Abstract
Upon storage, seeds inevitably age and lose their viability over time, which determines their longevity. Longevity correlates with successful seed germination and enhancing this trait is of fundamental importance for long-term seed storage (germplasm conservation) and crop improvement. Seed longevity is governed by a complex interplay between genetic factors and environmental conditions experienced during seed development and after-ripening that will shape seed physiology. Several factors have been associated with seed ageing such as oxidative stress responses, DNA repair enzymes, and composition of seed layers. Phytohormones, mainly abscisic acid, auxins, and gibberellins, have also emerged as prominent endogenous regulators of seed longevity, and their study has provided new regulators of longevity. Gaining a thorough understanding of how hormonal signalling genes and pathways are integrated with downstream mechanisms related to seed longevity is essential for formulating strategies aimed at preserving seed quality and viability. A relevant aspect related to research in seed longevity is the existence of significant differences between results depending on the seed equilibrium relative humidity conditions used to study seed ageing. Hence, this review delves into the genetic, environmental and experimental factors affecting seed ageing and longevity, with a particular focus on their hormonal regulation. We also provide gene network models underlying hormone signalling aimed to help visualize their integration into seed longevity and ageing. We believe that the format used to present the information bolsters its value as a resource to support seed longevity research for seed conservation and crop improvement.
1. Introduction
Loss of agrobiodiversity is a growing threat due to the abandonment of local varieties in favour of commercially driven, genetically uniform crops [1]. Now, a mere nine crops contribute to approximately 66% of global crop production [2]. Therefore, it is of paramount importance to preserve the genetic diversity of non-crop species for conservation purposes, but also as potential reservoirs of useful traits that could be reintroduced into elite cultivars. The establishment of germplasm banks, dedicated to storing seeds of various plant species and crop varieties, has become instrumental in safeguarding valuable genetic resources. The inevitable decline in seed viability with age is a critical factor determining their long-term storability, and thus, improving this characteristic is paramount for safeguarding wild species and crops’ genetic diversity and yield.
The period during which seeds maintain their viability determines longevity, which is significantly influenced by environmental conditions experienced during storage, including temperature, equilibrium relative humidity (RH), and oxygen pressure (Figure 1) [3,4,5,6,7,8]. Seeds can be classified into three primary categories based on their behaviour during storage: recalcitrant, intermediate, and orthodox [9,10]. Recalcitrant and intermediate seeds are extremely sensitive to desiccation and chilling, conditions routinely used for ex situ conservation of germplasm, thus making their storage problematic. Recalcitrant seeds have reduced longevity and are typically found in woody species from tropical or subtropical habitats [11,12]. Intermediate seeds, comprising around 10–15% of angiosperms worldwide, can tolerate higher levels of dehydration than recalcitrant seeds, but are not as resistant as orthodox seeds [10,13,14,15].
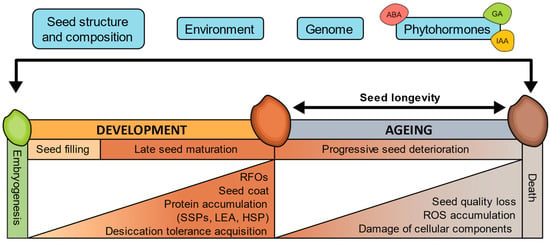
Figure 1.
Factors affecting seed longevity and processes associated with seed development and ageing. ABA, abscisic acid; HSP, heat shock protein; IAA, auxins; GA, gibberellins; LEA, late embryogenesis abundant protein; RFOs, raffinose family oligosaccharides; ROS, reactive oxygen species; SSPs, seed storage proteins.
Orthodox seeds, which include most crops, are characterized by their ability to tolerate desiccation to moisture contents of 10% on a fresh weight basis or lower, and endure subfreezing temperatures, typically as cold as −20 °C [16]. Dry orthodox mature seeds reach a state characterized by extreme cellular viscosity, called a glassy or vitrified state, during which their cellular activities and metabolism are greatly slowed down, including oxidation [8,17,18,19,20,21]. This mechanism represents an important protection against ageing, allowing orthodox seeds to survive for long periods with a vitrified cytoplasm [22,23,24]. However, predicting seed longevity is challenging due to considerable variations among species and storage conditions [25,26,27]. Also, seeds subjected to the same storage conditions can lose viability at vastly different rates due to variations in their physiological and genetic characteristics, with hormone signalling emerging as prominent regulators of seed longevity [3,25,26,28]. Understanding the factors that contribute to the longevity of seeds and how they interact can provide valuable insights for seed conservation and agricultural practices.
This review first explores the mechanisms of seed ageing in orthodox species and how they are influenced by seed storage conditions. The second part is focused on phytohormone signalling pathways that regulate seed ageing and provide a summary of genes involved in hormonal signalling and seed longevity. Thus, this review aims to shed light on the intricate mechanisms governing seed longevity and provide valuable insights for future research, leading to the improvement of this trait. Unless otherwise indicated, genes mentioned throughout this review are from Arabidopsis thaliana, and At in front of the gene names has been omitted for simplicity.
2. Seed Ageing Mechanisms
Seed ageing is a natural and irreversible process that leads to a progressive deterioration of seed quality. Initially, it manifests as a delay in the germination speed, followed by a gradual loss of viability evidenced by an increase in the percentage of seeds unable to germinate, and eventually culminates in the death of all seeds in the lot. This degenerative process occurs over time, even under optimal storage conditions [29]. Numerous physiological and biochemical changes have been linked to seed ageing, including the accumulation of reactive oxygen species (ROS), lipid peroxidation, loss of membrane phospholipids, reduced activity of antioxidant enzymes, and volatile production [30,31,32,33,34]. Additionally, ageing is associated with changes in transcript levels, impaired protein synthesis and post-translational modifications such as protein inactivation by carbonylation, hydrolysis, or alteration in enzyme activity [35,36,37]. So, ageing appears to result from a gradual decline in the enzymatic activities responsible for repairing damage, ultimately leading to the accumulation of unrepaired oxidative damage.
Recent genome-wide and reverse genetics studies on Arabidopsis thaliana highlighted the important role of oxidative stress on seed ageing, identifying genes involved in ROS metabolism and detoxification as related to seed longevity [38]. ROS originating from the partial reduction of oxygen can cause oxidative damage when their levels exceed the homeostatic capacity of the seed antioxidant system, particularly under stress conditions [39,40]. These highly reactive chemicals interact with cellular biomolecules, leading to severe oxidative damage in proteins, nucleic acids, and lipids [41]. The production of ROS in seeds depends on their metabolic and physiological state [42]. In hydrated seeds, the primary source of ROS is the mitochondrial respiratory chain, especially during germination when respiratory activity increases [43,44]. When seeds are in a dry, glassy state, such as during storage in dry conditions, the enzyme activities producing and scavenging ROS are extremely reduced. However, even in this state, ROS continue to accumulate due to non-enzymatic reactions, such as lipid peroxidation [45]. Although ROS accumulation is linked to seed ageing processes, cells are equipped with detoxifying enzymes and antioxidant compounds like glutathione to balance the effects of ROS and ensure seed survival [43]. When stored seeds are imbibed and set off to germinate, the accumulation of ROS is counteracted by the antioxidant system, and it has been recently identified that concentrations of glutathione before ageing provided a predictive indicator of seed longevity across a range of Arabidopsis wild-type accessions [46]. However, as seeds age, ROS-processing enzymes are themselves affected by oxidative damage, and the resulting decline in antioxidant enzyme activity compromises ROS detoxification, a situation that negatively affects seed longevity [47,48,49,50,51].
Among the various oxidative processes contributing to seed ageing, lipid peroxidation plays a crucial role. This process involves the oxidative degradation of lipids, particularly from the cell membrane, leading to cell damage and negatively impacting seed viability [42,52]. Studies based on the determination of malondialdehyde content have shown that lipid peroxidation is linked to seed ageing in different species [53,54]. However, contrasting results not finding a correlation between this compound and ageing have also been reported [47,49]. Since lipid peroxidation alters the membrane’s permeability, producing an increase in solute leaching, electrical conductivity measurements have also been used as a parameter to quantitate seed ageing in various species, specially within the Brassicaceae family [49,55,56,57,58,59,60]. Similarly, by measuring lipid peroxidation, some studies have not shown a direct correlation between electrical conductivity and seed ageing [61]. Since the rate of lipid peroxidation depends on the equilibrium RH and the seed lipid content, differences between species and experimental storage conditions may underly the contradictory results [32,33,62].
DNA and RNA integrity can also be affected during storage, especially due to oxidative stress. ROS are known to induce genotoxic effects in seeds, compromising the stability of DNA [34,63]. As seeds age, point mutations accumulate, and structural damage such as single and double-strand breaks could lead to DNA fragmentation [52,63,64,65,66,67,68]. Seeds may still be capable of germination despite genotoxic stress, but they must overcome damages in DNA to prevent mutations from being passed on to future generations [69,70,71]. To achieve this, DNA repair mechanisms are activated in the embryo during early germination stages, and DNA repair enzymes have been found to play a key role in seed longevity [71,72,73]. Recent studies highlight the crucial role of RNA metabolism in regulating seed longevity. Degradation processes of RNAs have been linked to seed ageing in various species [74,75,76]. In soybean seeds, it has been observed that lower viability is associated with RNA fragmentation during the ageing process [75]. This damage seems to occur randomly throughout the seed transcriptome, suggesting that RNA fragmentation could serve as a consistent molecular marker for tracking the progression of ageing in dry seeds. Moreover, the regulation of mRNA storage and processing has been identified as a significant factor in seed longevity. Specifically, factors related to RNA spliceosome subunits MOS4 or MAC3A/MAC3B have been highlighted and allow for the differentiation of accessions with high and low seed longevity [46].
The role of DNA methylation as a potential indicator of seed ageing and viability loss during seed storage has garnered attention, although investigations into epigenetic changes during this process remain limited [77]. Studies have revealed that drying has an impact on the global level of DNA methylation in seeds of certain tree species [78,79,80]. Also, seed ageing increased epigenetic instability in both seeds and seedlings in several species [81,82]. In mint seeds, methylation changes after storage were detected at a low rate, but changes increased in seedlings produced from aged seeds [82]. Supporting these results, rye seeds showed epigenetic changes in stored seeds and seedlings [81]. Understanding the impact of ageing on DNA and RNA integrity and their epigenetic marks, as well as the mechanisms involved in repairing and overcoming such damages, is critical for ensuring seed viability and preserving genetic stability in plants.
3. Seed Storage Conditions: Key Role of Water Content
Diverse experimental storage approaches have been used on seed ageing research, which led to variations in outcomes between different studies, making comparisons difficult. Equilibrium RH, temperature, and atmosphere are the main factors determining seed longevity during storage, and have been the focus of numerous studies since the 1990s [3,6,7,52,83,84]. The water content of seeds governs the biophysical and biochemical state of cells during storage and is recognized as a critical factor dictating the process of seed ageing [7,8,17,19,33,85,86,87,88,89,90]. Consequently, seed ageing mechanisms show substantial differences between storage under high or low equilibrium RH [32,33]. However, defining specific boundaries for what constitutes high or low humidity proves challenging, and terms like “accelerated ageing”, “controlled deterioration”, or “natural ageing” are rather subjective and often used imprecisely [91,92].
Another crucial aspect that contributes to the divergent outcomes in seed longevity studies is the choice of containers for storage. The importance of controlling humidity and storage atmospheres in deterioration experiments necessitates the use of hermetic containers. However, many lab containers fall short of providing sufficient hermeticity for extended periods of high humidity control [93]. Moreover, some experiments rely on hermetic storage using sealed containers, such aluminium foil bags or glass jars, while others employ open containers inside an incubator. The type of storage, whether hermetic or open storage, has been identified as a critical factor influencing seed longevity performance, likely due to variations in oxygen exposure, which is limited in hermetic conditions [84]. Also, studies have explored the impact of different gaseous environments, including air, vacuum, CO2, and N2, on seed viability during storage [94,95,96]. Oxygen presence in the storage environment has been identified as particularly harmful, leading to faster viability loss [4,97]. These findings indicate the importance of the storage containers and gas atmosphere as a factor affecting seed longevity.
Extrapolating findings obtained at one experimental condition to different storage settings further complicates matters. The rate and type of biochemical reactions might not be comparable when ageing is assayed at 100% or 75% equilibrium RH since, for example, the onset of respiration is at 90% RH (approximately water content of 0.25 g H2O g−1 dry weight) for a wide range of species [15,89]. Although many species age faster both at high RH and also in dry storage [25], some species exhibit unexpected faster ageing in the latter condition [62]. In Arabidopsis, relative longevity was compared among natural ecotypes, and similar results were found for two storage conditions below 75% RH, but not for storage above 75% RH [38,46]. In the case of studies with mutants, some of them rendered similar results when assayed under wet and dry conditions, while for heat shock protein mutants, the results were greatly influenced by storage conditions [38,98,99,100].
These observations highlight the significant disparity in ageing mechanisms depending on the storage conditions, thus limiting the predictive capacity of storage experiments for seed longevity. To address this issue, Hay et al. [91] proposed the use of ageing conditions that align with the intended future applications of the research results, and if possible, implement standardized conditions to facilitate comparison across studies. Following these recommendations, Zinsmeister et al. [28] coined three terms to classify seed ageing conditions based on association with a range of experimental values, instead of fixed values, for equilibrium relative humidity and temperature: (1) “Wet ageing”, which encompassed the accelerated ageing and controlled deterioration tests, storing seeds at high RH (>80%), and a wide range of temperatures (4–45 °C); (2) “Intermediate ageing”, which would allow the study of seed longevity under dry conditions within a reasonable timeframe by storing seeds at 75% RH and 30–35 °C; and (3) “Dry ageing”, representative of both natural ageing and seed bank conditions for long-term storage, storing seeds at low RH (<70%), and temperatures from 25 to −20 °C. In this latter case, given the wide range of temperatures associated to dry ageing, its selection is not trivial since it may introduce considerable variability in the experimentation. This classification will be used in this manuscript to categorize the storage conditions of different studies and allow comparisons.
4. Other Factors Affecting Seed Longevity
Seed longevity is a multifaceted trait influenced by the genome and seed structural composition, which are themselves subjected to environmental cues [25,26,101,102,103,104,105,106,107]. Interestingly, seed longevity exhibits similarities within some particular taxonomic families or genus, like the Apiaceae, being their seeds consistently short lived [25,26]. On the contrary, this trait can vary significantly between species within the same family [25,26], between accessions of the same cultivar [108,109,110], or even between seeds from the same plant [111,112]. It is of high interest to investigate whether those taxonomic-associated patterns of seed longevity are related to similarities or differences in seed structure and composition, including traits like lipid thermal behaviour, volatile production, and seed coat structure [25,32,33,55,113].
Longevity is progressively acquired during the final stages of orthodox seed development and maturation. These stages are associated with seed filling, acquisition of desiccation tolerance, loss of chlorophyll, and accumulation of the raffinose family oligosaccharides (RFOs) and seed storage proteins (SSPs) [114,115,116,117,118]. These processes have been related to seed longevity and are regulated by genetic programs that integrate hormonal and metabolic pathways mainly controlled by LAFL (i.e., LEC1, ABI3, FUS3, and LEC2) transcriptional regulators [115,119]. The LAFL regulatory network includes the family of B3 domain transcription factors ABI3 (ABSCISIC ACID INSENSITIVE3), FUS3 (FUSCA3), and LEC2 (LEAFY COTYLEDON2); and LEC1 (LEAFY COTYLEDON1), a member of the NFYB protein family [120]. Arabidopsis abi3 and lec1 mutants, in addition to showing a decrease in SSPs, RFOs, and chlorophyll degradation, also have reduced longevity [119]. Along with genetic regulation, environmental conditions during seed development have the potential to exert a profound influence on seed longevity [103,121]. Studies have demonstrated the significance of environmental parameters in shaping seed longevity traits, with light intensity and temperature during seed development showing positive correlations with longevity [122]. Consequently, species originating from cool, temperate climates tend to produce short-lived seeds, while those from warm, arid environments yield seeds with extended lifespans [25,26,102,121,123].
Seed coat structure plays an important role in seed longevity since it serves as a protective layer that shields the embryo from external abiotic and biotic factors [38,124]. Seeds of Arabidopsis transparent testa mutants lack proper regulation of testa composition and show reduced longevity [125]. Also, the identification of several seed-coat-related genes in regulating longevity supports its protecting role during storage [38,126]. Cell wall structure is crucial for seed viability maintenance, and recent studies suggest that genes involved in callose degradation can be related to both longevity and dormancy [127].
Seed composition, particularly lipid content, has been associated with variations in longevity among species. While the notion that high lipid content leads to lower longevity has been widespread [56], recent analyses have shown that the lipid composition plays a more significant role due to differences in susceptibility to oxidation or thermal behaviour [17,52,113,128]. For example, the energy associated with lipid melting has been used as a descriptor of viability loss [129]. Sugars and proteins also play a crucial role in seed longevity by stabilizing the glassy cytoplasm [20]. Non-reducing sugars, mainly sucrose and RFOs, heat shock proteins (HSPs), and late embryogenesis abundant (LEA) proteins, are synthesized during seed development and contribute to seed desiccation tolerance and longevity, likely preventing aggregation in the glassy-state and assisting in protein folding [116,118,130,131,132,133,134,135]. SSPs accumulate during seed filling and are major targets for oxidation and carbonylation [136]. This makes them an important part of the seed’s defence against oxidative damage, protecting other proteins from oxidation during storage. In Arabidopsis seeds, cruciferins and napins are the two main types of SSPs. Studies involving comparative proteomics and knockout mutants have identified these proteins, cruciferins in particular, as crucial for seed longevity [137,138].
In summary, it is evident that seed structure and chemical composition have a pivotal role as key endogenous factors influencing seed longevity. Therefore, gaining a better understanding of the factors that regulate these processes offers a new approach to bolster seed longevity.
5. Hormone-Mediated Regulation of Seed Longevity
In recent years, research on hormonal signalling pathways related to longevity has sparked increased interest. In particular, signalling mediated by abscisic acid (ABA), auxins, and gibberellins (GAs) have emerged as key players affecting this trait given their profound influence on seed development, composition, and structure. This section aims to explore the impact of hormonal regulation and signalling pathways on seed longevity and provide detailed information on the specific experimental conditions to better evaluate commonalities and differences between different plant species. Three different seed storage conditions have been used in the publications reviewed in this section that can be classified according to Zinsmeister et al. [28].
5.1. Abscisic Acid
ABA plays a significant role in enabling plants to resist various stresses, particularly those caused by abiotic factors, and is antagonized mainly by GAs [139,140,141,142]. ABA is known to repress seed germination, but it is required for crucial processes occurring during seed development, such as accumulation of seed-storage compounds, establishment of seed dormancy, and acquisition of desiccation tolerance [143,144,145,146]. Consequently, ABA plays a key role in seed longevity acquisition by regulating gene expression associated with seed dehydration tolerance and synthesis of RFOs, HSPs, and LEA proteins [135,140,147,148].
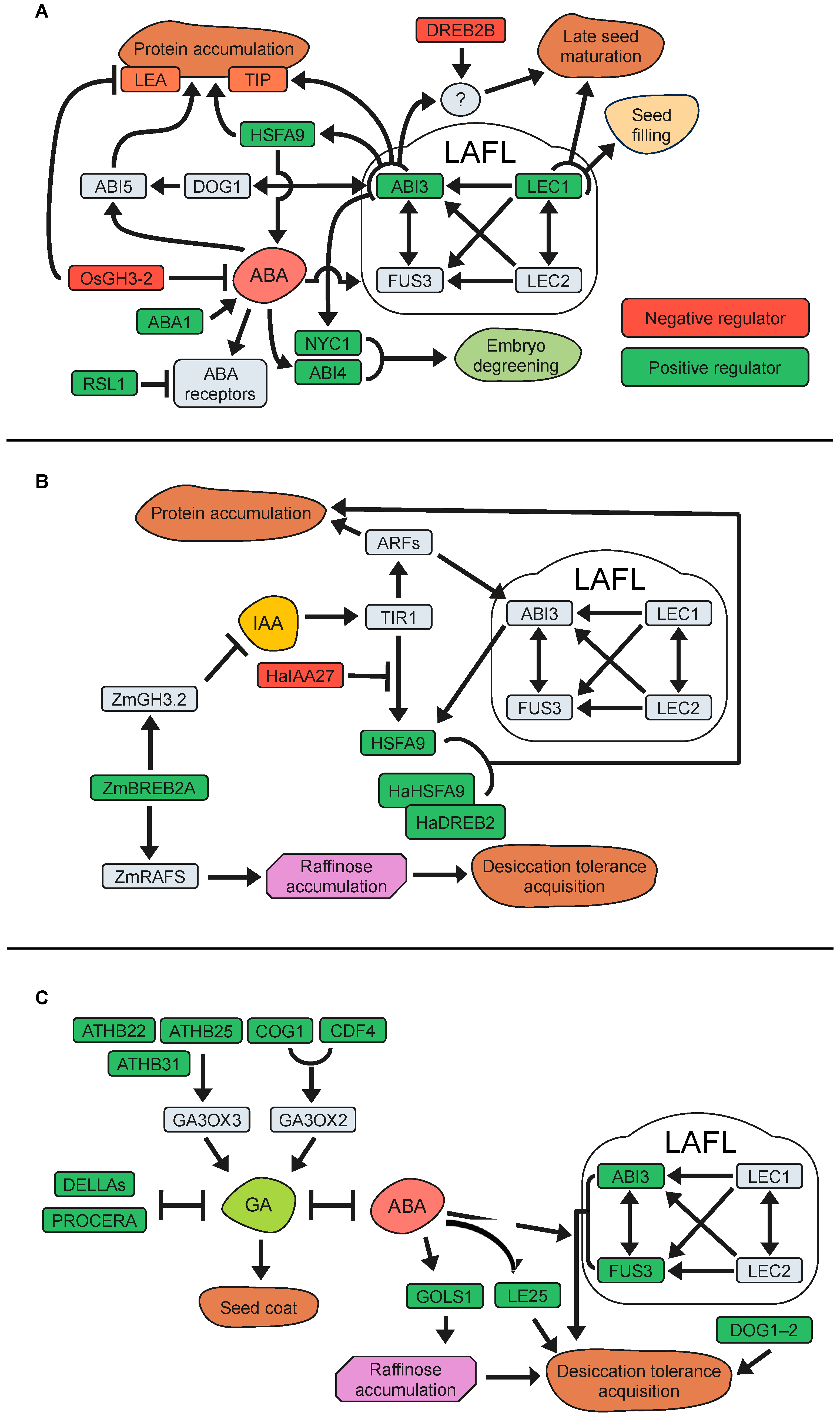
The ABA-signalling pathway relies on three key early components. Binding of ABA by the PYR/PYL/RCAR (pyrabactin resistance/pyrabactin resistance-like/regulatory component of ABA receptor) proteins triggers deactivation of the PP2Cs (type 2C Ser/Thr protein phosphatases). The absence of PP2Cs allows activation of the SnRK2 (Snf1-related protein kinase class 2) kinase that phosphorylate downstream signalling components [149,150]. The LAFL genes are master regulators controlling gene expression programs intimately linked to ABA signalling and essential for seed maturation, accumulation of storage compounds, and seed longevity. Their core function is well conserved among the spermatophytes, and although LAFL mutants alter seed longevity, they also compromise several other aspects related to their wide regulatory roles (i.e., seed filling, acquisition of desiccation tolerance, loss of chlorophyll, and accumulation of RFOs and SSPs). Several genes controlled by ABA/LAFL-signalling have been found to play a role in seed longevity. ABI3 is a transcription factor that positively regulates the ABA-signalling pathway, and ABI3 mutants show ABA insensitivity and reduced accumulation of seed storage proteins and seed longevity [119,143,151,152,153]. Clerkx et al. [154] reported that mutations such as abi3 or aba1 (abscisic acid deficient1; ABA biosynthetic mutant) led to reduced seed longevity under different ageing conditions. Tesnier et al. [155] and Sugliani et al. [119] also demonstrated poor longevity in ABA-related mutants, emphasizing the crucial role of ABI3 and LEC1 in maintaining seed viability in Arabidopsis during storage. Interestingly, co-expression network studies on the legume Medicago truncatula seeds have indicated that seed longevity is regulated by both MtABI3-dependent and independent pathways [115]. Downstream ABI3, the Arabidopsis transcription factor HSFA9 (HEAT SHOCK FACTOR A9) and its homologues in M. truncatula and soybean have also been linked to seed longevity [114,116,156,157,158,159]. In fact, heat shock transcription factors (HSFs) such as HSFA9 promote the expression of small heat shock protein genes (sHSP), contributing to a genetic program that regulates embryonic desiccation tolerance and seed longevity in different species [99,115,158,160,161,162,163]. In Arabidopsis, HSFA9 promotes the expression of HSPs and tonoplast intrinsic proteins (TSPs; aquaporins) mediating resistance against seed deterioration during storage [156,161,162]. Mutations in two Arabidopsis aquaporins localized in the seed protein storage vacuole membrane, tip3-1/tip3-2, exhibited reduced seed longevity after storage, and ABI3 was found to bind the TIP3 promoters and required for enhanced TIP3 mRNA and protein levels [152]. Moreover, hsfa9 mutant seeds of Arabidopsis, M. truncatula, and tobacco lost viability faster than wild-type seeds when stored at wet ageing and exhibited deregulation of ABA biosynthesis-related genes [99,158,161]. However, longevity of hsfa9 seeds was comparable to that of the wild-type seeds at intermediate ageing conditions, suggesting that HSFA9 plays a role in thermotolerance rather than in dry seed storage [99,158,161]. In summary, ABI3 and HSFA9 constitute an important ABA-related regulatory module for seed longevity that seems to be conserved in several plant species.
Seed longevity is established during the late maturation stage, coinciding with the process of seed degreening. It is long known that chlorophyll retention in mature seeds can be an undesirable trait, being related to low seed storability [164,165]. Chlorophyll degradation during seed maturation is also regulated by the ABA signalling pathway, and abi3 mutants are not able to degrade chlorophyll during maturation and remain green even after desiccation [143,151,166,167]. In this regard, the Arabidopsis nyc1 mutant (Non-yellow coloring1), encoding a chlorophyll b reductase isoform, showed increased seed chlorophyll content and exhibited a rapid decline in viability during storage at room temperature compared to wild-type seeds. Not surprisingly, NYC1 expression was repressed in the abi3 mutant, suggesting that ABA induces chlorophyll degradation during seed development to retain storability [166]. ABI4 is an AP2 domain-containing transcription factor and another core component of the ABA signalling pathway during seed development [168,169]. A role for ABI4 in longevity of M. truncatula and Solanum lycopersicum (tomato) seeds had previously been hinted at by gene co-expression analyses [116,148]. Indeed, Arabidopsis and M. truncatula abi4 mutants have reduced longevity compared to wild-type when stored at intermediate ageing conditions, and also showed elevated chlorophyll levels and reduced dormancy [170].
Additionally, another transcription factor of the ABA signalling pathway, ABI5, has been shown to be an important regulator of longevity in legume seeds [171]. In M. truncatula, MtABI5 is a positive regulator of seed longevity, dormancy, and storage proteins, and also act in the regulatory network of degreening during late seed maturation. Abi5 mutant seeds of pea and M. truncatula retained some chlorophyll and exhibited decreased longevity [171]. Moreover, the analysis of Mtabi5 Mtabi4 double mutants showed synergistic effects on chlorophyll retention and longevity, suggesting that they act via parallel pathways [170]. Consequently, ABI4 is hypothesized to coordinate the dismantling of chloroplasts during seed maturation and the subsequent attainment of seed longevity, acting in synergy with ABI5. In Arabidopsis, ABI5 controls the genes related to chlorophyll metabolism, similar to legumes. However, when ABI5 is not fully functional in seeds (abi5 mutants), seeds do not display green coloration or shortened longevity [171]. This might be attributed to the potential regulation of ABI5 by DOG1 (DELAY OF GERMINATION 1), ABI3, and a group of redundant bZIP (basic leucine zipper) transcription factors homologous to ABI5 (homologous ABF genes) [167,171]. All these regulatory proteins play a vital role in controlling the process of acquiring seed longevity.
As previously mentioned, dormancy is another process strongly related to longevity. The Arabidopsis DOG1 gene plays a crucial role in the establishment of seed dormancy during seed development [172]. Seeds of the dog1 loss-of-function mutants show reduced longevity under dry conditions [173]. Through transcriptomics and metabolomics approaches, Dekkers et al. [167] found that the DOG1 function is also required at later stages of seed development for seed longevity acquisition by promoting the expression of numerous genes, such as HSPs and LEAs, partly through positive control of ABI5 expression and in conjunction with ABI3 signalling. However, a negative correlation between seed longevity and seed dormancy was reported by analyses with inbred line populations [174]. The results revealed five loci (GAAS) that are collocated with DOG seed dormancy loci. Detailed analysis on the collocating GAAS5 and DOG1 quantitative trait loci revealed that the DOG1-Cape Verde Islands allele both reduces seed longevity and increases seed dormancy. These results suggest the existence of separate mechanisms for seed dormancy and longevity that may vary between accessions as a result of adaptation to its natural environment. Interestingly, we have just found that a DOG1 downstream target gene negatively feedback on DOG1 expression and dormancy, and enhances germination without altering seed longevity [175].
Several studies indicate that the role of ABA as a positive regulator of seed dormancy may not be indisputable. A negative regulator of ABA signalling was isolated as a dominant Arabidopsis mutant by screening an activation-tagging mutant collection [98]. This mutant showed increased seed longevity caused by a higher expression of RSL1 (Ring finger of Seed Longevity1), a gene encoding a RING-type zinc finger putative ubiquitin ligase. Interestingly, RSL1 reduced ABA sensitivity and altered regulation of ABA-responsive gene expression through the interaction with receptors PYR1 and PYL4 [176,177]. Although the seed’s longevity phenotype was unaffected by the specific ageing conditions used during the screening process (dry or wet ageing), reduced seed longevity for the rsl1 loss-of-function was only observed under wet ageing conditions [98], therefore highlighting the relevance of experimental conditions for assessing seed ageing. More evidence linking ABA-signalling with reduced longevity came from a study with Arabidopsis DREB2B and cotton orthologous GhDREB2B (Dehydration-Responsive Element-Binding Protein 2B) [178]. DREB2B belongs to a subgroup of the DREB transcription factor family and was found to function as a negative regulator of seed longevity after wet ageing. This function seemed to be under the control of an ABA-mediated pathway, since the abi3-16 mutant allele enhanced seed longevity of the dreb2b mutant, suggesting that ABI3/DREB2B negatively regulates seed longevity in both Arabidopsis and cotton species [178].
Regardless of seed longevity being under control of ABI3 (master regulator), bioinformatic and comparative analyses of expression during seed maturation data from M. truncatula and Arabidopsis suggested that at least half of the longevity-regulated genes are controlled by ABI3-independent pathway/s [115]. In support of this finding, Righetti et al. [115] identified two transcription factors (NFXL1 and WRKY3) whose mutants produced seeds with reduced permeability and longevity but no alterations in ABA sensitivity or ABI3-related traits (dry weight, chlorophyll degradation, accumulation of non-reducing sugar contents) [115]. In addition, these two transcription factors do not seem to be targets of ABI3, LEC1, and LEC2 [179,180,181]. Also, a transcriptomic study [148] characterizing the acquisition of longevity during seed maturation revealed 14 genes whose expression strongly correlated with longevity. Among them, Bizouerne et al. [148] found orthologs of known Arabidopsis regulators of ABA signalling pathways, such as PYL5, an ABA receptor, and RAV1 (related to ABI3/VIP1), a B3 transcription factor repressing the expression of ABI3, ABI4, and ABI5. Regarding the biochemical ageing mechanisms, seed deterioration is related to the accumulation of DNA damage, and recent findings point to the WHIRLY (WHY) family of DNA-binding proteins as important players in the maintenance of nuclear and organellar genomes. Longevity of why1 and why3 Arabidopsis mutants was lower than the wild type after wet ageing, and the double mutant why1why3 was highly sensitive to ageing [182]. In addition, seeds of the why1 mutants are insensitive to the phytohormones ABA and salicylic acid during germination [183].
In summary, seed longevity and the influence of important ABA-related genes have been studied in one or various plant species (Table 1, Figure 2A). Although most findings highlight the significance of ABA-related mechanisms as positive regulators of seed longevity across different plant species and storage conditions, some results deviate from this common thread, thus underscoring the complexity of this regulation.
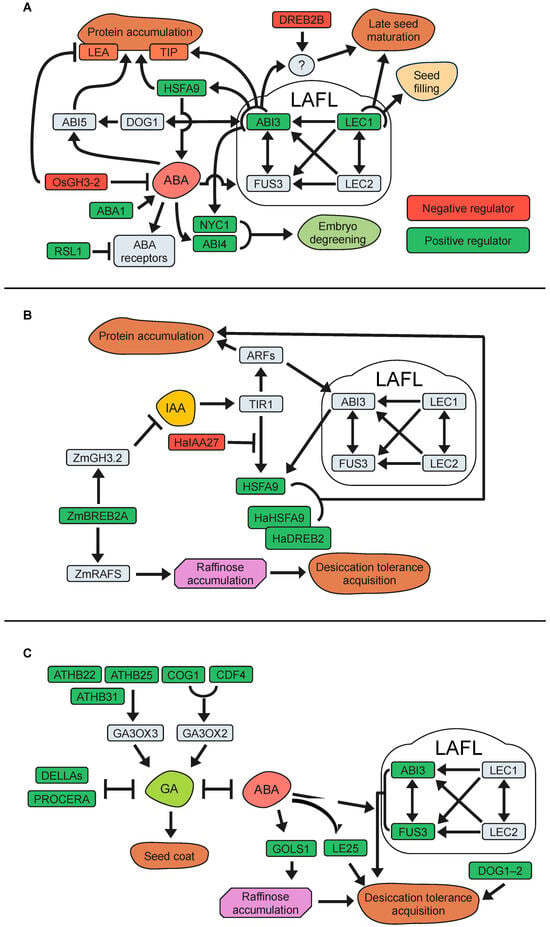
Figure 2.
Phytohormone-related genes with effects on seed longevity. (A) Graphic representation of genes related to abscisic acid (ABA); (B) genes related to auxins (IAA) and (C) genes related to gibberellins (GA) signalling pathway.
5.2. Auxins
Auxins are plant hormones whose primary function involves cell division, differentiation, fruit development, root formation, apical dominance, and leaf fall (abscission) [184]. Additionally, auxins are associated with critical aspects of seed characteristics, including size, weight, dormancy, and seed coat development [146,185,186]. One of the most significant natural hormones among auxins is the ß-indoleacetic acid, abbreviated as IAA (the main auxin in plants). The core components of the auxin-signalling machinery belong to three protein families: the F-box TIR1/AFB auxin co-receptors (TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX PROTEIN), the Aux/IAA transcriptional repressors (Auxin/INDOLE-3-ACETIC ACID), and the ARF transcription factors (AUXIN RESPONSE FACTOR). Auxin promotes an interaction between TIR1/AFB and Aux/IAA proteins, resulting in degradation of the Aux/IAAs and the release of ARF repression [187].
Using auxin biosensors, Pellizaro et al. [146] showed changes in auxin distribution and signalling, going from highly localized to widely spread throughout the embryo during the final stages of seed maturation. Also, using auxin single mutants, they found that seed longevity was affected in a dose-response manner depending on auxin levels. Moreover, the positive effect of auxins on seed longevity required ABI3 function, while the ABI3 gene and genes with promoters enriched in ARF-binding sequences were induced by auxin in developing embryos, pointing to an epistatic relationship between auxin and ABA in the control of seed longevity. This relationship was strengthened by employing a gene co-expression network analysis in M. truncatula and Arabidopsis. These investigations identified a high number of auxin- and HSP-related genes, suggesting the potential connection between seed longevity regulation and increased auxin levels during seed maturation, possibly through interactions with HSFA9, an ABI3-regulated gene [115]. In sunflowers (Helianthus annuus), a study examined the connection between auxins, seed longevity, and ABA by investigating the interactors of the HaHSFA9 protein [156]. The research revealed that the Aux/IAA protein HaIAA27 opposes the transcriptional activation mediated by HaHSFA9. This repression can be reversed by treating sunflower embryos with auxin. This finding suggests that, in addition to ARFs, the Aux/IAA-signalling pathway is recruiting an HSF protein induced by ABA to regulate seed longevity.
Regulation of IAA biosynthesis might have a potential role on seed longevity, as suggested by the positive association of an IAA-biosynthetic gene (CYP79B2) with seed longevity in Arabidopsis [146]. Moreover, IAAs can be inactivated by conjugation with amino acids catalysed by auxin-amido synthetases, and GH3-2 (GRETCHEN HAGEN3-2), a gene encoding one of these auxin-amido synthetases, has been negatively correlated with seed longevity [188,189,190]. In rice (Oryza sativa), functional analyses indicated that the OsGH3-2 encoded enzyme is involved in auxin inactivation, and its gene expression levels have a positive correlation with reduced ABA levels, ABA-related gene expression, and reduced seed longevity [189]. Additional research has shown that the overexpression of specific DREB family members, such as DREB1A (CBF3) in Arabidopsis and ZmDREB4.1 in tobacco, can decrease IAA accumulation in plant tissues [191,192]. Interestingly, experiments on maize (Zea mays) indicate that zmdreb2a mutants have reduced expression of ZmGH3.2, but increased IAA levels in their embryos and reduced seed longevity [190]. These apparently contradictory results could be explained by the simultaneous reduction of raffinose content in the zmdreb2a embryo, attributable to the lack of direct DREB2A transcriptional stimulation of ZmRAFS (RAFFINOSE SYNTHASES). These results suggest that the transcription factor ZmDREB2A regulates the expression of ZmRAFS and therefore the accumulation of RFOs, which can influence seed longevity. However, the molecular mechanisms by which DREBs regulate IAA levels in plant cells are not yet fully understood, and data available so far emphasize the implication of multiple molecular interactions. The most important IAA-related genes and their relationship with seed longevity have been compiled in Table 1 and Figure 2B.
5.3. Gibberellins
GAs are compounds that belong to the tetracyclic diterpenoid family, some of which are bioactive growth regulators in plants, fungi, and bacteria [193]. In plants, GAs control diverse aspects of growth and development, and they have also been associated with agronomic traits such as seed size, weight, dormancy, and germination [194,195,196]. Genetic screenings have identified three main components in GA perception and early GA signalling: the GID1 GA receptors (GIBBERELLIN INSENSITIVE DWARF1) and the SLY1 F-box protein (SLEEPY1), which are positive regulators of GA signalling, and the DELLA proteins, which play a negative role in GA-regulated expression and are targeted for degradation in the presence of GA [197]. DELLA proteins belong to the GRAS (GIBBERELLIC ACID INSENSITIVE REPRESSOR OF ga1-3 SCARECROW) family of transcription factors, acting as pivotal repressors in the GA signalling pathway [198]. In Arabidopsis, five DELLA proteins have been described (RGA: REPRESSOR OF GA1-3; GAI: GA-INSENSITIVE; RGL1: RGA-LIKE 1; RGL2: RGA-LIKE 2; and RGL3: RGA-LIKE 3), while only one has been identified in tomato (PRO: PROCERA) and cereals [199,200,201].
The positive role of GAs in seed longevity was well highlighted in Arabidopsis, demonstrating an increase in viability after wet storage of seeds originating from GA3-treated plants or a DELLA mutant with constitutive GA-signalling [98]. The authors also found that over-expression of the transcription factor ATHB25 (ARABIDOPSIS THALIANA HOMEOBOX25) increases GA levels by enhancing the expression of GA3ox2 (GIBBERELLIC ACID3-OXIDASE2), encoding a GA biosynthetic enzyme. Previous observations suggested that GAs in the outer integument would be required for the normal formation of the Arabidopsis seed coat [202]. Interestingly, it was observed that ATHB25 has an impact on seed coat structure and permeability by regulating lipid accumulation in the testa, a function also present in tomato and wheat [38,98]. In addition, Bueso et al. [203] reported that the overexpression of the COG1 and CDF4 genes, two DOF transcription factors, improves seed longevity. In particular, COG1 is involved in seed coat development and increases the level of GA1 in siliques, probably due to the up-regulation of the GA3ox3 biosynthetic gene. This evidence has clearly connected GA-mediated seed coat development, changes in testa structure, and changes in composition with seed longevity.
The role of DELLA proteins and their involvement in seed dormancy and longevity has also been studied among different plant species. In these cases, a negative relationship between GA-signalling and longevity was found, suggesting that the positive effects mentioned above could result from a DELLA-independent mechanism. A study in tomato identified that mutant seeds with no DELLA activity (procera mutant) showed reduced longevity during dry and intermediate storage, pointing to the negative involvement of the GA-signalling pathway on seed longevity [148,201]. In addition, the procera mutant also showed a reduction in the expression of desiccation-tolerance-related genes known to be upregulated by ABA, thus underscoring that the antagonistic balance between ABA and GAs is also involved in the regulation of seed longevity [201]. PROCERA has also been shown to have a positive role on seed dormancy [201]. The Arabidopsis DOG1 gene also promotes dormancy and longevity, and its transcript abundance is positively correlated with dormancy and longevity [173,174]. Interestingly, DOG1 has been described to strengthen the seed coat through the modulation of GA metabolism genes [204]. These results suggest the existence of an intricate relationship between GA-signalling, dormancy, and longevity.
Lastly, another study with Arabidopsis mutants defective on gibberellins synthesis and signalling (GA-deficient ga1-3 and GA-insensitive gai) reports that the role of GA in longevity appeared to be inconclusive [154]. Therefore, the relationship between GA signalling and seed longevity needs more investigation. The effects on seed longevity of genes associated with GAs studied in different plant species are summarized in Table 1 and Figure 2C.


Table 1.
Phytohormone-related genes and their effects on seed longevity.
Table 1.
Phytohormone-related genes and their effects on seed longevity.
| Gene | Longevity Regulator | Related Phytohor-Mone | Species | Ageing Conditions of Study * | Reference |
|---|---|---|---|---|---|
| ABI3 | Positive | ABA | Arabidopsis thaliana | Wet | Sugliani et al. [119]; Clerkx et al. [154] |
| Positive | Solanum lycopersicum | Dry | Livne et al. [201] | ||
| ABI4 | Positive | ABA | Medicago truncatula | Wet, Dry, and Intermediate | Zinsmeister et al. [170] |
| ABI5 | Positive | ABA | Medicago truncatula | Dry and Intermediate | Zinsmeister et al. [171] |
| LEC1 | Positive | ABA | Arabidopsis thaliana | Wet | Sugliani et al. [119] |
| FUS3 | Positive | ABA | Solanum lycopersicum | Dry | Livne et al. [201] |
| ABA1 | Positive | ABA | Arabidopsis thaliana | Wet | Clerkx et al. [154] |
| HSFA9 | Positive | ABA/IAA | Arabidopsis thaliana | Wet | Zinsmeister et al. [99] |
| No effect | Intermediate | ||||
| Positive | Medicago truncatula | Wet | |||
| No effect | Intermediate | ||||
| Positive | Helianthus annuus | Dry | Carranco et al. [156] | ||
| DREB2B | Negative | ABA/IAA | Arabidopsis thaliana | Wet | Ali et al. [178] |
| Negative | Gossypium hirsutum | Wet | Ali et al. [178] | ||
| Positive | Helianthus annuus | Dry | Carranco et al. [156] | ||
| Positive | Zea mays | Wet | Han et al. [190] | ||
| NYC1 | Positive | ABA | Arabidopsis thaliana | Dry | Nakajima et al. [166] |
| TIP3 | Positive | ABA | Arabidopsis thaliana | Wet | Mao and Sun [152] |
| RSL1 | Positive | ABA | Arabidopsis thaliana | Wet and Dry | Bueso et al. [176,177] |
| WHY | Positive | ABA | Arabidopsis thaliana | Wet | Taylor et al. [182]; Isemer et al. [183] |
| IAA27 | Negative | IAA | Helianthus annuus | Dry | Carranco et al. [156] |
| GH3-2 | Negative | IAA | Oryza sativa (rice) | Wet and Dry | Yuan et al. [189] |
| ATHB25 | Positive | GA | Arabidopsis thaliana | Wet | Bueso et al. [98] |
| ATHB22 | Positive | GA | Arabidopsis thaliana | Wet | Bueso et al. [98] |
| ATHB31 | Positive | GA | Arabidopsis thaliana | Wet | Bueso et al. [98] |
| COG1 | Positive | GA | Arabidopsis thaliana | Wet | Bueso et al. [203] |
| CDF4 | Positive | GA | Arabidopsis thaliana | Wet | Bueso et al. [203] |
| LE25 | Positive | GA | Solanum lycopersicum | Dry | Livne et al. [201] |
| GOLS1 | Positive | GA | Solanum lycopersicum | Dry | Livne et al. [201] |
| DOG1 SlDOG1-2 | Positive | ABA/GA | Arabidopsis thaliana | Dry | Benksin et al. [173] |
| Solanum lycopersicum | Intermediate | Bizouerne et al. [148] | |||
| PROCERA | Positive | GA | Solanum lycopersicum | Intermediate | Bizouerne et al. [148] |
* Ageing conditions of study: wet ageing (wet; >80% RH); intermediate ageing (intermediate; 75% RH; 30–35 °C); and dry ageing (dry; <70% RH; 25 to −20 °C).
5.4. Brassinosteroids and Other Hormones
Brassinosteroids (BRs) are ubiquitously distributed across different plant organs and tissues, with significantly higher concentrations observed in seeds, pollen, and fruits [205]. These compounds play a multifaceted role in plant biology, impacting various aspects of growth and development, such as seed morphology and germination, and stress responses [206,207,208]. Recent investigations have unveiled their potential negative regulation of seed longevity through a technique known as priming. Priming involves controlled seed imbibition and subsequent drying treatments typically used to improve germination performance. However, the treatment sometimes reduces seed longevity, a side effect attributed to the increase in seed coat permeability. However, priming of BR-deficient mutants (cyp85a1/a2 and det2) resulted in significantly extended seed longevity [209,210]. This longevity extension in mutant seed was attributed, at least in part, to a reduction of seed coat permeability after priming, in agreement with the known positive effect of BR-signalling regulation on this process. Since BRs are hormones that regulate common physiological responses to those regulated by GAs [211,212], it would be interesting to study their possible antagonistic regulation on seed coat development.
Apart from BRs, there is little documented evidence on the role of other hormones in seed longevity. It is worth noting that mutant seeds that are ethylene-resistant1 (etr1) and jasmonic acid-resistant1 (jar1-1) have exhibited no significant loss in viability even after prolonged dry storage periods of up to four years [154], suggesting that these hormones, even being involved in seed germination [213], may not have a relevant role in ageing. Despite the growing body of research in recent years, the dearth of knowledge in the area of seed longevity and its regulation by hormones still presents a substantial scope for exploration and discovery.
6. Conclusions
Research on seed longevity has provided important components with roles in several cases conserved across species. However, the intricate interplay between genetics and environmental factors underlying the multifaceted nature of seed longevity requires a profound analysis of the connections between different gene regulatory networks. In addition, since seed ageing is particularly prone to variations depending on experimental set ups, it is advisable to test different storage conditions for a robust assessment of the regulatory breadth of the identified gene. Recent studies have shed light on the role of hormone signalling in the acquisition of longevity and ageing mechanisms of seeds, with a particular focus on the ABA signalling pathway, which has emerged as a significant positive regulator of seed longevity. An epistatic relationship between auxin and ABA in promoting seed longevity has been found, although it is important to consider that IAA can act as a negative regulator when interconnected pathways simultaneously reduce raffinose synthesis. Notably, despite the typical antagonistic relationship between GAs and ABA, various species have exhibited positive effects of GAs on seed longevity. Also, while GAs and BRs have been implicated in seed coat development, potentially influencing seed longevity, further research is needed to provide a comprehensive understanding of their roles. Future research directions in seed longevity will benefit by considering a global and integrated perspective on the various aspects exposed in this review, such as experimental design, species-specific traits related to seed structure and composition, and hormone metabolism and signalling.
Author Contributions
Conceptualization, S.M.; Writing—original draft, M.P. and S.M.; Writing—review & editing, L.O.-S. and S.M.; Visualization, I.F.-P.; Supervision, L.O.-S. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported with grants to LO-S (PID2019-109154RB-I00 and PID2022-142059OB-I00) both funded by MICIN/AEI/10.13039/501100011033 (Spain). M.P. is supported by a postdoctoral contract (Margarita Salas) of the Universidad Politécnica de Madrid funded by the Spanish Universities Ministry, and IF-P is supported by a PhD contract (FPI) funded by grant SEV-2016-0672 to the CBGP (Centre of Excellence Severo Ochoa Program of the Agencia Estatal de Investigación, Spain).
Data Availability Statement
Not applicable.
Acknowledgments
We thank G. Carrera-Castaño for critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khoury, C.; Brush, S.; Costich, D.; Curry, H.; Haan, S.; Engels, J.; Guarino, L.; Hoban, S.; Mercer, K.; Miller, A.; et al. Crop Genetic Erosion: Understanding and Responding to Loss of Crop Diversity. New Phytol. 2022, 233, 84–118. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, J.; Pilling, D. The State of the World’s Biodiversity for Food and Agriculture; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2019; ISBN 92-5-131270-2. [Google Scholar]
- Walters, C. Understanding the Mechanisms and Kinetics of Seed Aging. Seed Sci. Res. 1998, 8, 223–244. [Google Scholar] [CrossRef]
- Groot, S.P.C.; de Groot, L.; Kodde, J.; van Treuren, R. Prolonging the Longevity of Ex Situ Conserved Seeds by Storage under Anoxia. Plant Genet. Resour. Charact. Util. 2015, 13, 18–26. [Google Scholar] [CrossRef]
- Walters, C.; Hill, L.M.; Wheeler, L.J. Dying While Dry: Kinetics and Mechanisms of Deterioration in Desiccated Organisms. Integr. Comp. Biol. 2005, 45, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Nadarajan, J.; Walters, C.; Pritchard, H.W.; Ballesteros, D.; Colville, L. Seed Longevity-the Evolution of Knowledge and a Conceptual Framework. Plants 2023, 12, 471. [Google Scholar] [CrossRef] [PubMed]
- Hay, F.R.; Rezaei, S.; Buitink, J. Seed Moisture Isotherms, Sorption Models, and Longevity. Front. Plant Sci. 2022, 13, 891913. [Google Scholar] [CrossRef] [PubMed]
- Gerna, D.; Ballesteros, D.; Arc, E.; Stöggl, W.; Seal, C.E.; Marami-Zonouz, N.; Na, C.S.; Kranner, I.; Roach, T. Does Oxygen Affect Ageing Mechanisms of Pinus Densiflora Seeds? A Matter of Cytoplasmic Physical State. J. Exp. Bot. 2022, 73, 2631–2649. [Google Scholar] [CrossRef]
- Roberts, E.H. Predicting the Storage Life of Seeds. Seed Sci. Technol. 1973, 1, 499–514. [Google Scholar]
- Ellis, R.H.; Hong, T.D.; Roberts, E.H. An Intermediate Category of Seed Storage Behavior 1. Coffee. J. Exp. Bot. 1990, 41, 1167–1174. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H. Seeds: Physiology of Development, Germination and Dormancy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 1-4614-4692-9. [Google Scholar]
- Pammenter, N.W.; Berjak, P. Evolutionary and Ecological Aspects of Recalcitrant Seed Biology. Seed Sci. Res. 2000, 10, 301–306. [Google Scholar] [CrossRef]
- Pammenter, N.W.; Berjak, P. A Review of Recalcitrant Seed Physiology in Relation to Desiccation-Tolerance Mechanisms. Seed Sci. Res. 1999, 9, 13–37. [Google Scholar] [CrossRef]
- Dickie, J.B.; Pritchard, H.W. Systematic and Evolutionary Aspects of Desiccation Tolerance in Seeds. In Desiccation and Survival in Plants: Drying without Dying; CAB International: Wallingford, UK, 2002; pp. 239–259. [Google Scholar]
- Walters, C. Orthodoxy, Recalcitrance and in-between: Describing Variation in Seed Storage Characteristics Using Threshold Responses to Water Loss. Planta 2015, 242, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Dickie, J.B.; Ellis, R.H.; Kraak, H.L.; Ryder, K.; Tompsett, P.B. Temperature and Seed Storage Longevity. Ann. Bot. 1990, 65, 197–204. [Google Scholar] [CrossRef]
- Ballesteros, D.; Walters, C. Detailed Characterization of Mechanical Properties and Molecular Mobility within Dry Seed Glasses: Relevance to the Physiology of Dry Biological Systems. Plant J. 2011, 68, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; Leprince, O. Glass Formation in Plant Anhydrobiotes: Survival in the Dry State. Cryobiology 2004, 48, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Buitink, J.; Leprince, O. Intracellular Glasses and Seed Survival in the Dry State. C. R. Biol. 2008, 331, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Q. Glassy State and Seed Storage Stability: The WLF Kinetics of Seed Viability Loss at T > Tg and the Plasticization Effect of Water on Storage Stability. Ann. Bot. 1997, 79, 291–297. [Google Scholar] [CrossRef]
- Walters, C. Temperature Dependency of Molecular Mobility in Preserved Seeds. Biophys. J. 2004, 86, 1253–1258. [Google Scholar] [CrossRef]
- Toole, V.K. Ancient Seeds; Seed Longevity. J. Seed Technol. 1986, 10, 1–23. [Google Scholar]
- Roos, E.E. Precepts of Successful Seed Storage. Physiol. Seed Deterioration 1986, 11, 1–25. [Google Scholar]
- Roos, E.; Davidson, D. Record Longevities of Vegetable Seeds in Storage. HortScience 1992, 27, 393–396. [Google Scholar] [CrossRef]
- Probert, R.; Daws, M.; Hay, F. Ecological Correlates of Ex Situ Seed Longevity: A Comparative Study on 195 Species. Ann. Bot. 2009, 104, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.; Wheeler, L.J.; Grotenhuis, J.M. Longevity of Seeds Stored in a Genebank: Species Characteristics. Seed Sci. Res. 2005, 15, 1–20. [Google Scholar] [CrossRef]
- Colville, L.; Pritchard, H.W. Seed Life Span and Food Security. New Phytol. 2019, 224, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and Environmental Factors Regulating Seed Longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, F.; González-Benito, M.E.; Gómez-Campo, C. Germination of Fourteen Endemic Species from the Iberian Peninsula, Canary and Balearic Islands after 32-34 Years of Storage at Low Temperature and Very Low Water Content. Seed Sci. Technol. 2008, 36, 407–422. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Mazuy, C.; Corbineau, F.; Bailly, C. DNA Alteration and Programmed Cell Death during Ageing of Sunflower Seed. J. Exp. Bot. 2011, 62, 5003–5011. [Google Scholar] [CrossRef]
- Kibinza, S.; Vinel, D.; Côme, D.; Bailly, C.; Corbineau, F. Sunflower Seed Deterioration as Related to Moisture Content during Ageing, Energy Metabolism and Active Oxygen Species Scavenging. Physiol. Plant. 2006, 128, 496–506. [Google Scholar] [CrossRef]
- Mira, S.; Hill, L.M.; Gonzalez-Benito, M.E.; Ibanez, M.A.; Walters, C. Volatile Emission in Dry Seeds as a Way to Probe Chemical Reactions during Initial Asymptomatic Deterioration. J. Exp. Bot. 2016, 67, 1783–1793. [Google Scholar] [CrossRef]
- Mira, S.; González-Benito, M.E.; Hill, L.M.; Walters, C. Characterization of Volatile Production during Storage of Lettuce (Lactuca sativa) Seeds. J. Exp. Bot. 2010, 61, 3915–3924. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Bray, C.M.; West, C.E. The Importance of Safeguarding Genome Integrity in Germination and Seed Longevity. J. Exp. Bot. 2015, 66, 3549–3558. [Google Scholar] [CrossRef]
- Fu, Y.B.; Ahmed, Z.; Diederichsen, A. Towards a Better Monitoring of Seed Ageing under Ex Situ Seed Conservation. Conserv. Physiol. 2015, 3, cov026. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Huo, H.; Mao, P. Antioxidant Response and Related Gene Expression in Aged Oat Seed. Front. Plant Sci. 2015, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Lovigny, Y.; Groot, S.P.C.; Belghaz, M.; Job, C.; Job, D. Proteome-Wide Characterization of Seed Aging in Arabidopsis: A Comparison between Artificial and Natural Aging Protocols. Plant Physiol. 2008, 148, 620–641. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Niñoles, R.; Martínez-Almonacid, I.; Gayubas, B.; Mateos-Fernández, R.; Bissoli, G.; Bueso, E.; Serrano, R.; Gadea, J. Identification of Novel Seed Longevity Genes Related to Oxidative Stress and Seed Coat by Genome-wide Association Studies and Reverse Genetics. Plant Cell Environ. 2020, 43, 2523–2539. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Minibayeva, F.V.; Beckett, R.P.; Seal, C.E. What Is Stress? Concepts, Definitions and Applications in Seed Science. New Phytol. 2010, 188, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The Roles of Reactive Oxygen Metabolism in Drought: Not so Cut and Dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Kurek, K.; Plitta-Michalak, B.; Ratajczak, E. Reactive Oxygen Species as Potential Drivers of the Seed Aging Process. Plants 2019, 8, 174. [Google Scholar] [CrossRef]
- Bailly, C. Active Oxygen Species and Antioxidants in Seed Biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Law, S.R.; Narsai, R.; Whelan, J. Mitochondrial Biogenesis in Plants during Seed Germination. Mitochondrion 2014, 19, 214–221. [Google Scholar] [CrossRef]
- Bailly, C.; El-Maarouf-Bouteau, H.; Corbineau, F. From Intracellular Signaling Networks to Cell Death: The Dual Role of Reactive Oxygen Species in Seed Physiology. C. R. Biol. 2008, 331, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Niñoles, R.; Planes, D.; Arjona, P.; Ruiz-Pastor, C.; Chazarra, R.; Renard, J.; Bueso, E.; Forment, J.; Serrano, R.; Kranner, I.; et al. Comparative Analysis of Wild-type Accessions Reveals Novel Determinants of Arabidopsis Seed Longevity. Plant Cell Environ. 2022, 45, 2708–2728. [Google Scholar] [CrossRef] [PubMed]
- Lehner, A.; Mamadou, N.; Poels, P.; Côme, D.; Bailly, C.; Corbineau, F. Changes in Soluble Carbohydrates, Lipid Peroxidation and Antioxidant Enzyme Activities in the Embryo during Ageing in Wheat Grains. J. Cereal Sci. 2008, 47, 555–565. [Google Scholar] [CrossRef]
- Merritt, D.J.; Senaratna, T.; Touchell, D.H.; Dixon, K.W.; Sivasithamparam, K. Seed Ageing of Four Western Australian Species in Relation to Storage Environment and Seed Antioxidant Activity. Seed Sci. Res. 2003, 13, 155–165. [Google Scholar] [CrossRef]
- Mira, S.; Estrelles, E.; Gonzalez-Benito, M.E.; Corbineau, F. Biochemical Changes Induced in Seeds of Brassicaceae Wild Species during Ageing. Acta Physiol. Plant. 2011, 33, 1803–1809. [Google Scholar] [CrossRef]
- Pamplona, R. Membrane Phospholipids, Lipoxidative Damage and Molecular Integrity: A Causal Role in Aging and Longevity. Biochim. Biophys. Acta BBA Bioenerg. 2008, 1777, 1249–1262. [Google Scholar] [CrossRef] [PubMed]
- Mansouri-Far, C.; Goodarzian-Ghahfarokhi, M.; Saeidi, M.; Abdoli, M. Antioxidant Enzyme Activity and Germination Characteristics of Different Maize Hybrid Seeds during Ageing. Environ. Exp. Biol. 2015, 13, 177–182. [Google Scholar]
- McDonald, M.B. Seed Deterioration: Physiology, Repair and Assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Bailly, C.; Benamar, A.; Corbineau, F.; Côme, D. Changes in Malondialdehyde Content and in Superoxide Dismutase, Catalase and Glutathione Reductase Activities in Sunflower Seeds as Related to Deterioration during Accelerated Aging. Physiol. Plant 1996, 97, 104–110. [Google Scholar] [CrossRef]
- Lazar, S.L.; Mira, S.; Pamfil, D.; Martinez-Laborde, J.B. Germination and Electrical Conductivity Tests on Artificially Aged Seed Lots of 2 Wall-Rocket Species. Turk. J. Agric. For. 2014, 38, 857–864. [Google Scholar] [CrossRef]
- Mira, S.; Veiga-Barbosa, L.; Pérez-García, F. Seed Dormancy and Longevity Variability of Hirschfeldia incana L. during Storage. Seed Sci. Res. 2019, 29, 97–103. [Google Scholar] [CrossRef]
- Priestley, D.A. Seed Aging. In Implications of Seed Storage and Persistence in the Soil; Comstock Publishing: Ithaca, NY, USA; London, UK, 1986; ISBN 0-8014-1865-8. [Google Scholar]
- Hsu, F.-H.; Lin, J.-B.; Chang, S.-R. Effects of Waterlogging on Seed Germination, Electric Conductivity of Seed Leakage and Developments of Hypocotyl and Radicle in Sudangrass. Bot. Bull. Acad. Sin. 2000, 41, 267–273. [Google Scholar]
- Milošević, M.; Vujaković, M.; Karagić, Đ. Vigour Tests as Indicators of Seed Viability. Genetika 2010, 42, 103–118. [Google Scholar] [CrossRef]
- Al-Maskri, A.; Khan, M.; Khan, I.; Al-Habsi, K. Effect of Accelerated Ageing on Viability, Vigor (RGR), Lipid Peroxidation and Leakage in Carrot (Daucus carota L.) Seeds. Int. J. Agric. Biol. 2003, 5, 580–584. [Google Scholar]
- Matthews, S.; Powell, A. Electrical Conductivity Vigour Test: Physiological Basis and Use. Seed Test. Int. 2006, 131, 32–35. [Google Scholar]
- Mira, S.; Veiga-Barbosa, L.; Gonzalez-Benito, M.E.; Perez-Garcia, F. Conductivity Test in Seeds of Different Passion Flower Species. Pesqui. Agropecu. Bras. 2015, 50, 510–513. [Google Scholar] [CrossRef]
- Mira, S.; Estrelles, E.; Gonzalez-Benito, M.E. Effect of Water Content and Temperature on Seed Longevity of Seven Brassicaceae Species after 5 Years of Storage. Plant Biol. 2015, 17, 153–162. [Google Scholar] [CrossRef]
- Tuteja, N.; Singh, M.B.; Misra, M.K.; Bhalla, P.L.; Tuteja, R. Molecular Mechanisms of DNA Damage and Repair: Progress in Plants. Crit. Rev. Biochem. Mol. Biol. 2001, 36, 337–397. [Google Scholar] [CrossRef]
- Navashin, N.; Shkvarnikov, P. Process of Mutation in Resting Seeds Accelerated by Increased Temperature. Nature 1933, 132, 482–483. [Google Scholar] [CrossRef]
- Roberts, E. Mutations during Seed Storage; Oxford Academy: Oxford, UK, 1977; pp. 279–282. [Google Scholar]
- Osborne, D.J. Hazards of a Germinating Seed: Available Water and the Maintenance of Genomic Integrity. Isr. J. Plant Sci. 2000, 48, 173–179. [Google Scholar] [CrossRef]
- Kranner, I.; Chen, H.Y.; Pritchard, H.W.; Pearce, S.R.; Birtic, S. Inter-Nucleosomal DNA Fragmentation and Loss of RNA Integrity during Seed Ageing. Plant Growth Regul. 2011, 63, 63–72. [Google Scholar] [CrossRef]
- Dona, M.; Balestrazzi, A.; Mondoni, A.; Rossi, G.; Ventura, L.; Buttafava, A.; Macovei, A.; Sabatini, M.E.; Valassi, A.; Carbonera, D. DNA Profiling, Telomere Analysis and Antioxidant Properties as Tools for Monitoring Ex Situ Seed Longevity. Ann. Bot. 2013, 111, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, W.M.; West, C.E. Genome Damage Accumulated in Seed Ageing Leads to Plant Genome Instability and Growth Inhibition. Biochem. J. 2023, 480, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, W.M.; Latham, R.; Wang, D.; Alsharif, M.; West, C.E. Seed DNA Damage Responses Promote Germination and Growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2022, 119, e2202172119. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, W.M.; Bray, C.M.; West, C.E. Seeds and the Art of Genome Maintenance. Front. Plant Sci. 2019, 10, 706. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, W.M.; Masnavi, G.; Bhardwaj, R.M.; Jiang, Q.; Bray, C.M.; West, C.E. A Plant DNA Ligase Is an Important Determinant of Seed Longevity. Plant J. 2010, 63, 848–860. [Google Scholar] [CrossRef]
- Waterworth, W.M.; Drury, G.E.; Bray, C.M.; West, C.E. Repairing Breaks in the Plant Genome: The Importance of Keeping It Together. New Phytol. 2011, 192, 805–822. [Google Scholar] [CrossRef]
- Fleming, M.B.; Patterson, E.L.; Reeves, P.A.; Richards, C.M.; Gaines, T.A.; Walters, C. Exploring the Fate of mRNA in Aging Seeds: Protection, Destruction, or Slow Decay? J. Exp. Bot. 2018, 69, 4309–4321. [Google Scholar] [CrossRef]
- Fleming, M.B.; Richards, C.M.; Walters, C. Decline in RNA Integrity of Dry-Stored Soybean Seeds Correlates with Loss of Germination Potential. J. Exp. Bot. 2017, 68, 2219–2230. [Google Scholar] [CrossRef]
- Fleming, M.B.; Hill, L.M.; Walters, C. The Kinetics of Ageing in Dry-Stored Seeds: A Comparison of Viability Loss and RNA Degradation in Unique Legacy Seed Collections. Ann. Bot. 2019, 123, 1133–1146. [Google Scholar] [CrossRef]
- González-Benito, M.E.; Ibáñez, M.Á.; Pirredda, M.; Mira, S.; Martín, C. Application of the MSAP Technique to Evaluate Epigenetic Changes in Plant Conservation. Int. J. Mol. Sci. 2020, 21, 7459. [Google Scholar] [CrossRef] [PubMed]
- Plitta, B.P.; Michalak, M.; Bujarska-Borkowska, B.; Barciszewska, M.Z.; Barciszewski, J.; Chmielarz, P. Effect of Desiccation on the Dynamics of Genome-Wide DNA Methylation in Orthodox Seeds of Acer platanoides L. Plant Physiol. Biochem. 2014, 85, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Plitta-Michalak, B.P.; Naskret-Barciszewska, M.Z.; Kotlarski, S.; Tomaszewski, D.; Tylkowski, T.; Barciszewski, J.; Chmielarz, P.; Michalak, M. Changes in Genomic 5-Methylcytosine Level Mirror the Response of Orthodox (Acer platanoides L.) and Recalcitrant (Acer pseudoplatanus L.) Seeds to Severe Desiccation. Tree Physiol. 2018, 38, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Barciszewska, M.Z.; Barciszewski, J.; Plitta, B.P.; Chmielarz, P. Global Changes in DNA Methylation in Seeds and Seedlings of Pyrus Communis after Seed Desiccation and Storage. PLoS ONE 2013, 8, e70693. [Google Scholar] [CrossRef] [PubMed]
- Pirredda, M.; González-Benito, M.E.; Martín, C.; Mira, S. Genetic and Epigenetic Stability in Rye Seeds under Different Storage Conditions: Ageing and Oxygen Effect. Plants 2020, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Mira, S.; Pirredda, M.; Martín-Sánchez, M.; Marchessi, J.E.; Martín, C. DNA Methylation and Integrity in Aged Seeds and Regenerated Plants. Seed Sci. Res. 2020, 30, 92–100. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. Improved Equations for the Prediction of Seed Longevity. Ann. Bot. 1980, 45, 13–30. [Google Scholar] [CrossRef]
- Ellis, R.H.; Hong, T.D. Seed Longevity—Moisture Content Relationships in Hermetic and Open Storage. Seed Sci. Technol. 2007, 35, 423–431. [Google Scholar] [CrossRef]
- Walters, C. Water Properties and Cell Longevity. In Water Properties of Food, Pharmaceutical and Biological Materials; del Pilar Buera, M., Welti-Chanes, J., Lillford, P.J., Corti, H.R., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 191–204. [Google Scholar]
- Walters, C.; Ballesteros, D.; Vertucci, V.A. Structural Mechanics of Seed Deterioration: Standing the Test of Time. Plant Sci. 2010, 179, 565–573. [Google Scholar] [CrossRef]
- Roberts, E.H.; Ellis, R.H. Water and Seed Survival. Ann. Bot. 1989, 63, 39–52. [Google Scholar] [CrossRef]
- Leopold, A.C.; Vertucci, C.W. Moisture as a Regulator of Physiological Reactions in Seeds. In Seed Moisture; Stanwood, P.C., McDonald, M.B., Eds.; CSSA Special Publication Number 14; Wiley Online Library: Hoboken, NJ, USA, 1989; pp. 51–69. [Google Scholar]
- Vertucci, C.W.; Roos, E.E. Theoretical Basis of Protocols for Seed Storage. Plant Physiol. 1990, 94, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Vertucci, C.W.; Roos, E.E.; Crane, J. Theoretical Basis of Protocols for Seed Storage. III. Optimum Moisture Contents for Pea Seeds Stored at Different Temperatures. Ann. Bot. 1994, 74, 531–540. [Google Scholar] [CrossRef]
- Hay, F.R.; Valdez, R.; Lee, J.-S.; Sta. Cruz, P.C. Seed Longevity Phenotyping: Recommendations on Research Methodology. J. Exp. Bot. 2018, 70, 425–434. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association. Seed Vigour Testing. In International Rules for Seed Testing; ISTA: Bassersdorf, Switzerland, 2018. [Google Scholar]
- Gómez-Campo, C. Long Term Seed Preservation: The Risk of Selecting Inadequate Containers Is Very High. Monogr. ETSIA Univ. Politéc. Madr. 2002, 163, 1–10. [Google Scholar]
- Barzali, M.; Lohwasser, U.; Niedzielski, M.; Borner, A. Effects of Different Temperatures and Atmospheres on Seed and Seedling Traits in a Long-Term Storage Experiment on Rye (Secale cereale L.). Seed Sci. Technol. 2005, 33, 713–721. [Google Scholar] [CrossRef]
- Gonzalez-Benito, M.E.; Perez-Garcia, F.; Tejeda, G.; Gomez-Campo, C. Effect of the Gaseous Environment and Water Content on Seed Viability of Four Brassicaceae Species after 36 Years Storage. Seed Sci. Technol. 2011, 39, 443–451. [Google Scholar] [CrossRef]
- Tompsett, P.B. The Influence of Gaseous Environment on the Storage Life of Araucaria hunsteinii Seed. Ann. Bot. 1983, 52, 229–237. [Google Scholar] [CrossRef]
- Groot, S.P.C.; Surki, A.A.; de Vos, R.C.H.; Kodde, J. Seed Storage at Elevated Partial Pressure of Oxygen, a Fast Method for Analysing Seed Ageing under Dry Conditions. Ann. Bot. 2012, 110, 1149–1159. [Google Scholar] [CrossRef]
- Bueso, E.; Munoz-Bertomeu, J.; Campos, F.; Brunaud, V.; Martinez, L.; Sayas, E.; Ballester, P.; Yenush, L.; Serrano, R. Arabidopsis thaliana Homeobox25 Uncovers a Role for Gibberellins in Seed Longevity. Plant Physiol. 2014, 164, 999–1010. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Berriri, S.; Basso, D.P.; Ly-Vu, B.; Dang, T.; Lalanne, D.; Da Silva, E.A.A.; Leprince, O.; Buitink, J. The Seed-specific Heat Shock Factor A9 Regulates the Depth of Dormancy in Medicago truncatula ABA Signalling. Plant Cell Environ. 2020, 43, 2508–2522. [Google Scholar] [CrossRef]
- Hay, F.R.; Mead, A.; Manger, K.; Wilson, F.J. One-Step Analysis of Seed Storage Data and the Longevity of Arabidopsis thaliana Seeds. J. Exp. Bot. 2003, 54, 993–1011. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, J.; Steadman, K.J.; Probert, R.J.; Adkins, S.W. Parental Effects Modulate Seed Longevity: Exploring Parental and Offspring Phenotypes to Elucidate Pre-Zygotic Environmental Influences. New Phytol. 2011, 191, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Mondoni, A.; Probert, R.J.; Rossi, G.; Vegini, E.; Hay, F.R. Seeds of Alpine Plants Are Short Lived: Implications for Long-Term Conservation. Ann. Bot. 2011, 107, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Mondoni, A.; Orsenigo, S.; Dona, M.; Balestrazzi, A.; Probert, R.J.; Hay, F.R.; Petraglia, A.; Abeli, T. Environmentally Induced Transgenerational Changes in Seed Longevity: Maternal and Genetic Influence. Ann. Bot. 2014, 113, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Probert, R.; Adams, J.; Coneybeer, J.; Crawford, A.; Hay, F. Seed Quality for Conservation Is Critically Affected by Pre-Storage Factors. Aust. J. Bot. 2007, 55, 326–335. [Google Scholar] [CrossRef]
- Sano, N.; Rajjou, L.; North, H.M.; Debeaujon, I.; Marion-Poll, A.; Seo, M. Staying Alive: Molecular Aspects of Seed Longevity. Plant Cell Physiol. 2016, 57, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Schwember, A.R.; Bradford, K.J. Quantitative Trait Loci Associated with Longevity of Lettuce Seeds under Conventional and Controlled Deterioration Storage Conditions. J. Exp. Bot. 2010, 61, 4423–4436. [Google Scholar] [CrossRef]
- Hay, F.R.; Klin, J.; Probert, R.J. Can a Post-Harvest Ripening Treatment Extend the Longevity of Rhododendron L. Seeds? Sci. Hortic. 2006, 111, 80–83. [Google Scholar] [CrossRef]
- Niedzielski, M.; Walters, C.; Luczak, W.; Hill, L.M.; Wheeler, L.J.; Puchalski, J. Assessment of Variation in Seed Longevity within Rye, Wheat and the Intergeneric Hybrid Triticale. Seed Sci. Res. 2009, 19, 213–224. [Google Scholar] [CrossRef]
- Guzzon, F.; Gianella, M.; Velazquez Juarez, J.A.; Sanchez Cano, C.; Costich, D.E. Seed Longevity of Maize Conserved under Germplasm Bank Conditions for up to 60 Years. Ann. Bot. 2021, 127, 775–785. [Google Scholar] [CrossRef]
- Gianella, M.; Doria, E.; Dondi, D.; Milanese, C.; Gallotti, L.; Börner, A.; Zannino, L.; Macovei, A.; Pagano, A.; Guzzon, F.; et al. Physiological and Molecular Aspects of Seed Longevity: Exploring Intra-Species Variation in Eight Pisum sativum L. Accessions. Physiol. Plant. 2022, 174, e13698. [Google Scholar] [CrossRef] [PubMed]
- Guzzon, F.; Orsenigo, S.; Gianella, M.; Muller, J.V.; Vagge, I.; Rossi, G.; Mondoni, A. Seed Heteromorphy Influences Seed Longevity in Aegilops. Seed Sci. Res. 2018, 28, 277–285. [Google Scholar] [CrossRef]
- Gianella, M.; Balestrazzi, A.; Ravasio, A.; Mondoni, A.; Börner, A.; Guzzon, F. Comparative Seed Longevity under Genebank Storage and Artificial Ageing: A Case Study in Heteromorphic Wheat Wild Relatives. Plant Biol. 2022, 24, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Mira, S.; Nadarajan, J.; Liu, U.; González-Benito, M.E.; Pritchard, H.W. Lipid Thermal Fingerprints of Long-Term Stored Seeds of Brassicaceae. Plants 2019, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Pereira Lima, J.J.; Buitink, J.; Lalanne, D.; Rossi, R.F.; Pelletier, S.; Da Silva, E.A.A.; Leprince, O. Molecular Characterization of the Acquisition of Longevity during Seed Maturation in Soybean. PLoS ONE 2017, 12, e0180282. [Google Scholar] [CrossRef] [PubMed]
- Righetti, K.; Vu, J.L.; Pelletier, S.; Vu, B.L.; Glaab, E.; Lalanne, D.; Pasha, A.; Patel, R.V.; Provart, N.J.; Verdier, J.; et al. Inference of Longevity-Related Genes from a Robust Coexpression Network of Seed Maturation Identifies Regulators Linking Seed Storability to Biotic Defense-Related Pathways. Plant Cell 2015, 27, 2692–2708. [Google Scholar] [CrossRef]
- Verdier, J.; Lalanne, D.; Pelletier, S.; Torres-Jerez, I.; Righetti, K.; Bandyopadhyay, K.; Leprince, O.; Chatelain, E.; Vu, B.L.; Gouzy, J.; et al. A Regulatory Network-Based Approach Dissects Late Maturation Processes Related to the Acquisition of Desiccation Tolerance and Longevity of Medicago truncatula Seeds. PLANT Physiol. 2013, 163, 757–774. [Google Scholar] [CrossRef]
- Holdsworth, M.J.; Bentsink, L.; Soppe, W.J.J. Molecular Networks Regulating Arabidopsis Seed Maturation, after-Ripening, Dormancy and Germination. New Phytol. 2008, 179, 33–54. [Google Scholar] [CrossRef]
- Buitink, J.; Hemminga, M.A.; Hoekstra, F.A. Is There a Role for Oligosaccharides in Seed Longevity? An Assessment of Intracellular Glass Stability. Plant Physiol. 2000, 122, 1217–1224. [Google Scholar] [CrossRef]
- Sugliani, M.; Rajjou, L.; Clerkx, E.J.M.; Koornneef, M.; Soppe, W.J.J. Natural Modifiers of Seed Longevity in the Arabidopsis Mutants Abscisic Acid Insensitive3-5 (Abi3-5) and Leafy Cotyledon1-3 (Lec1-3). New Phytol. 2009, 184, 898–908. [Google Scholar] [CrossRef]
- Lepiniec, L.; Devic, M.; Roscoe, T.J.; Bouyer, D.; Zhou, D.-X.; Boulard, C.; Baud, S.; Dubreucq, B. Molecular and Epigenetic Regulations and Functions of the LAFL Transcriptional Regulators That Control Seed Development. Plant Reprod. 2018, 31, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, J.; Buckley, Y.M.; Probert, R.J.; Adkins, S.W.; Steadman, K.J. Pre-Zygotic Parental Environment Modulates Seed Longevity. Austral. Ecol. 2010, 35, 837–848. [Google Scholar] [CrossRef]
- He, H.Z.; Vidigal, D.D.; Snoek, L.B.; Schnabel, S.; Nijveen, H.; Hilhorst, H.; Bentsink, L. Interaction between Parental Environment and Genotype Affects Plant and Seed Performance in Arabidopsis. J. Exp. Bot. 2014, 65, 6603–6615. [Google Scholar] [CrossRef] [PubMed]
- Franzese, J.; Ghermandi, L. Seed Longevity and Fire: Germination Responses of an Exotic Perennial Herb in NW Patagonian Grasslands (Argentina). Plant Biol. 2011, 13, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Rajjou, L.; Debeaujon, I. Seed Longevity: Survival and Maintenance of High Germination Ability of Dry Seeds. C. R. Biol. 2008, 331, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Debeaujon, I.; Leon-Kloosterziel, K.M.; Koornneef, M. Influence of the Testa on Seed Dormancy, Germination, and Longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Bissoli, G.; Planes, M.D.; Gadea, J.; Naranjo, M.Á.; Serrano, R.; Ingram, G.; Bueso, E. Endosperm Persistence in Arabidopsis Results in Seed Coat Fractures and Loss of Seed Longevity. Plants 2023, 12, 2726. [Google Scholar] [CrossRef]
- Wang, C.; Lyu, Y.; Zhang, Q.; Guo, H.; Chen, D.; Chen, X. Disruption of BG14 Results in Enhanced Callose Deposition in Developing Seeds and Decreases Seed Longevity and Seed Dormancy in Arabidopsis. Plant J. 2023, 113, 1080–1094. [Google Scholar] [CrossRef]
- Buitink, J.; Leprince, O.; Hemminga, M.A.; Hoekstra, F.A. Molecular Mobility in the Cytoplasm: An Approach to Describe and Predict Lifespan of Dry Germplasm. Proc. Natl. Acad. Sci. USA 2000, 97, 2385–2390. [Google Scholar] [CrossRef]
- Walters, C.; Wheeler, L.J.; Stanwood, P.C. Longevity of Cryogenically Stored Seeds. Cryobiology 2004, 48, 229–244. [Google Scholar] [CrossRef]
- Bentsink, L.; Alonso-Blanco, C.; Vreugdenhil, D.; Tesnier, K.; Groot, S.P.C.; Koornneef, M. Genetic Analysis of Seed-Soluble Oligosaccharides in Relation to Seed Storability of Arabidopsis. Plant Physiol. 2000, 124, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Salvi, P.; Varshney, V.; Majee, M. Raffinose Family Oligosaccharides (RFOs): Role in Seed Vigor and Longevity. Biosci. Rep. 2022, 42, BSR20220198. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Hundertmark, M.; Leprince, O.; Gall, S.L.; Satour, P.; Deligny-Penninck, S.; Rogniaux, H.; Buitink, J. Temporal Profiling of the Heat-stable Proteome during Late Maturation of Medicago truncatula Seeds Identifies a Restricted Subset of Late Embryogenesis Abundant Proteins Associated with Longevity. Plant Cell Environ. 2012, 35, 1440–1455. [Google Scholar] [CrossRef] [PubMed]
- Hundertmark, M.; Buitink, J.; Leprince, O.; Hincha, D.K. The Reduction of Seed-Specific Dehydrins Reduces Seed Longevity in Arabidopsis thaliana. Seed Sci. Res. 2011, 21, 165–173. [Google Scholar] [CrossRef]
- Kalemba, E.; Pukacka, S. Possible Roles of LEA Proteins and sHSPs in Seed Protection: A Short Review. Biol. Lett. 2007, 44, 3–16. [Google Scholar]
- Leprince, O.; Pellizzaro, A.; Berriri, S.; Buitink, J. Late Seed Maturation: Drying without Dying. J. Exp. Bot. 2017, 68, 827–841. [Google Scholar] [CrossRef] [PubMed]
- Wakasa, Y.; Takaiwa, F. Seed Storage Proteins. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 346–348. ISBN 978-0-08-096156-9. [Google Scholar]
- Nguyen, P.T.; Cueff, G.; Hegedus, D.D.; Rajjou, L.; Bentsink, L. A Role for Seed Storage Proteins in Arabidopsis Seed Longevity. J. Exp. Bot. 2015, 66, 6399–6413. [Google Scholar] [CrossRef]
- Niñoles, R.; Ruiz-Pastor, C.M.; Arjona-Mudarra, P.; Casañ, J.; Renard, J.; Bueso, E.; Mateos, R.; Serrano, R.; Gadea, J. Transcription Factor DOF4.1 Regulates Seed Longevity in Arabidopsis via Seed Permeability and Modulation of Seed Storage Protein Accumulation. Front. Plant Sci. 2022, 13, 915184. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic Acid Signaling in Seeds and Seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant Hormone Interactions during Seed Dormancy Release and Germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Antagonistic Regulation of ABA and GA in Metabolism and Signaling Pathways. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Ooms, J.J.J.; Leon-Kloosterziel, K.M.; Bartels, D.; Koornneef, M.; Karssen, C.M. Acquisition of Desiccation Tolerance and Longevity in Seeds of Arabidopsis thaliana. Plant Physiol. 1993, 102, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated Role of ABA in Seed Maturation, Dormancy, and Germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Kermode, A.R. Role of Abscisic Acid in Seed Dormancy. J. Plant Growth Regul. 2005, 24, 319–344. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Neveu, M.; Lalanne, D.; Ly Vu, B.; Kanno, Y.; Seo, M.; Leprince, O.; Buitink, J. A Role for Auxin Signaling in the Acquisition of Longevity during Seed Maturation. New Phytol. 2020, 225, 284–296. [Google Scholar] [CrossRef]
- Busk, P.K.; Pagès, M. Regulation of Abscisic Acid-Induced Transcription. Plant Mol. Biol. 1998, 37, 425–435. [Google Scholar] [CrossRef]
- Bizouerne, E.; Buitink, J.; Vu, B.L.; Vu, J.L.; Esteban, E.; Pasha, A.; Provart, N.; Verdier, J.; Leprince, O. Gene Co-Expression Analysis of Tomato Seed Maturation Reveals Tissue-Specific Regulatory Networks and Hubs Associated with the Acquisition of Desiccation Tolerance and Seed Vigour. BMC Plant Biol. 2021, 21, 124. [Google Scholar] [CrossRef]
- Komatsu, K.; Takezawa, D.; Sakata, Y. Decoding ABA and Osmostress Signalling in Plants from an Evolutionary Point of View. Plant Cell Environ. 2020, 43, 2894–2911. [Google Scholar] [CrossRef]
- Fujii, H.; Chinnusamy, V.; Rodrigues, A.; Rubio, S.; Antoni, R.; Park, S.-Y.; Cutler, S.R.; Sheen, J.; Rodriguez, P.L.; Zhu, J.-K. In Vitro Reconstitution of an Abscisic Acid Signalling Pathway. Nature 2009, 462, 660–664. [Google Scholar] [CrossRef]
- Clerkx, E.J.M.; Blankestijn-De Vries, H.; Ruys, G.J.; Groot, S.P.C.; Koornneef, M. Characterization of Green Seed, an Enhancer of Abi3-1 in Arabidopsis That Affects Seed Longevity. Plant Physiol. 2003, 132, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Sun, W. Arabidopsis Seed-Specific Vacuolar Aquaporins Are Involved in Maintaining Seed Longevity under the Control of Abscisic Acid Insensitive 3. J. Exp. Bot. 2015, 66, 4781–4794. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.A.R.; Afzal, I.; Börner, A. Genetic Aspects and Molecular Causes of Seed Longevity in Plants—A Review. Plants 2022, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Clerkx, E.J.M.; Blankestijn-De Vries, H.; Ruys, G.J.; Groot, S.P.C.; Koornneef, M. Genetic Differences in Seed Longevity of Various Arabidopsis Mutants. Physiol. Plant 2004, 121, 448–461. [Google Scholar] [CrossRef]
- Tesnier, K.; Strookman-Donkers, H.M.; Van Pijlen, J.G.; Van der Geest, A.H.M.; Bino, R.J.; Groot, S.P.C. A Controlled Deterioration Test for Arabidopsis thaliana Reveals Genetic Variation in Seed Quality. Seed Sci. Technol. 2002, 30, 149–165. [Google Scholar]
- Carranco, R.; Espinosa, J.M.; Prieto-Dapena, P.; Almoguera, C.; Jordano, J. Repression by an Auxin/Indole Acetic Acid Protein Connects Auxin Signaling with Heat Shock Factor-Mediated Seed Longevity. Proc. Natl. Acad. Sci. USA 2010, 107, 21908–21913. [Google Scholar] [CrossRef] [PubMed]
- Carranco, R.; Prieto-Dapena, P.; Almoguera, C.; Jordano, J. SUMO-Dependent Synergism Involving Heat Shock Transcription Factors with Functions Linked to Seed Longevity and Desiccation Tolerance. Front. Plant Sci. 2017, 8, 974. [Google Scholar] [CrossRef]
- Tejedor-Cano, J.; Prieto-Dapena, P.; Almoguera, C.; Carranco, R.; Hiratsu, K.; Ohme-Takagi, M.; Jordano, J. Loss of Function of the HSFA9 Seed Longevity Program. Plant Cell Environ. 2010, 33, 1408–1417. [Google Scholar] [CrossRef]
- Personat, J.-M.; Tejedor-Cano, J.; Prieto-Dapena, P.; Almoguera, C.; Jordano, J. Co-Overexpression of Two Heat Shock Factors Results in Enhanced Seed Longevity and in Synergistic Effects on Seedling Tolerance to Severe Dehydration and Oxidative Stress. BMC Plant Biol. 2014, 14, 56. [Google Scholar] [CrossRef]
- Almoguera, C.; Rojas, A.; Diaz-Martin, J.; Prieto-Dapena, P.; Carranco, R.; Jordano, J. A Seed-Specific Heat-Shock Transcription Factor Involved in Developmental Regulation during Embryogenesis in Sunflower. J. Biol. Chem. 2002, 277, 43866–43872. [Google Scholar] [CrossRef]
- Prieto-Dapena, P.; Castaño, R.; Almoguera, C.; Jordano, J. Improved Resistance to Controlled Deterioration in Transgenic Seeds. Plant Physiol. 2006, 142, 1102–1112. [Google Scholar] [CrossRef]
- Prieto-Dapena, P.; Castaño, R.; Almoguera, C.; Jordano, J. The Ectopic Overexpression of a Seed-specific Transcription Factor, HaHSFA9, Confers Tolerance to Severe Dehydration in Vegetative Organs. Plant J. 2008, 54, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Kotak, S.; Vierling, E.; Bäumlein, H.; Koskull-Döring, P.V. A Novel Transcriptional Cascade Regulating Expression of Heat Stress Proteins during Seed Development of Arabidopsis. Plant Cell 2007, 19, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Parcy, F. The Abscisic Acid-Lnsensitive3, FUSCA3, and Leafy Cotyledonf Loci Act in Concert to Control Multiple Aspects of Arabidopsis Seed Development. Plant Cell 1997, 9, 1265–1277. [Google Scholar] [PubMed]
- Delmas, F.; Sankaranarayanan, S.; Deb, S.; Widdup, E.; Bournonville, C.; Bollier, N.; Northey, J.G.B.; McCourt, P.; Samuel, M.A. ABI3 Controls Embryo Degreening through Mendel’s I Locus. Proc. Natl. Acad. Sci. USA 2013, 110, E3888–E3894. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Ito, H.; Tanaka, R.; Tanaka, A. Chlorophyll b Reductase Plays an Essential Role in Maturation and Storability of Arabidopsis Seeds. Plant Physiol. 2012, 160, 261–273. [Google Scholar] [CrossRef]
- Dekkers, B.J.W.; He, H.Z.; Hanson, J.; Willems, L.A.J.; Jamar, D.C.L.; Cueff, G.; Rajjou, L.; Hilhorst, H.W.M.; Bentsink, L. The Arabidopsis Delay of Germination 1 Gene Affects Abscisic Acid Insensitive 5 (ABI5) Expression and Genetically Interacts with ABI3 during Arabidopsis Seed Development. Plant J. 2016, 85, 451–465. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic Acid: Emergence of a Core Signaling Network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Umezawa, T.; Nakashima, K.; Miyakawa, T.; Kuromori, T.; Tanokura, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Molecular Basis of the Core Regulatory Network in ABA Responses: Sensing, Signaling and Transport. Plant Cell Physiol. 2010, 51, 1821–1839. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Lalanne, D.; Ly Vu, B.; Schoefs, B.; Marchand, J.; Dang, T.T.; Buitink, J.; Leprince, O. Abscisic Acid Insensitive 4 Coordinates Eoplast Formation to Ensure Acquisition of Seed Longevity during Maturation in Medicago truncatula. Plant J. 2023, 113, 934–953. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Lalanne, D.; Terrasson, E.; Chatelain, E.; Vandecasteele, C.; Vu, B.L.; Dubois-Laurent, C.; Geoffriau, E.; Signor, C.L.; Dalmais, M. ABI5 Is a Regulator of Seed Maturation and Longevity in Legumes. Plant Cell 2016, 28, 2735–2754. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Blanco, C.; Bentsink, L.; Hanhart, C.J.; Vries, H.B.; Koornneef, M. Analysis of Natural Allelic Variation at Seed Dormancy Loci of Arabidopsis thaliana. Genetics 2003, 164, 711–729. [Google Scholar] [CrossRef] [PubMed]
- Bentsink, L.; Jowett, J.; Hanhart, C.J.; Koornneef, M. Cloning of DOG1, a Quantitative Trait Locus Controlling Seed Dormancy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 17042–17047. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Keizer, P.; van Eeuwijk, F.; Smeekens, S.; Bentsink, L. Natural Variation for Seed Longevity and Seed Dormancy Are Negatively Correlated in Arabidopsis. Plant Physiol. 2012, 160, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Cartaño, G.; Mira Sara, S.; Fañanás-Pueyo, I.; Sánchez-Montesino, R.; Contreras, A.; Weiste, C.; Dröge-Laser, W.; Gómez, L.; Oñate-Sánchez, L. Complex Control of Seed Germination Timing by ERF50 Involves RGL2 Antagonism and Negative Feedback Regulation of DOG1. New Phytologist 2023. submitted. [Google Scholar]
- Bueso, E.; Rodriguez, L.; Lorenzo-Orts, L.; Gonzalez-Guzman, M.; Sayas, E.; Muñoz-Bertomeu, J.; Ibañez, C.; Serrano, R.; Rodriguez, P.L. The Single-subunit RING -type E3 Ubiquitin Ligase RSL 1 Targets PYL 4 and PYR 1 ABA Receptors in Plasma Membrane to Modulate Abscisic Acid Signaling. Plant J. 2014, 80, 1057–1071. [Google Scholar] [CrossRef]
- Bueso, E.; Ibañez, C.; Sayas, E.; Muñoz-Bertomeu, J.; Gonzalez-Guzmán, M.; Rodriguez, P.L.; Serrano, R. A Forward Genetic Approach in Arabidopsis thaliana Identifies a RING-Type Ubiquitin Ligase as a Novel Determinant of Seed Longevity. Plant Sci. 2014, 215–216, 110–116. [Google Scholar] [CrossRef]
- Ali, F.; Wei, Z.; Li, Y.; Gan, L.; Yang, Z.; Li, F.; Wang, Z. An Uncanonical Transcription Factor-DREB2B Regulates Seed Vigor Negatively through ABA Pathway. bioRxiv 2020. [Google Scholar] [CrossRef]
- Braybrook, S.A.; Stone, S.L.; Park, S.; Bui, A.Q.; Le, B.H.; Fischer, R.L.; Goldberg, R.B.; Harada, J.J. Genes Directly Regulated by Leafy Cotyledon2 Provide Insight into the Control of Embryo Maturation and Somatic Embryogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 3468–3473. [Google Scholar] [CrossRef]
- Junker, A.; Mönke, G.; Rutten, T.; Keilwagen, J.; Seifert, M.; Thi, T.M.N.; Renou, J.; Balzergue, S.; Viehöver, P.; Hähnel, U. Elongation-related Functions of Leafy Cotyledon1 during the Development of Arabidopsis thaliana. Plant J. 2012, 71, 427–442. [Google Scholar] [CrossRef]
- Mönke, G.; Seifert, M.; Keilwagen, J.; Mohr, M.; Grosse, I.; Hähnel, U.; Junker, A.; Weisshaar, B.; Conrad, U.; Bäumlein, H. Toward the Identification and Regulation of the Arabidopsis thaliana ABI3 Regulon. Nucleic Acids Res. 2012, 40, 8240–8254. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Waterworth, W.; West, C.E.; Foyer, C.H. WHIRLY Proteins Maintain Seed Longevity by Effects on Seed Oxygen Signalling during Imbibition. Biochem. J. 2023, 480, 941–956. [Google Scholar] [CrossRef] [PubMed]
- Isemer, R.; Krause, K.; Grabe, N.; Kitahata, N.; Asami, T.; Krupinska, K. Plastid Located WHIRLY1 Enhances the Responsiveness of Arabidopsis Seedlings toward Abscisic Acid. Front. Plant Sci. 2012, 3, 283. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, S.; Qi, Y. Advances in Structure and Function of Auxin Response Factor in Plants. J. Integr. Plant Biol. 2023, 65, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Matilla, A.J. Auxin: Hormonal Signal Required for Seed Development and Dormancy. Plants 2020, 9, 705. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, D.D.; Köhler, C. Auxin: A Molecular Trigger of Seed Development. Genes Dev. 2018, 32, 479–490. [Google Scholar] [CrossRef]
- Lavy, M.; Estelle, M. Mechanisms of Auxin Signaling. Development 2016, 143, 3226–3229. [Google Scholar] [CrossRef]
- Jez, J.M. Connecting Primary and Specialized Metabolism: Amino Acid Conjugation of Phytohormones by Gretchen HAGEN 3 (GH3) Acyl Acid Amido Synthetases. Curr. Opin. Plant Biol. 2022, 66, 102194. [Google Scholar] [CrossRef]
- Yuan, Z.; Fan, K.; Wang, Y.; Tian, L.; Zhang, C.; Sun, W.; He, H.; Yu, S. OsGRETCHENHAGEN3-2 Modulates Rice Seed Storability via Accumulation of Abscisic Acid and Protective Substances. Plant Physiol. 2021, 186, 469–482. [Google Scholar] [CrossRef]
- Han, Q.; Chen, K.; Yan, D.; Hao, G.; Qi, J.; Wang, C.; Dirk, L.M.A.; Bruce Downie, A.; Gong, J.; Wang, J.; et al. ZmDREB2A Regulates ZmGH3.2 and ZmRAFS, Shifting Metabolism towards Seed Aging Tolerance over Seedling Growth. Plant J. 2020, 104, 268–282. [Google Scholar] [CrossRef]
- Li, A.; Zhou, M.; Wei, D.; Chen, H.; You, C.; Lin, J. Transcriptome Profiling Reveals the Negative Regulation of Multiple Plant Hormone Signaling Pathways Elicited by Overexpression of C-Repeat Binding Factors. Front. Plant Sci. 2017, 8, 1647. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Q.; Zhu, D.; Yu, J. A DREB-like Transcription Factor from Maize (Zea mays), ZmDREB4. 1, Plays a Negative Role in Plant Growth and Development. Front. Plant Sci. 2018, 9, 395. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Sponsel, V. A Century of Gibberellin Research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, A.; Aoyanagi, T. Molecular Aspects of Seed Development Controlled by Gibberellins and Abscisic Acids. Int. J. Mol. Sci. 2022, 23, 1876. [Google Scholar] [CrossRef] [PubMed]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and Seed Germination. Annu. Plant Rev. Gibberellins 2016, 49, 253–284. [Google Scholar]
- Yamaguchi, S.; Kamiya, Y.; Nambara, E. Regulation of ABA and GA Levels during Seed Development and Germination in Arabidopsis. Annu. Plant Rev. Seed Dev. Dormancy Germination 2007, 27, 224–247. [Google Scholar]
- Davière, J.-M.; Achard, P. Gibberellin Signaling in Plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hirano, K.; Sato, T.; Mitsuda, N.; Nomoto, M.; Maeo, K.; Koketsu, E.; Mitani, R.; Kawamura, M.; Ishiguro, S.; et al. DELLA Protein Functions as a Transcriptional Activator through the DNA Binding of the Indeterminate Domain Family Proteins. Proc. Natl. Acad. Sci. USA 2014, 111, 7861–7866. [Google Scholar] [CrossRef]
- Ikeda, A.; Ueguchi-Tanaka, M.; Sonoda, Y.; Kitano, H.; Koshioka, M.; Futsuhara, Y.; Matsuoka, M.; Yamaguchi, J. Slender Rice, a Constitutive Gibberellin Response Mutant, Is Caused by a Null Mutation of the SLR1 Gene, an Ortholog of the Height-Regulating Gene GAI/RGA/RHT/D8. Plant Cell 2001, 13, 999–1010. [Google Scholar] [CrossRef]
- Jasinski, S.; Tattersall, A.; Piazza, P.; Hay, A.; Martinez-Garcia, J.F.; Schmitz, G.; Theres, K.; McCormick, S.; Tsiantis, M. PROCERA Encodes a DELLA Protein That Mediates Control of Dissected Leaf Form in Tomato. Plant J. 2008, 56, 603–612. [Google Scholar] [CrossRef]
- Livne, S.; Lor, V.S.; Nir, I.; Eliaz, N.; Aharoni, A.; Olszewski, N.E.; Eshed, Y.; Weiss, D. Uncovering DELLA-Independent Gibberellin Responses by Characterizing New Tomato Procera Mutants. Plant Cell 2015, 27, 1579–1594. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-C.; Nakajima, M.; Nakayama, A.; Yamaguchi, I. Contribution of Gibberellins to the Formation of Arabidopsis Seed Coat through Starch Degradation. Plant Cell Physiol. 2005, 46, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Bueso, E.; Muñoz-Bertomeu, J.; Campos, F.; Martínez, C.; Tello, C.; Martínez-Almonacid, I.; Ballester, P.; Simón-Moya, M.; Brunaud, V.; Yenush, L.; et al. Arabidopsis COGWHEEL 1 Links Light Perception and Gibberellins with Seed Tolerance to Deterioration. Plant J. 2016, 87, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Graeber, K.; Linkies, A.; Steinbrecher, T.; Mummenhoff, K.; Tarkowská, D.; Turečková, V.; Ignatz, M.; Sperber, K.; Voegele, A.; de Jong, H.; et al. Delay of Germination 1 Mediates a Conserved Coat-Dormancy Mechanism for the Temperature- and Gibberellin-Dependent Control of Seed Germination. Proc. Natl. Acad. Sci. USA 2014, 111, E3571–E3580. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y. Roles of Brassinosteroids in Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef]
- Planas-Riverola, A.; Gupta, A.; Betegón-Putze, I.; Bosch, N.; Ibañes, M.; Caño-Delgado, A.I. Brassinosteroid Signaling in Plant Development and Adaptation to Stress. Development 2019, 146, dev151894. [Google Scholar] [CrossRef] [PubMed]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) Role in Plant Development and Coping with Different Stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Li, J.; Chory, J. Brassinosteroid Actions in Plants. J. Exp. Bot. 1999, 50, 275–282. [Google Scholar] [CrossRef]
- Sano, N.; Kim, J.-S.; Onda, Y.; Nomura, T.; Mochida, K.; Okamoto, M.; Seo, M. RNA-Seq Using Bulked Recombinant Inbred Line Populations Uncovers the Importance of Brassinosteroid for Seed Longevity after Priming Treatments. Sci. Rep. 2017, 7, 8095. [Google Scholar] [CrossRef]
- Fabrissin, I.; Sano, N.; Seo, M.; North, H.M. Ageing Beautifully: Can the Benefits of Seed Priming Be Separated from a Reduced Lifespan Trade-Off? J. Exp. Bot. 2021, 72, 2312–2333. [Google Scholar] [CrossRef]
- Leubner-Metzger, G. Brassinosteroids and Gibberellins Promote Tobacco Seed Germination by Distinct Pathways. Planta 2001, 213, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-F.; He, J.-X. Mechanisms of Signaling Crosstalk between Brassinosteroids and Gibberellins. Plant Signal. Behav. 2013, 8, e24686. [Google Scholar] [CrossRef] [PubMed]
- Linkies, A.; Leubner-Metzger, G. Beyond Gibberellins and Abscisic Acid: How Ethylene and Jasmonates Control Seed Germination. Plant Cell Rep. 2012, 31, 253–270. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).