Genome-Wide Identification and Expression Analysis of the Dof Transcription Factor in Annual Alfalfa Medicago polymorpha
Abstract
:1. Introduction
2. Results
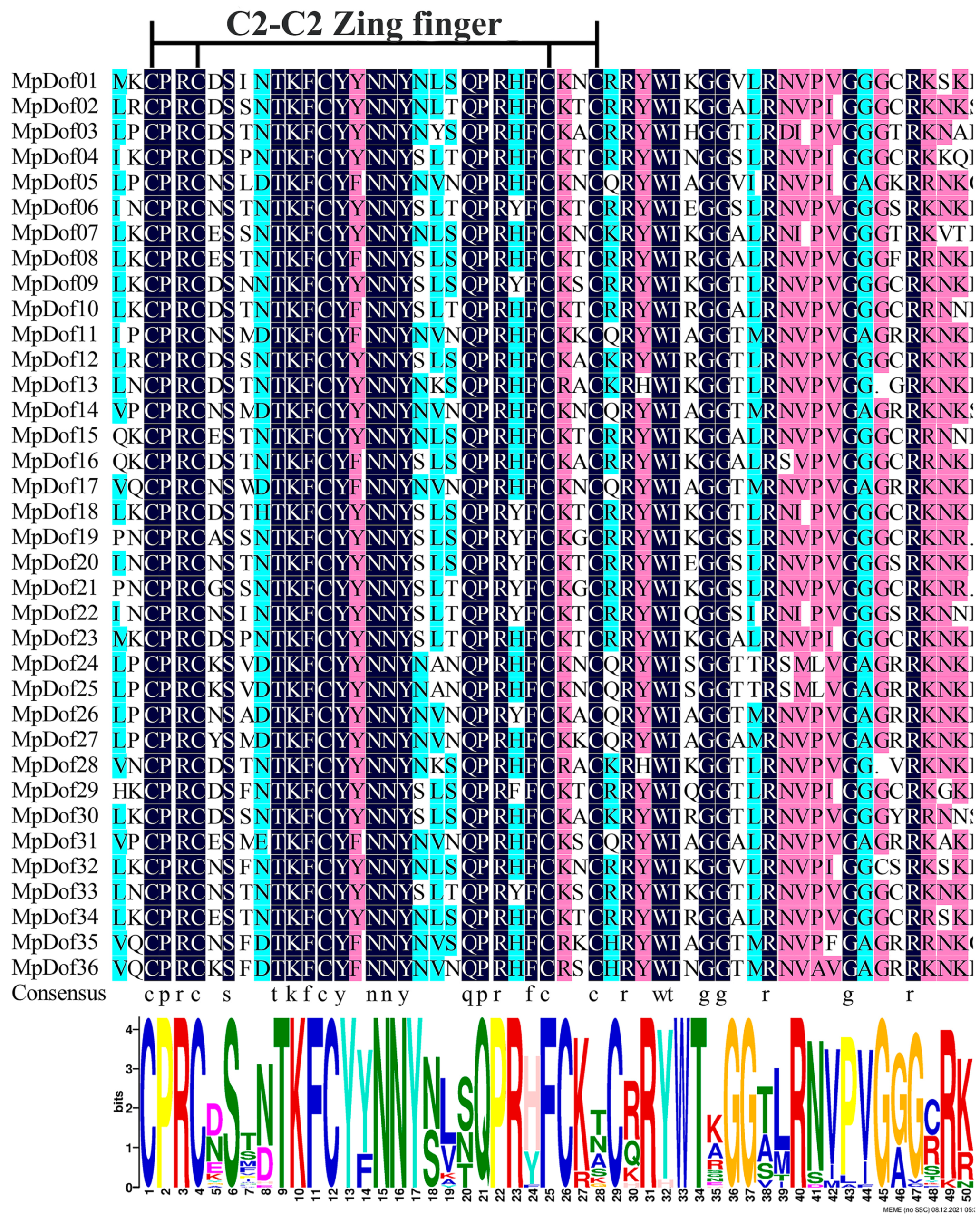
2.1. Identification and Characteristics of the MpDof Gene Family
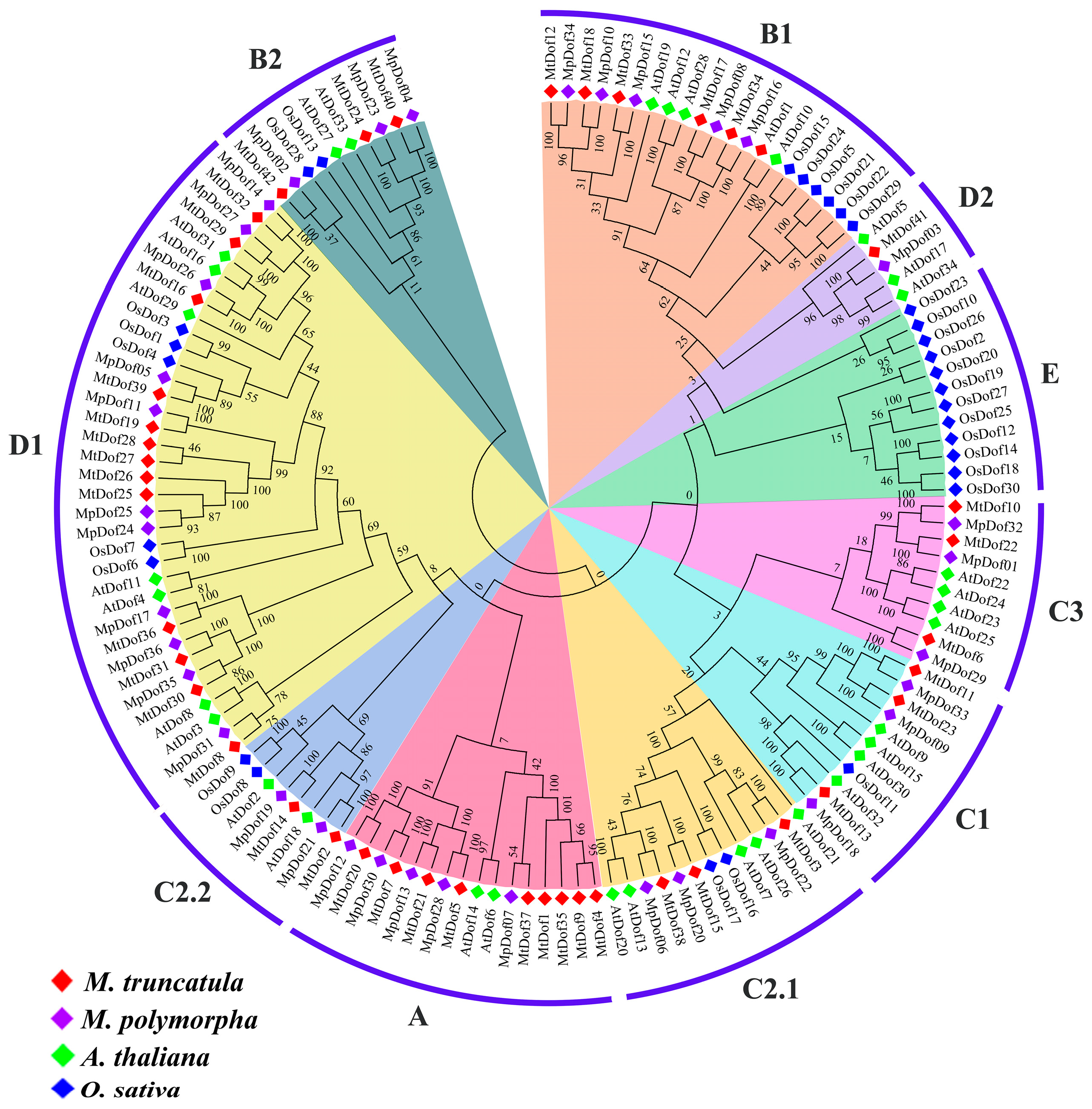
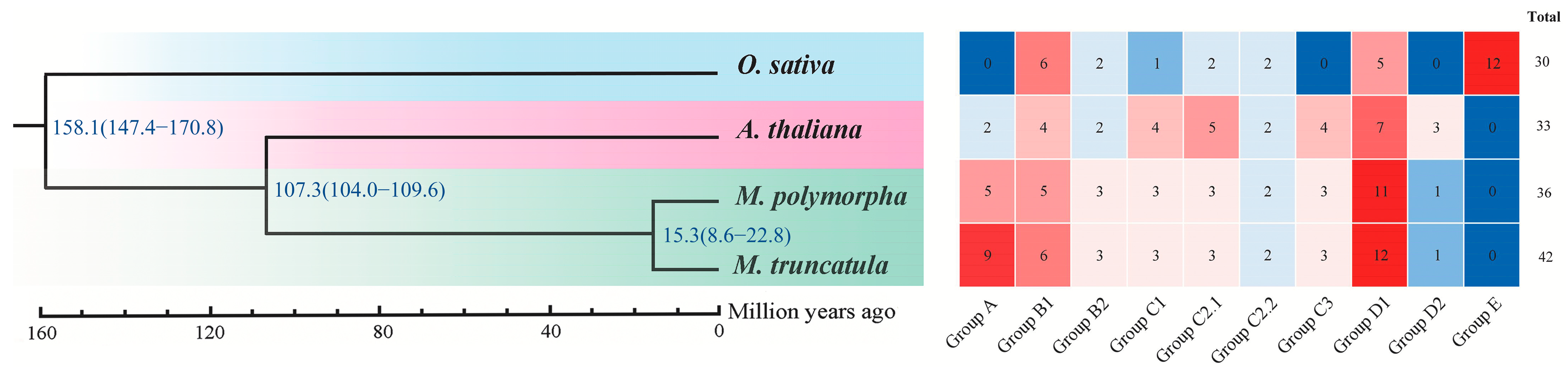
2.2. Comparative Phylogenetic Analysis of Dof Genes in M. polymorpha
2.3. Gene Structure and Conserved Motifs Analysis of the MpDofs
2.4. The Location on Chromosome, Gene Duplication, and Syntenic Analysis
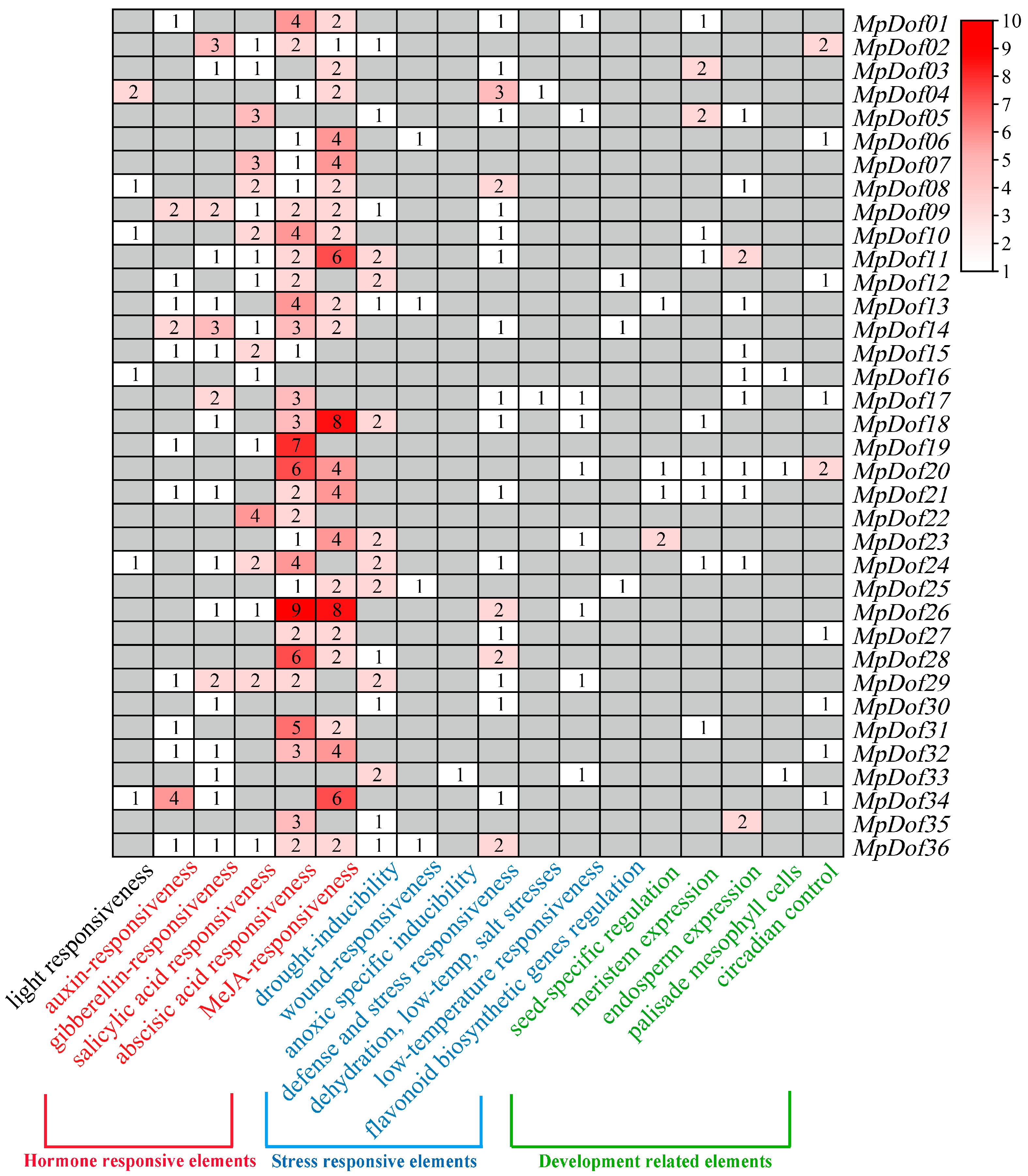
2.5. Cis-Acting Elements Analysis
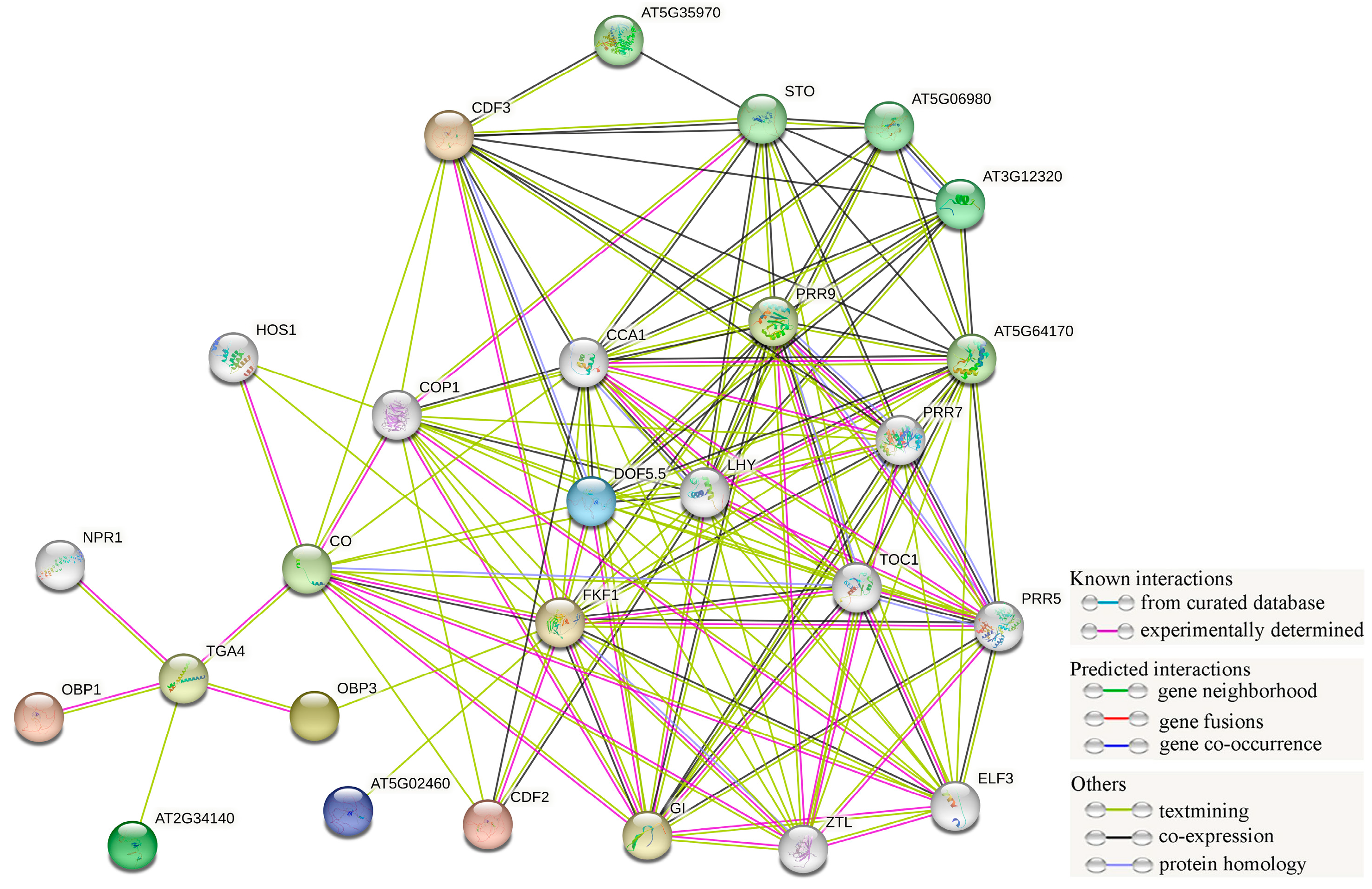
2.6. MpDofs Protein–Protein Interaction Network Analysis
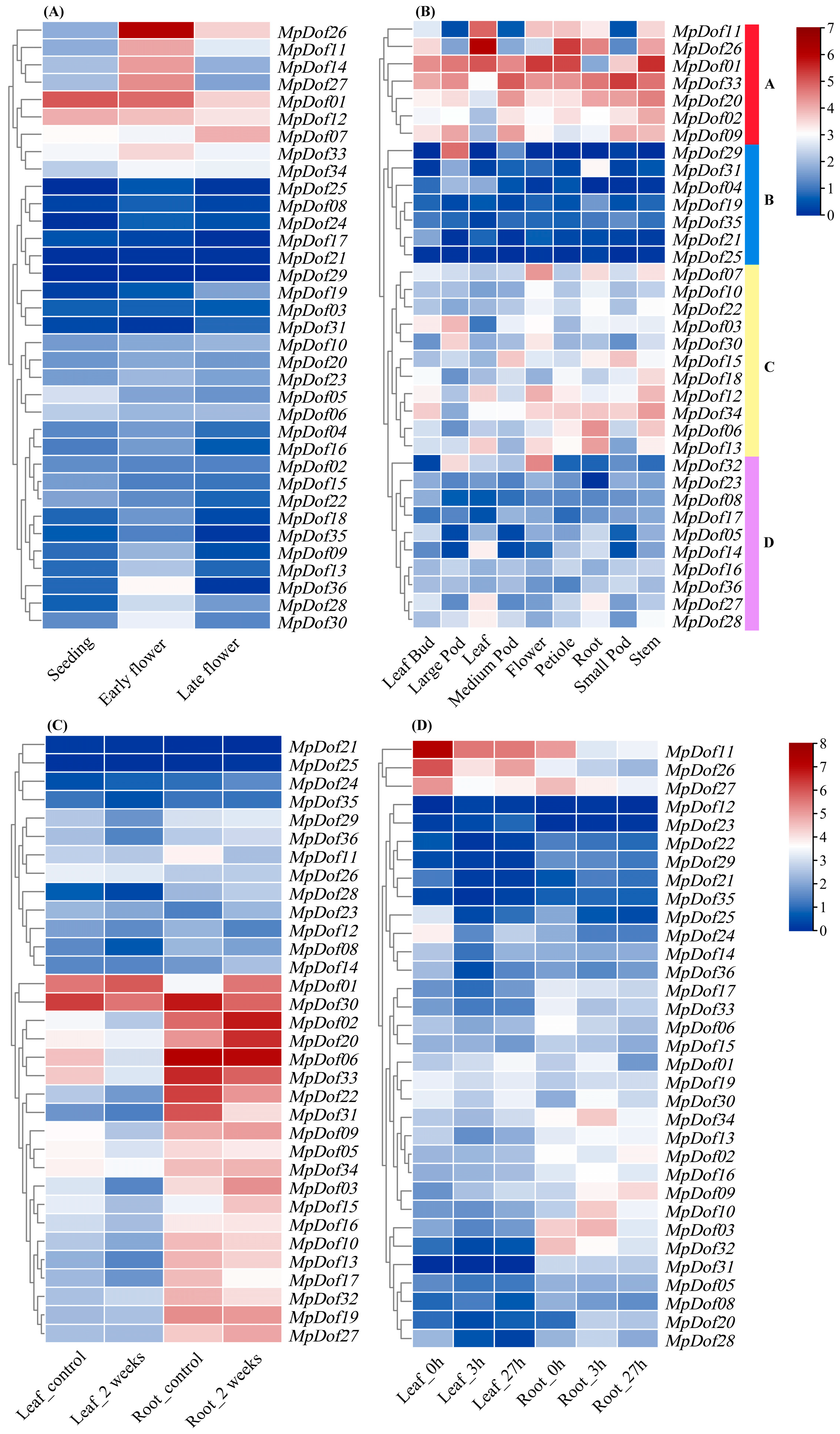
2.7. Expression Patterns of MpDof Genes at Different Development Stages, Tissues, and Abiotic Stresses in Medicago Plants
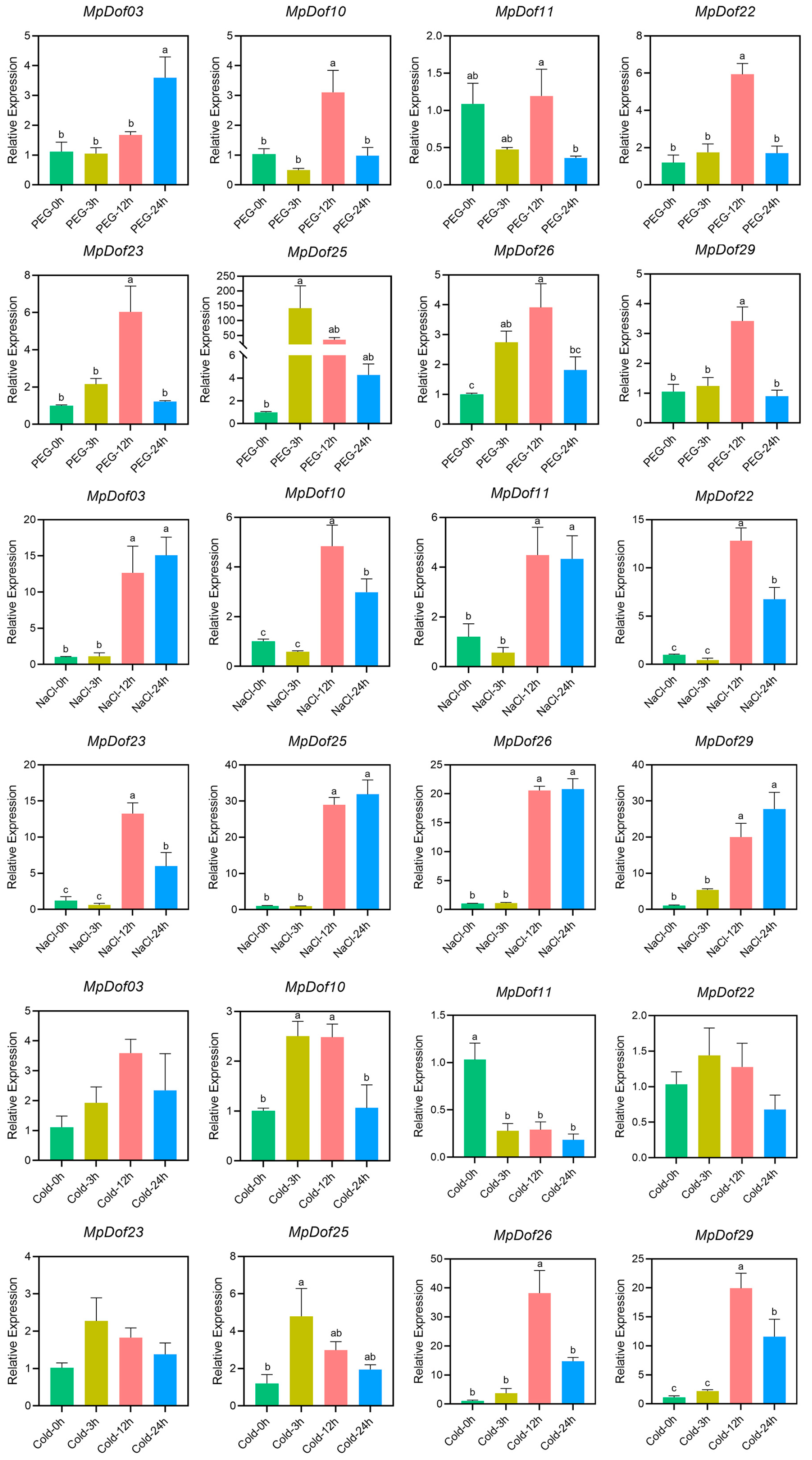
2.8. Validation of MpDof Genes Expression Patterns under Abiotic Stress
3. Discussion
4. Materials and Methods
4.1. Genome-Wide Identification of Dof Transcription Factor in M. polymorpha
4.2. Phylogenetic Analysis of Dof Family Members and Orthologous Groups Identification
4.3. Detection of Gene Structures and Conserved Motifs of MpDofs
4.4. Chromosomal Localization, Gene Duplication Events, and Synteny Analysis of the MpDof Genes
4.5. Identification of Promoter Cis-Acting Elements
4.6. Functional Protein Interaction Network Prediction of MpDofs
4.7. Expression Analysis of Dof Genes among Different Tissues at Different Development Stages
4.8. Expression Pattern Analysis of MpDof Genes under Abiotic Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qu, L.J.; Zhu, Y.X. Transcription factor families in Arabidopsis: Major progress and outstanding issues for future research. Curr. Opin. Plant Biol. 2006, 9, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Schmidt, R.J. Diversity and similarity among recognition sequences of Dof transcription factors. Plant J. 1999, 17, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Noguero, M.; Atif, R.M.; Ochatt, S.; Thompson, R.D. The role of the DNA-binding One Zinc Finger (DOF) transcription factor family in plants. Plant Sci. 2013, 209, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, M.-Y.; Wang, F.; Tang, J.; Xiong, A.-S. Genome-wide analysis of Dof family transcription factors and their responses to abiotic stresses in Chinese cabbage. BMC Genom. 2015, 16, 33. [Google Scholar] [CrossRef]
- Gupta, S.; Malviya, N.; Kushwaha, H.; Nasim, J.; Bisht, N.C.; Singh, V.; Yadav, D. Insights into structural and functional diversity of Dof (DNA binding with one finger) transcription factor. Planta 2015, 241, 549–562. [Google Scholar] [CrossRef]
- Yanagisawa, S.; Izui, K. Molecular cloning of two DNA-binding proteins of maize that are structurally different but interact with the same sequence motif. J. Biol. Chem. 1993, 268, 16028–16036. [Google Scholar] [CrossRef]
- Lohani, N.; Babaei, S.; Singh, M.B.; Bhalla, P.L. Genome-Wide In Silico Identification and Comparative Analysis of Dof Gene Family in Brassica napus. Plants 2021, 10, 709. [Google Scholar] [CrossRef]
- Moreno-Risueno, M.Á.; Martínez, M.; Vicente-Carbajosa, J.; Carbonero, P. The family of DOF transcription factors: From green unicellular algae to vascular plants. Mol. Genet. Genom. 2007, 277, 379–390. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, G.; Gu, H.; Qu, L.J. Dof5.6/HCA2, a Dof transcription factor gene, regulates interfascicular cambium formation and vascular tissue development in Arabidopsis. Plant Cell 2009, 21, 3518–3534. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.; Chen, M.; Zhou, W.; Dong, Q.; Jiang, H.; Cheng, B. The DOF-domain transcription factor ZmDOF36 positively regulates starch synthesis in transgenic maize. Front. Plant Sci. 2019, 10, 465. [Google Scholar] [CrossRef]
- Molina-Hidalgo, F.J.; Medina-Puche, L.; Cañete-Gómez, C.; Franco-Zorrilla, J.M.; López-Vidriero, I.; Solano, R.; Caballero, J.L.; Rodríguez-Franco, A.; Blanco-Portales, R.; Muñoz-Blanco, J. The fruit-specific transcription factor FaDOF2 regulates the production of eugenol in ripe fruit receptacles. J. Exp. Bot. 2017, 68, 4529–4543. [Google Scholar] [CrossRef]
- Su, Y.; Liang, W.; Liu, Z.; Wang, Y.; Zhao, Y.; Ijaz, B.; Hua, J. Overexpression of GhDof1 improved salt and cold tolerance and seed oil content in Gossypium hirsutum. J. Plant Physiol. 2017, 218, 222–234. [Google Scholar] [CrossRef]
- Ramírez Gonzales, L.; Shi, L.; Bergonzi, S.B.; Oortwijn, M.; Franco-Zorrilla, J.M.; Solano-Tavira, R.; Visser, R.G.; Abelenda, J.A.; Bachem, C.W. Potato CYCLING DOF FACTOR 1 and its lncRNA counterpart StFLORE link tuber development and drought response. Plant J. 2021, 105, 855–869. [Google Scholar] [CrossRef]
- Mena, M.; Vicente-Carbajosa, J.; Schmidt, R.J.; Carbonero, P. An endosperm-specific DOF protein from barley, highly conserved in wheat, binds to and activates transcription from the prolamin-box of a native B-hordein promoter in barley endosperm. Plant J. 1998, 16, 53–62. [Google Scholar] [CrossRef]
- Vicente-Carbajosa, J.; Moose, S.P.; Parsons, R.L.; Schmidt, R.J. A maize zinc-finger protein binds the prolamin box in zein gene promoters and interacts with the basic leucine zipper transcriptional activator Opaque2. Proc. Natl. Acad. Sci. USA 1997, 94, 7685–7690. [Google Scholar] [CrossRef]
- Diaz, I.; Vicente-Carbajosa, J.; Abraham, Z.; Martínez, M.; Isabel-La Moneda, I.; Carbonero, P. The GAMYB protein from barley interacts with the DOF transcription factor BPBF and activates endosperm-specific genes during seed development. Plant J. 2002, 29, 453–464. [Google Scholar] [CrossRef]
- Mena, M.; Cejudo, F.J.; Isabel-Lamoneda, I.; Carbonero, P. A role for the DOF transcription factor BPBF in the regulation of gibberellin-responsive genes in barley aleurone. Plant Physiol. 2002, 130, 111–119. [Google Scholar] [CrossRef]
- Santopolo, S.; Boccaccini, A.; Lorrai, R.; Ruta, V.; Capauto, D.; Minutello, E.; Serino, G.; Costantino, P.; Vittorioso, P. DOF AFFECTING GERMINATION 2 is a positive regulator of light-mediated seed germination and is repressed by DOF AFFECTING GERMINATION 1. BMC Plant Biol. 2015, 15, 72. [Google Scholar] [CrossRef]
- Martín, G.; Veciana, N.; Boix, M.; Rovira, A.; Henriques, R.; Monte, E. The photoperiodic response of hypocotyl elongation involves regulation of CDF1 and CDF5 activity. Physiol. Plant. 2020, 169, 480–490. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Wu, X.; Meng, L.; Zou, Z.; Bao, E.; Bian, Z.; Cao, K. Tomato SlCDF3 Delays Flowering Time by Regulating Different FT-Like Genes Under Long-Day and Short-Day Conditions. Front. Plant Sci. 2021, 12, 826. [Google Scholar] [CrossRef]
- Li, H.; Huang, W.; Liu, Z.-W.; Wang, Y.-X.; Zhuang, J. Transcriptome-based analysis of Dof family transcription factors and their responses to abiotic stress in tea plant (Camellia sinensis). Int. J. Genom. 2016, 2016, 5614142. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhang, C.; Shu, W.; Ye, Z.; Li, H.; Zhang, Y. The transcription factor SlDof22 involved in ascorbate accumulation and salinity stress in tomato. Biochem. Biophys. Res. Commun. 2016, 474, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dai, H. Brassica napus Cycling Dof Factor1 (BnCDF1) is involved in flowering time and freezing tolerance. Plant Growth Regul. 2016, 80, 315–322. [Google Scholar] [CrossRef]
- Sun, S.; Wang, B.; Jiang, Q.; Li, Z.; Jia, S.; Wang, Y.; Guo, H. Genome-wide analysis of BpDof genes and the tolerance to drought stress in birch (Betula platyphylla). PeerJ 2021, 9, e11938. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Reichelt, M.; Burow, M.; Birkemeyer, C.; Rolcik, J.; Kopka, J.; Zanor, M.I.; Gershenzon, J.; Strnad, M.; Szopa, J. DOF transcription factor AtDof1. 1 (OBP2) is part of a regulatory network controlling glucosinolate biosynthesis in Arabidopsis. Plant J. 2006, 47, 10–24. [Google Scholar] [CrossRef]
- Chen, W.; Chao, G.; Singh, K.B. The promoter of a H2O2-inducible, Arabidopsis glutathione S-transferase gene contains closely linked OBF-and OBP1-binding sites. Plant J. 1996, 10, 955–966. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Q.; Zhu, Y.; Li, T. Identification of MdDof genes in apple and analysis of their response to biotic or abiotic stress. Funct. Plant Biol. 2017, 45, 528–541. [Google Scholar] [CrossRef]
- Ewing, M. Annual pasture legumes: A vital component stabilizing and rehabilitating low-rainfall Mediterranean ecosystems. Arid Soil Res. Rehabil. 1999, 13, 327–342. [Google Scholar] [CrossRef]
- Del Pozo, A.; Ovalle, C.; Aronson, J.; Avendano, J. Ecotypic differentiation in Medicago polymorpha L. along an environmental gradient in central Chile. I. Phenology, biomass production and reproductive patterns. Plant Ecol. 2002, 159, 119–130. [Google Scholar] [CrossRef]
- Cui, J.; Wang, X.; Wei, Z.; Jin, B. Medicago truncatula (model legume), Medicago sativa (alfalfa), Medicago polymorpha (bur clover), and Medicago ruthenica. Trends Genet. 2022, 38, 782–783. [Google Scholar] [CrossRef]
- Cui, J.; Lu, Z.; Wang, T.; Chen, G.; Mostafa, S.; Ren, H.; Liu, S.; Fu, C.; Wang, L.; Zhu, Y. The genome of Medicago polymorpha provides insights into its edibility and nutritional value as a vegetable and forage legume. Hortic. Res. 2021, 8, 47. [Google Scholar] [CrossRef]
- Chattha, W.S.; Atif, R.M.; Iqbal, M.; Shafqat, W.; Farooq, M.A.; Shakeel, A. Genome-wide identification and evolution of Dof transcription factor family in cultivated and ancestral cotton species. Genomics 2020, 112, 4155–4170. [Google Scholar] [CrossRef]
- Khan, I.; Khan, S.; Zhang, Y.; Zhou, J. Genome-wide analysis and functional characterization of the Dof transcription factor family in rice (Oryza sativa L.). Planta 2021, 253, 101. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, W.; Hu, T.; Hu, H.; Mao, W.; Zhu, Q.; Bao, C. Genome-wide identification and characterization of Dof transcription factors in eggplant (Solanum melongena L.). PeerJ 2018, 6, e4481. [Google Scholar] [CrossRef]
- Malviya, N.; Gupta, S.; Singh, V.; Yadav, M.; Bisht, N.; Sarangi, B.; Yadav, D. Genome wide in silico characterization of Dof gene families of pigeonpea (Cajanus cajan (L.) Millsp.). Mol. Biol. Rep. 2015, 42, 535–552. [Google Scholar] [CrossRef]
- Tokunaga, S.; Sanda, S.; Uraguchi, Y.; Nakagawa, S.; Sawayama, S. Overexpression of the DOF-type transcription factor enhances lipid synthesis in Chlorella vulgaris. Appl. Biochem. Biotechnol. 2019, 189, 116–128. [Google Scholar] [CrossRef]
- Feng, B.-H.; Han, Y.-C.; Xiao, Y.-Y.; Kuang, J.-F.; Fan, Z.-Q.; Chen, J.-Y.; Lu, W.-J. The banana fruit Dof transcription factor MaDof23 acts as a repressor and interacts with MaERF9 in regulating ripening-related genes. J. Exp. Bot. 2016, 67, 2263–2275. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Wei, J.; Yoon, H.; An, G. Transcription factor OsDOF18 controls ammonium uptake by inducing ammonium transporters in rice roots. Mol. Cells 2017, 40, 178. [Google Scholar]
- Shu, Y.; Song, L.; Zhang, J.; Liu, Y.; Guo, C. Genome-wide identification and characterization of the Dof gene family in Medicago truncatula. Genet. Mol. Res. 2015, 14, 10645–10657. [Google Scholar] [CrossRef]
- Lijavetzky, D.; Carbonero, P.; Vicente-Carbajosa, J. Genome-wide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families. BMC Evol. Biol. 2003, 3, 17. [Google Scholar] [CrossRef]
- Cao, B.; Cui, Y.; Lou, K.; Luo, D.; Liu, Z.; Zhou, Q. Genome-wide identification and expression analysis of the Dof gene family in Medicago sativa L. under various abiotic stresses. DNA Cell Biol. 2020, 39, 1976–1989. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, H.; Gupta, S.; Singh, V.K.; Rastogi, S.; Yadav, D. Genome wide identification of Dof transcription factor gene family in sorghum and its comparative phylogenetic analysis with rice and Arabidopsis. Mol. Biol. Rep. 2011, 38, 5037–5053. [Google Scholar] [CrossRef] [PubMed]
- Shigyo, M.; Tabei, N.; Yoneyama, T.; Yanagisawa, S. Evolutionary processes during the formation of the plant-specific Dof transcription factor family. Plant Cell Physiol. 2007, 48, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Lin, X.; Chan, T.F.; Imtiaz, M.; Rehman, H.M.; Ali, M.A.; Baloch, F.S.; Atif, R.M.; Yang, S.H.; Chung, G. Characterization of Cellulose Synthase A (CESA) Gene Family in Eudicots. Biochem. Genet. 2019, 57, 248–272. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Li, C.; Zhang, J.; Tian, Y.; Wang, H.; Zhang, Y.; Zhang, Z.; Xiang, Q.; Han, X.; Zhang, L. Genome-wide identification and expression analysis of the Dof gene family under drought stress in tea (Camellia sinensis). PeerJ 2020, 8, e9269. [Google Scholar] [CrossRef]
- Hahn, M.W.; De Bie, T.; Stajich, J.E.; Nguyen, C.; Cristianini, N. Estimating the tempo and mode of gene family evolution from comparative genomic data. Genome Res. 2005, 15, 1153–1160. [Google Scholar] [CrossRef]
- Gabriele, S.; Rizza, A.; Martone, J.; Circelli, P.; Costantino, P.; Vittorioso, P. The Dof protein DAG1 mediates PIL5 activity on seed germination by negatively regulating GA biosynthetic gene AtGA3ox1. Plant J. 2010, 61, 312–323. [Google Scholar] [CrossRef]
- Derbyshire, P.; Drea, S.; Shaw, P.J.; Doonan, J.H.; Dolan, L. Proximal-distal patterns of transcription factor gene expression during Arabidopsis root development. J. Exp. Bot. 2008, 59, 235–245. [Google Scholar] [CrossRef]
- Imaizumi, T.; Schultz, T.F.; Harmon, F.G.; Ho, L.A.; Kay, S.A. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 2005, 309, 293–297. [Google Scholar] [CrossRef]
- Fornara, F.; Panigrahi, K.C.; Gissot, L.; Sauerbrunn, N.; Rühl, M.; Jarillo, J.A.; Coupland, G. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev. Cell 2009, 17, 75–86. [Google Scholar] [CrossRef]
- Corrales, A.R.; Carrillo, L.; Lasierra, P.; Nebauer, S.G.; Dominguez-Figueroa, J.; Renau-Morata, B.; Pollmann, S.; Granell, A.; Molina, R.V.; Vicente-Carbajosa, J. Multifaceted role of cycling DOF factor 3 (CDF3) in the regulation of flowering time and abiotic stress responses in Arabidopsis. Plant Cell Environ. 2017, 40, 748–764. [Google Scholar] [CrossRef]
- Fornara, F.; de Montaigu, A.; Sánchez-Villarreal, A.; Takahashi, Y.; Ver Loren van Themaat, E.; Huettel, B.; Davis, S.J.; Coupland, G. The GI-CDF module of Arabidopsis affects freezing tolerance and growth as well as flowering. Plant J. 2015, 81, 695–706. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Xu, L.; Wang, C.; Luo, Y.; Feng, S.; Yuan, Y.; Yang, Q.; Feng, B. Genome-Wide Identification of DNA Binding with One Finger (Dof) Gene Family in Tartary Buckwheat (Fagopyrum tataricum) and Analysis of Its Expression Pattern after Exogenous Hormone Stimulation. Biology 2022, 11, 173. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Morgan, C.C.; Loughran, N.B.; Walsh, T.A.; Harrison, A.J.; O’Connell, M.J. Positive selection neighboring functionally essential sites and disease-implicated regions of mammalian reproductive proteins. BMC Evol. Biol. 2010, 10, 39. [Google Scholar] [CrossRef]
- Zou, Z.; Zhu, J.; Zhang, X. Genome-wide identification and characterization of the Dof gene family in cassava (Manihot esculenta). Gene 2019, 687, 298–307. [Google Scholar] [CrossRef]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.F.; Lindquist, E.A.; Kamisugi, Y.; et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef]
- Liu, H.; Lyu, H.M.; Zhu, K.; Van de Peer, Y.; Cheng, Z.M. The emergence and evolution of intron-poor and intronless genes in intron-rich plant gene families. Plant J. 2021, 105, 1072–1082. [Google Scholar] [CrossRef]
- Corrales, A.-R.; Nebauer, S.G.; Carrillo, L.; Fernandez-Nohales, P.; Marques, J.; Renau-Morata, B.; Granell, A.; Pollmann, S.; Vicente-Carbajosa, J.; Molina, R.-V.; et al. Characterization of tomato Cycling Dof Factors reveals conserved and new functions in the control of flowering time and abiotic stress responses. J. Exp. Bot. 2014, 65, 995–1012. [Google Scholar] [CrossRef]
- Liu, J.; Meng, Q.; Xiang, H.; Shi, F.; Ma, L.; Li, Y.; Liu, C.; Liu, Y.; Su, B. Genome-wide analysis of Dof transcription factors and their response to cold stress in rice (Oryza sativa L.). BMC Genom. 2021, 22, 800. [Google Scholar] [CrossRef]
- Guo, T.; Wang, S.; Zhang, T.; Xu, L.; Li, Y.; Chao, Y.; Han, L. Expression of the Medicago truncatula MtDof32 transcription factor regulates plant growth and enhances abiotic stress tolerances in transgenic Arabidopsis. Environ. Exp. Bot. 2021, 183, 104339. [Google Scholar] [CrossRef]
- Wei, J.T.; Zhao, S.P.; Zhang, H.Y.; Jin, L.G.; Yu, T.F.; Zheng, L.; Ma, J.; Chen, J.; Zhou, Y.B.; Chen, M.; et al. GmDof41 regulated by the DREB1-type protein improves drought and salt tolerance by regulating the DREB2-type protein in soybean. Int. J. Biol. Macromol. 2023, 230, 123255. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qiu, L.-J. Genome-wide analysis of the Dof transcription factor gene family reveals soybean-specific duplicable and functional characteristics. PLoS ONE 2013, 8, e76809. [Google Scholar] [CrossRef] [PubMed]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 2015, 16, 157. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, L.; He, Y.; Zhang, H.; Li, W.; Chen, H.; Ma, D.; Yin, J. Genome-wide identification and transcriptional expression analysis of superoxide dismutase (SOD) family in wheat (Triticum aestivum). PeerJ 2019, 7, e8062. [Google Scholar] [CrossRef]
- Liu, H.-L.; Wu, M.; Li, F.; Gao, Y.-M.; Chen, F.; Xiang, Y. TCP transcription factors in moso bamboo (Phyllostachys edulis): Genome-wide identification and expression analysis. Front. Plant Sci. 2018, 9, 1263. [Google Scholar] [CrossRef]
- Blanc, G.; Wolfe, K.H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 2004, 16, 1667–1678. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Benedito, V.A.; Torres-Jerez, I.; Murray, J.D.; Andriankaja, A.; Allen, S.; Kakar, K.; Wandrey, M.; Verdier, J.; Zuber, H.; Ott, T.; et al. A gene expression atlas of the model legume Medicago truncatula. Plant J. 2008, 55, 504–513. [Google Scholar] [CrossRef]



| Gene Name | Gene ID | Chromosome Location | Protein Length (aa) | Protein GRAVY 1 | Isoelectric Point (pI) | Protein Molecular Weight (kDa) | DNA Molecular Weight (Da) | Orthologous |
|---|---|---|---|---|---|---|---|---|
| MpDof01 | Mpo1G10010 | Chr1.1: 11559125-11560093: + | 322 | −0.804 | 6.73 | 34.64 | 299,476.22 | AT5G60850.1 |
| MpDof02 | Mpo1G16410 | Chr1.1: 20059424-20060476: − | 350 | −0.651 | 8.48 | 37.93 | 324,040.69 | AT5G65590.1 |
| MpDof03 | Mpo1G16460 | Chr1.1: 20147292-20148017: + | 241 | −0.447 | 8.49 | 25.28 | 224,442.30 | AT3G50410.1 |
| MpDof04 | Mpo1G20440 | Chr1.1: 25979040-25980044: − | 334 | −0.638 | 8.26 | 36.64 | 310,622.49 | AT5G65590.1 |
| MpDof05 | Mpo1G25080 | Chr1.1: 37942310-37943488: − | 392 | −0.648 | 8.05 | 43.28 | 364,921.47 | AT5G39660.1 |
| MpDof06 | Mpo1G32290 | Chr1.1: 45817030-45817836: + | 268 | −0.865 | 8.76 | 29.47 | 249,549.77 | AT3G61850.4 |
| MpDof07 | Mpo1G29540 | Chr1.1: 50547300-50547953: + | 217 | −0.853 | 8.05 | 23.59 | 202,271.63 | AT1G51700.1 |
| MpDof08 | Mpo2G2730 | Chr2.1: 8158099-8158155: + | 363 | −0.657 | 9.61 | 38.92 | 337,721.50 | AT3G55370.1 |
| MpDof09 | Mpo2G35080 | Chr2.1: 28281501-28282337: − | 289 | −0.841 | 7.67 | 32.21 | 269,054.23 | AT2G28510.1 |
| MpDof10 | Mpo2G34260 | Chr2.1: 29860047-29860100: + | 332 | −0.765 | 9.60 | 36.38 | 307,822.14 | AT3G55370.1 |
| MpDof11 | Mpo2G27320 | Chr2.1: 38774383-38774523: + | 465 | −0.632 | 7.20 | 50.66 | 431,187.36 | AT3G47500.1 |
| MpDof12 | Mpo2G24260 | Chr2.1: 42219587-42219643: + | 377 | −0.790 | 8.33 | 40.96 | 350,193.07 | AT5G65590.1 |
| MpDof13 | Mpo2G23730 | Chr2.1: 42814492-42815355: + | 287 | −0.791 | 8.38 | 32.19 | 266,526.77 | AT1G28310.2 |
| MpDof14 | Mpo3G1810 | Chr3.1: 2401530-2401697: + | 483 | −0.976 | 6.64 | 54.01 | 448,411.65 | AT5G39660.1 |
| MpDof15 | Mpo3G5380 | Chr3.1: 8429491-8430576: − | 379 | −0.724 | 9.06 | 41.04 | 352,447.53 | AT3G55370.2 |
| MpDof16 | Mpo3G8630 | Chr3.1: 22387948-22388001: + | 349 | −0.801 | 9.50 | 37.60 | 323,816.27 | AT3G55370.1 |
| MpDof17 | Mpo3G16790 | Chr3.1: 35965767-35965865: + | 426 | −0.742 | 6.98 | 46.80 | 396,324.71 | AT5G39660.1 |
| MpDof18 | Mpo3G50430 | Chr3.1: 72788559-72789509: − | 336 | −0.733 | 7.17 | 37.24 | 313,075.94 | AT5G62940.1 |
| MpDof19 | Mpo3G44960 | Chr3.1: 78940187-78941191: − | 334 | −0.831 | 4.46 | 37.50 | 310,811.41 | AT3G52440.1 |
| MpDof20 | Mpo3G44270 | Chr3.1: 79688172-79688195: + | 303 | −0.868 | 8.67 | 33.31 | 282,187.94 | AT3G61850.4 |
| MpDof21 | Mpo4G36990 | Chr4.1: 30377373-30378218: + | 281 | −0.718 | 6.83 | 31.91 | 261,086.23 | AT3G52440.1 |
| MpDof22 | Mpo4G30010 | Chr4.1: 41059335-41059358: + | 271 | −0.728 | 7.44 | 30.08 | 252,867.00 | AT4G24060.1 |
| MpDof23 | Mpo5G11780 | Chr5.1: 15229952-15230992: + | 346 | −0.905 | 9.47 | 37.66 | 321,294.41 | AT5G65590.1 |
| MpDof24 | Mpo5G15010 | Chr5.1: 20897867-20898073: + | 383 | −0.722 | 7.54 | 42.10 | 355,269.3 | AT5G62430.1 |
| MpDof25 | Mpo5G15000 | Chr5.1: 20907162-20907368: + | 371 | −0.758 | 7.75 | 41.06 | 344,143.11 | AT5G62430.1 |
| MpDof26 | Mpo5G19310 | Chr5.1: 39023975-39025201: − | 471 | −0.987 | 5.39 | 52.22 | 438,204.34 | AT3G47500.1 |
| MpDof27 | Mpo5G25900 | Chr5.1: 45067640-45068944: − | 495 | −0.779 | 5.07 | 54.15 | 459,584.49 | AT5G39660.1 |
| MpDof28 | Mpo6G12720 | Chr6.1: 4264598-4265392: + | 264 | −0.846 | 8.36 | 29.71 | 245,622.01 | AT5G66940.1 |
| MpDof29 | Mpo6G12400 | Chr6.1: 4662282-4663304: + | 340 | −0.449 | 9.83 | 34.86 | 315,965.02 | AT5G60850.1 |
| MpDof30 | Mpo6G12390 | Chr6.1: 4682171-4683061: − | 317 | −0.720 | 8.45 | 34.93 | 294,262.61 | AT1G28310.2 |
| MpDof31 | Mpo6G11570 | Chr6.1: 5683475-5683948: − | 157 | −0.841 | 8.58 | 17.66 | 147,333.83 | AT1G29160.1 |
| MpDof32 | Mpo6G0728L | Chr6.1: 32599558-32600103: + | 181 | −0.919 | 9.21 | 20.62 | 167,666.87 | AT5G60850.1 |
| MpDof33 | Mpo6G21710 | Chr6.1: 49927718-49927744: + | 292 | −0.934 | 7.64 | 32.48 | 272,496.68 | AT2G28510.1 |
| MpDof34 | Mpo6G20540 | Chr6.1: 51515673-51515723: + | 302 | −0.762 | 9.55 | 33.42 | 280,022.16 | AT3G55370.1 |
| MpDof35 | Mpo7G21240 | Chr7.1: 3813449-3814384: − | 339 | −0.849 | 8.12 | 37.71 | 315,443.59 | AT3G47500.1 |
| MpDof36 | Mpo7G21250 | Chr7.1: 3820233-3821276: − | 383 | −0.700 | 8.32 | 41.99 | 356,634.29 | AT3G47500.1 |
| Gene Pairs | Duplication Type | Ka 1 | Ks 2 | Ka/Ks | Duplication Time/(Million Years) |
|---|---|---|---|---|---|
| MpDof01 and MpDof32 | Segmental | 0.322414 | 1.063264 | 0.30323 | 35.44 |
| MpDof04 and MpDof23 | Segmental | 0.302491 | 0.936352 | 0.323053 | 31.21 |
| MpDof06 and MpDof20 | Segmental | 0.354475 | 0.786864 | 0.450491 | 26.23 |
| MpDof13 and MpDof28 | Segmental | 0.192986 | 0.643507 | 0.299897 | 21.45 |
| MpDof12 and MpDof30 | Segmental | 0.284244 | 0.691025 | 0.411337 | 23.03 |
| MpDof11 and MpDof14 | Segmental | 0.426891 | 2.090931 | 0.204163 | 69.70 |
| MpDof11 and MpDof24 | Segmental | 0.323181 | 1.270375 | 0.254398 | 42.35 |
| MpDof11 and MpDof27 | Segmental | 0.420527 | 1.795379 | 0.234228 | 59.85 |
| MpDof10 and MpDof34 | Segmental | 0.329122 | 0.759796 | 0.433171 | 25.33 |
| MpDof09 and MpDof33 | Segmental | 0.187997 | 0.777575 | 0.241774 | 25.92 |
| MpDof17 and MpDof35 | Segmental | 0.309166 | 1.261554 | 0.245068 | 42.05 |
| MpDof14 and MpDof25 | Segmental | 0.40667 | 2.800724 | 0.145202 | 93.36 |
| MpDof14 and MpDof27 | Segmental | 0.251136 | 1.038649 | 0.241791 | 34.62 |
| MpDof24 and MpDof25 | Tandem | 0.033197 | 0.053723 | 0.617927 | 1.79 |
| MpDof35 and MpDof36 | Tandem | 0.102767 | 0.293985 | 0.349567 | 9.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Min, X.; Wei, Z.; Liu, N.; Li, J.; Zhang, Y.; Yang, Y. Genome-Wide Identification and Expression Analysis of the Dof Transcription Factor in Annual Alfalfa Medicago polymorpha. Plants 2023, 12, 1831. https://doi.org/10.3390/plants12091831
Yang L, Min X, Wei Z, Liu N, Li J, Zhang Y, Yang Y. Genome-Wide Identification and Expression Analysis of the Dof Transcription Factor in Annual Alfalfa Medicago polymorpha. Plants. 2023; 12(9):1831. https://doi.org/10.3390/plants12091831
Chicago/Turabian StyleYang, Linghua, Xueyang Min, Zhenwu Wei, Nana Liu, Jiaqing Li, Youxin Zhang, and Yuwei Yang. 2023. "Genome-Wide Identification and Expression Analysis of the Dof Transcription Factor in Annual Alfalfa Medicago polymorpha" Plants 12, no. 9: 1831. https://doi.org/10.3390/plants12091831






