MtWOX2 and MtWOX9-1 Effects on the Embryogenic Callus Transcriptome in Medicago truncatula
Abstract
:1. Introduction
2. Results
2.1. MtWOX2 Expression Pattern during Somatic Embryogenesis
2.2. MtWOX2 Overexpression Increases Callus Weight in M. truncatula
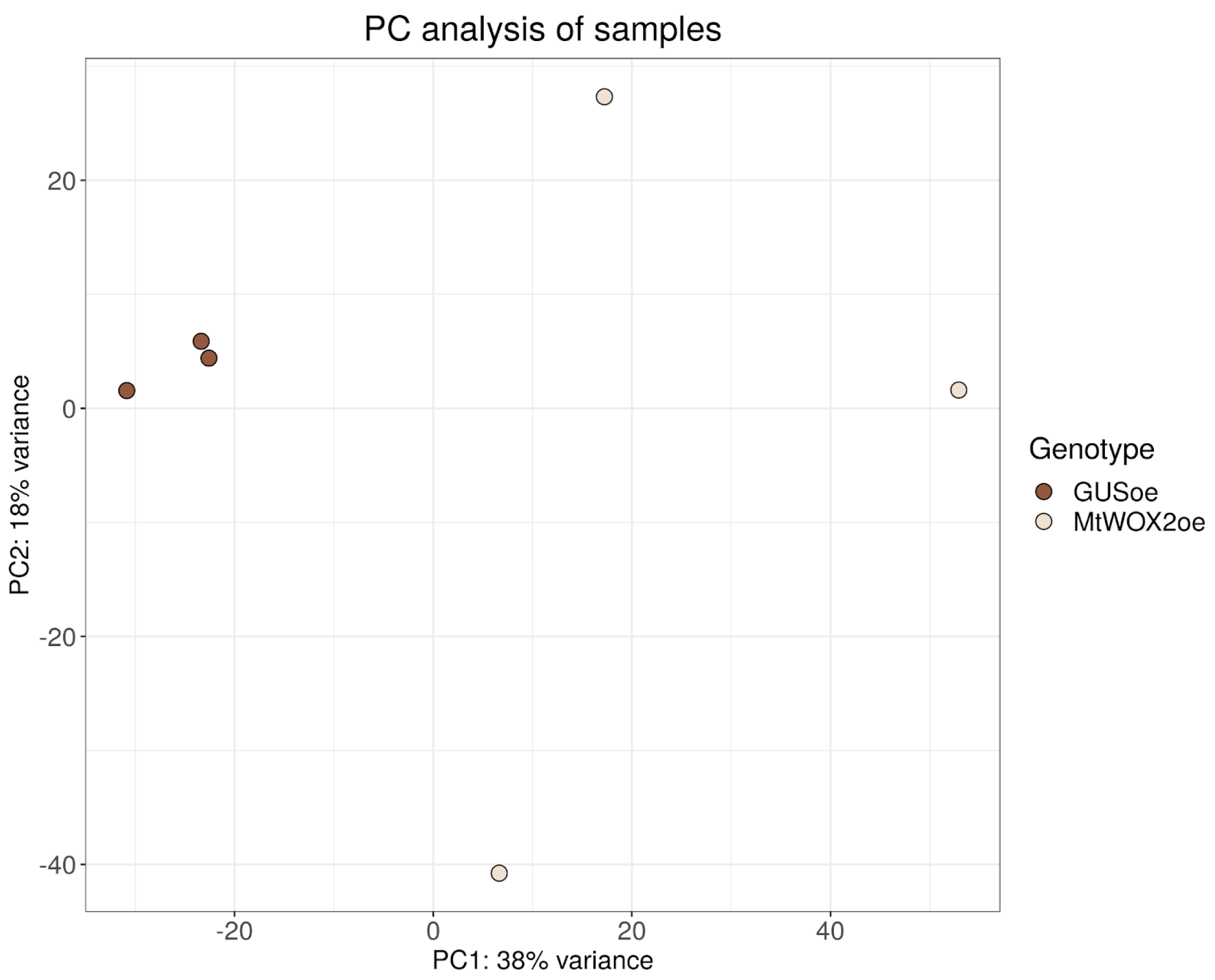
2.3. Transcriptomic Analysis of MtWOX2 Overexpressing Calli
3. Discussion
4. Materials and Methods
4.1. Plant Material and Bacterial Strains
4.2. Plant Cultivation Conditions
4.3. Microorganism Cultivation Conditions
4.4. Molecular Cloning and qPCR Analysis
4.5. Transcriptome Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. An Introduction to Plant Tissue Culture: Advances and Perspectives. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 3–13. ISBN 978-1-4939-8594-4. [Google Scholar]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New Insights into Tissue Culture Plant-Regeneration Mechanisms. Front. Plant Sci. 2022, 13, 926752. [Google Scholar] [CrossRef] [PubMed]
- Tvorogova, V.E.; Krasnoperova, E.Y.; Potsenkovskaia, E.A.; Kudriashov, A.A.; Dodueva, I.E.; Lutova, L.A. What Does the WOX Say? Review of Regulators, Targets, Partners. Mol. Biol. 2021, 55, 311–337. [Google Scholar] [CrossRef]
- Haecker, A.; Gross-Hardt, R.; Geiges, B.; Sarkar, A.; Breuninger, H.; Herrmann, M.; Laux, T. Expression Dynamics of WOX Genes Mark Cell Fate Decisions during Early Embryonic Patterning in Arabidopsis Thaliana. Development 2004, 131, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Zhang, Z.; Laux, T. Transcriptional Activation of Arabidopsis Axis Patterning Genes WOX8/9 Links Zygote Polarity to Embryo Development. Dev. Cell 2011, 20, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Breuninger, H.; Rikirsch, E.; Hermann, M.; Ueda, M.; Laux, T. Differential Expression of WOX Genes Mediates Apical-Basal Axis Formation in the Arabidopsis Embryo. Dev. Cell 2008, 14, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, X.; Ma, D.; Liu, C. Identification and Evolutionary Analysis of Cotton (Gossypium hirsutum) WOX Family Genes and Their Potential Function in Somatic Embryogenesis. Int. J. Mol. Sci. 2023, 24, 11077. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Lou, X.; Zhang, Y.; Han, X.; Yang, Q.; Tong, Z.; Zhang, J.; Zhang, M.; Chen, X.; et al. Identification of WUSCHEL-Related Homeobox (WOX) Gene Family Members and Determination of Their Expression Profiles during Somatic Embryogenesis in Phoebe bournei. For. Res. 2023, 3, 5. [Google Scholar] [CrossRef]
- Qi, S.; Zhao, R.; Yan, J.; Fan, Y.; Huang, C.; Li, H.; Chen, S.; Zhang, T.; Kong, L.; Zhao, J.; et al. Global Transcriptome and Coexpression Network Analyses Reveal New Insights into Somatic Embryogenesis in Hybrid Sweetgum (Liquidambar styraciflua × Liquidambar formosana). Front. Plant Sci. 2021, 12, 751866. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Liu, Z.; Zhang, Z.; XuHan, X.; Lin, Y.; Lai, Z. Global Scale Transcriptome Analysis Reveals Differentially Expressed Genes Involve in Early Somatic Embryogenesis in Dimocarpus longan Lour. BMC Genom. 2020, 21, 4. [Google Scholar] [CrossRef]
- Tvorogova, V.E.; Lebedeva, M.A.; Lutova, L.A. Expression of WOX and PIN Genes during Somatic and Zygotic Embryogenesis in Medicago truncatula. Russ. J. Genet. 2015, 51, 1189–1198. [Google Scholar] [CrossRef]
- Wu, X.; Chory, J.; Weigel, D. Combinations of WOX Activities Regulate Tissue Proliferation during Arabidopsis Embryonic Development. Dev. Biol. 2007, 309, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.B.; Trontin, J.-F.; Raschke, J.; Zoglauer, K.; Rupps, A. Constitutive Overexpression of a Conifer WOX2 Homolog Affects Somatic Embryo Development in Pinus Pinaster and Promotes Somatic Embryogenesis and Organogenesis in Arabidopsis Seedlings. Front. Plant Sci. 2022, 13, 838421. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Egertsdotter, U. In Silico Characterization of Putative Gene Homologues Involved in Somatic Embryogenesis Suggests That Some Conifer Species May Lack LEC2, One of the Key Regulators of Initiation of the Process. BMC Genom. 2021, 22, 392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Qi, S.; Cui, Y.; Gao, Y.; Jiang, S.; Zhao, J.; Zhang, J.; Kong, L. Transcriptomic and Physiological Analysis Identifies a Gene Network Module Highly Associated with Brassinosteroid Regulation in Hybrid Sweetgum Tissues Differing in the Capability of Somatic Embryogenesis. Hortic. Res. 2022, 9, uhab047. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wu, H.; Zheng, R.; Li, R.; Zhu, Z.; Chen, Y.; Lu, Y.; Cheng, T.; Shi, J.; Chen, J. The Plant Peptide Hormone Phytosulfokine Promotes Somatic Embryogenesis by Maintaining Redox Homeostasis in Cunninghamia lanceolata. Plant J. 2023, 113, 716–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-X.; Shang, G.-D.; Wu, L.-Y.; Xu, Z.-G.; Zhao, X.-Y.; Wang, J.-W. Chromatin Accessibility Dynamics and a Hierarchical Transcriptional Regulatory Network Structure for Plant Somatic Embryogenesis. Dev. Cell 2020, 54, 742–757.e8. [Google Scholar] [CrossRef] [PubMed]
- Godel-Jedrychowska, K.; Kulinska-Lukaszek, K.; Horstman, A.; Soriano, M.; Li, M.; Malota, K.; Boutilier, K.; Kurczynska, E.U. Symplasmic Isolation Marks Cell Fate Changes during Somatic Embryogenesis. J. Exp. Bot. 2020, 71, 2612–2628. [Google Scholar] [CrossRef] [PubMed]
- Samakovli, D.; Tichá, T.; Vavrdová, T.; Závorková, N.; Pecinka, A.; Ovečka, M.; Šamaj, J. HEAT SHOCK PROTEIN 90 Proteins and YODA Regulate Main Body Axis Formation during Early Embryogenesis. Plant Physiol. 2021, 186, 1526–1544. [Google Scholar] [CrossRef]
- Kyo, M.; Maida, K.; Nishioka, Y.; Matsui, K. Coexpression of WUSCHEL Related Homeobox (WOX) 2 with WOX8 or WOX9 Promotes Regeneration from Leaf Segments and Free Cells in Nicotiana tabacum L. Plant Biotechnol. 2018, 35, 23–30. [Google Scholar] [CrossRef]
- Lie, C.; Kelsom, C.; Wu, X. WOX2 and STIMPY-LIKE/WOX8 Promote Cotyledon Boundary Formation in Arabidopsis. Plant J. 2012, 72, 674–682. [Google Scholar] [CrossRef]
- Tvorogova, V.E.; Fedorova, Y.A.; Potsenkovskaya, E.A.; Kudriashov, A.A.; Efremova, E.P.; Kvitkovskaya, V.A.; Wolabu, T.W.; Zhang, F.; Tadege, M.; Lutova, L.A. The WUSCHEL-Related Homeobox Transcription Factor MtWOX9-1 Stimulates Somatic Embryogenesis in Medicago truncatula. Plant Cell Tiss. Organ. Cult. 2019, 138, 517–527. [Google Scholar] [CrossRef]
- Tvorogova, V.E.; Fedorova, Y.A.; Zhang, F.; Lutova, L.A. STENOFOLIA Gene and Regulation of Somatic Embryogenesis in Medicago truncatula. Russ. J. Plant Physiol. 2016, 63, 811–821. [Google Scholar] [CrossRef]
- Kadri, A.; Grenier De March, G.; Guerineau, F.; Cosson, V.; Ratet, P. WUSCHEL Overexpression Promotes Callogenesis and Somatic Embryogenesis in Medicago truncatula Gaertn. Plants 2021, 10, 715. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.J.; Nolan, K.E.; Bicego, L. The Development of the Highly Regenerable Seed Line Jemalong 2HA for Transformation of Medicago truncatula—Implications for Regenerability via Somatic Embryogenesis. J. Plant Physiol. 1999, 155, 788–791. [Google Scholar] [CrossRef]
- Zhai, N.; Xu, L. Pluripotency Acquisition in the Middle Cell Layer of Callus Is Required for Organ Regeneration. Nat. Plants 2021, 7, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Tvorogova, V.E.; Krasnoperova, E.Y.; Kudriashov, A.A.; Kuznetsova, K.A.; Potsenkovskaya, E.A.; Fedorova, Y.A. Ludmila Lutova Transcriptomic Analysis of Medicago truncatula Calli with MtWOX9-1 Overexpression. Vavilov J. Genet. Breed. 2019, 23, 691–699. [Google Scholar] [CrossRef]
- Nalapalli, S.; Tunc-Ozdemir, M.; Sun, Y.; Elumalai, S.; Que, Q. Morphogenic Regulators and Their Application in Improving Plant Transformation. Methods Mol. Biol. 2021, 2238, 37–61. [Google Scholar] [CrossRef]
- Carrere, S.; Verdier, J.; Gamas, P. MtExpress, a Comprehensive and Curated RNAseq-Based Gene Expression Atlas for the Model Legume Medicago truncatula. Plant Cell Physiol. 2021, 62, 1494–1500. [Google Scholar] [CrossRef]
- Zhou, X.; Guo, Y.; Zhao, P.; Sun, M.-X. Comparative Analysis of WUSCHEL-Related Homeobox Genes Revealed Their Parent-of-Origin and Cell Type-Specific Expression Pattern During Early Embryogenesis in Tobacco. Front. Plant Sci. 2018, 9, 311. [Google Scholar] [CrossRef]
- Kudriashov, A.A.; Zlydneva, N.S.; Efremova, E.P.; Tvorogova, V.E.; Lutova, L.A. MtCLE08, MtCLE16, and MtCLE18 Transcription Patterns and Their Possible Functions in the Embryogenic Calli of Medicago truncatula. Plants 2023, 12, 435. [Google Scholar] [CrossRef]
- Pecrix, Y.; Staton, S.E.; Sallet, E.; Lelandais-Brière, C.; Moreau, S.; Carrère, S.; Blein, T.; Jardinaud, M.-F.; Latrasse, D.; Zouine, M.; et al. Whole-Genome Landscape of Medicago truncatula Symbiotic Genes. Nat. Plants 2018, 4, 1017. [Google Scholar] [CrossRef]
- Fåhraeus, G. The Infection of Clover Root Hairs by Nodule Bacteria Studied by a Simple Glass Slide Technique. J. Gen. Microbiol. 1957, 16, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Trinh, T.H.; Leung, J.; Kondorosi, A.; Kondorosi, E. A New Medicago truncatula Line with Superior in Vitro Regeneration, Transformation, and Symbiotic Properties Isolated Through Cell Culture Selection. Mol. Plant-Microbe Interact. 1997, 10, 307–315. [Google Scholar] [CrossRef]
- Potsenkovskaia, E.; Tvorogova, V.; Yakovleva, D.; Zlydneva, N.; Lutova, L. Novel NF-Y Genes Expressed during Somatic Embryogenesis in Medicago truncatula. Plant Gene 2022, 31, 100364. [Google Scholar] [CrossRef]
- Green, M.; Sambrook, J. Molecular Cloning: Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2012. [Google Scholar]
- Sambrook, J.; Russell, D.W. Preparation and Transformation of Competent E. coli Using Calcium Chloride. Cold Spring Harb. Protoc. 2006, 2006, pdb.prot3932. [Google Scholar] [CrossRef]
- Jyothishwaran, G.; Kotresha, D.; Selvaraj, T.; Srideshikan, S.H.; Rajvanshi, P.K.; Jayabaskaran, C. A Modified Freeze–Thaw Method for Efficient Transformation of Agrobacterium Tumefaciens. Curr. Sci. 2007, 93, 770–772. [Google Scholar]
- Hartley, J.L.; Temple, G.F.; Brasch, M.A. DNA Cloning Using in Vitro Site-Specific Recombination. Genome Res. 2000, 10, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. A Gateway Cloning Vector Set for High-Throughput Functional Analysis of Genes in Planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ariel, F.; Diet, A.; Verdenaud, M.; Gruber, V.; Frugier, F.; Chan, R.; Crespi, M. Environmental Regulation of Lateral Root Emergence in Medicago truncatula Requires the HD-Zip I Transcription Factor HB1. Plant Cell 2010, 22, 2171–2183. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M. UGENE team Unipro UGENE: A Unified Bioinformatics Toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New Capabilities and Interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC A Quality Control Tool for High Throughput Sequence Data. 2014. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 October 2023).
- Bushnell, B. BBMap: A Fast, Accurate, Splice-Aware Aligner; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2014. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie Enables Improved Reconstruction of a Transcriptome from RNA-Seq Reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasnoperova, E.Y.; Tvorogova, V.E.; Smirnov, K.V.; Efremova, E.P.; Potsenkovskaia, E.A.; Artemiuk, A.M.; Konstantinov, Z.S.; Simonova, V.Y.; Brynchikova, A.V.; Yakovleva, D.V.; et al. MtWOX2 and MtWOX9-1 Effects on the Embryogenic Callus Transcriptome in Medicago truncatula. Plants 2024, 13, 102. https://doi.org/10.3390/plants13010102
Krasnoperova EY, Tvorogova VE, Smirnov KV, Efremova EP, Potsenkovskaia EA, Artemiuk AM, Konstantinov ZS, Simonova VY, Brynchikova AV, Yakovleva DV, et al. MtWOX2 and MtWOX9-1 Effects on the Embryogenic Callus Transcriptome in Medicago truncatula. Plants. 2024; 13(1):102. https://doi.org/10.3390/plants13010102
Chicago/Turabian StyleKrasnoperova, Elizaveta Y., Varvara E. Tvorogova, Kirill V. Smirnov, Elena P. Efremova, Elina A. Potsenkovskaia, Anastasia M. Artemiuk, Zakhar S. Konstantinov, Veronika Y. Simonova, Anna V. Brynchikova, Daria V. Yakovleva, and et al. 2024. "MtWOX2 and MtWOX9-1 Effects on the Embryogenic Callus Transcriptome in Medicago truncatula" Plants 13, no. 1: 102. https://doi.org/10.3390/plants13010102
APA StyleKrasnoperova, E. Y., Tvorogova, V. E., Smirnov, K. V., Efremova, E. P., Potsenkovskaia, E. A., Artemiuk, A. M., Konstantinov, Z. S., Simonova, V. Y., Brynchikova, A. V., Yakovleva, D. V., Pavlova, D. B., & Lutova, L. A. (2024). MtWOX2 and MtWOX9-1 Effects on the Embryogenic Callus Transcriptome in Medicago truncatula. Plants, 13(1), 102. https://doi.org/10.3390/plants13010102






