Macro and Micro-Nutrient Accumulation and Partitioning in Soybean Affected by Water and Nitrogen Supply
Abstract
1. Introduction
2. Results
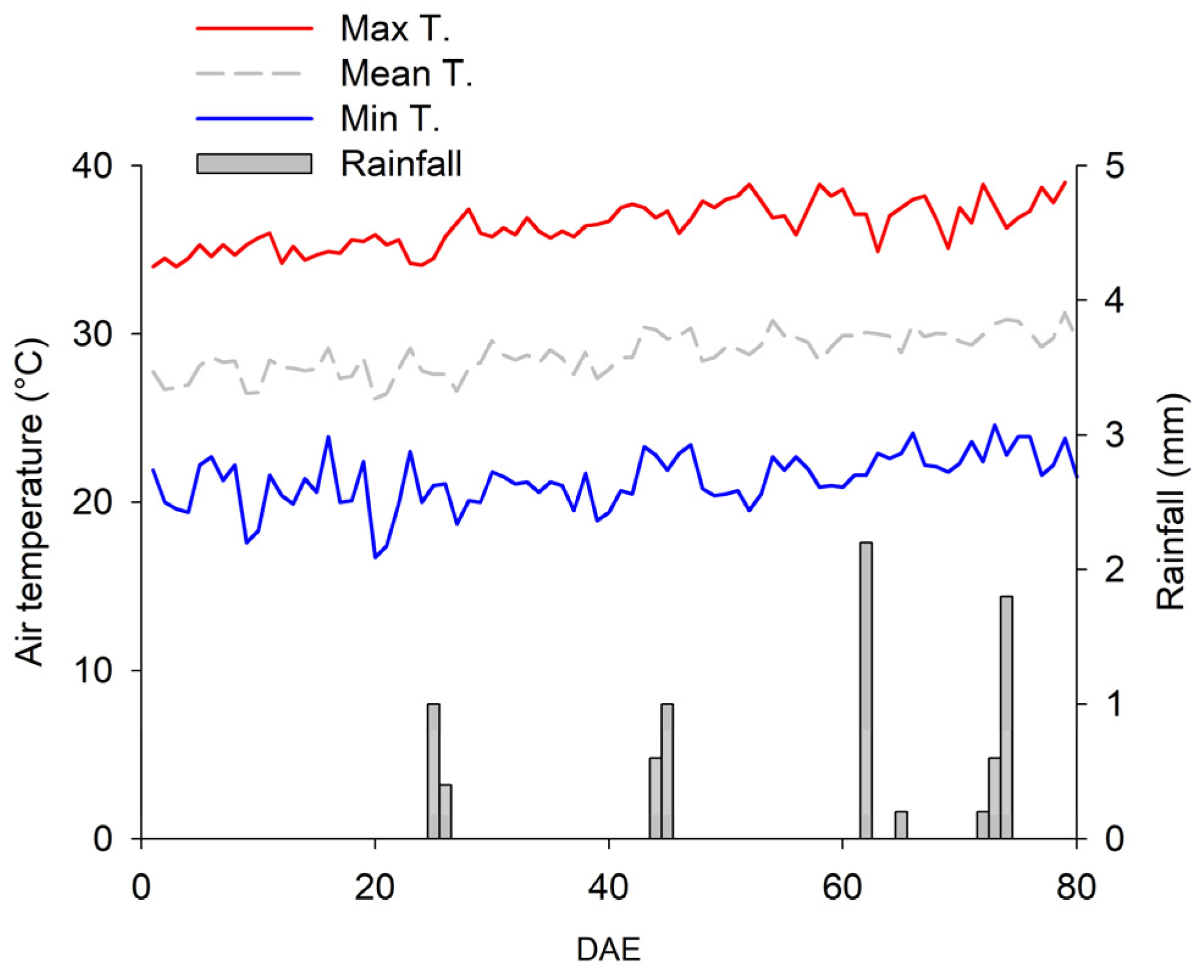
2.1. Water Regimes and Soil Moisture
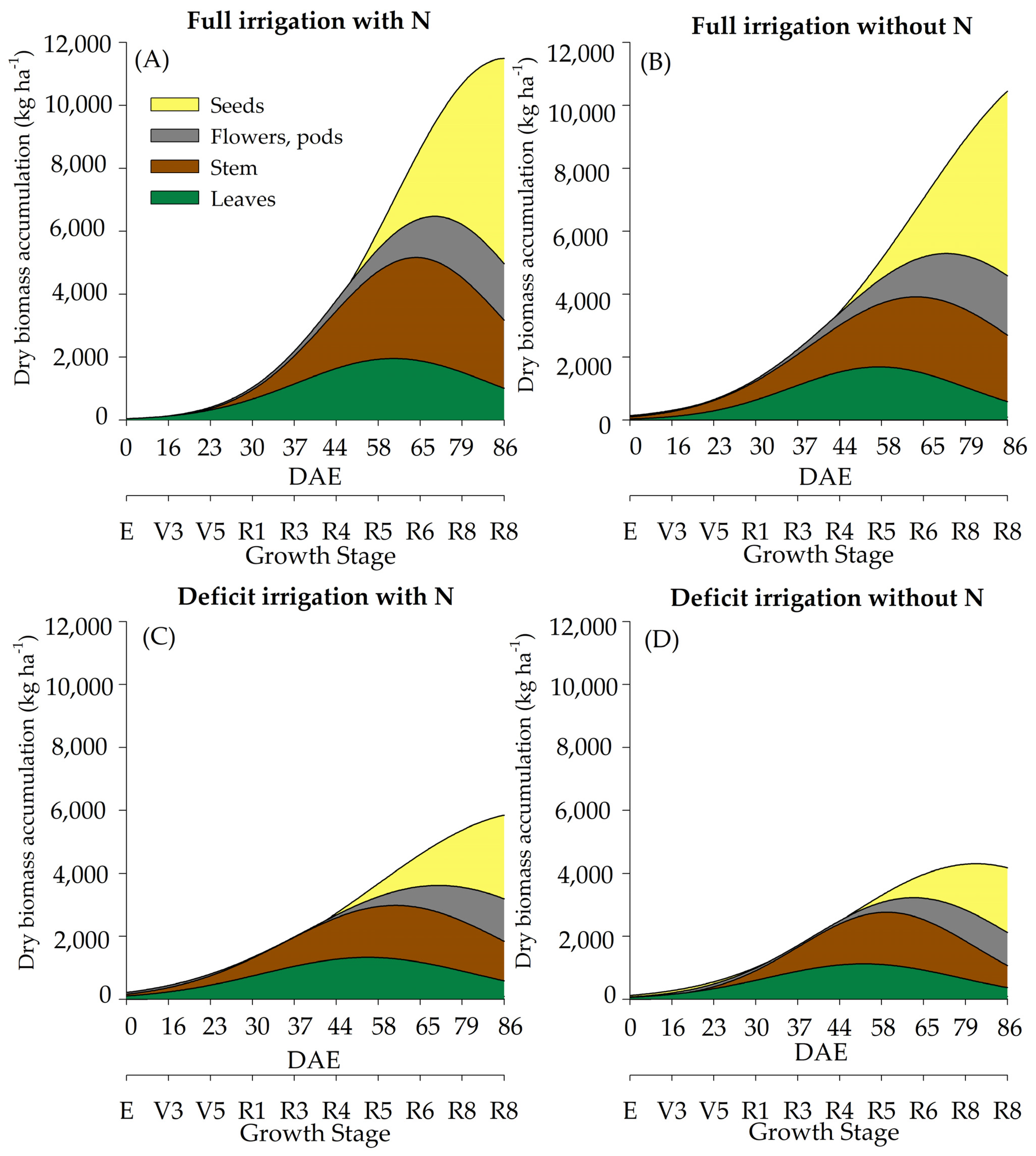
2.2. Dry Biomass Accumulation
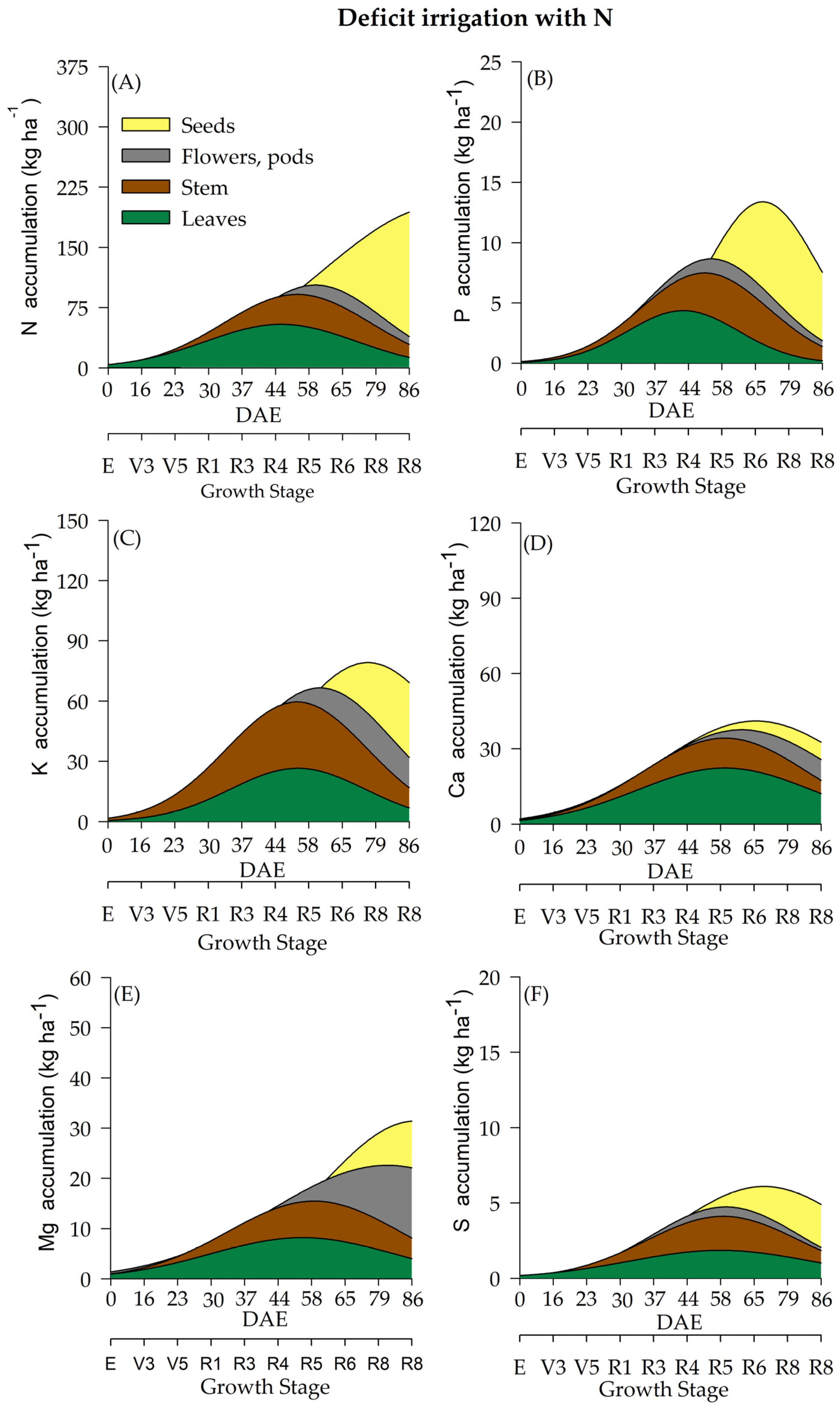
2.3. Macronutrient Accumulation
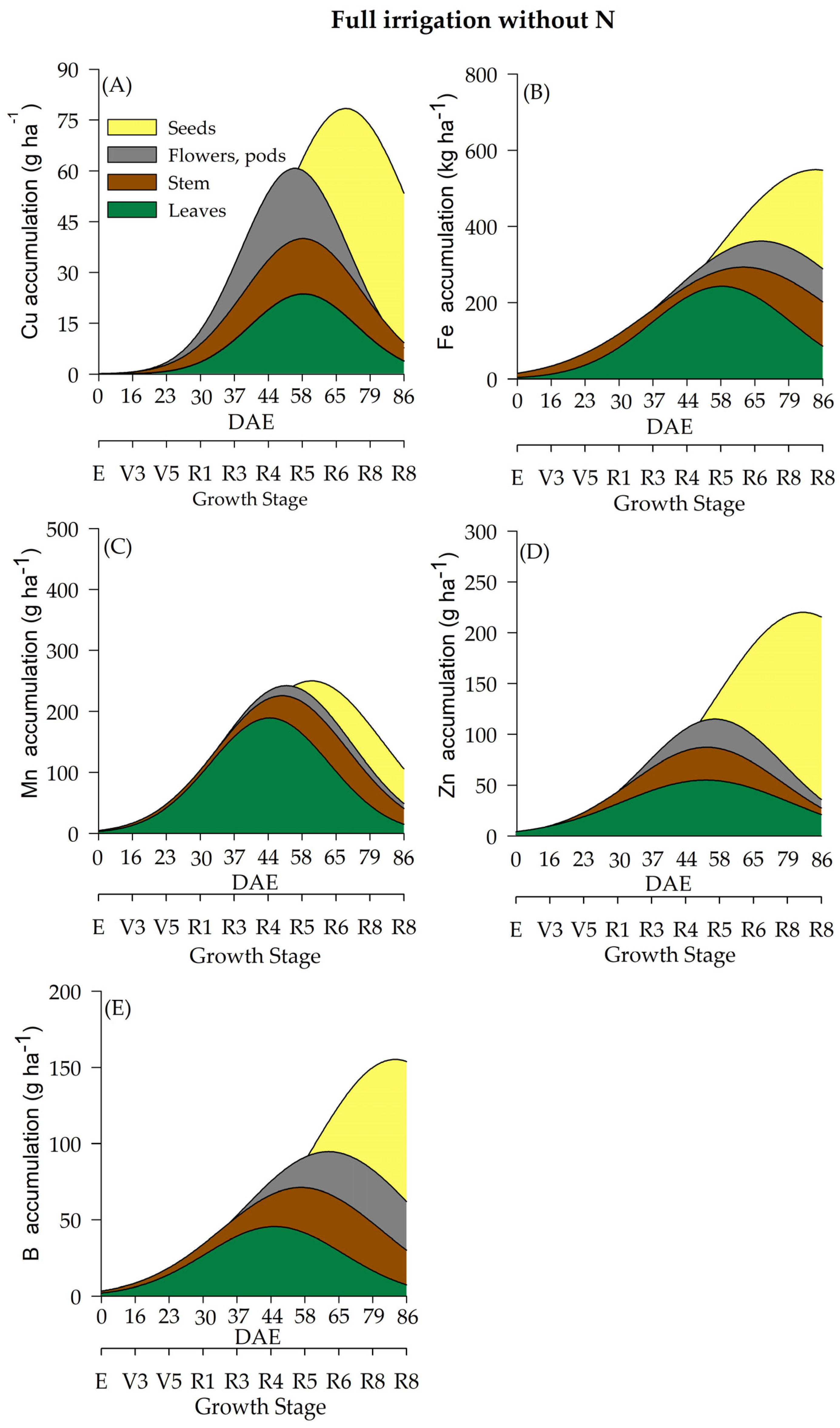
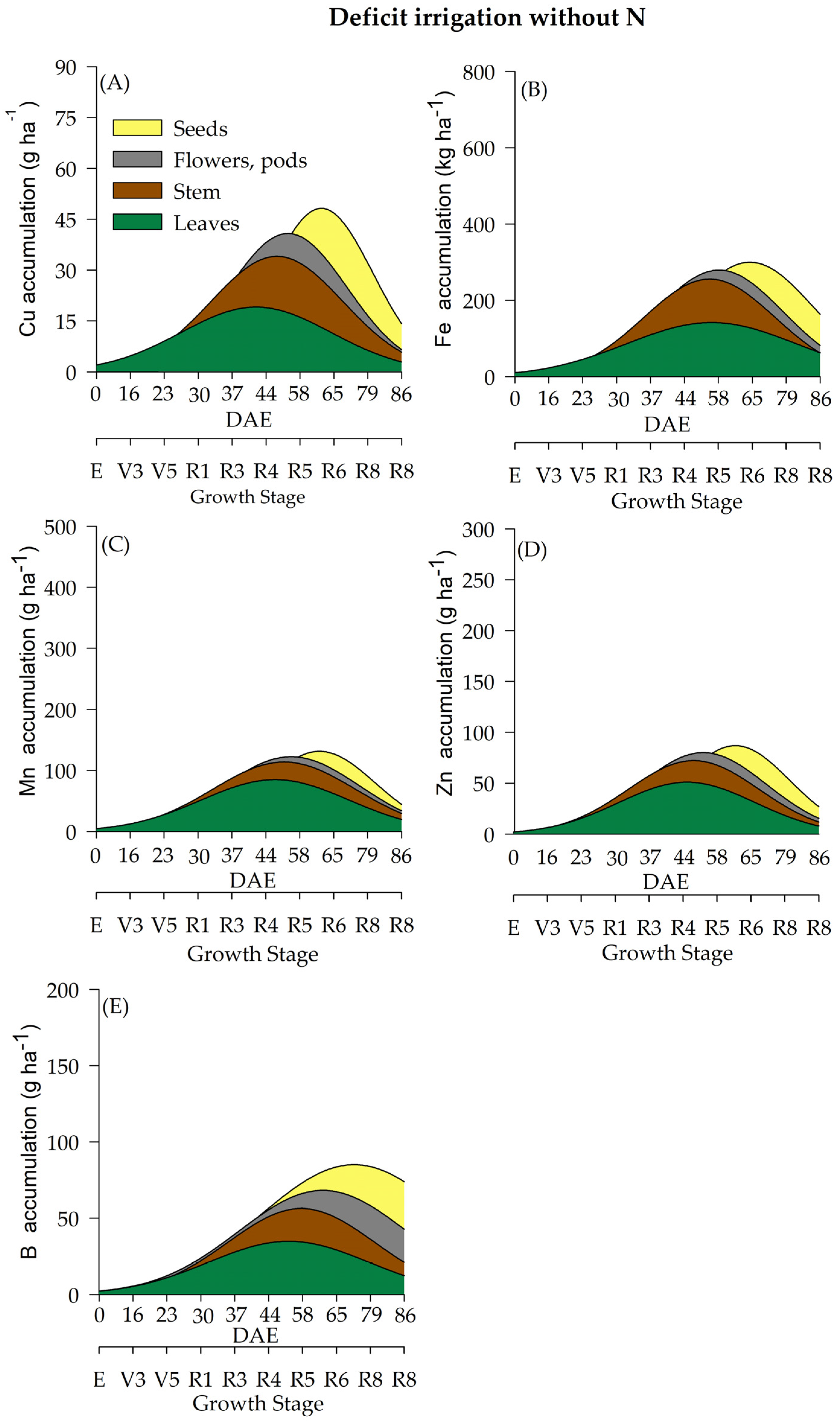
2.4. Micronutrient Accumulation
2.5. Seed Yield, Protein and Oil Percentages
3. Discussion
4. Materials and Methods
4.1. Growth Conditions and Treatments
4.2. Plant Harvests and Trials
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias, L.P.; de Oliveira-Busatto, L.A.; Bodanese-Zanettini, M.H. The Differential Expression of Soybean [ Glycine Max (L.) Merrill] WRKY Genes in Response to Water Deficit. Plant Physiol. Biochem. 2016, 107, 288–300. [Google Scholar] [CrossRef]
- United States Department of Agriculture Foreign Agricultural Service—USDA. Available online: https://www.fas.usda.gov/data (accessed on 26 February 2023).
- Taiz, L.; Zeiger, E.; max Moller, I.; Murphy, A. Physiology and Plant Development Plant Diversity, 6th ed.; Oficina de Textos: São Paulo, SP, Brazil, 2016; ISBN 978-85-8271-366-2. Available online: https://www.ofitexto.com.br/fisiologia-e-desenvolvimento-vegetal/p (accessed on 27 March 2023).
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walters, D.T.; Weiss, A.; Dobermann, A. Nitrogen Uptake, Fixation and Response to Fertilizer N in Soybeans: A Review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Cafaro La Menza, N.; Monzon, J.P.; Specht, J.E.; Grassini, P. Is Soybean Yield Limited by Nitrogen Supply? Field Crops Res. 2017, 213, 204–212. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Wang, X.; Fu, H.; Zhao, M.; Wang, H.; Shi, L. Photosynthetic Characteristics and Metabolic Analyses of Two Soybean Genotypes Revealed Adaptive Strategies to Low-Nitrogen Stress. J. Plant Physiol. 2018, 229, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Córdova, S.C.; Castellano, M.J.; Dietzel, R.; Licht, M.A.; Togliatti, K.; Martinez-Feria, R.; Archontoulis, S.V. Soybean Nitrogen Fixation Dynamics in Iowa, USA. Field Crops Res. 2019, 236, 165–176. [Google Scholar] [CrossRef]
- Barzotto, F.; Robaina, A.D.; Peiter, M.X.; Torres, R.R.; Kirchber, J.H.; Rosso, R.B.; Giradi, L.B.; Mezzomo, W. Effect of Irrigation and Nitrogen Fertilization on Developmental Parameters and Yield Components of Soybean Crops. Rev. Espac. 2016, 37, 10. [Google Scholar]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G.; Wang, H. Integrated Use of Straw Mulch with Nitrogen Fertilizer Improves Soil Functionality and Soybean Production. Environ. Int. 2019, 132, 105092. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.R.; Haegele, J.W.; Below, F.E. Nutrient Uptake, Partitioning, and Remobilization in Modern Soybean Varieties. Agron. J. 2015, 107, 563–573. [Google Scholar] [CrossRef]
- Bortolon, L.; Bortolon, E.S.O.; Camargo, F.P.d; Seraglio, N.A.; Lima, A.d.O.; Rocha, P.H.F.; Souza, J.P.d.; Sousa, W.C.; Tomazzi, M.; Lago, B.C.; et al. Yield and Nutrient Uptake of Soybean Cultivars Under Intensive Cropping Systems. J. Agric. Sci. 2018, 10, 344. [Google Scholar] [CrossRef]
- de Souza, P.J.O.P.; Ortega-Farias, S.; da Rocha, E.J.P.; de Sousa, A.M.L.; de Souza, E.B. Water consumption of soybean in the northeast of Pará. IRRIGA 2018, 1, 218. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Bellaloui, N. Soybean Seed Physiology, Quality, and Chemical Composition under Soil Moisture Stress. Food Chem. 2019, 278, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ciampitti, I.A.; Salvagiotti, F. New Insights into Soybean Biological Nitrogen Fixation. Agron. J. 2018, 110, 1185–1196. [Google Scholar] [CrossRef]
- Cafaro La Menza, N.; Monzon, J.P.; Lindquist, J.L.; Arkebauer, T.J.; Knops, J.M.H.; Unkovich, M.; Specht, J.E.; Grassini, P. Insufficient Nitrogen Supply from Symbiotic Fixation Reduces Seasonal Crop Growth and Nitrogen Mobilization to Seed in Highly Productive Soybean Crops. Plant Cell Env. 2020, 43, 1958–1972. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Khan, A.; Ren, G.; Afridi, M.Z.; Feng, Y.; Yang, G. Wheat Straw Mulching Offset Soil Moisture Deficient for Improving Physiological and Growth Performance of Summer Sown Soybean. Agric. Water Manag. 2019, 211, 16–25. [Google Scholar] [CrossRef]
- Lumactud, R.A.; Dollete, D.; Liyanage, D.K.; Szczyglowski, K.; Hill, B.; Thilakarathna, M.S. The Effect of Drought Stress on Nodulation, Plant Growth, and Nitrogen Fixation in Soybean during Early Plant Growth. J. Agron. Crop Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Pierozan Junior, C.; Favarin, J.L.; Baptistella, J.L.C.; de Almeida, R.E.M.; Maciel de Oliveira, S.; Lago, B.C.; Tezotto, T. Controlled Release Urea Increases Soybean Yield without Compromising Symbiotic Nitrogen Fixation. Exp. Agric. 2023, 59, e1. [Google Scholar] [CrossRef]
- Companhia Nacional De Abastecimento—CONAB. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 31 May 2020).
- Giménez, L.; Paredes, P.; Pereira, L.S. Water Use and Yield of Soybean under Various Irrigation Regimes and Severe Water Stress. Application of AquaCrop and SIMDualKc Models. Water 2017, 9, 393. [Google Scholar] [CrossRef]
- Monzon, J.P.; Cafaro La Menza, N.; Cerrudo, A.; Canepa, M.; Rattalino Edreira, J.I.; Specht, J.; Andrade, F.H.; Grassini, P. Critical Period for Seed Number Determination in Soybean as Determined by Crop Growth Rate, Duration, and Dry Matter Accumulation. Field Crops Res. 2021, 261, 108016. [Google Scholar] [CrossRef]
- Silva, B.M. Soil Water Availability: Estimation Methods and Management Implications for Coffee Plants in the Cerrado Region; Universidade Federal de Lavras: Lavras, MG, USA, 2014. [Google Scholar]
- Cafaro La Menza, N.; Monzon, J.P.; Specht, J.E.; Lindquist, J.L.; Arkebauer, T.J.; Graef, G.; Grassini, P. Nitrogen Limitation in High-Yield Soybean: Seed Yield, N Accumulation, and N-Use Efficiency. Field Crops Res. 2019, 237, 74–81. [Google Scholar] [CrossRef]
- Adeboye, O.B.; Schultz, B.; Adekalu, K.O.; Prasad, K. Crop Water Productivity and Economic Evaluation of Drip-Irrigated Soybeans (Glyxine Max L. Merr.). Agric. Food Secur. 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Gaspar, A.P.; Laboski, C.A.M.; Naeve, S.L.; Conley, S.P. Dry Matter and Nitrogen Uptake, Partitioning, and Removal across a Wide Range of Soybean Seed Yield Levels. Crop Sci. 2017, 57, 2170–2182. [Google Scholar] [CrossRef]
- Santachiara, G.; Borrás, L.; Salvagiotti, F.; Gerde, J.A.; Rotundo, J.L. Relative Importance of Biological Nitrogen Fixation and Mineral Uptake in High Yielding Soybean Cultivars. Plant Soil 2017, 418, 191–203. [Google Scholar] [CrossRef]
- Marques Pires, M.d.F.; de Souza, H.A.; Medeiros, J.C.; Dalla Rosa, J.; de Souza Martins, R.V.; Sobral, A.H.S.; Pereira Carvalho, S.; de Sousa Vera, G.; de Melo Jorge Vieira, P.F.; Sagrilo, E. Nutrient Uptake by Soybean Plants in Succession of Cover Crops in Northeast of Brazil. Commun. Soil Sci. Plant Anal. 2022, 54, 1–19. [Google Scholar] [CrossRef]
- Vinha, A.P.C.; Carrara, B.H.; Souza, E.F.S.; dos Santos, J.A.F.; Arantes, S.A.C.M. Phosphorus Adsorption in Soils of Tropical Regions. Nativ.-Pesqui. Agrárias E Ambient. 2021, 9, 30–35. [Google Scholar] [CrossRef]
- Catuchi, T.A.; Guidorizzi, F.V.C.; Guidorizi, K.A.; Barbosa, A.d.M.; Souza, G.M. Physiological Responses of Soybean Cultivars to Potassium Fertilization under Different Water Regimes. Pesqui. Agropecu. Bras. 2012, 47, 519–527. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6—Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. ISBN 978-0-12-384905-2. [Google Scholar]
- de Oliveira Junior, A.; de Castro, C.; de Oliveira, F.A.; Klepker, D. Soil Fertility and Nutritional Status Assessment of Soybeans. In Soybean Production Technology; Production Systems; Neumaier, N., Balbinot Junior, A.A., Krzyzanowski, F.F., Leite, R.M.V.B.d.C., Eds.; Embrapa Soja: Londrina, Brazil, 2020; pp. 133–184. [Google Scholar]
- Rodrigues, V.A.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Portugal, J.R.; Mundt, T.T.; de Oliveira, S.L.; Garcia, A.; Calonego, J.C.; Lollato, R.P. Magnesium Foliar Supplementation Increases Grain Yield of Soybean and Maize by Improving Photosynthetic Carbon Metabolism and Antioxidant Metabolism. Plants 2021, 10, 797. [Google Scholar] [CrossRef]
- Whigham, D.K.; Minor, H.C. 4—Agronomic Characteristics and Environmental Stress. In Soybean Physiology, Agronomy, and Utilization; Norman, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1978; pp. 77–118. ISBN 978-0-12-521160-4. [Google Scholar]
- Zafar, S.; Li, Y.-L.; Li, N.-N.; Zhu, K.-M.; Tan, X.-L. Recent Advances in Enhancement of Oil Content in Oilseed Crops. J. Biotechnol. 2019, 301, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Meteorologia—INMET. Available online: https://portal.inmet.gov.br/dadoshistoricos (accessed on 14 December 2019).
- Melo, F.d.B.; de Andrade Junior, A.S.; Pessoa, B.L.d.O. Levantamento, Zoneamento e Mapeamento Pedológico Detalhado Da Área Experimental Da Embrapa Meio-Norte Em Teresina [Detailed Pedological Survey, Zoning and Mapping of the Experimental Area of Embrapa Mid-North in Teresina]; Embrapa Meio-Norte: Teresina, PI, Brazil, 2014; 47p. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotraspiration Guidelines for Computing Crop Water Requirements; FAO: Rome, Italy, 1998; ISBN 92-5-104219-5. [Google Scholar]
- Fehr, W.R.; Caviness, C.E. “Stages of Soybean Development” (1977). Special Report. 80. Iowa State Univ. 1977, 80, 1–12. [Google Scholar]
- Hungria, M.; Campo, R.J.; Mendes, I.C. Fixação Biológica Do Nitrogênio Na Cultura Da Soja [Biological Nitrogen Fixation in Soybean Crop]. Biol. Nitrogen Fixat. Soybean Crops 2001, 31, 143–154. [Google Scholar]
- Souza, J.R.D.; Lemos, L.B.; Leal, F.T.; Magalhães, R.S. Volatilization of Ammonia from Conventional Sources of Nitrogen and Compacted Urea under Controlled Conditions. Rev. Bras. De Ciências Agrárias 2020, 15, 2–7. [Google Scholar] [CrossRef]
- Boaretto, A.E.; Raij, B.V.; Silva, F.C.d; Chitolina, J.C.; Tedesco, M.J.; Santana do Carmo, C.A.F. Sampling, Packaging and Preparation of Plant Samples for Chemical Analysis. In Manual de Análises Químicas de Solos, Plantas e Fertilizantes [Manual of Chemical Analyses of Soils, Plants and Fertilizers]; Fábio Cesar da Silva, Ed.; Embrapa Informação Tecnológica: Brasília, DF, USA, 2009; p. 627. ISBN 978-85-7383-430-7. [Google Scholar]
- Miyazawa, M.; Pavan, M.A.; Muraoka, T.; Carmo, C.A.; Melo, W.J.D. Análise química de tecido vegetal [Chemical analysis of plant tissue]. Manual de Análises Químicas de Solos, Plantas e Fertilizantes [Manual of Chemical Analyses of Soils, Plants and Fertilizers]; Silva, F.C., Ed.; Embrapa-Informação Tecnológica: Brasília, DF, Brazil, 2009; pp. 191–233. ISBN 978-85-7383-430-7. [Google Scholar]
- Silva, D.J.; Queiroz, A.C. Análise de Alimentos [Food Analysis], 3rd ed.; Editora UFV: Viçosa, MG, Brazil, 2006; ISBN 85-7269-105-7. [Google Scholar]






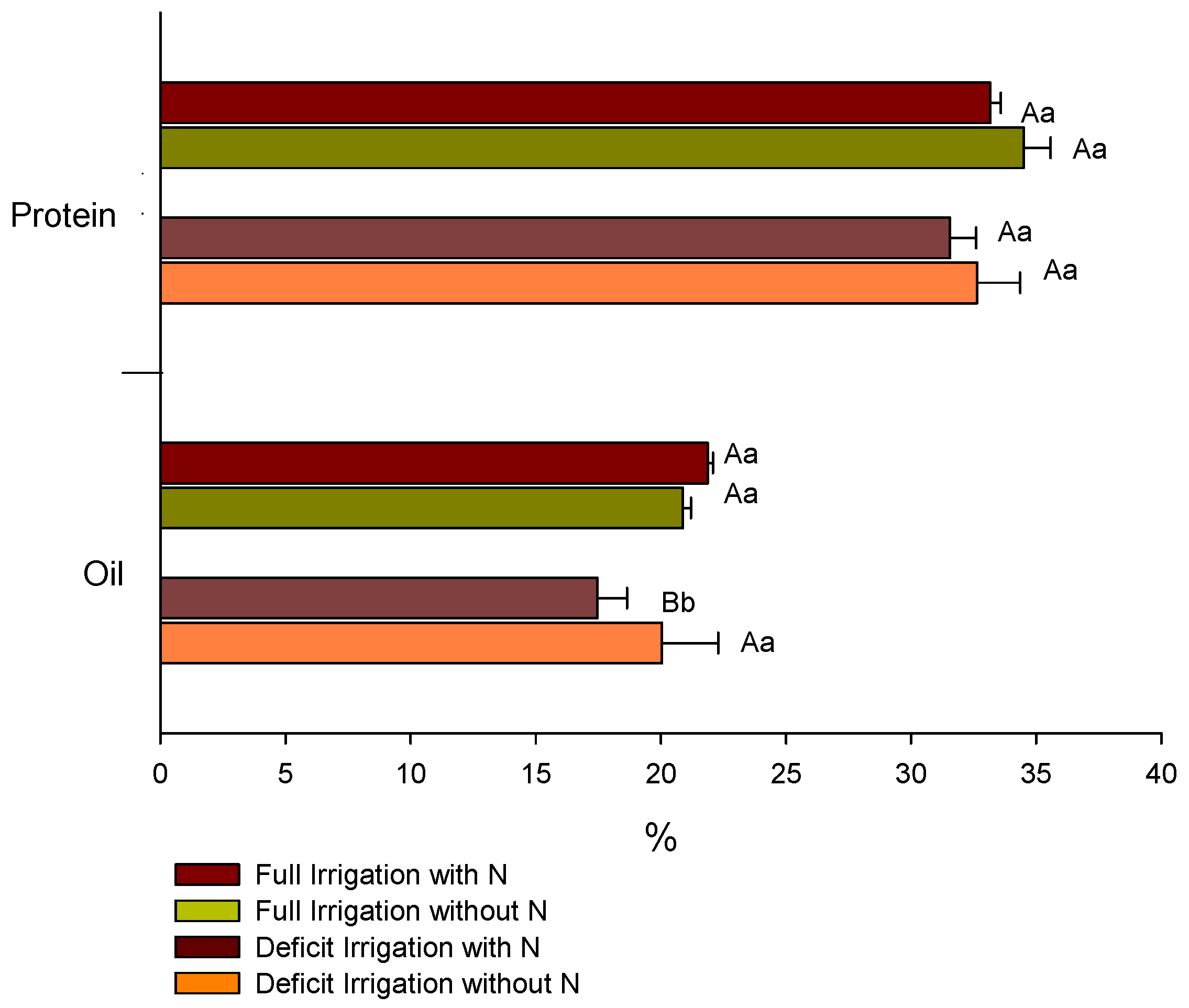
| Source of Variation | ADM | N | P | K | Ca | Mg | S | Seed Yield | Seed Protein | Seed Oil | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Design | df | F | F | F | F | F | F | F | df | F | F | F |
| Blocks | 2 | 1.79 | 2.66 | 0.64 | 0.35 | 3.31 * | 0.20 | 1.47 | 2 | 0.88 | 0.24 | 1.11 |
| Treatment | ||||||||||||
| WR | 1 | 1028.53 ** | 7908.64 ** | 8247.47 ** | 479.93 * | 414.15 * | 1014.90 ** | 64.62 * | 1 | 220.03 ** | 1.63 | 8.45 |
| Error1 | 2 | 2 | ||||||||||
| N | 1 | 44.07 * | 36.47 * | 0.91 | 2.69 | 16.07 | 158.89 * | 22.99 * | 1 | 0.69 | 3.41 | 2.72 |
| Error2 | 2 | 4 | ||||||||||
| DAE | 8 | 485.87 ** | 392.25 ** | 401.13 ** | 323.88 ** | 195.78 ** | 408.48 ** | 143.66 ** | - | |||
| WR × N | 1 | 0.05 | 1.26 | 17.24 ** | 10.39 * | 0.97 | 0.24 | 11.60 ** | 1 | 3.07 | 0.01 | 13.66 ** |
| WR × DAE | 8 | 71.24 ** | 74.81 ** | 73.94 ** | 46.16 ** | 46.57 ** | 71.08 ** | 19.98 ** | - | |||
| N × DAE | 8 | 7.76 ** | 3.71 ** | 1.05 ** | 2.44 * | 5.44 ** | 17.41 ** | 7.23 ** | - | |||
| WR × N × DAE | 8 | 2.04 * | 3.87 ** | 10.51 ** | 3.12 * | 3.31 * | 7.23 ** | 3.69 ** | - | |||
| Error3 | 66 | |||||||||||
| Error | MS | MS | MS | MS | MS | MS | MS | MS | MS | MS | ||
| Blocks × Treatment | 107 | 216,303.78 | 260.63 | 0.99 | 60.69 | 30.66 | 6.58 | 0.69 | 11 | 206,608.00 | 6.12 | 4.68 |
| CV 1 (%) 1 = | 8.55 | 3.34 | 3.30 | 11.47 | 13.94 | 8.71 | 30.25 | 10.27 | 4.97 | 7.80 | ||
| CV 2 (%) 2 = | 10.22 | 17.36 | 17.40 | 12.07 | 25.20 | 11.72 | 22.78 | 11.42 | 4.93 | 4.16 | ||
| CV 3 (%) 3 = | 12.84 | 14.64 | 12.44 | 13.14 | 17.33 | 14.04 | 20.96 | - | - | - |
| Source of Variation | Cu | Fe | Mn | Zn | B | |
|---|---|---|---|---|---|---|
| Design | df | F | F | F | F | F |
| Blocks | 2 | 0.67 | 1.63 ** | 0.59 | 1.70 | 2.41 |
| Treatment | ||||||
| WR | 1 | 854.53 ** | 5337.30 *** | 207.09 * | 6428.20 ** | 844.62 ** |
| Error1 | 2 | |||||
| N | 1 | 39.39 * | 216.97 * | 594.98 *** | 87.93 * | 9.39 * |
| Error2 | 2 | |||||
| DAE | 7 | 484.09 *** | 504.01 *** | 231.31 *** | 274.35 *** | 657.51 *** |
| WR × N | 1 | 0.02 | 0.09 | 1.18 | 28.46 *** | 24.49 *** |
| WR × DAE | 7 | 78.67 *** | 99.79 *** | 44.31 *** | 80.08 *** | 88.41 *** |
| N × DAE | 7 | 4.69 *** | 15.47 *** | 22.95 *** | 10.40 *** | 3.08 * |
| WR × N × DAE | 7 | 17.65 *** | 13.54 *** | 17.74 *** | 41.41 ** | 10.26 *** |
| Error3 | 58 | |||||
| Error | MS | MS | MS | MS | MS | |
| Blocks × Treatment | 95 | 17.75 | 934.52 | 388.64 | 164.77 | 18.80 |
| CV 1 (%) 1 = | 9.74 | 3.44 | 22.09 | 4.31 | 7.20 | |
| CV 2 (%) 2 = | 7.98 | 8.08 | 8.98 | 10.24 | 10.05 | |
| CV 3 (%) 3 = | 12.42 | 11.00 | 14.62 | 13.72 | 10.31 |
| Depth | pH | OM | N | P | K+ | Na+ | Ca2+ | Mg2+ | Al3+ |
|---|---|---|---|---|---|---|---|---|---|
| (m) | (CaCl2) | |-----g kg−1 -----| | mg dm−3 | |------------------ cmolc dm−3 -------------------| | |||||
| 0.0 a 0.2 | 5.4 | 10.5 | 1.1 | 9.0 | 0.2 | 0.3 | 2.4 | 0.8 | 0.0 |
| 0.2 a 0.4 | 5.3 | 9.2 | 0.9 | 4.7 | 0.2 | 0.1 | 2.7 | 0.8 | 0.0 |
| Depth | H + Al | SB | CEC | BS | Clay | Silt | Sand | ||
| (m) | |------- cmolc dm−3 ------| | |-%-| |------ (g kg−1) -----| | |||||||
| 0.0 a 0.2 | 1.3 | 3.7 | 5.0 | 74 | 150 | 110 | 730 | ||
| 0.2 a 0.4 | 1.4 | 3.9 | 5.3 | 73 | 240 | 100 | 660 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setubal, I.S.; Andrade Júnior, A.S.d.; Silva, S.P.d.; Rodrigues, A.C.; Bonifácio, A.; Silva, E.H.F.M.d.; Vieira, P.F.d.M.J.; Miranda, R.d.S.; Cafaro La Menza, N.; Souza, H.A.d. Macro and Micro-Nutrient Accumulation and Partitioning in Soybean Affected by Water and Nitrogen Supply. Plants 2023, 12, 1898. https://doi.org/10.3390/plants12091898
Setubal IS, Andrade Júnior ASd, Silva SPd, Rodrigues AC, Bonifácio A, Silva EHFMd, Vieira PFdMJ, Miranda RdS, Cafaro La Menza N, Souza HAd. Macro and Micro-Nutrient Accumulation and Partitioning in Soybean Affected by Water and Nitrogen Supply. Plants. 2023; 12(9):1898. https://doi.org/10.3390/plants12091898
Chicago/Turabian StyleSetubal, Ingrid Silva, Aderson Soares de Andrade Júnior, Silvestre Paulino da Silva, Artenisa Cerqueira Rodrigues, Aurenívia Bonifácio, Evandro Henrique Figueiredo Moura da Silva, Paulo Fernando de Melo Jorge Vieira, Rafael de Souza Miranda, Nicolas Cafaro La Menza, and Henrique Antunes de Souza. 2023. "Macro and Micro-Nutrient Accumulation and Partitioning in Soybean Affected by Water and Nitrogen Supply" Plants 12, no. 9: 1898. https://doi.org/10.3390/plants12091898
APA StyleSetubal, I. S., Andrade Júnior, A. S. d., Silva, S. P. d., Rodrigues, A. C., Bonifácio, A., Silva, E. H. F. M. d., Vieira, P. F. d. M. J., Miranda, R. d. S., Cafaro La Menza, N., & Souza, H. A. d. (2023). Macro and Micro-Nutrient Accumulation and Partitioning in Soybean Affected by Water and Nitrogen Supply. Plants, 12(9), 1898. https://doi.org/10.3390/plants12091898







