Metabolic Discrimination between Adventitious Roots and Standard Medicinal Part of Atractylodes macrocephala Koidz. Using FT-IR Spectroscopy
Abstract
1. Introduction
2. Results
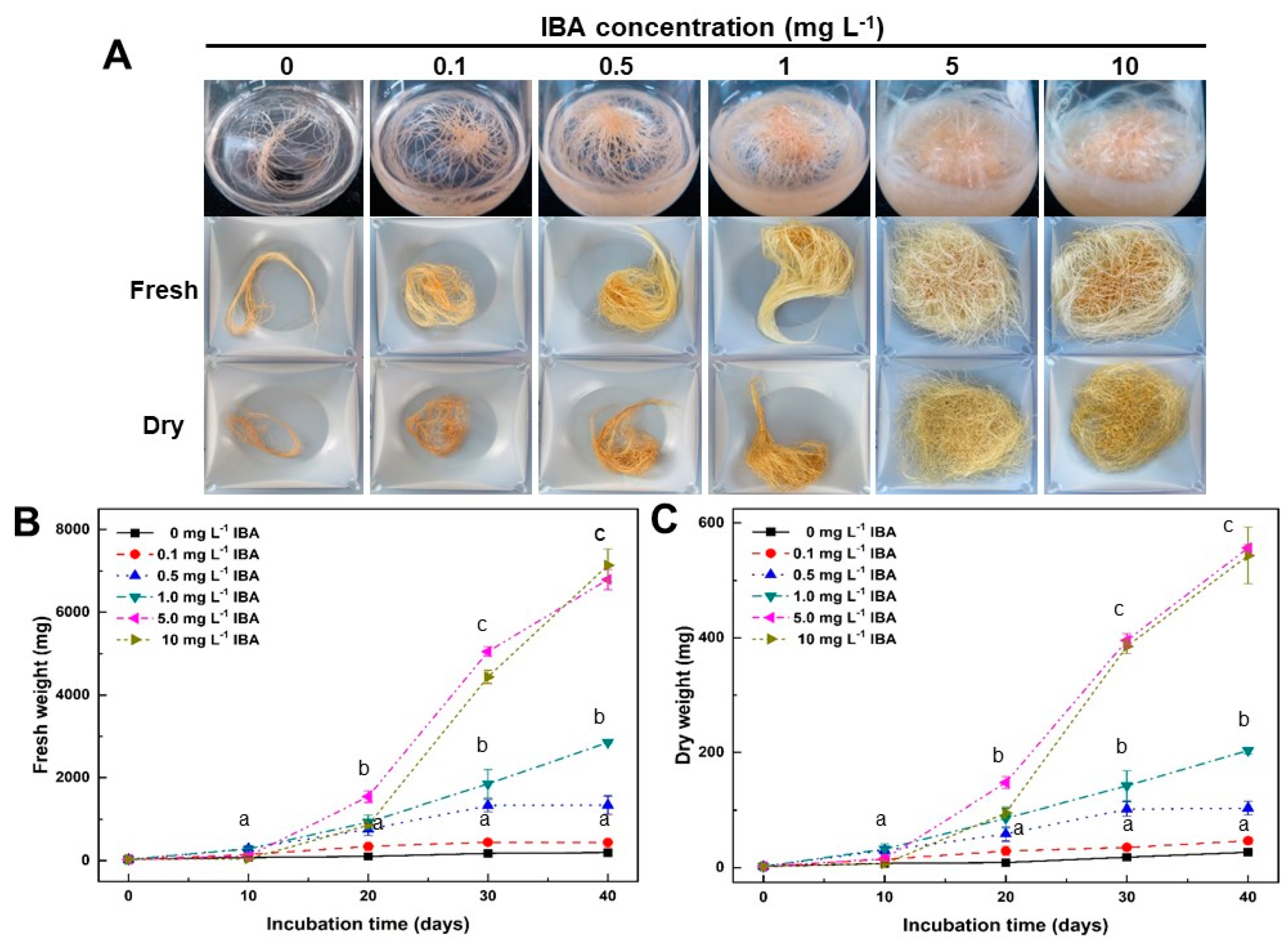
2.1. Effect of IBA Concentrations on Growth of Adventitious Roots
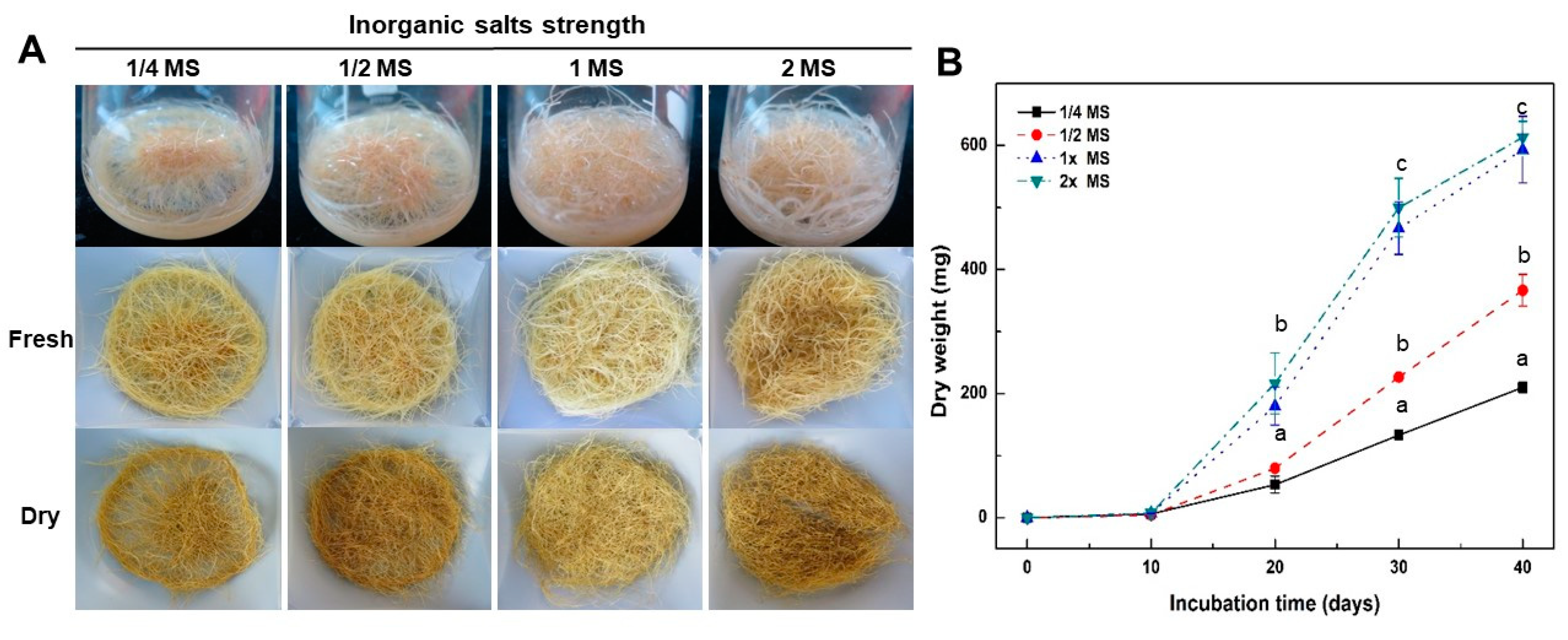
2.2. Effect of Culture Media and Inorganic Salt Concentrations in MS Medium on Growth of Adventitious Roots
2.3. Effect of Elicitor Types and Concentrations on Growth and Metabolic Change in Adventitious Roots
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Induction of Adventitious Roots from Leaf Explants
4.2. Effect of IBA Concentrations, Types of Culture Media, and Inorganic Salt Concentrations in Culture Medium on Growth of Adventitious Roots
4.3. Effect of Elicitor Types and Concentrations on Growth and Metabolic Change in Adventitious Roots
4.4. Quantification of Atractylenolide from Adventitious Roots by HPLC Analysis
4.5. Whole Cell Extract Preparation for FT-IR Spectroscopy
4.6. FT-IR Spectroscopy and Multivariate Statistical Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koo, W.-L.; Cho, J.-H.; Park, C.-G.; Ahn, Y.-S.; Park, C.-B. Effect of plant growth regulators on in vitro cultured Atractylodes hybrid ‘Dachul’ (A. macrocephala × A. japonica). Korean J. Plant Resour. 2011, 24, 591–598. [Google Scholar] [CrossRef]
- Sakurai, T.; Yamada, H.; Saito, K.; Kano, Y. Enzyme inhibitory activities of acetylene and sesquiterpene compounds in Atractylodes rhizome. Biol. Pharm. Bull. 1993, 16, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Jun, X.; Fu, P.; Lei, Y.; Cheng, P. Pharmacological effects of medicinal components of Atractylodes lancea (Thunb.) DC. Chin. Med. 2018, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhao, Z.; Chang, L.; Cao, Y.; Wang, S.; Kang, C.; Wang, H.; Zhou, L.; Huang, L.; Guo, L. Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J. Ethnopharmacol. 2021, 266, 113415. [Google Scholar] [CrossRef]
- Chung, H.G.; Bang, K.H.; Bang, J.K.; Lee, S.E.; Seong, N.S.; Cho, J.H.; Han, B.S.; Kim, S.M. Comparison of volatile components in essential oil from different origin of Atractylodes spp. Korean J. Med. Crop Sci. 2004, 12, 149–153. [Google Scholar]
- Zhu, B.; Zhang, Q.L.; Hua, J.W.; Cheng, W.L.; Qin, L.-P. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: A review. J. Ethnopharmacol. 2018, 226, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.H.; Seo, J.W.; Park, B.J.; Han, K.J.; Lee, J.G.; Kim, N.Y.; Kim, M.J.; Seong, E.S. Evaluation of growth characters and biological activities of ‘Dachul’, a hybrid medicinal plant of Atractylodes macrocephala × Atractylodes japonica, under different artificial light sources. Plants 2022, 11, 2035. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Kim, Y.W.; Bang, K.H.; Park, C.G.; Seong, N.S. Isolation of the Phytophthora root rot pathogen of Atractylodes macrocephala, Phytophthora drechsleri, and bioassay of the isolates with seedlings. Korean J. Med. Crop Sci. 2002, 10, 155–161. [Google Scholar]
- Murthy, H.N.; Dandin, V.S.; Paek, K.-Y. Tools for biotechnological production of useful phytochemicals from adventitious root cultures. Phytochem. Rev. 2016, 15, 129–145. [Google Scholar] [CrossRef]
- Hiraoka, N.; Yamada, N.; Kodama, T.; Tomita, Y. In vitro propagation of Atractylodes lancea. Plant Cell Rep. 1984, 3, 85–87. [Google Scholar] [CrossRef]
- Mao, B.; He, B.; Chen, Z.; Wang, B.; Pan, H.; Li, D. Effects of plant growth regulators on the rapid proliferation of shoots and root induction in the Chinese traditional medicinal plant Atractylodes macrocephala. Front. Biol. China 2009, 4, 217–221. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, S.L.; Lu, F.; Yang, S.H. Establishment of culture system of Atractylodes Koidz. ez Kitam. hairy roots and determination of polysaccharide. J. Chin. Pharm. Sci. 2014, 49, 1386–1392. [Google Scholar]
- Goodacre, R.; Timmins, É.M.; Burton, R.; Kaderbhai, N.; Woodward, A.M.; Kell, D.B.; Rooney, P.J. Rapid identification of urinary tract infection bacteria using hyperspectral whole-organism fingerprinting and artificial neural networks. Microbiology 1998, 144, 1157–1170. [Google Scholar] [CrossRef]
- Ward, J.L.; Harris, C.; Lewis, J.; Beale, M.H. Assessment of 1H NMR spectroscopy and multivariate analysis as a technique for metabolite fingerprinting of Arabidopsis thaliana. Phytochemistry 2003, 62, 949–957. [Google Scholar] [CrossRef]
- Timmins, É.M.; Howell, S.A.; Alsberg, B.K.; Noble, W.C.; Goodacre, R. Rapid differentiation of closely related Candida species and strains by pyrolysis-mass spectrometry and fourier transform-infrared spectroscopy. J. Clin. Microbiol. 1998, 36, 367–374. [Google Scholar] [CrossRef]
- Wenning, M.; Seiler, H.; Scherer, S. Fourier-transform infrared microspectroscopy, a novel and rapid tool for identification of yeasts. Appl. Environ. Microbiol. 2002, 68, 4717–4721. [Google Scholar] [CrossRef]
- Kim, S.W.; Ban, S.H.; Chung, H.; Cho, S.H.; Chung, H.J.; Choi, P.S.; Yoo, O.J.; Liu, J.R. Taxonomic discrimination of higher plants by multivariate analysis of Fourier transform infrared spectroscopy data. Plant Cell Rep. 2004, 23, 246–250. [Google Scholar] [CrossRef]
- Kim, S.W.; Min, S.R.; Kim, J.; Park, S.K.; Kim, T.I.; Liu, J.R. Rapid discrimination of commercial strawberry cultivars using Fourier transform infrared spectroscopy data combined by multivariate analysis. Plant Biotechnol. Rep. 2009, 3, 87–93. [Google Scholar] [CrossRef]
- Kwon, Y.-K.; Ahn, M.S.; Park, J.S.; Liu, J.R.; In, D.S.; Min, B.W.; Kim, S.W. Discrimination of cultivation ages and cultivars of ginseng leaves using Fourier transform infrared spectroscopy combined with multivariate analysis. J. Ginseng Res. 2014, 38, 52–58. [Google Scholar] [CrossRef]
- Ahn, M.S.; Min, S.R.; Jie, E.Y.; So, E.J.; Choi, S.Y.; Moon, B.C.; Kang, Y.M.; Park, S.-Y.; Kim, S.W. Rapid comparison of metabolic equivalence of standard medicinal parts from medicinal plants and their in vitro-generated adventitious roots using FT-IR spectroscopy. J. Plant Biotechnol. 2015, 42, 257–264. [Google Scholar] [CrossRef]
- Ahn, M.S.; So, E.J.; Jie, E.Y.; Choi, S.Y.; Park, S.U.; Moon, B.C.; Kang, Y.M.; Min, S.R.; Kim, S.W. Metabolic comparison between standard medicinal parts and their adventitious roots of Cynanchum wilfordii (Maxim.) Hemsl. using FT-IR spectroscopy after IBA and elicitor treatment. J. Plant Biotechnol. 2018, 45, 250–256. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1971, 50, 199–204. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirement of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Rahmat, E.; Kang, Y. Adventitious root culture for secondary metabolite production in medicinal plants: A review. J. Plant Biotechnol. 2019, 46, 143–157. [Google Scholar] [CrossRef]
- Pop, T.I.; Pamfil, D.; Bellini, C. Auxin control in the formation of adventitious roots. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Auxin-induced adventitious roots formation in nodal cuttings of Camellia sinensis. Int. J. Mol. Sci. 2019, 20, 4817. [Google Scholar] [CrossRef]
- Manokari, M.; Shekhawat, M. Implications of auxins in induction of adventitious roots from leaf explants of cannon ball tree (Couroupita guianensis Aubl.). World Sci. News. 2016, 33, 109–121. [Google Scholar]
- Cui, X.-H.; Murthy, H.N.; Wu, C.-H.; Paek, K.-Y. Sucrose-induced osmotic stress affects biomass, metabolite, and antioxidant levels in root suspension cultures of Hypericum perforatum L. Plant Cell Tiss. Organ Cult. 2010, 103, 7–14. [Google Scholar] [CrossRef]
- Ling, A.P.K.; Kok, K.M.; Hussein, S.; Ong, S.L. Effects of plant growth regulators on adventitious roots induction from different explants of Orthosiphon stamineus. Am.-Eurasian J. Sustain. Agric. 2009, 3, 493–501. [Google Scholar]
- Liu, H.; Wang, J.; Gao, W.; Wang, Q.; Zhang, L.; Man, S. Optimization and quality assessment of adventitious roots culture in Panax quinquefolius L. Acta Physiol. Plant. 2014, 36, 713–719. [Google Scholar] [CrossRef]
- Wu, C.-H.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Establishment of adventitious root co-culture of Ginseng and Echinacea for the production of secondary metabolites. Acta Physiol. Plant. 2008, 30, 891–896. [Google Scholar] [CrossRef]
- Chang, S.W.; Kim, I.H.; Han, T.J. Anthraquinone productivity by the cultures of adventitious roots and hairy roots from curled dock (Rumex crispus). Korean J. Plant Tiss. Cult. 1999, 26, 7–14. [Google Scholar]
- Wu, C.-H.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Improved production of caffeic acid derivatives in suspension cultures of Echinacea purpurea by medium replenishment strategy. Arch. Pharm. Res. 2007, 30, 945–949. [Google Scholar] [CrossRef]
- Sharma, S.N.; Jha, Z.; Sinha, R.K. Establishment of in vitro adventitious root cultures and analysis of andrographolide in Andrographis paniculata. Nat. Prod. Commun. 2013, 8, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Jung, H.Y.; Min, J.Y.; Chung, Y.G.; Lee, C.H.; Choi, M.S. Production of tropane alkaloids by two-stage culture of Scopolia parviflora Nakai adventitious root. Korean J. Med. Crop Sci. 2004, 12, 372–377. [Google Scholar]
- Jiang, X.L.; Jin, M.Y.; Piao, X.C.; Yin, C.R.; Lian, M.L. Fed-batch culture of Oplopanax elatus adventitious roots: Feeding medium selection through comprehensive evaluation using an analytic hierarchy process. Biochem. Eng. J. 2021, 167, 107927. [Google Scholar] [CrossRef]
- Wu, C.-H.; Dewir, Y.H.; Hahn, E.-J.; Paek, K.-Y. Optimizing of culturing conditions for the production of biomass and phenolics from adventitious roots of Echinacea angustifolia. J. Plant Biol. 2006, 49, 193–199. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Yu, X.-K.; Lian, M.-L.; Park, S.-Y.; Piao, X.-C. Several factors affecting hypericin production of Hypericum perforatum during adventitious root culture in airlift bioreactors. Acta Physiol. Plant. 2014, 36, 975–981. [Google Scholar] [CrossRef]
- Sivakumar, G.; Paek, K.Y. Methyl jasmonate induce enhanced production of soluble biophenols in Panax ginseng adventitious roots from commercial scale bioreactors. Chem. Nat. Compd. 2005, 41, 669–673. [Google Scholar] [CrossRef]
- Ho, T.-T.; Lee, J.-D.; Jee, C.-S.; Paek, K.-Y.; Park, S.-Y. Improvement of biosynthesis and accumulation of bioactive compounds by elicitation in adventitious root cultures of Polygonum multiforum. Appl. Microbiol. Biotechnol. 2018, 102, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Jung, H.-Y.; Kang, Y.-M.; Yun, D.-J.; Bahk, J.-D.; Yang, J.-K.; Choi, M.-S. Effects of methyl jasmonate and salicylic acid on the production of tropane alkaloids and the expression of PMT and H6H in adventitious root cultures of Scopolia parviflora. Plant Sci. 2004, 166, 745–751. [Google Scholar] [CrossRef]
- Zhao, J.; Davis, L.C.; Verpoorte, R. Elicitors signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [Google Scholar]
- Baranski, R.; Baranska, M.; Schulz, H.; Simon, P.W.; Nothnagel, T. Single seed Raman measurements allow taxonomical discrimination of Apiaceae accessions collected in gene banks. Biopolymers 2006, 81, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Schwinté, P.; Foerstendorf, H.; Hussain, Z.; Gärtner, W.; Mronginski, M.-A.; Hildebrandt, P.; Siebert, F. FTIR study of the photoinduced processes of plant phytochrome phyA using isotope-labeled bilins and density functional theory calculations. Biophys. J. 2008, 95, 1256–1267. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.Y.; Ku, S.S.; Ahn, M.S.; So, E.J.; Kim, H.; Park, S.U.; Lee, M.-S.; Kang, Y.M.; Min, S.R.; Kim, S.W. Metabolic Discrimination between Adventitious Roots and Standard Medicinal Part of Atractylodes macrocephala Koidz. Using FT-IR Spectroscopy. Plants 2023, 12, 1821. https://doi.org/10.3390/plants12091821
Choi SY, Ku SS, Ahn MS, So EJ, Kim H, Park SU, Lee M-S, Kang YM, Min SR, Kim SW. Metabolic Discrimination between Adventitious Roots and Standard Medicinal Part of Atractylodes macrocephala Koidz. Using FT-IR Spectroscopy. Plants. 2023; 12(9):1821. https://doi.org/10.3390/plants12091821
Chicago/Turabian StyleChoi, So Yeon, Seong Sub Ku, Myung Suk Ahn, Eun Jin So, HyeRan Kim, Sang Un Park, Moon-Soon Lee, Young Min Kang, Sung Ran Min, and Suk Weon Kim. 2023. "Metabolic Discrimination between Adventitious Roots and Standard Medicinal Part of Atractylodes macrocephala Koidz. Using FT-IR Spectroscopy" Plants 12, no. 9: 1821. https://doi.org/10.3390/plants12091821
APA StyleChoi, S. Y., Ku, S. S., Ahn, M. S., So, E. J., Kim, H., Park, S. U., Lee, M.-S., Kang, Y. M., Min, S. R., & Kim, S. W. (2023). Metabolic Discrimination between Adventitious Roots and Standard Medicinal Part of Atractylodes macrocephala Koidz. Using FT-IR Spectroscopy. Plants, 12(9), 1821. https://doi.org/10.3390/plants12091821




