | 0 | 28,712 | 0.0000004 | |
| cci-miR396a-3p | GTTCAATAAAGCTGTGGGAAG | 21 | ath-miR396a-3p | 0 | 6661 | 0.0000003 | |
| MIR397 | cci-miR397 | ATTGAGTGCAGCGTTGATGA | 20 | lja-miR397 | 0 | 21 | 0.0000006 |
| MIR398 | cci-miR398a-3p | TGTGTTCTCAGGTCACCCCTT | 21 | ath-miR398a-3p | 0 | 85 | 0.0000002 |
| cci-miR398c | TGTGTTCTCAGGTCGCCCCTG | 21 | gma-miR398c | 0 | 81 | 0.0000001 | |
| cci-miR398a-5p | GGAGTGTCATGGGAACACA | 19 | aly-miR398a-5p | 1 | 4 | 0.0006 | |
| MIR399 | cci-miR399i | TGCCAAAGGAGAATTGCCCTG | 21 | gma-miR399i | 0 | 15 | 0.0000002 |
| cci-miR399i | TGCCAAAGGAGAGTTGCCCTA | 21 | ptc-miR399i | 0 | 15 | 0.0000002 | |
| cci-miR399d | TGCCAAAGGAGATTTGCCCCG | 21 | ath-miR399d | 0 | 1 | 0.0000002 | |
| MIR403 | cci-miR403-3p | TTAGATTCACGCACAAACTCG | 21 | ath-miR403-3p | 0 | 4500 | 0.0000003 |
| cci-miR403a | TTAGATTCACGCACAAACTT | 20 | gma-miR403a | 0 | 471 | 0.0000009 | |
| cci-miR403-5p | TTAGATTCACGCACAAAA | 18 | bra-miR403-5p | 0 | 7 | 0.00001 | |
| MIR408 | cci-miR408 | TGCACTGCCTCTTCCCTGG | 19 | cpa-miR408 | 0 | 35 | 0.000003 |
| MIR414 | cci-miR414 | TGACGATGATGATGATGATG | 20 | ath-miR414 | 1 | 1 | 0.0002 |
| MIR437 | cci-miR437x-5p | GTTTGACTTAGGACAACTCTA | 21 | sbi-miR437x-5p | 0 | 2 | 0.0000002 |
| MIR444 | cci-miR444b.2 | TGCAGTTGTTGTCTCAAGCTT | 21 | osa-miR444b.2 | 0 | 2 | 0.0000002 |
| cci-miR444b | CTTGAGACAGCAACTGCA | 18 | hvu-miR444b | 0 | 1 | 0.00001 | |
| cci-miR444d.3 | TTGTGGCTTTCTTGCAAGTTG | 21 | osa-miR444d.3 | 0 | 1 | 0.0000002 | |
| MIR477 | cci-miR477-5p | ACTCTCCCTCAAAGGCTTC | 19 | ppe-miR477-5p | 0 | 455 | 0.000005 |
| cci-miR477a | ACTCTCCCTCAAGGGCTTCTG | 21 | nta-miR477a | 0 | 265 | 0.0000003 | |
| cci-miR477a-5p | TCTCCCTCAGAGGCTTCC | 18 | ptc-miR477a-5p | 0 | 1 | 0.000009 | |
| MIR482 | cci-miR2118 | TTTCCTATTCCACCCATCCCAT | 22 | pgi-miR2118 | 0 | 43 | 0.00000004 |
| MIR529 | cci-miR529-5p | AGAAGAGAGAGAGTACAGCCT | 21 | zma-miR529-5p | 0 | 1 | 0.0000002 |
| MIR774 | cci-miR774b-5p | GTCATCCAAACCTTCATCT | 19 | aly-miR774b-5p | 1 | 1 | 0.0006 |
| MIR818 | cci-miR1130b-3p | TTATATTAAGGGACGGAGG | 19 | tae-miR1130b-3p | 1 | 15 | 0.0007 |
| cci-miR1436 | ACATTATGAGACGGAGGGAGT | 21 | osa-miR1436 | 1 | 2 | 0.00004 | |
| cci-miR1439 | TTTTGGGACGGAGTGAGTA | 19 | osa-miR1439 | 1 | 1 | 0.0006 | |
| MIR821 | cci-miR821d | CAACTTTGTTGTTGTTGAC | 19 | sbi-miR821d | 1 | 1 | 0.0009 |
| cci-miR821e | AAGTCATCAAAACAAAAGT | 19 | sbi-miR821e | 1 | 1 | 0.0006 | |
| MIR827 | cci-miR827 | TTAGATGATCATCAGCAAACA | 21 | osa-miR827 | 1 | 5537 | 0.00007 |
| cci-miR827-5p | TTTGTTGGTGGTCATCTAA | 19 | bdi-miR827-5p | 1 | 251 | 0.0007 | |
| cci-miR827 | TTAGATGAACATCAGCAAACA | 21 | nta-miR827 | 1 | 3 | 0.00004 | |
| MIR828 | cci-miR828 | TCTTGCTTAAATGAGTGTTCCA | 22 | ath-miR828 | 1 | 24 | 0.000009 |
| MIR834 | cci-miR834 | TGGTAGCAGTAGTGGTGGT | 19 | ath-miR834 | 1 | 2 | 0.0006 |
| MIR835 | cci-miR835-5p | TTCTTGCATATGTTCTTT | 18 | ath-miR835-5p | 0 | 1 | 0.000009 |
| MIR845_3 | cci-miR845b | CAATTGGTATCAGAGCTA | 18 | vvi-miR845b | 0 | 2 | 0.00001 |
| MIR858 | cci-miR858a | TTTCGTTGTCTGTTCGACCTT | 21 | ath-miR858a | 0 | 1090 | 0.0000002 |
| cci-miR858b | TTCGCTGTCTGTTCGACCTTG | 21 | ath-miR858b | 1 | 3 | 0.00004 | |
| cci-miR858-3p | TTCGTTGTCTGCTCGACC | 18 | aly-miR858-3p | 0 | 2 | 0.000009 | |
| MIR862 | cci-miR862-3p | ATATGCTGGATTTACTTGAAG | 21 | ath-miR862-3p | 1 | 1 | 0.00004 |
| MIR1120 | cci-miR1120a | TTATATTATGAGACGGAG | 18 | tae-miR1120a | 0 | 5 | 0.00001 |
| MIR1122 | cci-miR1133 | GGACGGAGGGAGTATATG | 18 | tae-miR1133 | 0 | 3 | 0.00002 |
| cci-miR5281e | ATAAATAGAACCGGAGGGAG | 20 | mtr-miR5281e | 1 | 2 | 0.0001 | |
| MIR1511 | cci-miR1511 | ACCTAGCTCTGATACCATGA | 20 | mdm-miR1511 | 0 | 2 | 0.0000006 |
| MIR1520 | cci-miR1520q | ACCAATTAGAACATGACACA | 20 | gma-miR1520q | 1 | 1 | 0.0002 |
| MIR1863 | cci-miR1863b | AGCTCTGATACCATATTAACTG | 22 | osa-miR1863b | 1 | 4 | 0.00001 |
| MIR2084 | cci-miR2084 | CCTGCATTGGTGGATTGTG | 19 | ppt-miR2084 | 1 | 1 | 0.0006 |
| MIR2275 | cci-miR2275b-3p | AGATATTAGAGAAAACTGA | 19 | zma-miR2275b-3p | 1 | 3 | 0.0007 |
| MIR2275 | cci-miR2275d-5p | AGAGTTGGAGTAAAGAAAA | 19 | zma-miR2275d-5p | 1 | 1 | 0.0006 |
| MIR2646 | cci-miR2646b | ATGACATGTAGTGATGATGT | 20 | mtr-miR2646b | 1 | 1 | 0.0002 |
| MIR2673 | cci-miR2673b | CCTCTTCCTCTTCCTCTTCC | 20 | mtr-miR2673b | 0 | 2 | 0.0000009 |
| MIR2912 | cci-miR2912a | TCTAGAACTCCAGATATGG | 19 | peu-miR2912a | 1 | 3 | 0.0006 |
| MIR3630 | cci-miR3630-3p | TGGGAATCTCTTTGATGCAC | 20 | vvi-miR3630-3p | 1 | 3 | 0.0003 |
| MIR5067 | cci-miR5181-3p | CACTTATTTTGGAACGGAGGG | 21 | ata-miR5181-3p | 1 | 4 | 0.00004 |
| cci-miR5049d | ACAACTATTTAGGAACGGAG | 20 | hvu-miR5049d | 1 | 3 | 0.0002 | |
| cci-miR5181-5p | GACAATTATTCTGGATCGG | 19 | ata-miR5181-5p | 1 | 1 | 0.0006 | |
| MIR5298 | cci-miR5298a | TTCTTCATCTTCATCTCAT | 19 | mtr-miR5298a | 1 | 1 | 0.0006 |
| MIR5387 | cci-miR5387b | CTTTAGCACCGGCCAGAGCCAC | 22 | sbi-miR5387b | 1 | 1 | 0.00001 |
| MIR5564 | cci-miR5564a | TGGGGAAGCAATTCGTCGAACA | 22 | sbi-miR5564a | 0 | 23 | 0.00000004 |
| cci-miR5564b | GCAATTCGTCGAACAGCTTG | 20 | sbi-miR5564b | 0 | 15 | 0.0000006 | |
| MIR5565 | cci-miR5565b | TCGCATCAATCCACATGTGTT | 21 | sbi-miR5565b | 1 | 1 | 0.00004 |
| MIR5568 | cci-miR5568d-5p | TGGCTTTTCTAGACACATAGC | 21 | sbi-miR5568d-5p | 1 | 1 | 0.00004 |
| MIR6161 | cci-miR6161b | TGGACCAGTATACTTTGCT | 19 | nta-miR6161b | 1 | 1 | 0.0006 |
| MIR6476 | cci-miR6476a | TCAGTGGAGATGAAACATG | 19 | ptc-miR6476a | 0 | 97 | 0.000005 |
| MIR7982 | cci-miR7982a | TGGAGGATAATAATATATA | 19 | stu-miR7982a | 1 | 1 | 0.0006 |
| MIR7996 | cci-miR7996b | TGGTATATATGAAATTTGAA | 20 | stu-miR7996b | 1 | 1 | 0.0002 |
| MIR8762 | cci-miR8762c | CAACAAAGTTAGCAAACGT | 19 | gra-miR8762c | 1 | 2 | 0.0005 |
| NA | cci-miR6478 | CCGACCTTAGCTCAGTTGGT | 20 | ptc-miR6478 | 0 | 5673 | 0.000001 |
| cci-miR6300 | GTCGTTGTAGTATAGTGG | 18 | gma-miR6300 | 0 | 4672 | 0.00002 | |
| cci-miR894 | CGTTTCACGTCGGGTTCACC | 20 | ppt-miR894 | 0 | 744 | 0.000001 |
| Name | Sequence (5′–3′) | Length | Read Count | Strand | MFEI of Precursor |
|---|---|---|---|---|---|
| cci-miRN1-3p | UGCUCACUCUCUAUCUGUCACC | 22 | 8 | + | 1.41 |
| cci-miRN2-3p | GGCGUACCGUGAGCCAAGCAUGC | 23 | 975 | − | 0.77 |
| cci-miRN3-5p | UAGAAGUGUGACCUGUCUUGCAU | 23 | 11 | − | 0.90 |
| cci-miRN4-3p | GCAAGGCAGGACACCUUCUAUG | 22 | 194 | − | 0.98 |
| cci-miRN5-5p | CAGAAGUGUGACCUGUCUUGCAU | 23 | 83 | − | 0.98 |
| cci-miRN6-5p | UGACAGAAGAGAGUGAGCACA | 21 | 6 | − | 0.98 |
| cci-miRN7-5p | UGACAGAAGAGAGUGAGCACA | 21 | 6 | − | 1.08 |
| cci-miRN8-5p | AUUUUAGCUGAAGUAUUUAGUAU | 23 | 5 | + | 0.75 |
| cci-miRN9-3p | UAGGCAGGCAUUUUUGGCUAGC | 22 | 6 | + | 0.94 |
| cci-miRN10-3p | UUUAUGGUGAUUUAUUUGUGUGG | 23 | 1382 | − | 1.18 |
| cci-miRN11-5p | AAACAAAUUCAUCACCAUAAGGA | 23 | 10 | − | 1.18 |
| cci-miRN12-3p | UUAUGGUGAUUUAUUUGUGUGGG | 23 | 20 | − | 1.04 |
| cci-miRN13-3p | GAGUGAAUGAAGCGGGAGACUUAU | 24 | 2 | − | 0.99 |
| cci-miRN14-5p | UAGCUGCUGACUCAUUCAUCCAA | 23 | 12 | − | 0.99 |
| cci-miRN15-3p | UCAUCUACGCUGCACUCAAUCAU | 23 | 224 | − | 0.95 |
| cci-miRN16-5p | GGAAUGUUGUCCGGCUCGAUGCU | 23 | 5 | − | 1.01 |
| cci-miRN17-3p | CUGAUGCAUGAUGUGAGAGCAA | 22 | 6 | − | 0.90 |
| cci-miRN18-5p | GCUGUCAUCUCAUGCAUUUGGU | 22 | 24 | − | 0.90 |
| cci-miRN19-3p | CUGAGCCGAACCAAUAUUACUC | 22 | 177 | + | 0.92 |
| cci-miRN20-5p | GCGUAAUAUUGCUCCGGCUCAGC | 23 | 262 | + | 0.92 |
| cci-miRN21-5p | AUUUGGCAUUCUGUUCACCUCCA | 23 | 35 | + | 1.05 |
| cci-miRN22-3p | UGGUGCCACACUGCUCGCGUUU | 22 | 40 | − | 1.00 |
| cci-miRN23-3p | GCGCUAUCUAUUCUGAGUUUCA | 22 | 134 | + | 1.00 |
| cci-miRN24-5p | UAGCUGCCGACUCAUUCACUCA | 22 | 8 | + | 0.89 |
| cci-miRN25-3p | UUUAGUUUUCUCCAAUUUCUCAU | 23 | 67 | − | 0.81 |
| cci-miRN26-3p | CUUAGUUUUCUCCGAUAUCUCAU | 23 | 10 | − | 1.12 |
| cci-miRN27-3p | GCAUGUGCUCUUGCUCUCCUGCU | 23 | 52 | + | 1.12 |
| cci-miRN28-5p | GCAUGCUCCCUUUUUUAUUGGC | 22 | 6 | − | 0.85 |
| cci-miRN29-3p | CAAGAUACUCUGAAGAAGCUAGC | 23 | 11 | − | 0.81 |
| cci-miRN30-5p | AUAUUGGCCGGGCUCACUCAGA | 22 | 6 | + | 0.93 |
| cci-miRN31-3p | UUAACUAUGCGGUCAAAACUCUU | 23 | 5 | − | 0.80 |
| cci-miRN32-5p | GUUCCCUUGACCACUUCAUUGG | 22 | 34 | − | 1.10 |
| cci-miRN33-3p | UCGAACGAUGCAGGGGUUGUGUU | 23 | 110 | − | 0.92 |
| cci-miRN34-3p | UACAUUGAGGGAAAUUGAGGGA | 22 | 83 | + | 1.17 |
| cci-miRN35-3p | AUCGGACCAGGCUUCAUUCCUC | 22 | 3 | − | 0.98 |
| cci-miRN36-5p | GGGAUGUUGGCUGGCUCGAUGC | 22 | 7 | − | 0.98 |
| cci-miRN37-3p | CAAUGUUUCAUUGCGGGUGUGA | 22 | 8 | − | 0.98 |
| cci-miRN38-5p | CCUCAAUUUCCCUCAGUGUAGU | 22 | 35 | − | 1.14 |
| cci-miRN39-5p | UGCUUUGUAUAUUUGGAUUUGAU | 23 | 5 | − | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Calderón, A.; Gutiérrez-García, C.; Urióstegui-Pena, A.G.; Srivastava, A.; Aguilar-Marcelino, L.; Iqbal, H.M.N.; Ahmed, S.S.S.J.; Paul, S.; Sharma, A. Identification of Cumin (Cuminum cyminum) MicroRNAs through Deep Sequencing and Their Impact on Plant Secondary Metabolism. Plants 2023, 12, 1756. https://doi.org/10.3390/plants12091756
Reyes-Calderón A, Gutiérrez-García C, Urióstegui-Pena AG, Srivastava A, Aguilar-Marcelino L, Iqbal HMN, Ahmed SSSJ, Paul S, Sharma A. Identification of Cumin (Cuminum cyminum) MicroRNAs through Deep Sequencing and Their Impact on Plant Secondary Metabolism. Plants. 2023; 12(9):1756. https://doi.org/10.3390/plants12091756
Chicago/Turabian StyleReyes-Calderón, Almendra, Claudia Gutiérrez-García, Andrea G. Urióstegui-Pena, Aashish Srivastava, Liliana Aguilar-Marcelino, Hafiz M. N. Iqbal, Shiek S. S. J. Ahmed, Sujay Paul, and Ashutosh Sharma. 2023. "Identification of Cumin (Cuminum cyminum) MicroRNAs through Deep Sequencing and Their Impact on Plant Secondary Metabolism" Plants 12, no. 9: 1756. https://doi.org/10.3390/plants12091756








