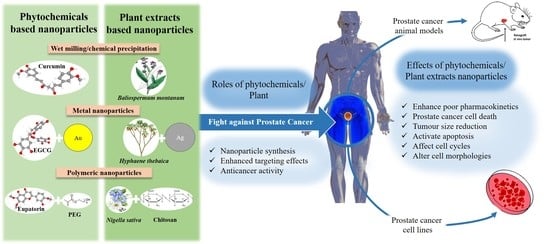
Reports of Plant-Derived Nanoparticles for Prostate Cancer Therapy
Abstract
:1. Introduction
2. Results
2.1. EGCG-Based NPs
2.2. Noscapine Based NPs
2.3. Curcumin Based NPs
2.4. Berberine-Based NPs
2.5. Eupatorin-Based NPs
2.6. Plant-Extract-Based NPs
2.7. Plant-Derived Metallic NPs
2.8. Loading Plant Extracts into NPs
2.9. Reported Toxicity of the Reviewed NPs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| 22Rν1 | Human prostate cancer cells |
| 67LR | 67-kDa laminin receptors |
| AO | Acridine orange dye |
| AuNPs | Gold nanoparticles |
| CHO | Chinese hamster ovary cells |
| CuO NPs | Copper oxide nanoparticles |
| DLS | Dynamic light scattering |
| DMPC | Dimyristoyl phosphatidyl choline |
| Du145 | Androgen-insensitive human prostate cancer cell line |
| FeO NPs | Iron oxide nanoparticles |
| fR2 | Normal human breast epithelial cells |
| HA | Hyaluronic acid |
| hATF | Human-type ATF |
| HepG2 | Human hepatic cancer cells |
| HUVECs | Human umbilical vein endothelial cells |
| LNCaP | Androgen-sensitive human prostate cancer cell line |
| MCF7 | Human breast cancer cells |
| MTT | Tetrazolium-dye-based assay |
| N/A | Not applicable |
| NIH3T3 | Normal mouse embryonic fibroblast |
| NR | Not reported |
| PBL | Human peripheral blood lymphocytes |
| PC | Prostate cancer |
| PC3 | Androgen-insensitive human prostate cancer cells |
| PCL | Poly-e-caprolactone |
| PEG | Polyethyleneglycol |
| PEG-PE | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] |
| PI | Propidium iodide dye |
| PLGA | Poly-d,l-lactide-co-glycolide |
| PNT1A | Normal prostate epithelium cells |
| PSMA | Prostate-specific membrane antigen |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TPGS | D-α-tocopheryl polyethylene glycol 1000 succinate |
| uPA | Urokinase-type plasminogen activator |
| ZnO NPs | Zinc oxide nanoparticles |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, J.; Qiu, W.; Lin, S.; Chen, Y.; Liang, G.; Fang, Y. Safety and Efficacy of First-Line Treatments for Chemotherapy-Naive Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Indirect Comparison. BioMed Res. Int. 2017, 2017, 3941217. [Google Scholar] [CrossRef] [PubMed]
- Formenti, A.M.; Volta, A.D.; di Filippo, L.; Berruti, A.; Giustina, A. Effects of Medical Treatment of Prostate Cancer on Bone Health. Trends Endocrinol. Metab. 2021, 32, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.K.; Patel, A.P. Passive Targeting of Nanoparticles to Cancer. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak, Y.V., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 125–143. [Google Scholar]
- Sun, W.; Deng, Y.; Zhao, M.; Jiang, Y.; Gou, J.; Wang, Y.; Yin, T.; Zhang, Y.; He, H.; Tang, X. Targeting therapy for prostate cancer by pharmaceutical and clinical pharmaceutical strategies. J. Control. Release 2021, 333, 41–64. [Google Scholar] [CrossRef]
- Peng, Z.-H.; Sima, M.; Salama, M.E.; Kopečková, P.; Kopeček, J. Spacer length impacts the efficacy of targeted docetaxel conjugates in prostate-specific membrane antigen expressing prostate cancer. J. Drug Target. 2013, 21, 968–980. [Google Scholar] [CrossRef]
- Chang, S.S. Overview of Prostate-Specific Membrane Antigen. Rev. Urol. 2004, 6 (Suppl. S10), S13–S18. [Google Scholar]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef]
- Choong, P.F.M.; Nadesapillai, A.P.W. Urokinase plasminogen activator system: A multifunctional role in tumor progression and metastasis. Clin. Orthop. Relat. Res. 2003, 415, S46–S58. [Google Scholar] [CrossRef]
- Pulukuri, S.M.; Gondi, C.S.; Lakka, S.S.; Jutla, A.; Estes, N.; Gujrati, M.; Rao, J.S. RNA Interference-directed Knockdown of Urokinase Plasminogen Activator and Urokinase Plasminogen Activator Receptor Inhibits Prostate Cancer Cell Invasion, Survival, and Tumorigenicity in Vivo. J. Biol. Chem. 2005, 280, 36529–36540. [Google Scholar] [CrossRef]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.G.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006, 25, 1696–1708. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Zulkipli, I.N.; David, S.R.; Rajabalaya, R.; Idris, A. Medicinal Plants: A Potential Source of Compounds for Targeting Cell Division. Drug Target Insights 2015, 9, 9–19. [Google Scholar] [CrossRef]
- Kai On, C.; Calvin, C.P.P. Pharmacokinetics and Disposition of Green Tea Catechins. In Pharmacokinetics and Adverse Effects of Drugs; Ntambwe, M., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Ai, X.; Yu, P.; Peng, L.; Luo, L.; Liu, J.; Li, S.; Lai, X.; Luan, F.; Meng, X. Berberine: A Review of its Pharmacokinetics Properties and Therapeutic Potentials in Diverse Vascular Diseases. Front. Pharmacol. 2021, 12, 762654. [Google Scholar] [CrossRef]
- Seufferlein, T.; Ettrich, T.J.; Menzler, S.; Messmann, H.; Kleber, G.; Zipprich, A.; Frank-Gleich, S.; Algül, H.; Metter, K.; Odemar, F.; et al. Green Tea Extract to Prevent Colorectal Adenomas, Results of a Randomized, Placebo-Controlled Clinical Trial. Am. J. Gastroenterol. 2022, 117, 884–894. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Lee, R.-P.; Trang, A.; Husari, G.; Yang, J.; Grojean, E.M.; Ly, A.; Hsu, M.; Heber, D.; et al. Prospective randomized trial evaluating blood and prostate tissue concentrations of green tea polyphenols and quercetin in men with prostate cancer. Food Funct. 2020, 11, 4114–4122. [Google Scholar] [CrossRef]
- Passildas-Jahanmohan, J.; Eymard, J.-C.; Pouget, M.; Kwiatkowski, F.; Van Praagh, I.; Savareux, L.; Atger, M.; Durando, X.; Abrial, C.; Richard, D.; et al. Multicenter randomized phase II study comparing docetaxel plus curcumin versus docetaxel plus placebo in first-line treatment of metastatic castration-resistant prostate cancer. Cancer Med. 2021, 10, 2332–2340. [Google Scholar] [CrossRef]
- Abdifetah, O.; Na-Bangchang, K. Pharmacokinetic studies of nanoparticles as a delivery system for conventional drugs and herb-derived compounds for cancer therapy: A systematic review. Int. J. Nanomed. 2019, 14, 5659–5677. [Google Scholar] [CrossRef]
- Yazdanian, M.; Rostamzadeh, P.; Rahbar, M.; Alam, M.; Abbasi, K.; Tahmasebi, E.; Tebyaniyan, H.; Ranjbar, R.; Seifalian, A.; Yazdanian, A. The Potential Application of Green-Synthesized Metal Nanoparticles in Dentistry: A Comprehensive Review. Bioinorg. Chem. Appl. 2022, 2022, 2311910. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large Scale Screening of Southern African Plant Extracts for the Green Synthesis of Gold Nanoparticles Using Microtitre-Plate Method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef] [PubMed]
- Anadozie, S.O.; Adewale, O.B.; Sibuyi, N.R.; Fadaka, A.O.; Isitua, C.C.; Davids, H.; Roux, S. One-pot synthesis, characterisation and biological activities of gold nanoparticles prepared using aqueous seed extract of Garcinia kola. Process. Biochem. 2023, 128, 49–57. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current developments in green synthesis of metallic nanoparticles using plant extracts: A review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Karthik, C.; Suresh, S.; Mirulalini, S.; Kavitha, S. A FTIR approach of green synthesized silver nanoparticles by Ocimum sanctum and Ocimum gratissimum on mung bean seeds. Inorg. Nano-Metal Chem. 2020, 50, 606–612. [Google Scholar] [CrossRef]
- Sanna, V.; Pintus, G.; Roggio, A.M.; Punzoni, S.; Posadino, A.M.; Arca, A.; Marceddu, S.; Bandiera, P.; Uzzau, S.; Sechi, M. Targeted Biocompatible Nanoparticles for the Delivery of (−)-Epigallocatechin 3-Gallate to Prostate Cancer Cells. J. Med. Chem. 2011, 54, 1321–1332. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing Nanochemoprevention as a Novel Approach for Cancer Control: Proof of Principle with Green Tea Polyphenol Epigallocatechin-3-Gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef]
- Rocha, S.; Generalov, R.; Pereira, M.D.C.; Peres, I.; Juzenas, P.; Coelho, M.A. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine 2011, 6, 79–87. [Google Scholar] [CrossRef]
- Shafiei, S.S.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Kalantarinejad, R.; Asadi-Eydivand, M.; ABU Osman, N.A. Epigallocatechin Gallate/Layered Double Hydroxide Nanohybrids: Preparation, Characterization, and In Vitro Anti-Tumor Study. PLoS ONE 2015, 10, e0136530. [Google Scholar] [CrossRef]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin receptor specific therapeutic gold nanoparticles (198AuNP-EGCg) show efficacy in treating prostate cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2013, 35, 415–423. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Tsai, S.-C.; Ko, H.-Y.; Wu, C.-C.; Lin, Y.-H. Co-Delivery of Natural Compounds with a Dual-Targeted Nanoparticle Delivery System for Improving Synergistic Therapy in an Orthotopic Tumor Model. ACS Appl. Mater. Interfaces 2019, 11, 23880–23892. [Google Scholar] [CrossRef]
- Peng, S.-L.; Lai, C.-H.; Chu, P.-Y.; Hsieh, J.-T.; Tseng, Y.-C.; Chiu, S.-C.; Lin, Y.-H. Nanotheranostics With the Combination of Improved Targeting, Therapeutic Effects, and Molecular Imaging. Front. Bioeng. Biotechnol. 2020, 8, 570490. [Google Scholar] [CrossRef]
- Adahoun, M.A.; Al-Akhras, M.-A.H.; Jaafar, M.S.; Bououdina, M. Enhanced anti-cancer and antimicrobial activities of curcumin nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 45, 98–107. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Puri, A.; Tele, S.; Blumenthal, R.; Maheshwari, R.K. Evaluation of a nanotechnology-based carrier for delivery of curcumin in prostate cancer cells. Int. J. Oncol. 2008, 32, 1119–1123. [Google Scholar] [CrossRef]
- Narayanan, N.K.; Nargi, D.; Randolph, C.; Narayanan, B.A. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer 2009, 125, 1–8. [Google Scholar] [CrossRef]
- Abdalla, M.O.; Karna, P.; Sajja, H.K.; Mao, H.; Yates, C.; Turner, T.; Aneja, R. Enhanced noscapine delivery using uPAR-targeted optical-MR imaging trackable nanoparticles for prostate cancer therapy. J. Control. Release 2011, 149, 314–322. [Google Scholar] [CrossRef]
- Shen, R.; Kim, J.J.; Yao, M.; Elbayoumi, T.A. Development and evaluation of vitamin E D-α-tocopheryl polyethylene glycol 1000 succinate-mixed polymeric phospholipid micelles of berberine as an anticancer nanopharmaceutical. Int. J. Nanomed. 2016, 11, 1687–1700. [Google Scholar] [CrossRef]
- Tousi, M.S.; Sepehri, H.; Khoee, S.; Farimani, M.M.; Delphi, L.; Mansourizadeh, F. Evaluation of apoptotic effects of mPEG-b-PLGA coated iron oxide nanoparticles as a eupatorin carrier on DU-145 and LNCaP human prostate cancer cell lines. J. Pharm. Anal. 2020, 11, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.M.; Snima, K.; Kamath, C.R.; Nair, S.V.; Lakshmanan, V.-K. Effect of Baliospermum montanum nanomedicine apoptosis induction and anti-migration of prostate cancer cells. Biomed. Pharmacother. 2015, 71, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Nair, S.V.; Lakshmanan, V.-K. Leucas aspera Nanomedicine Shows Superior Toxicity and Cell Migration Retarded in Prostate Cancer Cells. Appl. Biochem. Biotechnol. 2016, 181, 1388–1400. [Google Scholar] [CrossRef] [PubMed]
- Bello, B.A.; Khan, S.A.; Khan, J.A.; Syed, F.Q.; Mirza, M.B.; Shah, L.; Khan, S.B. Anticancer, antibacterial and pollutant degradation potential of silver nanoparticles from Hyphaene thebaica. Biochem. Biophys. Res. Commun. 2017, 490, 889–894. [Google Scholar] [CrossRef]
- Bello, B.A.; Khan, S.A.; Khan, J.A.; Syed, F.Q.; Anwar, Y.; Khan, S.B. Antiproliferation and antibacterial effect of biosynthesized AgNps from leaves extract of Guiera senegalensis and its catalytic reduction on some persistent organic pollutants. J. Photochem. Photobiol. B Biol. 2017, 175, 99–108. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Wang, J.; Yang, Q.; Yao, B.; Zhao, Y.; Zhao, A.; Sun, W.; Zhang, Q. Synthesis, characterization and evaluation cytotoxic activity of silver nanoparticles synthesized by Chinese herbal Cornus officinalis via environment friendly approach. Environ. Toxicol. Pharmacol. 2017, 56, 56–60. [Google Scholar] [CrossRef]
- Bethu, M.S.; Netala, V.R.; Domdi, L.; Tartte, V.; Janapala, V.R. Potential anticancer activity of biogenic silver nanoparticles using leaf extract of Rhynchosia suaveolens: An insight into the mechanism. Artif. Cells Nanomed. Biotechnol. 2018, 46, 104–114. [Google Scholar] [CrossRef]
- Netala, V.R.; Bukke, S.; Domdi, L.; Soneya, S.; Reddy, S.G.; Bethu, M.S.; Kotakdi, V.S.; Saritha, K.V.; Tartte, V. Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif. Cells Nanomed. Biotechnol. 2018, 46, 1138–1148. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Ravi, S.O.A.S.; Ramachandran, A.; Ibrahim, I.A.A.; Nassir, A.M.; Yao, J. Synthesis of silver nanoparticles (AgNPs) from leaf extract of Salvia miltiorrhiza and its anticancer potential in human prostate cancer LNCaP cell lines. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2846–2854. [Google Scholar] [CrossRef]
- Kumari, R.; Saini, A.K.; Kumar, A.; Saini, R.V. Apoptosis induction in lung and prostate cancer cells through silver nanoparticles synthesized from Pinus roxburghii bioactive fraction. JBIC J. Biol. Inorg. Chem. 2019, 25, 23–37. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Maina, E.N.; El-Shemy, H.A.; Meroka, A.M.; Kangogo, G.K.; Magoma, G.; Wamunyokoli, F. Annona muricata silver nanoparticles exhibit strong anticancer activities against cervical and prostate adenocarcinomas through regulation of CASP9 and the CXCL1/CXCR2 genes axis. Tumor Biol. 2021, 43, 37–55. [Google Scholar] [CrossRef]
- Reddy, N.; Li, H.; Hou, T.; Bethu, M.S.; Ren, Z.; Zhang, Z. Phytosynthesis of Silver Nanoparticles Using Perilla frutescens Leaf Extract: Characterization and Evaluation of Antibacterial, Antioxidant, and Anticancer Activities. Int. J. Nanomed. 2021, 16, 15–29. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Thipe, V.C.; Panjtan-Amiri, K.; Meyer, M.; Katti, K.V. Green synthesis of gold nanoparticles using Acai berry and Elderberry extracts and investigation of their effect on prostate and pancreatic cancer cells. Nanobiomedicine 2021, 8, 1849543521995310. [Google Scholar] [CrossRef]
- Mohammad, G.R.K.S.; Karimi, E.; Oskoueian, E.; Homayouni-Tabrizi, M. Anticancer properties of green-synthesised zinc oxide nanoparticles using Hyssopus officinalis extract on prostate carcinoma cells and its effects on testicular damage and spermatogenesis in Balb/C mice. Andrologia 2019, 52, e13450. [Google Scholar] [CrossRef]
- Naz, S.; Tabassum, S.; Fernandes, N.F.; Mujahid, M.; Zia, M.; de Blanco, E.J.C. Anticancer and antibacterial potential of Rhus punjabensis and CuO nanoparticles. Nat. Prod. Res. 2018, 34, 720–725. [Google Scholar] [CrossRef]
- Dawaba, A.M.; Dawaba, H.M. Application of Optimization Technique to Develop Nano-Based Carrier of Nigella Sativa Essential Oil: Characterization and Assessment. Recent Pat. Drug Deliv. Formul. 2020, 13, 228–240. [Google Scholar] [CrossRef]
- Ribeiro, A.F.; Santos, J.F.; Mattos, R.R.; Barros, E.G.; Nasciutti, L.E.; Cabral, L.M.; De Sousa, V.P. Characterization and in vitro antitumor activity of polymeric nanoparticles loaded with Uncaria tomentosa extract. An. Acad. Bras. Ciências 2020, 92, e20190336. [Google Scholar] [CrossRef]
- Ahmad, N.; Mukhtar, H. Green Tea Polyphenols and Cancer: Biologic Mechanisms and Practical Implications. Nutr. Rev. 1999, 57, 78–83. [Google Scholar] [CrossRef]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.-D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Zhang, G.; Miura, Y.; Yagasaki, K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000, 159, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Dhatwalia, S.K.; Kumar, M.; Dhawan, D.K. Role of EGCG in Containing the Progression of Lung Tumorigenesis—A Multistage Targeting Approach. Nutr. Cancer 2018, 70, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.H.; Al Shabrmi, F.M.; Allemailem, K.S.; Aly, S.M.; Khan, M.A. Implications of Green Tea and Its Constituents in the Prevention of Cancer via the Modulation of Cell Signalling Pathway. BioMed Res. Int. 2015, 2015, 925640. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Gkiouras, K.; Papageorgiou, S.; Myrogiannis, I.; Mykoniatis, I.; Papamitsou, T.; Bogdanos, D.P.; Goulis, D.G. Dietary Factors and Supplements Influencing Prostate-Specific Antigen (PSA) Concentrations in Men with Prostate Cancer and Increased Cancer Risk: An Evidence Analysis Review Based on Randomized Controlled Trials. Nutrients 2020, 12, 2985. [Google Scholar] [CrossRef]
- Chow, H.-H.S.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of Dosing Condition on the Oral Bioavailability of Green Tea Catechins after Single-Dose Administration of Polyphenon E in Healthy Individuals. Clin. Cancer Res. 2005, 11, 4627–4633. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.-Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef]
- Li, W.; Qian, L.; Lin, J.; Huang, G.; Hao, N.; Wei, X.; Wang, W.; Liang, J. CD44 regulates prostate cancer proliferation, invasion and migration via PDK1 and PFKFB4. Oncotarget 2017, 8, 65143–65151. [Google Scholar] [CrossRef]
- Natoni, A.; Macauley, M.S.; O’Dwyer, M.E. Targeting Selectins and Their Ligands in Cancer. Front. Oncol. 2016, 6, 93. [Google Scholar] [CrossRef]
- Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green Tea Polyphenol EGCG Sensing Motif on the 67-kDa Laminin Receptor. PLoS ONE 2012, 7, e37942. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K. Scope of Nanotechnology in Nutraceuticals. In Nanotechnology Applications in Food; Oprea, A.E., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 43–63. [Google Scholar]
- Tomar, V.; Kumar, N.; Tomar, R.; Sood, D.; Dhiman, N.; Dass, S.K.; Prakash, S.; Madan, J.; Chandra, R. Biological Evaluation of Noscapine analogues as Potent and Microtubule-Targeted Anticancer Agents. Sci. Rep. 2019, 9, 19542. [Google Scholar] [CrossRef]
- Debono, A.; Capuano, B.; Scammells, P.J. Progress Toward the Development of Noscapine and Derivatives as Anticancer Agents. J. Med. Chem. 2015, 58, 5699–5727. [Google Scholar] [CrossRef]
- Andey, T.; Patel, A.R.; Marepally, S.; Chougule, M.B.; Spencer, S.D.; Rishi, A.K.; Singh, M. Formulation, Pharmacokinetic, and Efficacy Studies of Mannosylated Self-Emulsifying Solid Dispersions of Noscapine. PLoS ONE 2016, 11, e0146804. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Nagahama, K.; Utsumi, T.; Kumano, T.; Maekawa, S.; Oyama, N.; Kawakami, J. Discovery of a new function of curcumin which enhances its anticancer therapeutic potency. Sci. Rep. 2016, 6, 30962. [Google Scholar] [CrossRef]
- Wang, J.-B.; Qi, L.-L.; Zheng, S.-D.; Wu, T.-X. Curcumin induces apoptosis through the mitochondria-mediated apoptotic pathway in HT-29 cells. J. Zhejiang Univ. B 2009, 10, 93–102. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Hibiya, Y.; Mutoh, M.; Koshiji, M.; Akao, S.; Fujiwara, H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J. Ethnopharmacol. 1999, 66, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Chuang, K.-H.; Fang, K.; Lee, Y.; Chung, J.; Chuang, Y. Effect of berberine on activity and mRNA expression of N-acetyltransferase in human lung cancer cell line A549. J. Tradit. Chin. Med. 2014, 34, 302–308. [Google Scholar] [CrossRef]
- Sun, Y.; Xun, K.; Wang, Y.; Chen, X. A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anti-Cancer Drugs 2009, 20, 757–769. [Google Scholar] [CrossRef]
- Cui, H.-X.; Hu, Y.-N.; Li, J.-W.; Yuan, K.; Guo, Y. Preparation and Evaluation of Antidiabetic Agents of Berberine Organic Acid Salts for Enhancing the Bioavailability. Molecules 2018, 24, 103. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- De Matteis, V.; Rojas, M.; Cascione, M.; Mazzotta, S.; Di Sansebastiano, G.P.; Rinaldi, R. Physico-Chemical Properties of Inorganic NPs Influence the Absorption Rate of Aquatic Mosses Reducing Cytotoxicity on Intestinal Epithelial Barrier Model. Molecules 2021, 26, 2885. [Google Scholar] [CrossRef]
- Rai, M.; Kon, K.; Ingle, A.; Duran, N.; Galdiero, S.; Galdiero, M. Broad-spectrum bioactivities of silver nanoparticles: The emerging trends and future prospects. Appl. Microbiol. Biotechnol. 2014, 98, 1951–1961. [Google Scholar] [CrossRef]
- Taha, G.A.; Abdel-Farid, I.B.; Elgebaly, H.A.; Mahalel, U.A.; Sheded, M.G.; Bin-Jumah, M.; Mahmoud, A.M. Metabolomic Profiling and Antioxidant, Anticancer and Antimicrobial Activities of Hyphaene thebaica. Processes 2020, 8, 266. [Google Scholar] [CrossRef]
- Adebayo, I.A.; Gagman, H.A.; Balogun, W.G.; Adam, M.A.A.; Abas, R.; Hakeem, K.R.; Him, N.A.I.I.B.N.; Bin Samian, M.R.; Arsad, H. Detarium microcarpum, Guiera senegalensis, and Cassia siamea Induce Apoptosis and Cell Cycle Arrest and Inhibit Metastasis on MCF7 Breast Cancer Cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 6104574. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus Officinalis—Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Huang, Y.; Bu, Q. Adverse Effects of Phytochemicals. In Nutritional Toxicology; Zhang, L., Ed.; Springer Nature Singapore: Singapore, 2022; pp. 355–384. [Google Scholar]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of silver ions, metallic silver, and silver nanoparticle materials after in vivo dermal and mucosal surface exposure: A review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef]
- Nair, H.; Snima, K.; Kamath, R.; Nair, S.; Lakshmanan, V.K. Plumbagin Nanoparticles Induce Dose and pH Dependent Toxicity on Prostate Cancer Cells. Curr. Drug Deliv. 2015, 12, 709–716. [Google Scholar] [CrossRef]
| NPs Type | NPs Composition | Shape | Size (nm) | Size Determination Technique | Encapsulation Efficiency % | Anticancer Effect | References |
|---|---|---|---|---|---|---|---|
| Epigallocatehin Gallate (EGCG)-based NPs | poly-d,l-lactide-co-glycolide/polyethyleneglycol (PLGA-PEG) NPs conjugated with anti PSMA | Spherical | 80.53 ± 15.0 | Scanning electron microscopy (SEM) | 9.61 ± 0.7 | Increased cytotoxicity activity against androgen-sensitive human prostate cancer cell line (LNCaP). | [29] |
| Polylactic acid-PEG NPs | Not reported (NR) | 260 | Dynamic light scattering (DLS) | NR | Increased cytotoxicity against androgen-insensitive human prostate cancer cells (PC3); induction of apoptosis; antiangiogenesis effect; prostate tumour size reduction in mice. | [30] | |
| Gum Arabic–maltodextrin matrix | Spherical | 120 | DLS | 85 ± 3 | Increased cytotoxicity against androgen-insensitive human prostate cancer cells (Du145). | [31] | |
| Inorganic NPs | Nano-flakes of hexagonal shapes | 10 | Transmission electron microscopy (TEM) | NR | Increased cytotoxicity against PC3; induction of apoptosis. | [32] | |
| Gold nanoparticles (AuNPs) | Spherical | 40–55 | TEM | NR | Increased cytotoxicity against PC3 in vivo. | [33] | |
| Chitosan NPs | Spherical | 150–200 | TEM | 10% | Increased cytotoxicity against human prostate cancer cells (22Rν1) implanted in mice. | [34] | |
| Lipid NPs (glycerol monostearate/stearic acid/soya lecithin) | NR | 157 | DLS | 67.2 ± 4.5 | Increased cytotoxicity against Du145 cells; apoptosis induction. | [35] | |
| PEG-Gelatine NPs conjugated with hyaluronic acid (HA) and fucoidan. EGCG was loaded in combination with curcumin | Spherical | 197.73 ± 18.53 | TEM | 46.01 ± 1.96 (EGCG), 67.76 ± 6.67 (curcumin) | Increased cytotoxicity against Luc PC3 both in vitro and in vivo. | [36] | |
| PLGA-PEG-Gelatine NPs conjugated with HA and fucoidan. | Spherical | 217.19 ± 11.37 | DLS | 19.67 ± 2.48 | Increased cytotoxicity against PC3 both in vitro and in vivo; upregulation of caspases; reduction in G0/G1 and increase in S-phase population. | [37] | |
| Curcumin-based NPs | Curcumin NPs | NR | 34–359.4 | SEM | Not applicable (N/A) | Increased cytotoxicity against PC3. | [38] |
| Liposome NPs | NR | 100–150 | DLS | 0.125 mg/ml | Increased cytotoxicity against LNCaP. | [39] | |
| Liposome NPs | NR | NR | NR | NR | Reduction in the prostate weight and the number of adenocarcinomas in prostate-specific PTEN knockout mice; apoptosis induction; increase in the pre-G1 population; downregulation of p-Akt, cyclin D1, AR, and mTOR from PTEN-Cap8 cells. | [40] | |
| Noscapine-based NPs | Iron oxide nanoparticles (FeO NPs) loaded with urokinase-type plasminogen activator(uPA) targeting moiety (human-type ATF (hATF) and fluorescent dye (cy5.5) | Spherical | 35.62 ± 4.1 | DLS | 88.2 ± 2.3 | Increased cytotoxicity and targeting towards PC3 cells. | [41] |
| Berberine-based NPs | Mixed polymeric phospholipid micelles of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethyleneglycol)-2000] (PEG-PE) and D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) | Micelles | 24.1 ± 1.8 | DLS | 95.7 ± 3.6 | Increased cytotoxicity against PC3 and LNCaP cells. | [42] |
| Eupatorin-based NPs | Eupatorin loaded on copolymer of magnetic PEG and PLGA | Spherical | 58.5 ± 4 | DLS | 90.99 ± 2.1 | Antiproliferation activity against Du145 and LNCaP; apoptosis induction; activation of sub-G1-phase population; upregulation of caspase 3 and Bax/Bcl-2 ratio. | [43] |
| Plant-extract-based NPs | Baliospermum montanum-based NPs via precipitation method | Irregular | 211.3 and 233 for NPs from the aqueous and ethanolic extracts, respectively | DLS | N/A | Increased cytotoxicity against PC3 cells. | [44] |
| Leucas aspera-based NPs via precipitation method | Spherical | 200–400 | DLS | N/A | Increased cytotoxicity against PC3 cells. | [45] | |
| Silver nanoparticles (AgNPs) using Hyphaene thebaica aqueous extract | Spherical | 20 | TEM | N/A | Cytotoxic activity against PC3. | [46] | |
| AgNPs using Guiera senegalensis aqueous extract | Spherical | 50 | TEM | N/A | Cytotoxic activity against PC3. | [47] | |
| AgNPs using Cornus Officinalis fruit aqueous extract | Quasi-spherical | 11.7 | TEM | N/A | Cytotoxic activity against PC3. | [48] | |
| AgNPs using aqueous leaf extract of Rhynchosia suaveolens | Spherical | 10–30 | TEM | N/A | Selective cytotoxic activity against PC3 and DU145 cells. | [49] | |
| AgNPs using aqueous leaf extract of Indigofera hisruta | Spherical | 5–10 | TEM | N/A | Selective cytotoxic activity against PC3 cells. | [50] | |
| AgNPs from Salvia miltiorrhiza leaf aqueous extract | Different shapes | 12–80 | TEM | N/A | Cytotoxic activity against LNCaP; apoptosis induction. Increased Reactive oxygen species (ROS) production. | [51] | |
| AgNPs from the butanol fraction of Pinus roxburghii needles. | Spherical | 80 | TEM | N/A | Increased cytotoxicity against PC3; apoptosis induction. Increased ROS production and mitochondrial dysfunction; increased cells in the sub-G1 phase. | [52] | |
| AgNPs from the ethanolic fruits and leaves extracts of Annona muricata. | NR | NR | NR | N/A | Increased and selective cytotoxic activity against PC3. | [53] | |
| AgNPs from the aqueous leaf extract of Perilla frutescens | Different shapes | 20–50 | TEM | N/A | Cytotoxic activity against LNCaP. | [54] | |
| AuNPs from different solvent extracts of Euterpe oleraceae and Sambucus nigra | Different shapes | Variable according to the type of extract used | DLS/TEM | N/A | Cytotoxic activity against PC3. | [55] | |
| Zinc oxide NPs (ZnO NPs) from the aqueous leaf extract of Hyssopus officinalis | NR | NR | NR | N/A | Cytotoxic activity against PC3; apoptosis induction. | [56] | |
| Copper oxide nanoparticles (CuO NPs) from the aqueous leaf extract of Rhus punjabensis. | Spherical | 31.27 | SEM | N/A | Cytotoxic activity against PC3; inhibition of NF-κB signalling. | [57] | |
| Loaded Nigella sativa essential oil in chitosan NPs connected to different ratios of benzoic and cinnamic acids. | Spherical | 341 | TEM | 98 | Increased cytotoxic activity against PC3. | [58] | |
| PLGA and poly-e-caprolactone (PCL) NPs loaded with Uncaria tomentosa extract | Spherical | 247.3 ± 9.9 for PCL-based NPs, and 107.4 ± 3.0 for PLGA-based NPs | DLS | 81.6 ± 0.7% for PCL-based NPs and 64.6 ± 2.0% for the PLGA-based NPs | Cytotoxic activity against LNCaP and Du145. | [59] |
| Type of NPs | Toxicity Summary | References |
|---|---|---|
| EGCG NPs | EGCG polymeric NPs showed selective toxicity against LNCaP, while no cytotoxicity was exhibited against human umbilical vein endothelial cells (HUVECs). | [29] |
| Curcumin NPs | The curcumin NPs showed selective cytotoxicity against PC3 (IC50 = 121.92 µM) as compared to the mammalian cell line (HEK 293) (IC50 = 292.88 µM).The curcumin NPs showed comparable haemolysis % to the parent curcumin with concentrations up to 600 µM. | [38] |
| A seven-week administration of liposomal curcumin, liposomal resveratrol, and their co-administration (at a dosage of 50 mg/kg and 25 mg/kg, respectively) did not cause any significant toxicity or weight changes in mice. | [40] | |
| Berberine NPs | The micelles loaded with berberine showed higher hemocompatibility as compared to the free berberine. | [42] |
| Plumbagin NPs | The plumbagin NPs were less toxic to normal cells as compared to the crude extract. The NPs also showed high blood biocompatibility in vivo. | [101] |
| Eupatorin NPs | The polymeric NPs loaded with eupatorin exhibited selective cytotoxicity against Du145 and LNCaP cell lines, while not exhibiting any cytotoxic effect on HUVECs. | [43] |
| Plant extract NPs | The B. montanum extract NPs were cytotoxic against PC3 cells but did not show growth inhibition against normal mouse embryonic fibroblasts (NIH3T3). In addition, mixing the extract NPs with blood did not exhibit significant haemolysis. | [44] |
| The L. aspera extract NPs were blood biocompatible, as no agglomeration of different blood cells was detected at a concentration of 0.25 mg/mL. | [45] | |
| The AgNPs from R. suaveolens exhibited 16 times higher toxicity against PC3 and DU145 cancer cells compared to Chinese hamster ovary (CHO) cells. | [49] | |
| The AgNPs derived from I. hisruta did not exhibit cytotoxicity against CHO cells at the highest concentration tested, in contrast to their cytotoxic effect against PC3 cells. | [50] | |
| The AgNPs derived from P. roxburghii demonstrated selective antiproliferation activity against two cancer cell lines, while showing no activity on two normal human cell lines, including normal human breast epithelial cells (fR2) and human peripheral blood lymphocytes (PBL). | [52] | |
| The AgNPs synthesized from the fruits and leaves of A. muricata demonstrated a selective index of 7.8 and 2.26, respectively, against the PC3 cell line as compared to the normal prostate epithelium (PNT1A) cell line. | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbagory, A.M.; Hull, R.; Meyer, M.; Dlamini, Z. Reports of Plant-Derived Nanoparticles for Prostate Cancer Therapy. Plants 2023, 12, 1870. https://doi.org/10.3390/plants12091870
Elbagory AM, Hull R, Meyer M, Dlamini Z. Reports of Plant-Derived Nanoparticles for Prostate Cancer Therapy. Plants. 2023; 12(9):1870. https://doi.org/10.3390/plants12091870
Chicago/Turabian StyleElbagory, Abdulrahman M., Rodney Hull, Mervin Meyer, and Zodwa Dlamini. 2023. "Reports of Plant-Derived Nanoparticles for Prostate Cancer Therapy" Plants 12, no. 9: 1870. https://doi.org/10.3390/plants12091870