Comparative Transcriptome Analysis Provides Insights into the Effect of Epicuticular Wax Accumulation on Salt Stress in Coconuts
Abstract
:1. Introduction
2. Results
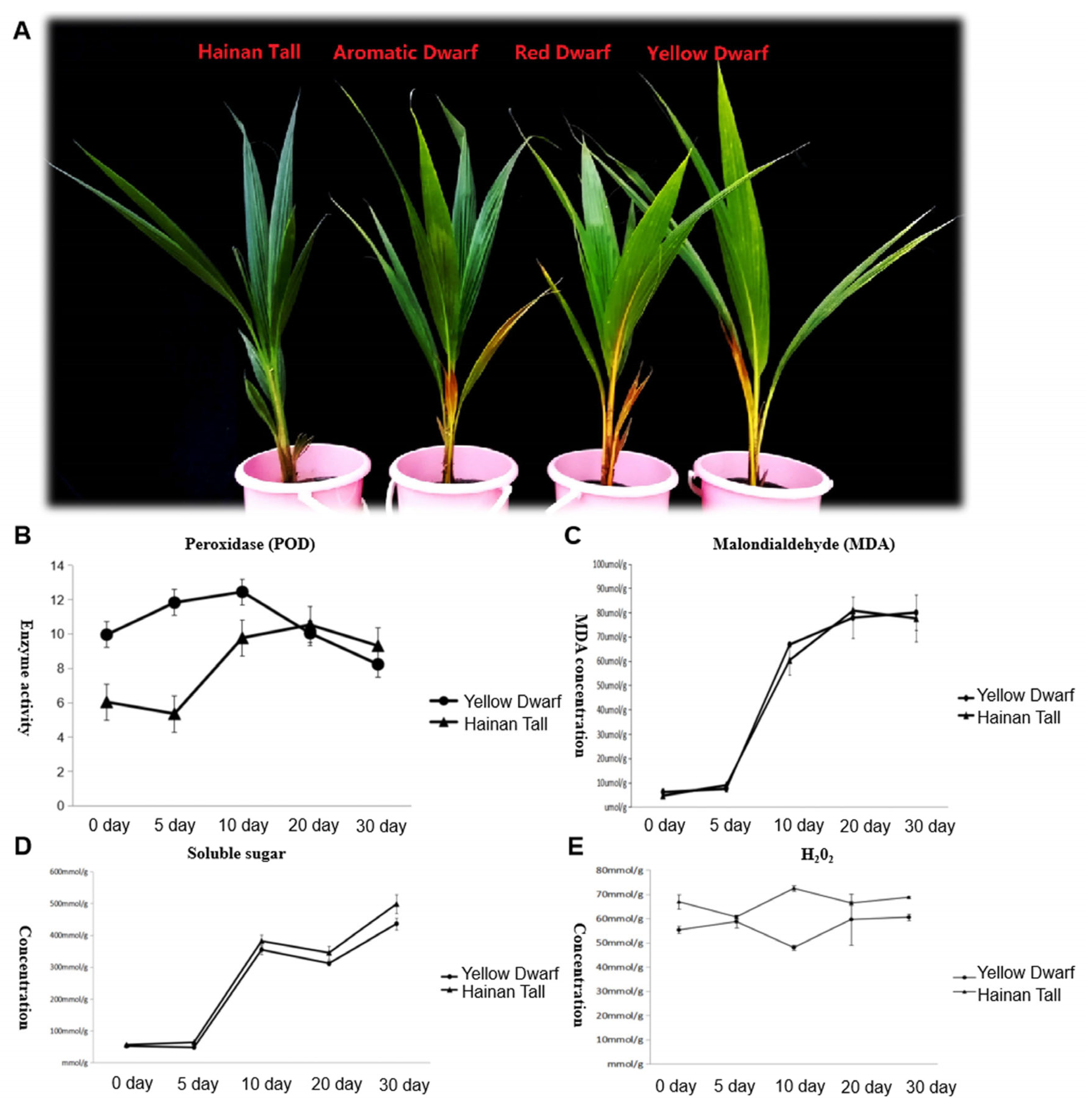
2.1. Physiological Changes in Four Coconut Cultivars under Salt Stress
2.2. Transcriptome Sequencing
2.3. Gene Expression Pattern after Salt Treatment
2.4. Enrichment Analysis
2.5. Cutin, Suberine, and Wax Biosynthesis
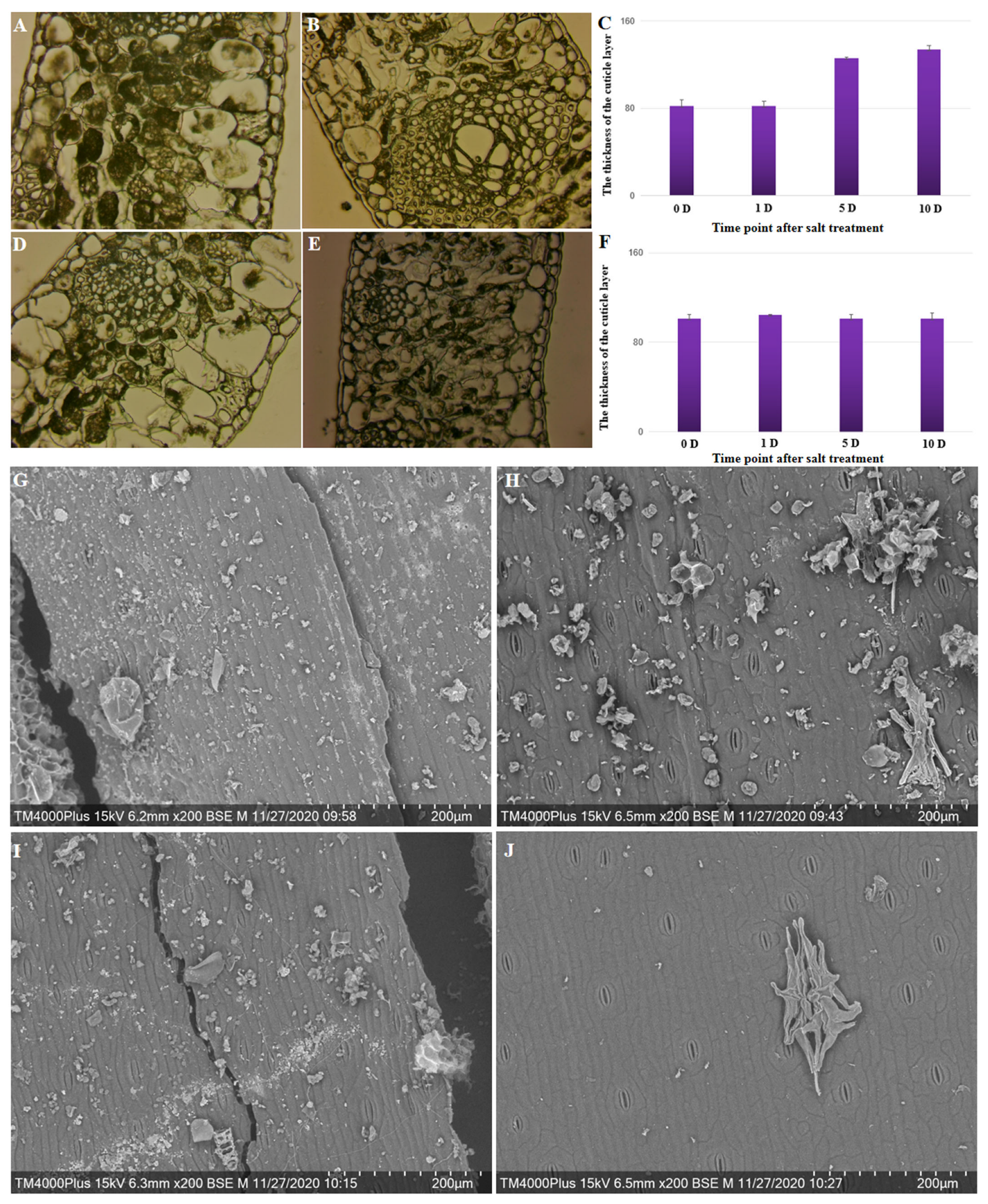
2.6. Cytological Examination of the Cuticle and Epicuticular Layer
3. Discussion
4. Material and Methods
4.1. Plant Materials
4.2. RNA Extraction
4.3. Illumina Sequencing and De Novo Assembly
4.4. Calculating Gene Expression Level
4.5. Cytological Observation
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, Y.; Xu, P.; Fan, H.; Baudouin, L.; Xia, W.; Bocs, S.; Xu, J.; Li, Q.; Guo, A.; Zhou, L.; et al. The genome draft of coconut (Cocos nucifera). GigaScience 2017, 6, gix095. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, W.A.; Dissanayake, A.S. Coconut fats. Ceylon Med. J. 2006, 51, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Protyusha, G.B.; Sabarinath, B. Coconut water. Br. Dent. J. 2021, 231, 268. [Google Scholar] [CrossRef] [PubMed]
- Ajien, A.; Idris, J.; Md Sofwan, N.; Husen, R.; Seli, H. Coconut shell and husk biochar: A review of production and activation technology, economic, financial aspect and application. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2023, 41, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.; Benjakul, S. Coconut Milk and Coconut Oil: Their Manufacture Associated with Protein Functionality. J. Food Sci. 2018, 83, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- DebMandal, M.; Mandal, S. Coconut (Cocos nucifera L.: Arecaceae): In health promotion and disease prevention. Asian Pac. J. Trop. Med. 2011, 4, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Chanakya, H.N.; Khuntia, H.K.; Mukherjee, N.; Aniruddha, R.; Mudakavi, J.R.; Thimmaraju, P. The physicochemical characteristics and anaerobic degradability of desiccated coconut industry waste water. Environ. Monit. Assess. 2015, 187, 772. [Google Scholar] [CrossRef]
- Brantson, E.T.; Osei, H.; Aidoo, M.S.K.; Appau, P.O.; Issaka, F.N.; Liu, N.; Ejeh, C.J.; Kouamelan, K.S. Coconut oil and fermented palm wine biodiesel production for oil spill cleanup: Experimental, numerical, and hybrid metaheuristic modeling approaches. Environ. Sci. Pollut. Res. Int. 2022, 29, 50147–50165. [Google Scholar] [CrossRef]
- Batugal, P.A.; Rao, V.R.; Jeffrey, O. Coconut Genetic Resources; International Plant Genetic Resources Institute: Rome, Italy, 2005. [Google Scholar]
- Available online: www.fao.org/faostat/en/ (accessed on 2 May 2017).
- Braconnier, S.; Bonneau, X. Effects of chlorine deficiency in the field on leaf gas exchanges in the PB121 coconut hybrid. Agronomie 1998, 18, 563–572. [Google Scholar] [CrossRef]
- Magat, S.; Margate, R.; Habana, J. Effect of increasing rates of sodium chloride (common salt) fertilization on coconut palms grown under an inland soil (tropudalfs) of Mindanao, Philippines. Oléagineux 1988, 43, 14–19. [Google Scholar]
- Negacz, K.; Malek, Z.; De Vos, A.; Vellinga, P. Saline soils worldwide: Identifying the most promising areas for saline agriculture. J. Arid Environ. 2022, 203, 104775. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef]
- Yang, Y.; Bocs, S.; Fan, H.; Armero, A.; Baudouin, L.; Xu, P.; Xu, J.; This, D.; Hamelin, C.; Iqbal, A.; et al. Coconut genome assembly enables evolutionary analysis of palms and highlights signaling pathways involved in salt tolerance. Commun. Biol. 2021, 4, 105. [Google Scholar] [CrossRef]
- Gong, S.; Chen, S. Changes of osmoregulatory substances contents in different varieties of coconut seedlings under salt stress. Chin. J. Trop. Agric. 2018, 38, 1–5. [Google Scholar]
- Perera, L.; Baudouin, L.; Mackay, I. Ssr markers indicate a common origin of self-pollinating dwarf coconut in south-east asia under domestication. Sci. Hortic. 2016, 211, 255–262. [Google Scholar] [CrossRef]
- Hossain, M.S. Present Scenario of Global Salt Affected Soils, its Management and Importance of Salinity Research. Int. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Lu, Y.; Fricke, W. Salt Stress-Regulation of Root Water Uptake in a Whole-Plant and Diurnal Context. Int. J. Mol. Sci. 2023, 24, 8070. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt stress resilience in plants mediated through osmolyte accumulation and its crosstalk mechanism with phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef]
- Cheng, X.; He, Q.; Tang, S.; Wang, H.; Zhang, X.; Lv, M.; Liu, H.; Gao, Q.; Zhou, Y.; Wang, Q.; et al. The miR172/IDS1 signaling module confers salt tolerance through maintaining ROS homeostasis in cereal crops. New Phytol. 2021, 230, 1017–1033. [Google Scholar] [CrossRef]
- Zhang, P.; Zou, Y.; Song, B.; Zhou, M.; He, J.; Chen, Y.; Zhou, Y.; Xu, X. Cuticular lipids and associated gene expression analysis under NaCl stress in Thellungiella salsuginea. Physiol. Plant. 2022, 174, e13625. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Roychoudhury, A. Gene regulation at transcriptional and post-transcriptional levels to combat salt stress in plants. Physiol. Plant. 2021, 173, 1556–1572. [Google Scholar] [CrossRef] [PubMed]
- Fich, E.A.; Segerson, N.A.; Rose, J.K. The Plant Polyester Cutin: Biosynthesis, Structure, and Biological Roles. Annu. Rev. Plant Biol. 2016, 67, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.; Shao, M.; Pahari, S.; Venglat, P.; Soolanayakanahally, R.; Qiu, X.; Rahman, A.; Tanino, K. Dissecting the roles of cuticular wax in plant resistance to shoot dehydration and low-temperature stress in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1554. [Google Scholar] [CrossRef]
- Bourdenx, B.; Bernard, A.; Domergue, F.; Pascal, S.; Léger, A.; Roby, D.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A.; et al. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol. 2011, 156, 29–45. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Kong, X.; Du, Z.; Zhou, H.; Yu, Z.; Qin, J.; Chen, C. Leaf cuticular transpiration barrier organization in tea tree under normal growth conditions. Front. Plant Sci. 2021, 12, 655799. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Xu, C.; Ren, J.; Liu, X.; Black, K.; Gai, X.; Wang, Q.; Ren, H. Cucumber ECERIFERUM1 (CsCER1), which influences the cuticle properties and drought tolerance of cucumber, plays a key role in VLC alkanes biosynthesis. Plant Mol. Biol. 2015, 87, 219–233. [Google Scholar] [CrossRef]
- Richardson, A.; Boscari, A.; Schreiber, L.; Kerstiens, G.; Jarvis, M.; Herzyk, P.; Fricke, W. Cloning and expression analysis of candidate genes involved in wax deposition along the growing barley (Hordeum vulgare) leaf. Planta 2007, 226, 1459–1473. [Google Scholar] [CrossRef]
- Lü, S.; Song, T.; Kosma, D.K.; Parsons, E.P.; Rowland, O.; Jenks, M.A. Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J. Cell Mol. Biol. 2009, 59, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dietrich, C.R.; Lessire, R.; Nikolau, B.J.; Schnable, P.S. The Endoplasmic reticulum-associated maize GL8 protein is a component of the acyl-coenzyme A elongase involved in the production of cuticular waxes. Plant Physiol. 2002, 128, 924–934. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, H.E.; Shin, J.J.; Bird, D.A.; Samuels, A.L. Arabidopsis ABCG transporters, which are required for export of diverse cuticular lipids, dimerize in different combinations. Plant Cell 2010, 22, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, H.; Wang, X.; Chang, C. Transcription Factor TaMYB30 Activates Wheat Wax Biosynthesis. Int. J. Mol. Sci. 2023, 24, 10235. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Go, Y.S.; Park, C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Djemal, R.; Khoudi, H. The barley SHN1-type transcription factor HvSHN1 imparts heat, drought and salt tolerances in transgenic tobacco. Plant Physiol. Biochem. 2021, 164, 44–53. [Google Scholar] [CrossRef]
- Pascal, S.; Bernard, A.; Deslous, P.; Gronnier, J.; Fournier-Goss, A.; Domergue, F.; Rowland, O.; Joubès, J. Arabidopsis CER1-LIKE1 Functions in a Cuticular Very-Long-Chain Alkane-Forming Complex. Plant Physiol. 2019, 179, 415–432. [Google Scholar] [CrossRef]
- Rao, G.G.; Basha, S.K.; Rao, G.R. Effect of NaCl salinity on amount and composition of cuticular wax and cuticular transpiration rate in peanut (Arachis hypogaea L.). Indian J. Exp. Biol. 1981, 19, 880–881. [Google Scholar]
- Mills, D.; Zhang, G.; Benzioni, A. Effect of different salts and of aba on growth and mineral uptake in jojoba shoots grown in vitro. J. Plant Physiol. 2001, 158, 1031–1039. [Google Scholar] [CrossRef]
- Liu, N.; Chen, J.; Wang, T.; Li, Q.; Cui, P.; Jia, C.; Hong, Y. Overexpression of WAX INDUCER1/SHINE1 Gene Enhances Wax Accumulation under Osmotic Stress and Oil Synthesis in Brassica napus. Int. J. Mol. Sci. 2019, 20, 4435. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, H.; Wang, X.; Qiu, Y.; Tian, L.; Qi, X.; Qu, L.Q. Cytochrome P450 family member CYP96B5 hydroxylates alkanes to primary alcohols and is involved in rice leaf cuticular wax synthesis. New Phytol. 2020, 225, 2094–2107. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Ren, L.; Lian, H.; Liu, Y.; Chen, H. Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Sci. Int. J. Exp. Plant Biol. 2016, 249, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Rostami, Z.; Fazeli, A.; Hojati, Z. The isolation and expression analysis of cinnamate 4-hydroxylase and chalcone synthase genes of Scrophularia striata under different abiotic elicitors. Sci. Rep. 2022, 12, 8128. [Google Scholar] [CrossRef] [PubMed]
- Diharce, J.; Bignon, E.; Fiorucci, S.; Antonczak, S. Exploring Dihydroflavonol-4-Reductase Reactivity and Selectivity by QM/MM-MD Simulations. Chembiochem A Eur. J. Chem. Biol. 2022, 23, e202100553. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yu, C.; Yan, J.; Wang, X.; Chen, F.; Zhao, Y.; Wei, W. Overexpression of the Jatropha curcas JcERF1 gene coding an AP2/ERF-type transcription factor increases tolerance to salt in transgenic tobacco. Biochemistry 2014, 79, 1226–1236. [Google Scholar] [CrossRef]
- Schmidt, R.; Mieulet, D.; Hubberten, H.M.; Obata, T.; Hoefgen, R.; Fernie, A.R.; Fisahn, J.; San Segundo, B.; Guiderdoni, E.; Schippers, J.H.; et al. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 2013, 25, 2115–2131. [Google Scholar] [CrossRef]
- Liu, X.; Hong, L.; Li, X.Y.; Yao, Y.; Hu, B.; Li, L. Improved drought and salt tolerance in transgenic Arabidopsis overexpressing a NAC transcriptional factor from Arachis hypogaea. Biosci. Biotechnol. Biochem. 2011, 75, 443–450. [Google Scholar] [CrossRef]
- Ferreira Neto, J.R.; Pandolfi, V.; Guimaraes, F.C.; Benko-Iseppon, A.M.; Romero, C.; Silva, R.L.; Rodrigues, F.A.; Abdelnoor, R.V.; Nepomuceno, A.L.; Kido, E.A. Early transcriptional response of soybean contrasting accessions to root dehydration. PLoS ONE 2013, 8, e83466. [Google Scholar] [CrossRef]
- Nakashima, K.; Tran, L.S.; Van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. Cell Mol. Biol. 2007, 51, 617–630. [Google Scholar] [CrossRef]
- Pauwels, L.; Morreel, K.; De Witte, E.; Lammertyn, F.; Van Montagu, M.; Boerjan, W.; Inzé, D.; Goossens, A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Davletova, S.; Schlauch, K.; Coutu, J.; Mittler, R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Roldan, M.; Dixon, R.A. Characterization of two TT2-type MYB transcription factors regulating proanthocyanidin biosynthesis in tetraploid cotton, Gossypium hirsutum. Planta 2017, 246, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Hossain, M.N.; Iqbal, M.A.; Oba, S. Bioactive Components and Radical Scavenging Activity in Selected Advance Lines of Salt-Tolerant Vegetable Amaranth. Front. Nutr. 2020, 7, 587257. [Google Scholar] [CrossRef]
- Yang, W.; Pollard, M.; Li-Beisson, Y.; Beisson, F.; Feig, M.; Ohlrogge, J. A distinct type of glycerol-3-phosphate acyltransferase with sn-2 preference and phosphatase activity producing 2-monoacylglycerol. Proc. Natl. Acad. Sci. USA 2010, 107, 12040–12045. [Google Scholar] [CrossRef]
- Beisson, F.; Li, Y.; Bonaventure, G.; Pollard, M.; Ohlrogge, J.B. The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 2007, 19, 351–368. [Google Scholar] [CrossRef]
- Lee, J.; Ridgway, N.D. Substrate channeling in the glycerol-3-phosphate pathway regulates the synthesis, storage and secretion of glycerolipids. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2020, 1865, 158438. [Google Scholar] [CrossRef]
- Bai, Y.; Shen, Y.; Zhang, Z.; Jia, Q.; Xu, M.; Zhang, T.; Fang, H.; Yu, X.; Li, L.; Liu, D.; et al. A GPAT1 mutation in Arabidopsis enhances plant height but impairs seed oil biosynthesis. Int. J. Mol. Sci. 2021, 22, 785. [Google Scholar] [CrossRef]
- Quiroga, I.Y.; Pellon-Maison, M.; Suchanek, A.L.; Coleman, R.A.; Gonzalez-Baro, M.R. Glycerol-3-phosphate acyltransferases 3 and 4 direct glycerolipid synthesis and affect functionality in activated macrophages. Biochem. J. 2019, 476, 85–99. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.B.; Kim, H.J.; Min, M.K.; Hwang, I.; Suh, M.C. Characterization of glycosylphosphatidylinositol-anchored lipid transfer protein 2 (LTPG2) and overlapping function between LTPG/LTPG1 and LTPG2 in cuticular wax export or accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2012, 53, 1391–1403. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Y.; Cao, H.; Fan, H.; Ma, Z.; Lei, X.; Mason, A.S.; Xia, Z.; Huang, X. Efficient isolation of high quality rna from tropical palms for rna-seq analysis. Plant Omics J. Plant Mol. Biol. Omics 2012, 5, 584–589. [Google Scholar]


| Rep 1 | Rep 2 | Rep 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Clean Read Number (Million) | Nucleotide Bases (Gb) | Q20 (%) | Clean Read Number (Million) | Nucleotide Bases (Gb) | Q20 (%) | Clean Read Number (Million) | Nucleotide Bases (Gb) | Q20 (%) | |
| Hainan_Tall_0_day | 19.83 | 5.93 | 97.9 | 19.18 | 5.73 | 98.08 | 18.53 | 5.54 | 97.95 |
| Red_Dwarf_0_day | 19.79 | 5.92 | 97.86 | 20.89 | 6.24 | 98.06 | 21.08 | 6.30 | 97.95 |
| Yellow_Dwarf_0_day | 21.31 | 6.37 | 98.14 | 22.03 | 6.59 | 97.89 | 17.38 | 5.20 | 98.12 |
| Aromatic_0_day | 20.71 | 6.19 | 98.01 | 18.94 | 5.67 | 97.86 | 17.49 | 5.23 | 97.93 |
| Hainan_Tall_1_day | 18.01 | 5.38 | 97.98 | 18.98 | 5.68 | 97.97 | 24.85 | 7.43 | 97.92 |
| Red_Dwarf_1_day | 19.56 | 5.85 | 98.03 | 18.27 | 5.46 | 98.16 | 22.76 | 6.80 | 98.04 |
| Yellow_Dwarf_1_day | 18.43 | 5.51 | 97.91 | 19.98 | 5.97 | 97.77 | 18.12 | 5.42 | 97.98 |
| Aromatic_1_day | 17.64 | 5.27 | 98.04 | 22.21 | 6.64 | 97.39 | 21.08 | 6.30 | 97.67 |
| Hainan_Tall_5_day | 18.08 | 5.41 | 97.87 | 20.15 | 6.03 | 97.79 | 17.26 | 5.17 | 97.94 |
| Red_Dwarf_5_day | 19.52 | 5.85 | 97.74 | 21.06 | 6.31 | 97.81 | 21.20 | 6.34 | 97.72 |
| Yellow_Dwarf_5_day | 20.34 | 6.09 | 97.81 | 16.99 | 5.09 | 97.66 | 18.66 | 5.59 | 97.85 |
| Aromatic_5_day | 19.37 | 5.80 | 97.78 | 18.76 | 5.62 | 97.83 | 17.88 | 5.36 | 97.87 |
| Hainan_Tall_10_day | 21.22 | 6.35 | 97.83 | 18.11 | 5.43 | 97.93 | 19.49 | 5.84 | 97.75 |
| Red_Dwarf_10_day | 18.16 | 5.44 | 97.81 | 18.47 | 5.53 | 97.6 | 17.73 | 5.31 | 97.82 |
| Yellow_Dwarf_10_day | 18.39 | 5.51 | 97.81 | 18.62 | 5.58 | 97 | 17.92 | 5.37 | 97.66 |
| Aromatic_10_day | 18.86 | 5.65 | 97.71 | 18.88 | 5.66 | 97.69 | 18.28 | 5.47 | 97.79 |
| Trend | Trend Fig. | Variety | Expression Number | Shared Gene Number | Venn Diagram |
|---|---|---|---|---|---|
| UUU |  | Hainan Tall | 914 | 6 |  |
| Aromatic | 1157 | ||||
| Red dwarf | 1068 | ||||
| Yellow dwarf | 967 | ||||
| UUD |  | Hainan Tall | 2808 | 22 |  |
| Aromatic | 2091 | ||||
| Red dwarf | 2302 | ||||
| Yellow dwarf | 2568 | ||||
| UDD |  | Hainan Tall | 2431 | 102 | 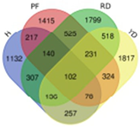 |
| Aromatic | 3148 | ||||
| Red dwarf | 3929 | ||||
| Yellow dwarf | 3606 | ||||
| UDU |  | Hainan Tall | 3991 | 57 |  |
| Aromatic | 4232 | ||||
| Red dwarf | 3221 | ||||
| Yellow dwarf | 4543 | ||||
| DDD |  | Hainan Tall | 2112 | 107 | 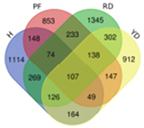 |
| Aromatic | 1087 | ||||
| Red dwarf | 2699 | ||||
| Yellow dwarf | 2028 | ||||
| DDU |  | Hainan Tall | 4770 | 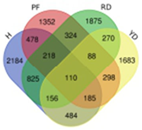 | |
| Aromatic | 3189 | ||||
| Red dwarf | 4003 | 110 | |||
| Yellow dwarf | 3403 | ||||
| DUD |  | Hainan Tall | 4471 | 62 |  |
| Aromatic | 5072 | ||||
| Red dwarf | 3584 | ||||
| Yellow dwarf | 4096 | ||||
| DUU |  | Hainan Tall | 2586 | 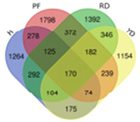 | |
| Aromatic | 3383 | ||||
| Red dwarf | 3118 | 170 | |||
| Yellow dwarf | 2576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Kaleri, G.A.; Mu, Z.; Feng, Y.; Yang, Z.; Zhong, Y.; Dou, Y.; Xu, H.; Zhou, J.; Luo, J.; et al. Comparative Transcriptome Analysis Provides Insights into the Effect of Epicuticular Wax Accumulation on Salt Stress in Coconuts. Plants 2024, 13, 141. https://doi.org/10.3390/plants13010141
Sun X, Kaleri GA, Mu Z, Feng Y, Yang Z, Zhong Y, Dou Y, Xu H, Zhou J, Luo J, et al. Comparative Transcriptome Analysis Provides Insights into the Effect of Epicuticular Wax Accumulation on Salt Stress in Coconuts. Plants. 2024; 13(1):141. https://doi.org/10.3390/plants13010141
Chicago/Turabian StyleSun, Xiwei, Ghulam Abid Kaleri, Zhihua Mu, Yalan Feng, Zhuang Yang, Yazhu Zhong, Yajing Dou, Hang Xu, Junjie Zhou, Jie Luo, and et al. 2024. "Comparative Transcriptome Analysis Provides Insights into the Effect of Epicuticular Wax Accumulation on Salt Stress in Coconuts" Plants 13, no. 1: 141. https://doi.org/10.3390/plants13010141






