Nitric Oxide and Strigolactone Alleviate Mercury-Induced Oxidative Stress in Lens culinaris L. by Modulating Glyoxalase and Antioxidant Defense System
Abstract
:1. Introduction
2. Results
2.1. Growth Parameters
2.2. Pigment Content
2.3. Osmolytes Content
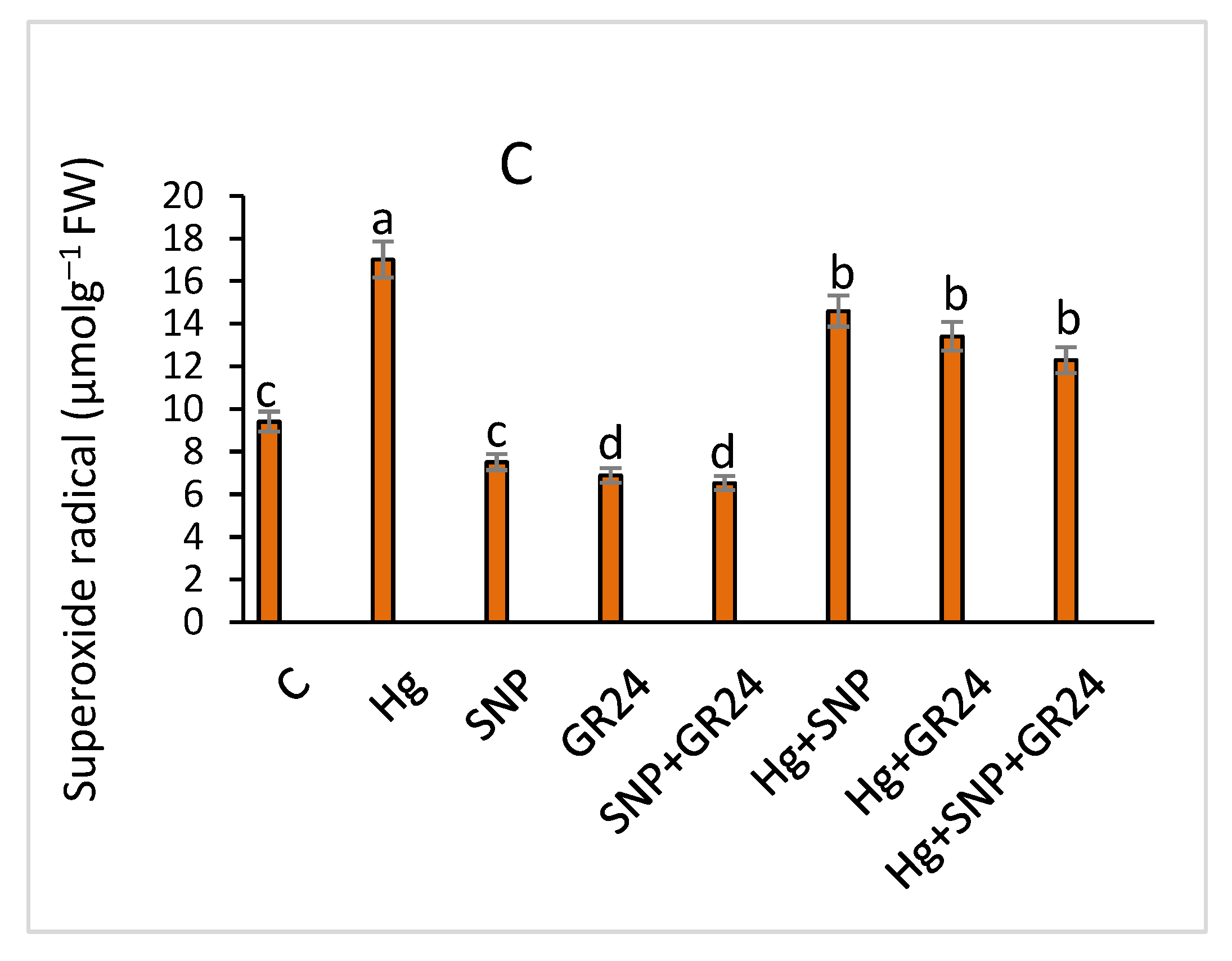
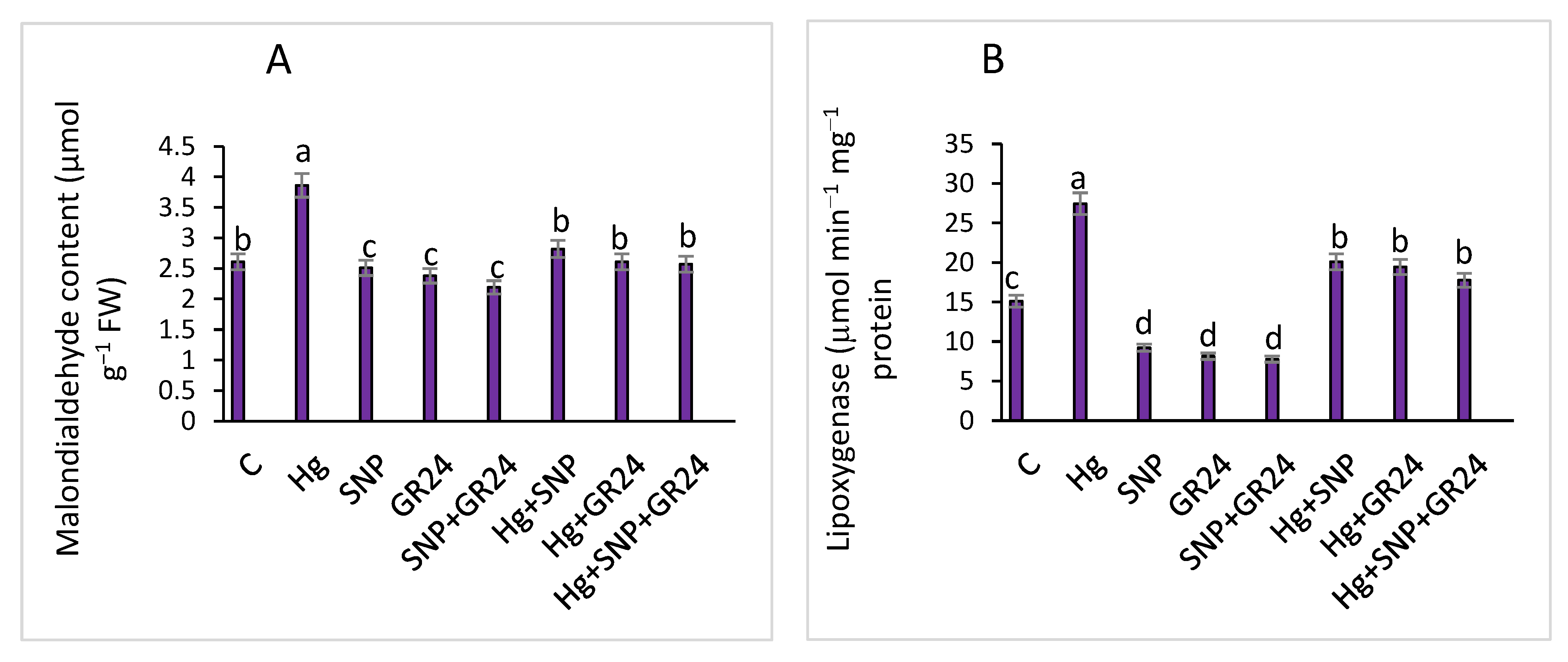
2.4. Oxidative Stress Markers
2.5. Accumulation of Hg and Other Inorganic Elements
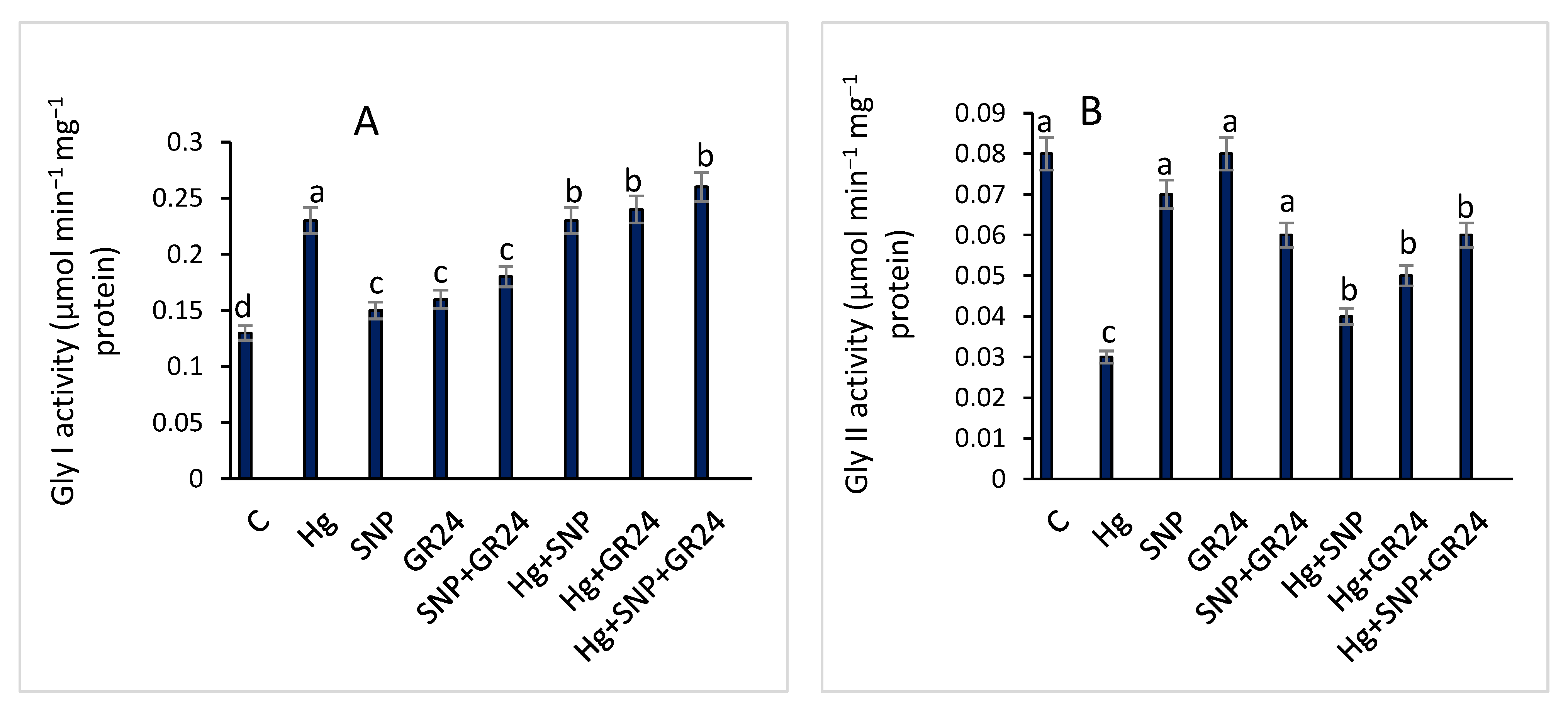
2.6. Methylglyoxal Content and Glyoxalase Enzyme Activity
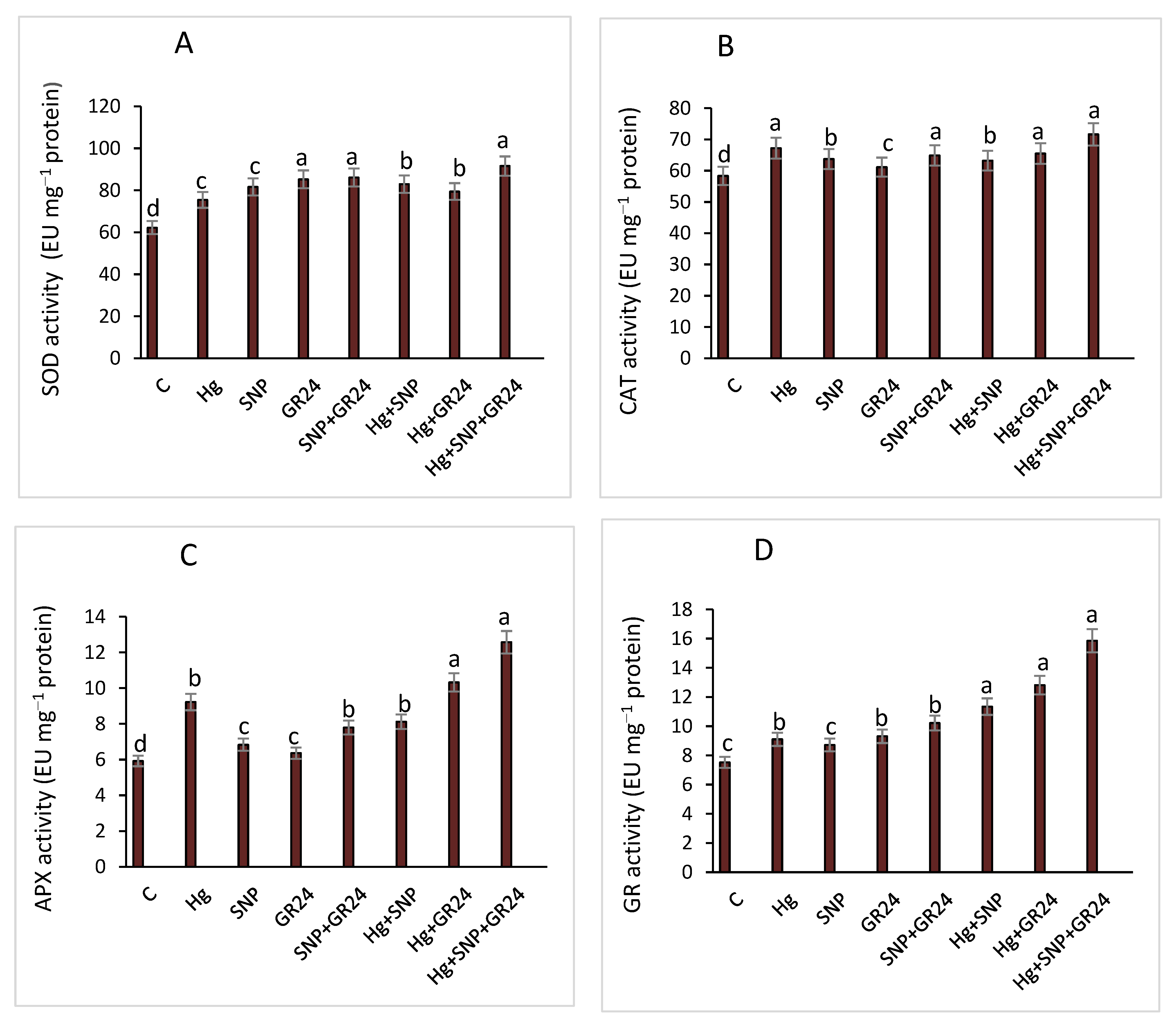
2.7. Antioxidant Enzymes
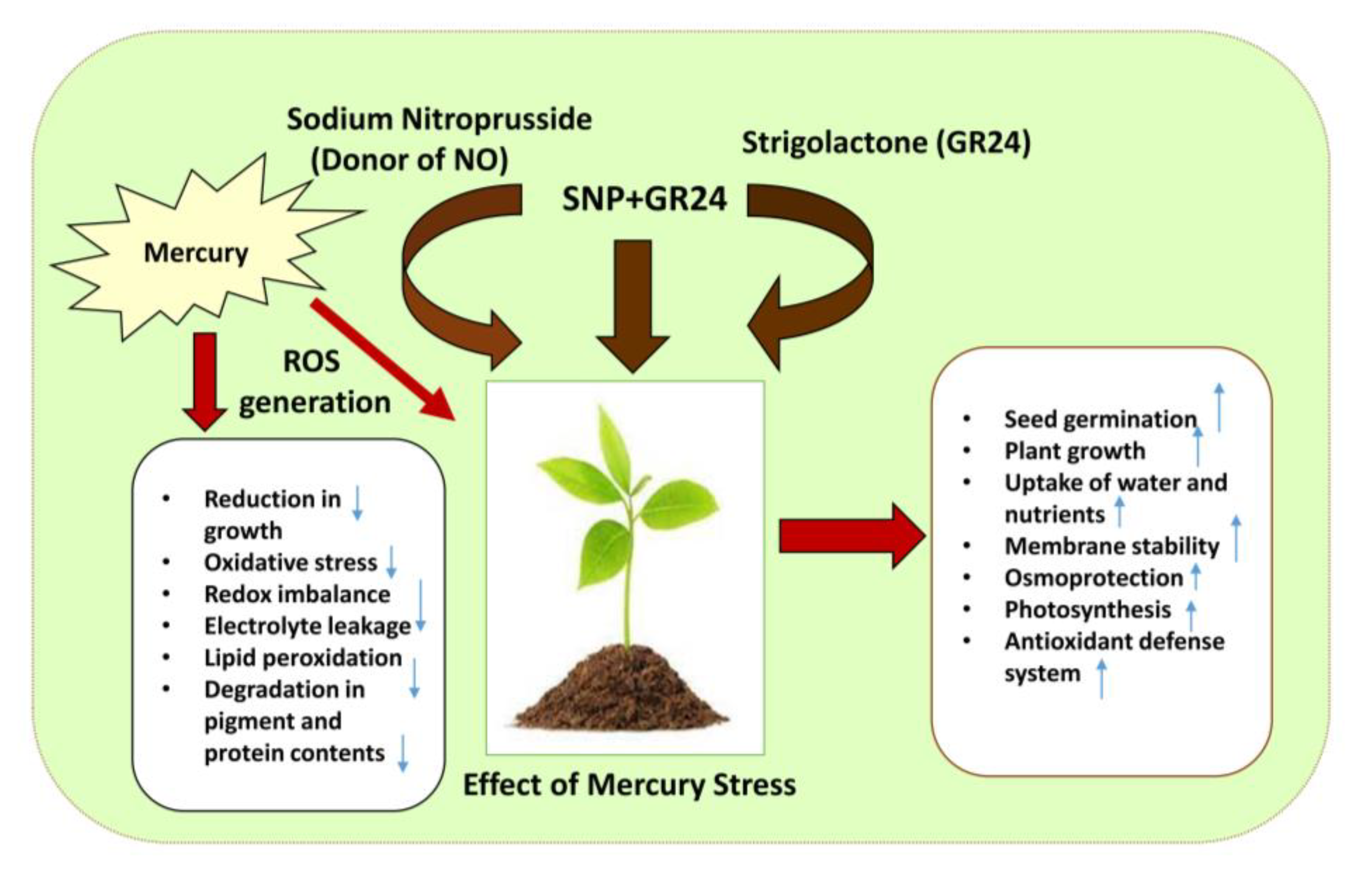
3. Discussion
4. Materials and Methods
4.1. Procurement of Chemicals
4.2. Cultivation of Plants and Stress Treatment
4.3. Growth and Relative Water Content
4.4. Pigment Content
4.5. Evaluation of Sugar, Proline, Glycine Betaine and Protein Contents
4.6. Measurement of Electrolyte Leakage, Hydrogen Peroxide, Superoxide Radical
4.7. Lipid Peroxidation and Lipoxygenase Activity
4.8. Assessment of Hg and Other Inorganic Elements
4.9. Antioxidant Enzymes
4.10. Superoxide Dismutase Assay
4.11. Catalase Assay
4.12. Ascorbate Peroxidase Assay
4.13. Glutathione Reductase
4.14. Assay of Glyoxalase I and II
4.15. Methylglyoxal Content
4.16. Determination of Ascorbate and Reduced Glutathione
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, K.K.; Pandey, N.; Meena, R.P.; Rai, S.P. Biotechnological strategies for enhancing heavy metal tolerance in neglected and underutilized legume crops: A comprehensive review. Ecotoxicol. Environ. Saf. 2021, 208, 111750. [Google Scholar] [CrossRef] [PubMed]
- Chaki, M.; Begara-Morales, J.C.; Barroso, J.B. Oxidative stress in plants. Antioxidants 2020, 9, 481. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive Review on the Heavy Metal Toxicity and Sequestration in Plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef]
- Du, J.; Guo, Z.; Li, R.; Ali, A.; Guo, D.; Lahori, A.H.; Wang, P.; Liu, X.; Wang, X.; Zhang, Z. Screening of Chinese mustard (Brassica juncea L.) cultivars for the phytoremediation of Cd and Zn based on the plant physiological mechanisms. Environ. Pollut. 2020, 261, 114213. [Google Scholar] [CrossRef]
- Ganguly, R.; Sarkar, A.; Acharya, K.; Keswani, C.; Minkina, T.; Mandzhieva, S.; Sushkova, S.; Chakraborty, N. The role of no in the amelioration of heavy metal stress in plants by individual application or in combination with phytohormones, especially auxin. Sustainability 2022, 14, 8400. [Google Scholar] [CrossRef]
- Zhao, S.; Terada, A.; Nakamura, K.; Nakashima, M.; Komai, T.; Riya, S.; Hosomi, M.; Hou, H. Significance of soil moisture on temperature dependence of Hg emission. J. Environ. Manag. 2021, 305, 114308. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.P.; Chang, T.C.; Chiu, C.H.; Hsi, H.C. Mercury speciation and mass distribution in cement production process of Taiwan. Aerosol Air Qual. Res. 2018, 18, 2801–2812. [Google Scholar] [CrossRef]
- Hao, R.; Yang, F.; Mao, X.; Mao, Y.; Zhao, Y.; Lu, Y. Emission factors of mercury and particulate matters, and in situ control of mercury during the co-combustion of anthracite and dried sawdust sludge. Fuel 2018, 230, 202–210. [Google Scholar] [CrossRef]
- Aysin, F.; Karaman, A.; Yilmaz, A.; Aksakal, O.; Gezgincioglu, E.; Kohnehshahri, S.M. Exogenous cysteine alleviates mercury stress by promoting antioxidant defence in maize (Zea mays L.) seedlings. Turkish J. Agric. For. 2020, 44, 506–516. [Google Scholar] [CrossRef]
- Han, Y.; Ni, Z.; Li, S.; Qu, M.; Tang, F.; Mo, R.; Ye, C.; Liu, Y. Distribution, relationship, and risk assessment of toxic heavy metals in walnuts and growth soil. Environ. Sci. Pollut. Res. 2018, 25, 17434–17443. [Google Scholar] [CrossRef]
- Campos, J.; Esbrí, J.; Madrid, M.; Naharro, R.; Peco, J.; García-Noguero, E. Does mercury presence in soils promote their microbial activity? The Almadenejos case (Almadén mercury mining district, Spain). Chemosphere 2018, 201, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.S.; Oliveira, J.R.; Curi, N.; Schulze, D.G.; Marques, J.J. Selenium and mercury in Brazilian Cerrado soils and their relationships with physical and chemical soil characteristics. Chemosphere 2019, 218, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zhu, Y.; Zhang, X.; Zhou, X.; Zhong, Z.; Li, H.; Li, Y.; Li, X.; Daud, M.K.; Chen, J.; et al. Mercury-induced phytotoxicity and responses in upland cotton (Gossypium hirsutum L.) seedlings. Plants 2021, 10, 1494. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Khalid, S.; Bibi, I.; Bundschuh, J.; Niazi, N.K.; Dumat, C. A critical review of mercury speciation, bioavailability, toxicity and detoxification in soil-plant environment: Ecotoxicology and health risk assessment. Sci. Total Environ. 2020, 711, 134749. [Google Scholar]
- Jyothi, N.R.; Farook, N.A.M. Mercury toxicity in public health. Heavy Met. Toxic. Public Health 2020, 54, 237-e1. [Google Scholar]
- Safari, F.; Akramian, M.; Salehi-Arjmand, H.; Khadivi, A. Physiological and molecular mechanisms underlying salicylic acid-mitigated mercury toxicity in lemon balm (Melissa officinalis L). Ecotoxicol. Environ. Saf. 2019, 183, 109542. [Google Scholar] [CrossRef]
- Tamashiro, H.; Arakaki, M.; Akagi, H.; Futatsuka, M.; Roht, L.H. Mortality and survival for minamata disease. Int. J. Epidemiol. 1985, 14, 582–588. [Google Scholar] [CrossRef]
- Piscopo, M.; Tenore, G.C.; Notariale, R.; Maresca, V.; Maisto, M.; De Ruberto, F.; Heydari, M.; Sorbo, S.; Basile, A. Antimicrobial and antioxidant activity of proteins from Feijoa sellowiana Berg. fruit before and after in vitro gastrointestinal digestion. Nat. Prod. Res. 2020, 34, 2607–2611. [Google Scholar] [CrossRef]
- Tortora, F.; Notariale, R.; Maresca, V.; Good, K.V.; Sorbo, S.; Basile, A.; Piscopo, M.; Manna, C. Phenol-rich Feijoa sellowiana (pineapple guava) extracts protect human red blood cells from mercury-induced cellular toxicity. Antioxidants 2019, 8, 220. [Google Scholar] [CrossRef]
- Henriques, M.C.; Loureiro, S.; Fardilha, M.; Herdeiro, M.T. Exposure to mercury and human reproductive health: A systematic review. Rep. Toxicol. 2019, 85, 93–103. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakraborty, N.; Acharya, K. Unraveling the role of nitric oxide in regulation of defense responses in chilli against Alternaria leaf spot disease. Physiol. Mol. Plant Pathol. 2021, 114, 101621. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to enhancing antioxidant defense in plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, H.; Ding, J.; Fu, W.; Gan, L.; Li, Y. Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Sci. Rep. 2017, 7, srep46545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lu, Q.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Mercury induced oxidative stress, DNA damage, and the activation of antioxidative system and Hsp 70 induction in duckweed (Lemna minor). Ecotoxicol. Environ. Saf. 2017, 143, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Feng, F.; Liu, J.; Zhao, Q. Nitric oxide affects rice root growth by regulating auxin transport under nitrate supply. Front. Plant Sci. 2018, 9, 659. [Google Scholar] [CrossRef]
- Zhang, H.; Huo, S.; Yeager, K.M.; Xi, B.; Zhang, J.; He, Z. Accumulation of arsenic, mercury and heavy metals in lacustrine sediment in relation to eutrophication: Impacts of sources and climate change. Ecol. Indic. 2018, 93, 771–780. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alam, P. Exogenous application of nitric oxide modulates osmolytes metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 2018, 255, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.; Rodriguez, E.; Mendes, R.J.; Mariz-Ponte, N.; Sario, S.; Lopes, J.C.; de Oliveira, J.M.P.F.; Santos, C. Inorganic Hg toxicity in plants: A comparison of different genotoxic parameters. Plant Physiol. Biochem. 2018, 125, 247–254. [Google Scholar] [CrossRef]
- Paul, A.; Sarkar, A.; Acharya, K.; Chakraborty, N. Fungal elicitor-mediated induction of innate immunity in Catharanthus roseus against leaf blight disease caused by Alternaria alternata. J. Plant Growth Regul. 2022, 6, 100124. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric Oxide Signaling and Its Crosstalk with Other Plant Growth Regulators in Plant Responses to Abiotic Stress. Environ. Sci. Pollut. Res. Int. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Ghosh, S.; Paul, S.; Mazumdar, S.; Das, G.; Das, S. Pre-treatment of seeds with salicylic acid attenuates cadmium chloride-induced oxidative damages in the seedlings of mungbean (Vigna radiata L. Wilczek). Acta Physiol. Plant 2016, 38, 11. [Google Scholar] [CrossRef]
- Keishama, M.; Jaina, P.; Singha, N.; Toerneb, C.V.; Bhatla, S.C.; Lindermayr, C. Deciphering the nitric oxide, cyanide and iron-mediated actions of sodium nitroprusside in cotyledons of salt stressed sunflower seedlings. Nitric Oxide 2019, 88, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A. Strigolactones: Multi-level regulation of biosynthesis and diverse responses in plant abiotic stresses. Acta Physiol. Plant 2018, 40, 86. [Google Scholar] [CrossRef]
- Jamil, M.; Kountche, B.A.; Haider, I.; Wang, J.Y.; Aldossary, F.; Zarban, R.A.; Jia, K.P.; Yonli, D.; Shahul Hameed, U.F.; Takahashi, I.; et al. Methylation at the c-3′ in d-ring of strigolactone analogs reduces biological activity in root parasitic plants and rice. Front Plant Sci. 2019, 10, 353. [Google Scholar] [CrossRef]
- Sun, H.; Tao, J.; Liu, S.; Huang, S.; Chen, S.; Xie, X.; Yoneyama, K.; Zhang, Y.; Xu, G. Strigolactones are involved in phosphate and nitrate-deficiency induced root development and auxin transport in rice. J. Exp. Bot. 2014, 65, 6735–6746. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ali, M.; Ahmad, M.Z.; Liang, G.; Zhao, J. Strigolactones promote rhizobia interaction and increase nodulation in soybean (Glycine max). Microb. Pathog. 2018, 114, 420–430. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef]
- Chelladurai, V.; Erkinbaev, C. Lentils. In Pulses; Manickavasagan, A., Thirunathan, P., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Kaur, C.; Sharma, S.; Hasan, M.R.; Pareek, A.; Singla-Pareek, S.L.; Sopory, S.K. Characteristic variations and similarities in biochemical, molecular, and functional properties of glyoxalases across prokaryotes and eukaryotes. Int. J. Mol. Sci. 2017, 18, 250–271. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Shafiq, M.; Athar, M. Phytotoxic effects of mercury on seed germination and seedling growth of Albizia lebbeck L. Benth. (Leguminosae). Adv. Environ. Res. 2014, 3, 207–216. [Google Scholar] [CrossRef]
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; AlKahtani, M.D.; Khan, M.S. Heavy metal induced stress on wheat: Phytotoxicity and microbiological management. RSC Adv. 2020, 10, 38379–38403. [Google Scholar] [CrossRef]
- Cui, W.; Fang, P.; Zhu, K.; Mao, Y.; Gao, C.; Xie, Y.; Wang, J.; Shen, W. Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol. Environ. Saf. 2014, 105, 103–111. [Google Scholar] [CrossRef]
- Magistrali, P.R.; Borges, E.E.D.E.; Oliveira, J.A.; Faria, J.M.R.; Ataide, G.D.; Nascimento, J.F. Mercury affects aquaporins activity and germination of the embryonic axis of Schizolobium parahyba (vell. Blake (fabaceae). Rev. Arvore 2019, 43, 430601. [Google Scholar] [CrossRef]
- Kim, Y.X.; Stumpf, B.; Sung, J.; Lee, S.J. The relationship between turgor pressure change and cell hydraulics of midrib parenchyma cells in the leaves of Zea mays. Cells 2018, 7, 180. [Google Scholar] [CrossRef]
- Basalah, M.O.; Ali, H.M.; Al-Whaibi, M.H.; Siddiqui, M.H.; Sakran, A.M.; Al Sahli, A.A. Oxide and Salicylic Acid Mitigate Cadmium Stress in. Wheat Seedlings. J. Pure. Appl. Microbiol. 2013, 7, 139–148. [Google Scholar]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd-Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef]
- Kolbert, Z. Implication of nitric oxide (no) in excess element-induced morphogenic responses of the root system. Plant Physiol. Biochem. 2016, 101, 149–161. [Google Scholar] [CrossRef]
- Bin-Jumah, M.; Abdel-Fattah, A.F.M.; Saied, E.M.; El-Seedi, H.R.; Abdel-Daim, M.M. Acrylamide-induced peripheral neuropathy: Manifestations, mechanisms, and potential treatment modalities. Environ. Sci. Pollut. Res. 2021, 28, 13031–13046. [Google Scholar] [CrossRef] [PubMed]
- Kharbech, O.; Sakouhi, L.; Ben Massoud, M.; Mur, L.A.; Corpas, F.J.; Djebali, W.; Chaoui, A. Nitric oxide and hydrogen sulfide protect plasma membrane integrity and mitigate chromium-induced methylglyoxal toxicity in maize seedlings. Plant Physiol. Biochem. 2020, 157, 1016. [Google Scholar] [CrossRef] [PubMed]
- Gaber, A.; Alsanie, W.F.; Kumar, D.N.; Refat, M.S.; Saied, E.M. Novel papaverine metal complexes with potential anticancer activities. Molecules 2020, 25, 5447. [Google Scholar] [CrossRef]
- Kharbech, O.; Houmani, H.; Chaoui, A.; Corpas, F.J. Alleviation of Cr(VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPH-recycling metabolisms. J. Plant Physiol. 2017, 219, 1016. [Google Scholar] [CrossRef]
- Rizwan, M.; Mostofa, M.G.; Mz, A. Nitric oxide induces rice tolerance to excessive nickel by regulating nickel uptake, reactive oxygen species detoxification and defense-related gene expression. Chemosphere 2018, 191, 1016. [Google Scholar] [CrossRef]
- Demecsova, L.; Bočova, B.; Zelinova, V.; Tamas, L. Enhanced nitric oxide generation mitigates cadmium toxicity via superoxide scavenging leading to the formation of peroxynitrite in barley root tip. J. Plant Physiol. 2019, 238, 1016. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z. Strigolactone-nitric oxide interplay in plants: The story has just begun. Physiol. Plant 2019, 165, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.Z.; Rehman, N.U.; Yu, S.; Zhou, Y.; Haq, B.U.; Wang, J.; Li, P.; Zeng, Z.; Zhao, J. Gm MAX2-D14 and -KAI interaction mediated SL and KAR signaling play essential roles in soybean root nodulation. Plant J. 2020, 101, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.C.; Lacerda, L.D.; Silva-Filho, E.V. Foliar mercury content from tropical trees and its correlation with physiological parameters in situ. Environ. Pollut. 2018, 242, 1050–1057. [Google Scholar] [CrossRef]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiol. Plant 2019, 168, 318–344. [Google Scholar] [CrossRef]
- Yang, J.; Li, G.; Bishopp, A.; Heenatigala, P.P.M.; Hu, S.; Chen, Y.; Wu, Z.; Kumar, S.; Duan, P.; Yao, L.; et al. A Comparison of growth on mercuric chloride for three lemnaceae species reveals differences in growth dynamics that effect their suitability for use in either monitoring or remediating ecosystems contaminated with mercury. Front. Chem. 2018, 6, 112. [Google Scholar] [CrossRef]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2018, 135, 11037. [Google Scholar] [CrossRef]
- Ben Massoud, M.; Sakouhi, L.; Karmous, K. Protective role of exogenous phytohormones on redox status in pea seedlings under copper stress. J. Plant Physiol. 2018, 221, 51–61. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Duarte, B.; Cesário, R.; Mendes, R.; Hintelmann, H.; Eckey, K.; Dimock, B.; Caçador, I.; Canário, J. Mercury mobility and effects in the salt-marsh plant Halimione portulacoides: Uptake, transport, and toxicity and tolerance mechanisms. Sci. Total Environ. 2019, 650, 111–120. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M. Exogenous application of nitric oxide promotes growth and oxidative defense system in highly boron stressed tomato plants bearing fruit. Sci. Hort. 2015, 185, 43–47. [Google Scholar] [CrossRef]
- Asada, K. Ascorbate peroxidase—A hydrogen peroxide-scavenging enzyme in plants. Physiol. Plantarum. 1992, 85, 235–241. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Jogeswar, G.; Rasineni, G.K.; Maheswari, M.; Reddy, A.R.; Varshney, R.K. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum. Moench. Plant Physiol. Biochem. 2015, 94, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Wani, K.I.; Naeem, M.; Khan, M.M.A.; Aftab, T. Cellular responses, osmotic adjustments, and role of osmolytes in providing salt stress resilience in higher plants: Polyamines and nitric oxide crosstalk. J. Plant Growth Regul. 2022, 42, 539–553. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Jamshed, M.; Kumar, A.; Skori, L.; Scandola, S.; Wang, T.; Spiegel, D.; Samuel, M.A. Glyoxalase goes green: The expanding roles of glyoxalase in plants. Int. J. Mol. Sci. 2017, 18, 898–913. [Google Scholar] [CrossRef]
- Suhartono, E.; Triawanti, T.; Setyo Leksono, A.; Sasmito Djati, M. The role of cadmium in proteinsglycation by glucose: Formation of methylglyoxal and hydrogenper- oxide invitro. J. Med. Bioeng. 2014, 3, 59–62. [Google Scholar]
- Chowardhara, B.; Borgohain, P.; Saha, B.; Awasthi, J.P.; Panda, S.K. Differential oxidative stress responses in Brassica juncea (L.) Czern and Coss cultivars induced by cadmium at germination and early seedling stage. Acta Physiol. Plant 2020, 42, 105. [Google Scholar] [CrossRef]
- Xu, L.L.; Fan, Z.Y.; Dong, Y.J.; Kong, J.; Bai, X.Y. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol. Plant. 2015, 59, 171–182. [Google Scholar] [CrossRef]
- Nahar, K.; Rhaman, M.S.; Parvin, K.; Bardhan, K.; Marques, D.N.; García-Caparrós, P.; Hasanuzzaman, M. Arsenic-induced oxidative stress and antioxidant defense in plants. Stresses 2022, 2, 179–209. [Google Scholar] [CrossRef]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Irfan, Q.M.; Rehman, R.U. Characterization of mercury-induced stress biomarkers in Fagopyrum tataricum plants. Inter. J. Phytoremed. 2018, 20, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Ben Massoud, M.; Kharbech, O.; Mahjoubi, Y.; Chaoui, A.; Wingler, A. Effect of exogenous treatment with nitric oxide (no) on redox homeostasis in barley seedlings (hordeum vulgare l.) under copper stress. J. Soil Sci. Plant Nutr. 2022, 22, 1604–1617. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Hefft, D.I.; Ahmad, A. Nitric oxide and spermidine alleviate arsenic-incited oxidative damage in Cicer arietinum by modulating glyoxalase and antioxidant defense system. Funct. Plant Biol. 2023, 50, 108–120. [Google Scholar]
- Alsahli, A.A.; Bhat, J.A.; Alyemeni, M.N. Hydrogen sulphide mitigates arsenic induced toxicity in pea plants by regulating osmoregulating osmoregulation, antioxidant defense system, ascorbate glutathione cycle and glyoxylase system. J. Plant Growth Regul. 2021, 40, 2015–2531. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids pigments of photosynthetic membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Hedge, J.E.; Hofreiter, B.T. Estimation of carbohydrate. In Methods in Carbohydrate Chemistry; Whistler, R.L., Wolfrom, M.L., Eds.; Academic Press: New York, NY, USA, 1962; pp. 17–22. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for the determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Fan, A.L.; Randall, R.I. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 131–139. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Yang, L.; Tian, D.; Todd, C.D.; Luo, Y.; Hu, X. Comparative proteome analyses reveal that nitric oxide is an important signal molecule in the response of rice to aluminum toxicity. J. Proteome Res. 2013, 12, 1316–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Leul, M. Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. Plant Growth Regul. 1998, 26, 41–47. [Google Scholar] [CrossRef]
- Doderer, A.; Kokkelink, I.; Vanderween, S.; Valk, B.; Schrom, A.W.; Douma, A.C. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim. Biophys. Acta 1992, 1120, 97–104. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Foster, J.S.; Hess, J.L. Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol. 1980, 66, 482–487. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef]
- Wild, R.; Ooi, L.; Srikanth, V.; Münch, G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: The N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 2012, 403, 2577–2581. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Choudhuri, M.A. Implications of water stressinduced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]


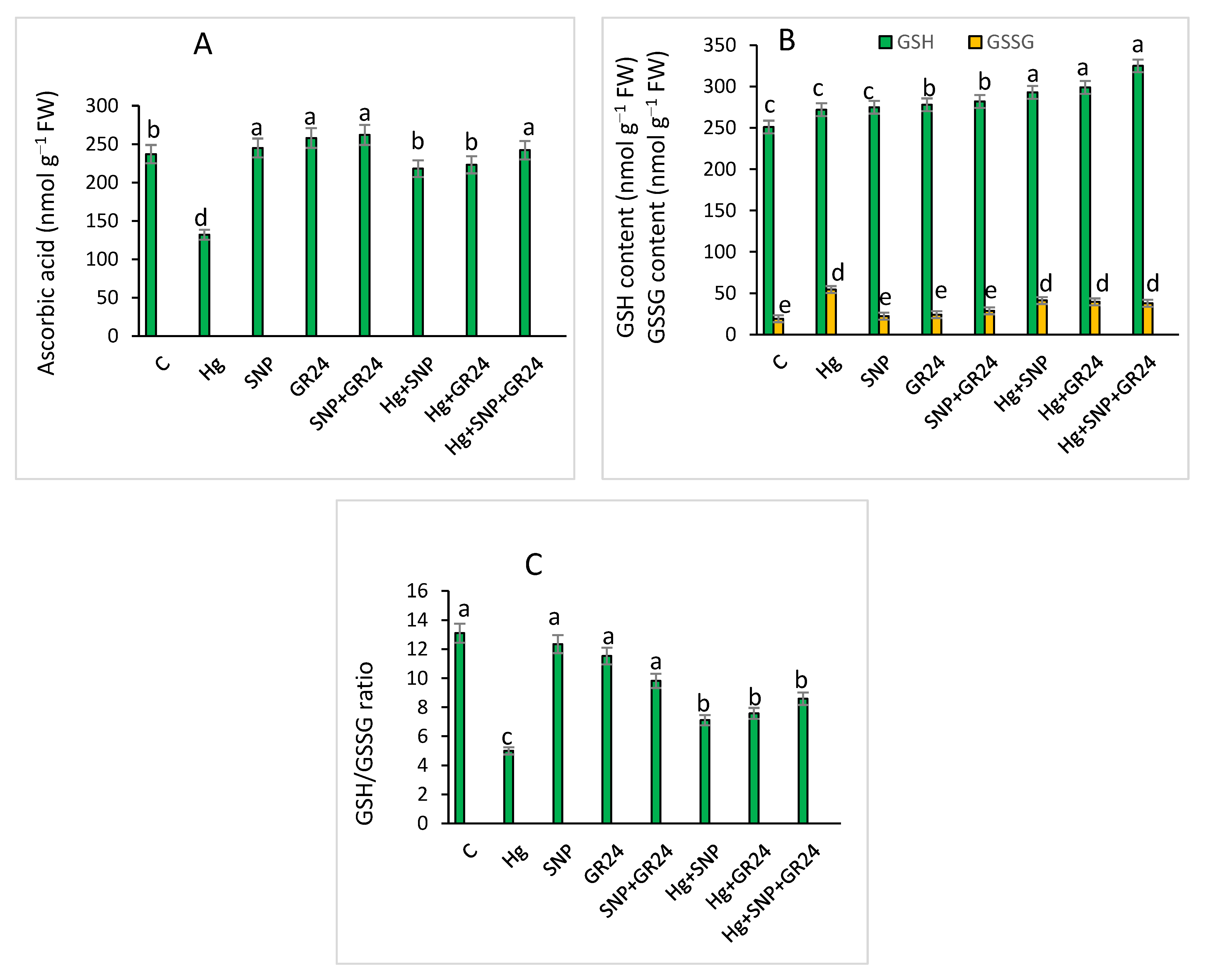
| Treatment | Root Length (cm) | Shoot Length (cm) | Fresh Weight (g) | Dry Weight (g) | Relative Water Content (%) |
|---|---|---|---|---|---|
| Control | 12.76 ± 0.36 a | 26.15 ± 0.17 a | 8.95 ± 0.05 b | 2.49 ± 0.23 a | 97.58 ± 0.15 a |
| Hg | 4.29 ± 0.24 c | 12.69 ± 0.36 c | 2.51 ± 0.06 d | 0.27 ± 0.05 c | 68.29 ± 0.35 c |
| SNP | 13.81 ± 0.23 a | 26.46 ± 0.39 a | 9.34 ± 0.34 a | 2.55 ± 0.19 a | 89.81 ± 0.06 b |
| GR24 | 14.92 ± 0.09 a | 27.70 ± 0.35 a | 10.02 ± 0.03 a | 2.67 ± 0.18 a | 92.45 ± 0.04 a |
| SNP + GR24 | 15.51 ± 0.35 a | 28.80 ± 0.24 a | 10.54 ± 0.32 a | 2.8 ± 0.07 a | 96.99 ± 0.35 a |
| Hg + SNP | 8.69 ± 0.13 b | 15.37 ± 0.45 b | 5.76 ± 0.29 c | 1.35 ± 0.09 b | 83.84 ± 0.38 b |
| Hg + GR24 | 9.58 ± 0.37 b | 16.73 ± 0.33 b | 6.61 ± 0.09 b | 1.54 ± 0.03 b | 88.32 ± 0.07 b |
| Hg + SNP + GR24 | 11.63 ± 0.22 b | 19.87 ± 0.15 b | 7.22 ± 0.19 b | 1.91 ± 0.07 b | 91.71 ± 0.08 a |
| Treatment | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Total Chlorophyll (a + b) (mg g−1 FW) | Carotenoids (mg g−1 FW) |
|---|---|---|---|---|
| Control | 1.61 ± 0.01 b | 0.76 ± 0.03 a | 2.37 ± 0.03 a | 0.49 ± 0.01 b |
| Hg | 1.17 ± 0.08 c | 0.22 ± 0.01 d | 1.39 ± 0.01 c | 0.43 ± 0.02 b |
| SNP | 1.72 ± 0.03 a | 0.77 ± 0.01 a | 2.49 ± 0.01 a | 0.53 ± 0.03 a |
| GR24 | 1.8 ± 0.07 a | 0.82 ± 0.02 a | 2.62 ± 0.04 a | 0.56 ± 0.02 a |
| SNP + GR24 | 1.86 ± 0.04 a | 0.86 ± 0.03 a | 2.72 ± 0.02 a | 0.58 ± 0.01 a |
| Hg + SNP | 1.2 ± 0.07 b | 0.48 ± 0.01 c | 1.68 ± 0.02 c | 0.48 ± 0.04 b |
| Hg + GR24 | 1.23 ± 0.11 b | 0.63 ± 0.01 c | 1.86 ± 0.03 b | 0.51 ± 0.01 a |
| Hg + SNP + GR24 | 1.45 ± 0.03 b | 0.71 ± 0.01 a | 2.16 ± 0.04 b | 0.52 ± 0.01 a |
| Treatment | Sugar (mg g−1 DW) | Proline (µM g−1 DW) | Glycine Betaine (µg g−1 DW) | Protein (mg g−1 FW) |
|---|---|---|---|---|
| Control | 4.15 ± 0.03 c | 15.25 ± 0.16 d | 2.33 ± 0.16 c | 15.29 ± 0.10 a |
| Hg | 3.06 ± 0.04 c | 19.32 ± 0.09 d | 6.17 ± 0.08 a | 9.34 ± 0.11 c |
| SNP | 4.89 ± 0.07 b | 25.42 ± 0.36 c | 2.77 ± 0.48 c | 13.66 ± 0.20 b |
| GR24 | 5.13 ± 0.04 b | 27.33 ± 0.22 c | 3.13 ± 0.11 b | 14.91 ± 0.05 b |
| SNP + GR24 | 6.75 ± 0.16 a | 34.69 ± 0.19 b | 3.67 ± 0.10 b | 17.14 ± 0.03 a |
| Hg + SNP | 5.43 ± 0.17 b | 37.38 ± 0.29 b | 6.5 ± 0.32 a | 12.95 ± 0.06 b |
| Hg + GR24 | 5.94 ± 0.05 b | 40.29 ± 0.27 a | 6.9 ± 0.21 a | 14.17 ± 0.08 b |
| Hg + SNP + GR24 | 7.16 ± 0.04 a | 48.11 ± 0.13 a | 7.8 ± 0.37 a | 16.64 ± 0.25 a |
| Treatment | Hg Content (µg g−1 DW) | Concentration Index (CI) | Translocation Factor (TF) | ||
|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | ||
| Control | 0.16 ± 0.01 c | 0.13 ± 0.004 c | 3.06 | 3.06 | 0.81 |
| Hg | 9.63 ± 0.04 a | 7.29 ± 0.07 a | 60.19 | 56.08 | 0.76 |
| SNP | 5.26 ± 0.01 b | 4.19 ± 0.06 b | 32.88 | 32.23 | 0.79 |
| GR24 | 4.95 ± 0.03 b | 3.82 ± 0.19 b | 30.94 | 29.38 | 0.77 |
| SNP + GR24 | 4.12 ± 0.09 b | 3.11 ± 0.07 b | 25.75 | 23.92 | 0.75 |
| Hg + SNP | 6.03 ± 0.03 a | 5.27 ± 0.05 a | 37.69 | 40.54 | 0.87 |
| Hg + GR24 | 6.82 ± 0.09 a | 6.11 ± 0.01 a | 42.63 | 47 | 0.89 |
| Hg + SNP + GR24 | 5.95 ± 0.04 a | 5.08 ± 0.07 a | 37.19 | 39.08 | 0.85 |
| Lentil Plant | Minerals (µg g−1 DW) | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Hg | SNP | GR24 | SNP + GR24 | Hg + SNP | Hg + GR24 | Hg + SNP + GR24 | ||
| Root | N | 827 ± 1.41 b | 524 ± 2.16 c | 884 ± 2.16 a | 903 ± 1.63 a | 933 ± 2.16 a | 653 ± 4.97 a | 567 ± 1.41 a | 689 ± 2.12 a |
| P | 384 ± 1.78 c | 121 ± 0.82 d | 420 ± 0.71 b | 424 ± 3.34 b | 452 ± 2.94 b | 346 ± 3.94 b | 321 ± 1.5 b | 366 ± 3.54 b | |
| K | 1324 ± 3.63 a | 705 ± 1.87 b | 1365 ± 15.20 a | 1406 ± 3.56 a | 1416 ± 5.72 a | 1219 ± 0.41 a | 1172 ± 2.48 a | 1294 ± 3.56 a | |
| Ca | 203 ± 1.77 b | 62 ± 0.41 d | 218 ± 0.41 c | 222 ± 4.32 b | 229 ± 4.49 b | 158 ± 2.55 c | 142 ± 0.82 c | 164 ± 1.47 c | |
| Mg | 264 ± 1.08 b | 83 ± 1.08 d | 269 ± 1.1 c | 274 ± 0.41 b | 281 ± 2.16 b | 198± 0.71 c | 155 ± 1.22 c | 203 ± 2.04 b | |
| Shoot | N | 731 ± 0.82 a | 421 ± 1.78 b | 778 ± 3.19 a | 804 ± 2.86 a | 833 ± 2.55 a | 689 ± 6.72 a | 632 ± 1.1 a | 703 ± 1.08 a |
| P | 285 ± 2.27 b | 93 ± 0.41 d | 289 ± 5.21 c | 296 ± 0.82 b | 308 ± 5.72 b | 252 ± 1.47 b | 248 ± 1.08 b | 268 ± 1.41 b | |
| K | 906 ± 1.1 a | 432 ± 1.47 b | 873 ± 1.08 a | 996 ± 1.77 a | 1013 ± 5.09 a | 862 ± 4.97 a | 721 ± 0.41 a | 675 ± 4.32 a | |
| Ca | 175 ± 0.41 c | 54 ± 1.41 d | 186 ± 0.82 d | 194 ± 3.89 c | 205 ± 1.47 b | 153 ± 1.78 c | 137 ± 3.54 c | 145 ± 3.54 c | |
| Mg | 214 ± 0.71 b | 63 ± 0.40 d | 222 ± 1.87 c | 237 ± 1.47 b | 244 ± 2.04 b | 172 ± 1.77 c | 139 ± 2.48 c | 181 ± 0.41 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, R.T.; Ahmad, A.; Shakoor, A.; Paray, B.A.; Ahmad, P. Nitric Oxide and Strigolactone Alleviate Mercury-Induced Oxidative Stress in Lens culinaris L. by Modulating Glyoxalase and Antioxidant Defense System. Plants 2023, 12, 1894. https://doi.org/10.3390/plants12091894
Kapoor RT, Ahmad A, Shakoor A, Paray BA, Ahmad P. Nitric Oxide and Strigolactone Alleviate Mercury-Induced Oxidative Stress in Lens culinaris L. by Modulating Glyoxalase and Antioxidant Defense System. Plants. 2023; 12(9):1894. https://doi.org/10.3390/plants12091894
Chicago/Turabian StyleKapoor, Riti Thapar, Ajaz Ahmad, Awais Shakoor, Bilal Ahamad Paray, and Parvaiz Ahmad. 2023. "Nitric Oxide and Strigolactone Alleviate Mercury-Induced Oxidative Stress in Lens culinaris L. by Modulating Glyoxalase and Antioxidant Defense System" Plants 12, no. 9: 1894. https://doi.org/10.3390/plants12091894
APA StyleKapoor, R. T., Ahmad, A., Shakoor, A., Paray, B. A., & Ahmad, P. (2023). Nitric Oxide and Strigolactone Alleviate Mercury-Induced Oxidative Stress in Lens culinaris L. by Modulating Glyoxalase and Antioxidant Defense System. Plants, 12(9), 1894. https://doi.org/10.3390/plants12091894








