Functional Quality and Radical Scavenging Activity of Selected Watermelon (Citrullus lanatus (Thunb.) Mansfeld) Genotypes as Affected by Early and Full Cropping Seasons
Abstract
1. Introduction
2. Results and Discussion
2.1. Weather Conditions
2.2. Carpometric Traits
2.2.1. Marketable Yield
2.2.2. Average Fruit Weight
2.2.3. Total Soluble Solids
2.2.4. Colour Indexes
2.3. Functional Quality Attributes
2.3.1. Citrulline Content
2.3.2. Carotenoid Content
2.3.3. Vitamin C Content
2.3.4. Total Phenolics
2.3.5. Flavonoid Content
2.4. Radical Scavenging Activity of the Hydrophilic and Lipophilic Fractions
2.5. Correlation
3. Materials and Methods
3.1. Plant Culture and Growth Conditions
3.2. Watermelon Harvest and Sampling
3.3. Carpometric Traits
3.4. Functional Quality Attributes
3.4.1. Determination of Citrulline Content
3.4.2. Determination of Total Vitamin C
3.4.3. Determination of Carotenoid Content
3.4.4. Determination of Total Phenols Content
3.4.5. Determination of Flavonoid Content
3.5. Determination of the Radical Scavenging Activity
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2021. Available online: http://www.http.fao.org (accessed on 2 January 2023).
- Weststrate, J.A.; Poppel, G.V.; Verschuren, P.M. Functional foods, trends, and future. Br. J. Nutr. 2002, 88, S233–S235. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Siddiqui, M.W.; Tlili, I.; Montefusco, A.; Piro, G.; Hdider, C.; Lenucci, M.S. When color really matters: Horticultural performance and functional quality of high-lycopene tomatoes. Crit. Rev. Plant Sci. 2018, 37, 15–53. [Google Scholar] [CrossRef]
- Leskovar, D.I.; Bang, H.; Crosby, K.M.; Maness, N.; Franco, J.A.; Perkins-Veazie, P. Lycopene, carbohydrates, ascorbic acid and yield components of diploid and triploid watermelon cultivars are affected by deficit irrigation. J. Hortic. Sci. Biotechnol. 2004, 79, 75–81. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Clevidence, B. Watermelons and health. Acta Hort. (ISHS) 2007, 731, 121–128. [Google Scholar] [CrossRef]
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Ilahy, R.; Jebari, H.; Dalessandro, G. Bioactive compounds and antioxidant activities of different watermelon (Citrullus lanatus (Thunb.) Mansfeld) cultivars as affected by fruit sampling area. J. Food. Compos. Anal. 2011, 24, 307–314. [Google Scholar] [CrossRef]
- Naz, A.; Butt, M.S.; Sultan, M.T.; Qayyum, M.M.N.; Niaz, R.S. Review article: Watermelon lycopene and allied health claims. EXCLI J. 2014, 13, 650–666. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care. 2017, 20, 92–98. [Google Scholar] [CrossRef]
- Maoto, M.M.; Beswa, D.; Jideani, A.I.O. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef]
- Manivannan, A.; Lee, E.-S.; Han, K.; Lee, H.-E.; Kim, D.-S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules 2020, 25, 5258. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Davis, A.R.; Webber III, C.L.; Fish, W.W. L-Citrulline Levels in Watermelon Cultigens Tested in Two Environments. Hortic. Sci. 2011, 46, 1572–1575. [Google Scholar] [CrossRef]
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Ilahy, R.; Jebari, H.; Dalessandro, G. Bioactive compounds and antioxidant activities during fruit ripening of watermelon cultivars. J. Food. Compos. Anal. 2011, 24, 923–928. [Google Scholar] [CrossRef]
- Rimando, A.M.; Perkins-Veazie, P. Determination of citrulline in watermelon rind. J. Chromatogr. 2005, 1078, 196–200. [Google Scholar] [CrossRef]
- Davis, A.R.; Fish, W.; Levi, A.; King, S.; Wehner, T.; Perkins-Veazie, P. L-citrulline levels in watermelon cultivars from three locations. Cucurbit Genet. Coop. Rpt. 2010, 33, 36–39. [Google Scholar]
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao-Martinsc, M.; Aguayoa, E. Bioactive compounds from flesh and by-product of fresh-cut watermelon cultivars. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, S.; Cheng, Z.; Wan, X.; Yan, Z.; King, S. Lycopene and citrulline contents in watermelon (Citrullus lanatus) fruit with different ploidy and changes during fruit development. Acta Hortic. 2010, 871, 543–550. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid content of 50 watermelon cultivars. J. Agric. Food Chem. 2006, 54, 2593–2597. [Google Scholar] [CrossRef]
- Naz, A.; Butt, M.S.; Pasha, I.; Nawaz, H. Antioxidant indices of watermelon juice and lycopene extract. Pak. J. Nutr. 2013, 12, 255–260. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, M.K.; Kim, S.K.; Cho, Y.H. Antioxidant capacity and anti-inflammatory activity of lycopene in watermelon. Int J. Food Sci. Technol. 2014, 49, 2083–2091. [Google Scholar] [CrossRef]
- Tlili, I.; Hdider, C.; Ilahy, R.; Jebari, H. Phytochemical Composition and Antioxidant Activity of Selected Watermelon Varieties Grown in Tunisia. Afr. J. Plant Sci. Biotechnol. 2010, 4, 68–71. [Google Scholar]
- Brat, P.; George, S.; Bellamy, A.; Du Chaffaut, L.; Scalbert, A.; Mennen, L.; Arnault, M.; Amiot, M.J. Daily polyphenol intake in France from fruit and vegetables. J. Nutr. Biochem. 2006, 136, 2368–2373. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Maness, N.; Roduner, R. Composition of orange, yellow and red-fleshed watermelons. In Cucurbitacea; ASHS Press: Alexandria, VA, USA, 2002; pp. 436–440. [Google Scholar]
- Dorais, M.; Papadopoulos, A.P.; Gosselin, A. Greenhouse tomato fruit quality. Hortic. Rev. 2001, 26, 239–319. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P.; Lister, C.E. Seasonal variations in the antioxidant composition of greenhouse grown tomatoes. J. Food. Compos. Anal. 2006, 19, 1–10. [Google Scholar] [CrossRef]
- Tlili, I.; Hdider, C.; Ilahy, R.; Rhim, T.; Jebari, H. Effect of growing period on the agronomic characteristics and phenolic content of different watermelon (Citrullus lanatus (Thunb.) Mansfeld) cultivars grown in Tunisia. Food 2013, 7, 22–26. [Google Scholar]
- Susila, T.; Amarender Reddy, S.; Rajkumar, M.; Padmaja, G.; Rao, P.V. Effects of Sowing Date and Spraying of Brassinosteroid on Yield and Fruit Quality Characters of Watermelon. World J. Agric. Sci. 2012, 8, 223–228. [Google Scholar]
- Alan, D.; Sen, F.; Duzyaman, E. The effectiveness of growth cycles on improving fruit quality for grafted watermelon combinations. Food Sci. Technol. 2017, 38, 270–277. [Google Scholar] [CrossRef]
- Winsor, G.W.; Adams, P. Changes in the composition and quality of tomato fruit throughout the season. Annu. Rep. Glasshouse Crops Res. Inst. 1976, 1975, 134. [Google Scholar]
- Heinonen, M.I.; Ollilainen, V.; Linkola, E.K.; Varo, P.T.; Koivistoinen, P.E. Carotenoids in Finnish foods: Vegetables, fruits, and berries. J. Agric. Food Chem. 1989, 37, 655–659. [Google Scholar] [CrossRef]
- Lester, G.E. Environmental regulation of human health nutrients (ascorbic acid, β-carotene, and folic acid) in fruits and vegetables. HortScience 2006, 41, 59–64. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and Beyond Color: Comparative Overview of Functional Quality of Tomato and Watermelon Fruits. Front. Plant Sci. 2019, 10, 769. [Google Scholar] [CrossRef]
- Mélo, E.A.; Lima, V.L.A.G.; Maciel, M.I.S.; Caetano, A.C.S.; Leal, F.L.L. Polyphenol, ascorbic acid and total carotenoid contents in common fruits and vegetables. Braz. J. Food Technol. 2006, 9, 89–94. [Google Scholar] [CrossRef]
- Wadas, W.; Mioduszewska, H. The effect of the sowing date on the content of carotenoids and l-ascorbic acid in spaghetti squash (Cucurbita pepo L.). Acta Sci. Pol. Hortorum Cultus 2011, 10, 41–48. [Google Scholar]
- Davies, J.N.; Hobson, G.E. The constituents of tomato fruit the influence of environment, nutrition, and genotype. CRC Crit. Rev. Food. Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef]
- Ilahy, R.; Siddiqui, M.W.; Piro, G.; Lenucci, M.S.; Hdider, C. Year-to-year variations in antioxidant components of high-lycopene tomato (Solanum lycopersicum L.) breeding lines. Turk. J. Agric. Sci. Technol. 2016, 4, 486–492. [Google Scholar] [CrossRef]
- Knipp, M.; Vašák, M.A. Colorimetric 96-Well Microtiter Plate Assay for the Determination of Enzymatically Formed Citrulline. Anal. Biochem. 2000, 286, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Daood, H.G.; Bencze, G.; Palotas, G.; Pék, Z.; Sidikov, A.; Helyes, L. HPLC analysis of carotenoids from tomatoes using cross-linked C18 column and MS detection. J. Chromatogr. Sci. 2014, 52, 985–991. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Influence of processing and storage on the phenolic composition of Thompson Seedless grape juice. J. Agric. Food. Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food. Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Pellegrini, N.; Colombi, B.; Salvatore, S.; Brenna, O.; Galaverna, G.; Del Rio, D. Evaluation of antioxidant capacity of some fruit and vegetable foods: Efficiency of extraction of a sequence of solvents. J. Sci. Food Agric. 2007, 87, 103–111. [Google Scholar] [CrossRef]

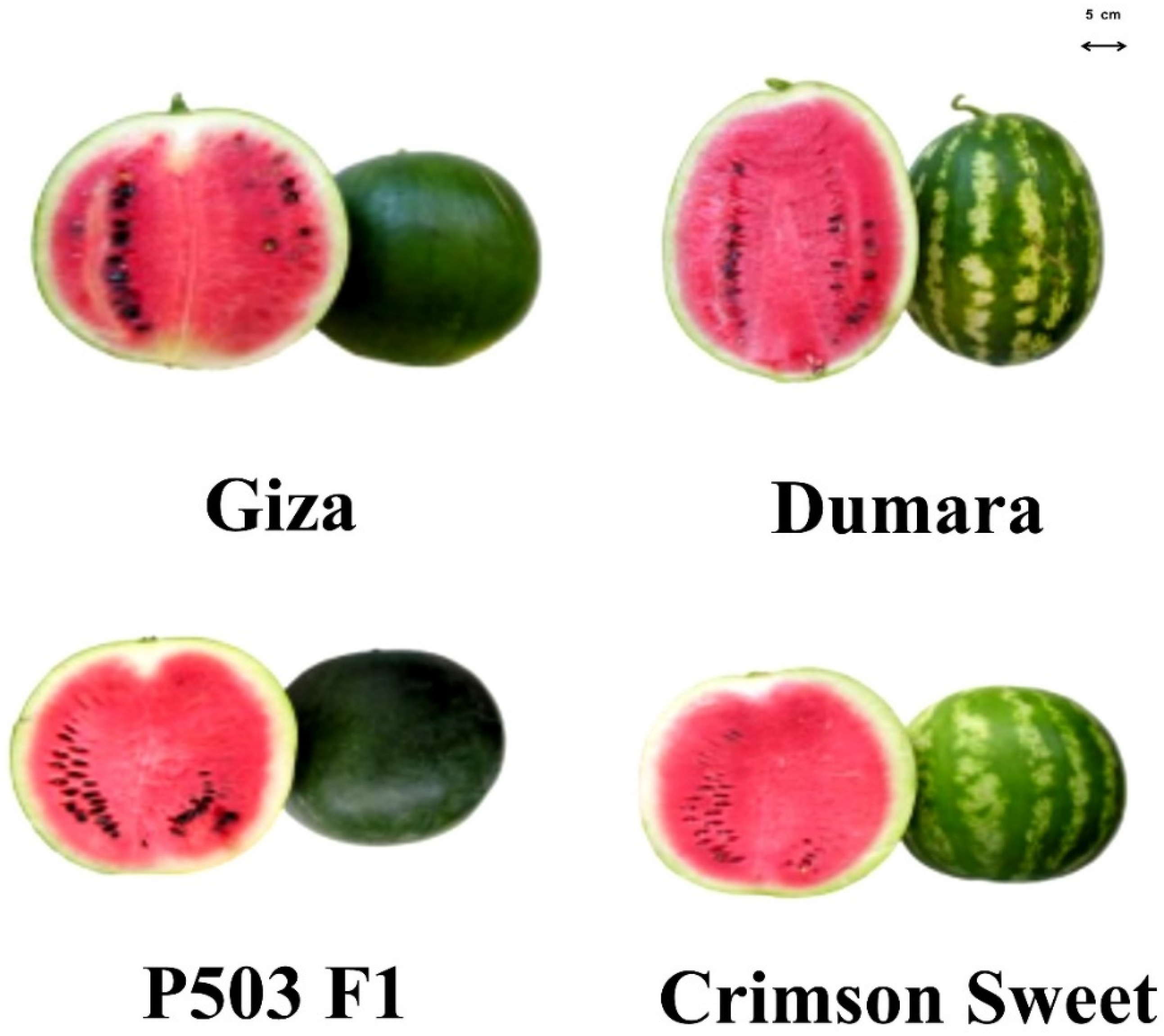
| Cultivar | Cropping Season | Marketable Yield (kg Plant−1) | Average Fruit Weight (kg) | Soluble Solids (°Brix) | Colour Indexes | |||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | a*/b* | |||||
| Crimson Sweet | ||||||||
| ECS | 5.94 ± 0.13 b | 5.12 ± 0.11 b | 9.17 ± 0.14 b | 37.00 ± 0.40 b | 18.44 ± 0.64 a | 10.31 ± 0.95 b | 1.82 ± 0.18 a | |
| FCS | 8.00 ± 0.14 a | 6.90 ± 0.17 a | 12.0 ± 0.12 a | 39.17 ± 0.86 a | 19.97 ± 0.50 a | 24.40 ± 0.66 a | 0.82 ± 0.03 b | |
| Mean | 6.97 B | 6.01 B | 10.58 A | 38.08 A | 19.20 B | 17.36 B | 1.32 B | |
| Giza | ||||||||
| ECS | 6.08 ± 0.27 b | 4.97 ± 0.24 b | 8.27 ± 0.18 b | 32.90 ± 1.22 b | 22.93 ± 0.55 b | 29.53 ± 0.53 b | 0.78 ± 0.03 a | |
| FCS | 7.92 ± 0.21 a | 6.27 ± 0.18 a | 10.60 ± 0.12 a | 35.23 ± 0.43 a | 27.59 ± 0.68 a | 34.66 ± 0.66 a | 0.80 ± 0.01 a | |
| Mean | 7.0 B | 5.62 B | 9.43 C | 34.05 B | 25.26 A | 32.09 A | 0.79 C | |
| Dumara | ||||||||
| ECS | 7.12 ± 0.22 b | 6.31 ± 0.47 b | 8.87 ± 0.12 b | 38.76 ± 1.50 b | 19.16 ± 0.43 b | 9.49 ± 0.18 b | 2.02 ± 0.09 a | |
| FCS | 11.13 ± 0.68 a | 7.65 ± 0.32 a | 11.36 ± 0.09 a | 40.93 ± 0.32 a | 21.52 ± 0.56 a | 18.74 ± 1.08 a | 1.16 ± 0.08 b | |
| Mean | 9.13 A | 6.98 A | 10.12 B | 39.85 A | 20.34 B | 14.12 C | 1.59 A | |
| P503 F1 | ||||||||
| ECS | 5.44 ± 0.20 b | 3.95 ± 0.16 b | 8.43 ± 0.12 b | 34.94 ± 0.35 b | 24.12 ± 0.77 a | 32.25 ± 0.56 a | 0.75 ± 0.01 a | |
| FCS | 6.90 ± 0.28 a | 5.29 ± 0.20 a | 10.53 ± 0.12 a | 43.32 ± 0.38 a | 24.63 ± 0.72 a | 32.18 ± 1.47 a | 0.77 ± 0.06 a | |
| Mean | 6.57 B | 4.62 C | 9.48 C | 39.13 A | 24.38 A | 32.22 A | 0.76 C | |
| Cropping Seasons | ||
|---|---|---|
| ECS | FCS | |
| Carpometric traits | ||
| Marketable yield (kg plant−1) | 6.16 b | 8.67 a |
| Average fruit weight (kg) | 5.09 b | 6.53 a |
| Total soluble solids (°Brix) | 8.68 b | 11.13 a |
| Colour indexes | ||
| L* | 35.89 b | 39.66 a |
| a* | 21.16 b | 23.42 a |
| b* | 20.39 b | 27.50 a |
| a*/b* | 0.89 b | 1.34 a |
| Functional quality attributes | ||
| Citrulline (mg g−1 fw) | 1.29 b | 2.48 a |
| Lycopene (mg kg−1 fw) | 60.48 b | 81.16 a |
| β-carotene (mg kg−1 fw) | 4.40 b | 6.31 a |
| γ-carotene | 0.47 b | 0.65 a |
| Total phenolics (mg GAE kg−1 fw) | 115.70 b | 197.97 a |
| Flavonoids (mg RE kg−1 fw) | 168.19 b | 235.65 a |
| Total vitamin C (mg kg−1 fw) | 142.58 b | 187.25 a |
| Radical scavenging activities TEAC (µmol TE 100 g−1 fw) | ||
| HRSA | 255.04 b | 348.33 a |
| LRSA | 232.90 b | 307.81 a |
| Cultivar | Growing Season | Citrulline (mg g−1 fw) | Lycopene (mg kg−1 fw) | β-Carotene (mg kg−1 fw) | γ-Carotene (mg kg−1 fw) | Total Phenolics (mg EAG kg−1 fw) | Flavonoids (mg ER kg−1 fw) | Total Vitamin C (mg kg−1 fw) |
|---|---|---|---|---|---|---|---|---|
| Crimson Sweet | ||||||||
| ECS | 2.03± 0.03 b | 44.50± 0.98 b | 1.54 ± 0.06 b | 0.23 ± 0.01 b | 118.12 ± 1.13 b | 180.47 ± 3.57 b | 139.52 ± 5.92 b | |
| FCS | 3.21± 0.01 a | 56.53± 3.35 a | 2.64 ± 0.10 a | 0.44 ± 0.01 a | 171.18 ± 3.41 a | 235.27 ± 1.32 a | 186.43 ± 5.36 a | |
| Mean | 2.62 A | 50.51 C | 2.087 B | 0.33 C | 144.65 C | 207.87 B | 162.98 C | |
| Giza | ||||||||
| ECS | 1.23± 0.00 b | 72.55± 1.53 b | 7.05 ± 0.43 b | 0.58 ± 0.02 b | 153.68 ± 3.19 b | 183.88 ± 4.83 b | 179.36 ± 1.59 b | |
| FCS | 2.31 ± 0.02 a | 107.70 ± 3.85 a | 10.39 ± 0.50 a | 1.00 ± 0.07 a | 243.52 ± 3.90 a | 277.08 ± 5.21 a | 206.49 ± 4.94 a | |
| Mean | 1.77 C | 90.12 B | 8.72 A | 0.80 B | 198.60 A | 230.48 A | 192.92 A | |
| Dumara | ||||||||
| ECS | 0.47± 0.01 b | 47.05± 0.72 a | 1.95 ± 0.13 b | 0.22 ± 0.01 a | 111.43 ± 1.62 b | 121.15 ± 0.48 b | 113.44 ± 0.54 b | |
| FCS | 1.88± 0.04 a | 44.29± 1.25 a | 2.28 ± 0.10 a | 0.25 ± 0.01 a | 192.10 ± 4.27 a | 198.05 ± 3.61 a | 241.17 ± 1.07 a | |
| Mean | 1.17 D | 45.66 C | 2.12 B | 0.24 D | 151.77 B | 159.60 C | 177.30 B | |
| P503 F1 | ||||||||
| ECS | 1.40± 0.02 b | 77.83± 3.34 b | 7.05 ± 0.23 b | 0.84 ± 0.04 a | 79.55 ± 4.16 b | 187.26 ± 5.75 b | 138.01 ± 4.73 a | |
| FCS | 2.51 ± 0.02 a | 116.13± 2.1 a | 9.93 ± 0.52 a | 0.91 ± 0.02 a | 185.07 ± 1.21 a | 232.22 ± 2.59 a | 114.94 ± 3.90 b | |
| Mean | 1.95 B | 96.98 A | 8.49 A | 0.87 A | 132.31 D | 209.74 B | 126.48 D | |
| Cultivar | Cropping Season | TEAC Assay (µmol TE 100 g−1 fw) | |
|---|---|---|---|
| HRSA | LRSA | ||
| Crimson Sweet | |||
| ECS | 313.25 ± 7.97 b | 245.33 ± 3.03 b | |
| FCS | 537.38 ± 15.2 a | 438.01 ± 21.37 a | |
| Mean | 425.31 A | 341.67 A | |
| Giza | |||
| ECS | 255.27 ± 9.93 b | 247.19 ± 2.40 b | |
| FCS | 291.28 ± 4.7 a | 230.06 ± 3.60 a | |
| Mean | 273.27 B | 238.62 C | |
| Dumara | |||
| ECS | 231.03 ± 1.64 b | 231.95 ± 5.08 b | |
| FCS | 255.29 ± 0.83 a | 304.90 ± 4.22 a | |
| Mean | 243.16 C | 268.42 B | |
| P503 F1 | |||
| ECS | 220.60 ± 3.63 b | 207.15 ± 4.80 b | |
| FCS | 309.36 ± 13.01 a | 258.27 ± 8.31 a | |
| Trait | TSS | L* | a* | b* | a*/b* | Citr | TVC | TPC | Flav | Lyc | β-Car | ϒ-Car | HRSA | LRSA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSS | 1 | 0.57 ** | 0.53 ns | 0.10 ns | −0.26 ns | 0.78 ** | 0.52 ** | 0.68 ** | 0.60 ** | 0.56 ns | −0.07 ns | −0.018 ns | 0.67 ** | 0.75 ** |
| L* | 1 | −0.18 ns | −0.19 ns | 0.12 ns | 0.33 ns | −0.11 ns | 0.20 ns | 0.07 ns | 0.03 ns | −0.15 ns | −0.16 ns | 0.24 ns | 0.32 ns | |
| a* | 1 | 0.84 ** | 0.65 ** | 0.16 ns | 0.21 ns | 0.50 * | 0.64 ** | 0.84 ** | 0.89 ** | 0.89 ** | −0.23 ns | −0.33 ns | ||
| b* | 1 | 0.92 ** | 0.35 ns | 0.18 ns | 0.40 * | 0.69 ** | 0.82 ** | 0.88 ** | 0.91 ** | 0.03 ns | 0.07 ns | |||
| a*/b* | 1 | 0.48 * | −0.33 ns | −0.40 * | −0.69 ** | −0.63 ** | −0.68 ** | −0.72 ** | −0.20 ns | −0.19 ns | ||||
| Citr | 1 | 0.31 ns | 0.54 ** | 0.80 ** | 0.29 ns | 0.16 ns | 0.27 ns | 0.80 ** | 0.66 ** | |||||
| TVC | 1 | 0.61 ** | 0.45 * | −0.14 ns | −0.57 ns | −0.04 ns | 0.17 ns | 0.37 ns | ||||||
| TPC | 1 | 0.77 ** | 0.46 * | 0.42 * | 0.35 ns | 0.27 ns | 0.28 ns | |||||||
| Flav | 1 | 0.65 ** | 0.60 ** | 0.66 ** | 0.44 * | 0.28 ns | ||||||||
| Lyc | 1 | 0.95 ** | 0.93 ** | −0.07 ns | −0.29 ns | |||||||||
| β-Car | 1 | 0.94 ** | −0.20 ns | −0.38 ns | ||||||||||
| ϒ-Car | 1 | −0.09 ns | −0.29 ns | |||||||||||
| HRSA | 1 | 0.89 ** | ||||||||||||
| LRSA | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tlili, I.; Ilahy, R.; Romdhane, L.; R’him, T.; Ben Mohamed, H.; Zgallai, H.; Rached, Z.; Azam, M.; Henane, I.; Saïdi, M.N.; et al. Functional Quality and Radical Scavenging Activity of Selected Watermelon (Citrullus lanatus (Thunb.) Mansfeld) Genotypes as Affected by Early and Full Cropping Seasons. Plants 2023, 12, 1805. https://doi.org/10.3390/plants12091805
Tlili I, Ilahy R, Romdhane L, R’him T, Ben Mohamed H, Zgallai H, Rached Z, Azam M, Henane I, Saïdi MN, et al. Functional Quality and Radical Scavenging Activity of Selected Watermelon (Citrullus lanatus (Thunb.) Mansfeld) Genotypes as Affected by Early and Full Cropping Seasons. Plants. 2023; 12(9):1805. https://doi.org/10.3390/plants12091805
Chicago/Turabian StyleTlili, Imen, Riadh Ilahy, Leila Romdhane, Thouraya R’him, Hatem Ben Mohamed, Hatem Zgallai, Zouhair Rached, Muhammad Azam, Imen Henane, Mohamed Najib Saïdi, and et al. 2023. "Functional Quality and Radical Scavenging Activity of Selected Watermelon (Citrullus lanatus (Thunb.) Mansfeld) Genotypes as Affected by Early and Full Cropping Seasons" Plants 12, no. 9: 1805. https://doi.org/10.3390/plants12091805
APA StyleTlili, I., Ilahy, R., Romdhane, L., R’him, T., Ben Mohamed, H., Zgallai, H., Rached, Z., Azam, M., Henane, I., Saïdi, M. N., Pék, Z., Daood, H. G., Helyes, L., Hdider, C., & Lenucci, M. S. (2023). Functional Quality and Radical Scavenging Activity of Selected Watermelon (Citrullus lanatus (Thunb.) Mansfeld) Genotypes as Affected by Early and Full Cropping Seasons. Plants, 12(9), 1805. https://doi.org/10.3390/plants12091805









