Role of Acetic Acid and Nitric Oxide against Salinity and Lithium Stress in Canola (Brassica napus L.)
Abstract
:1. Introduction
2. Results
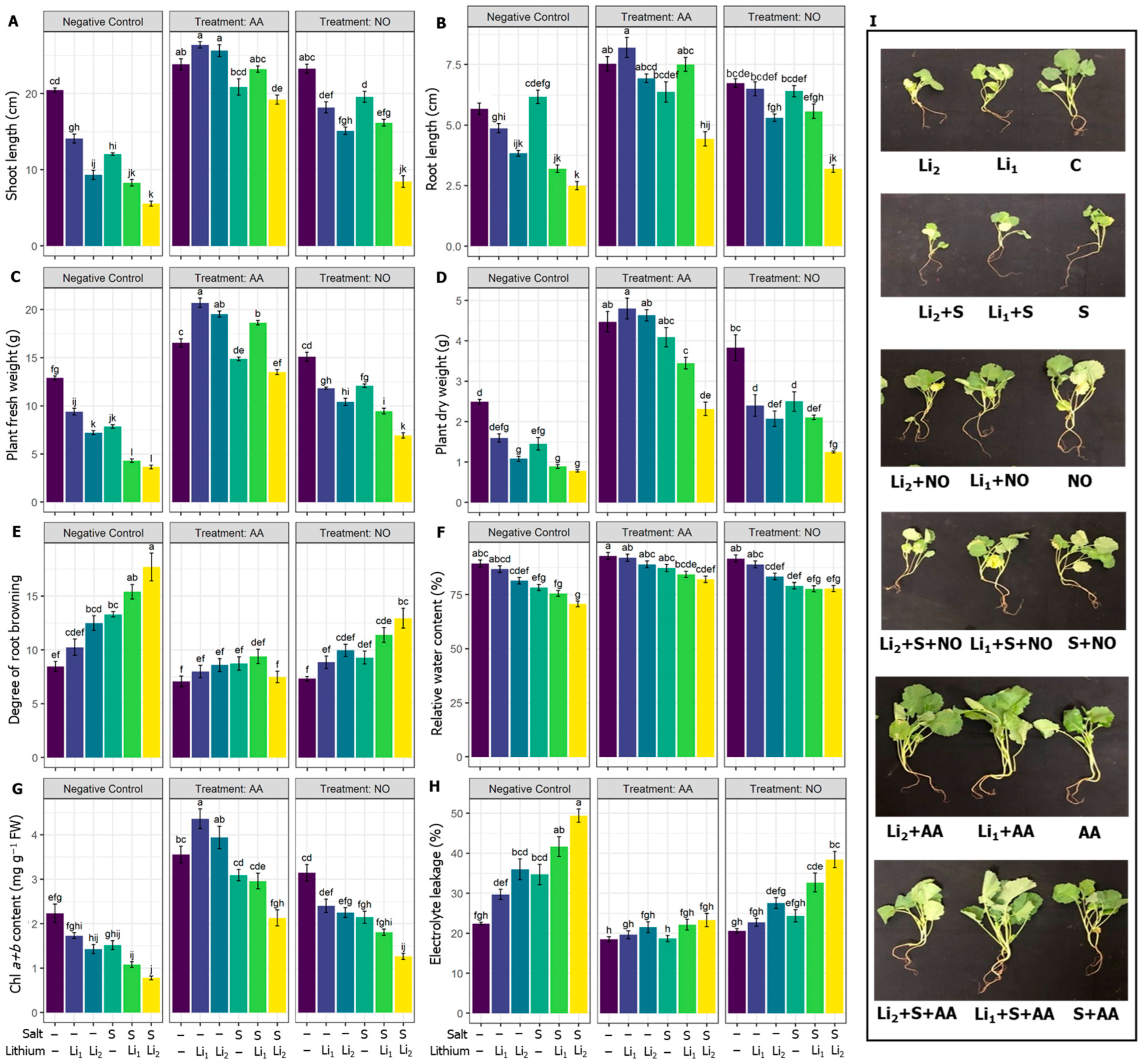
2.1. Effects of Exogenous AA or NO on Growth, Photosynthetic Pigments, and Water Relations
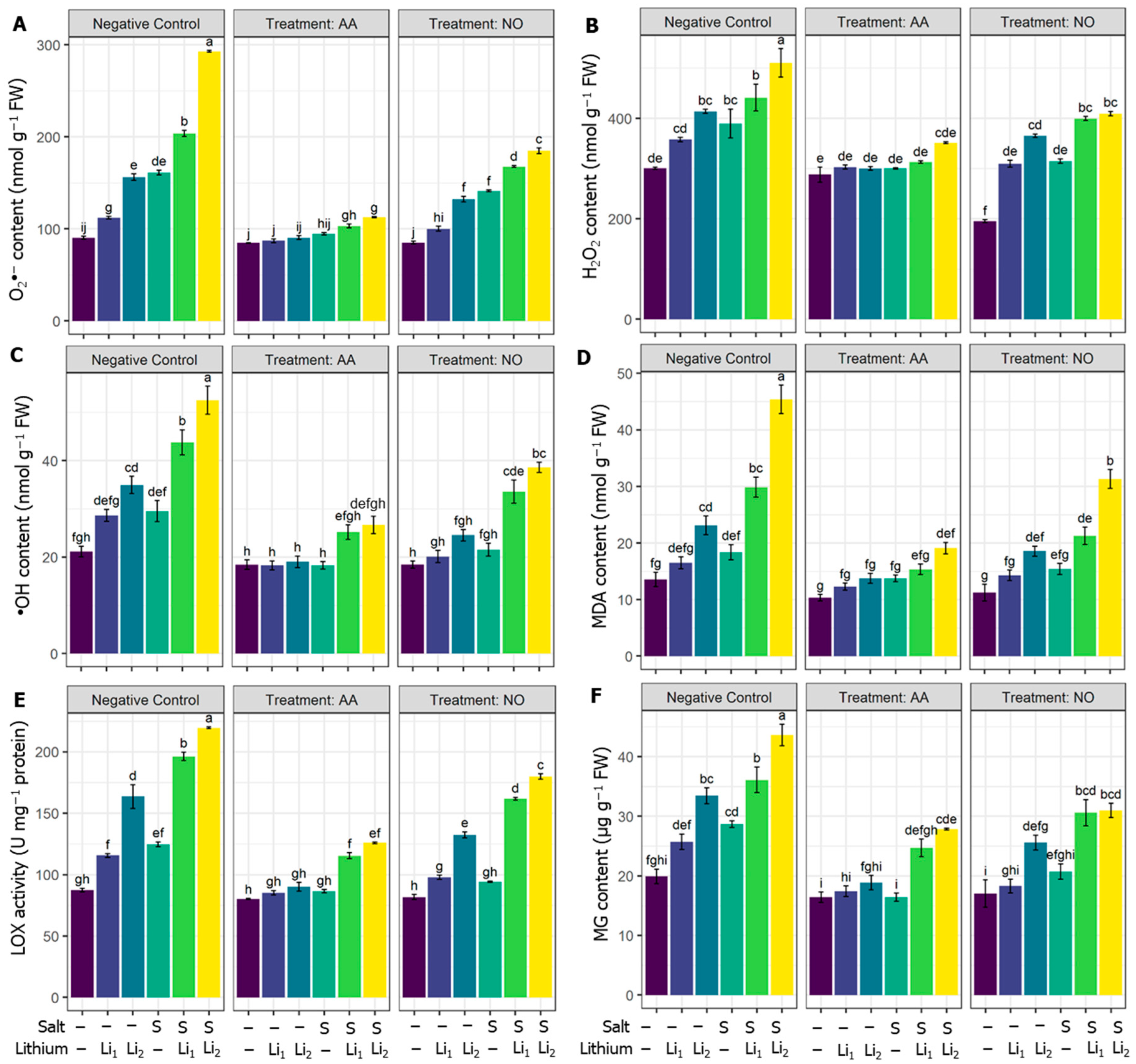
2.2. Effects of AA or NO on ROS Contents and Membrane Protection
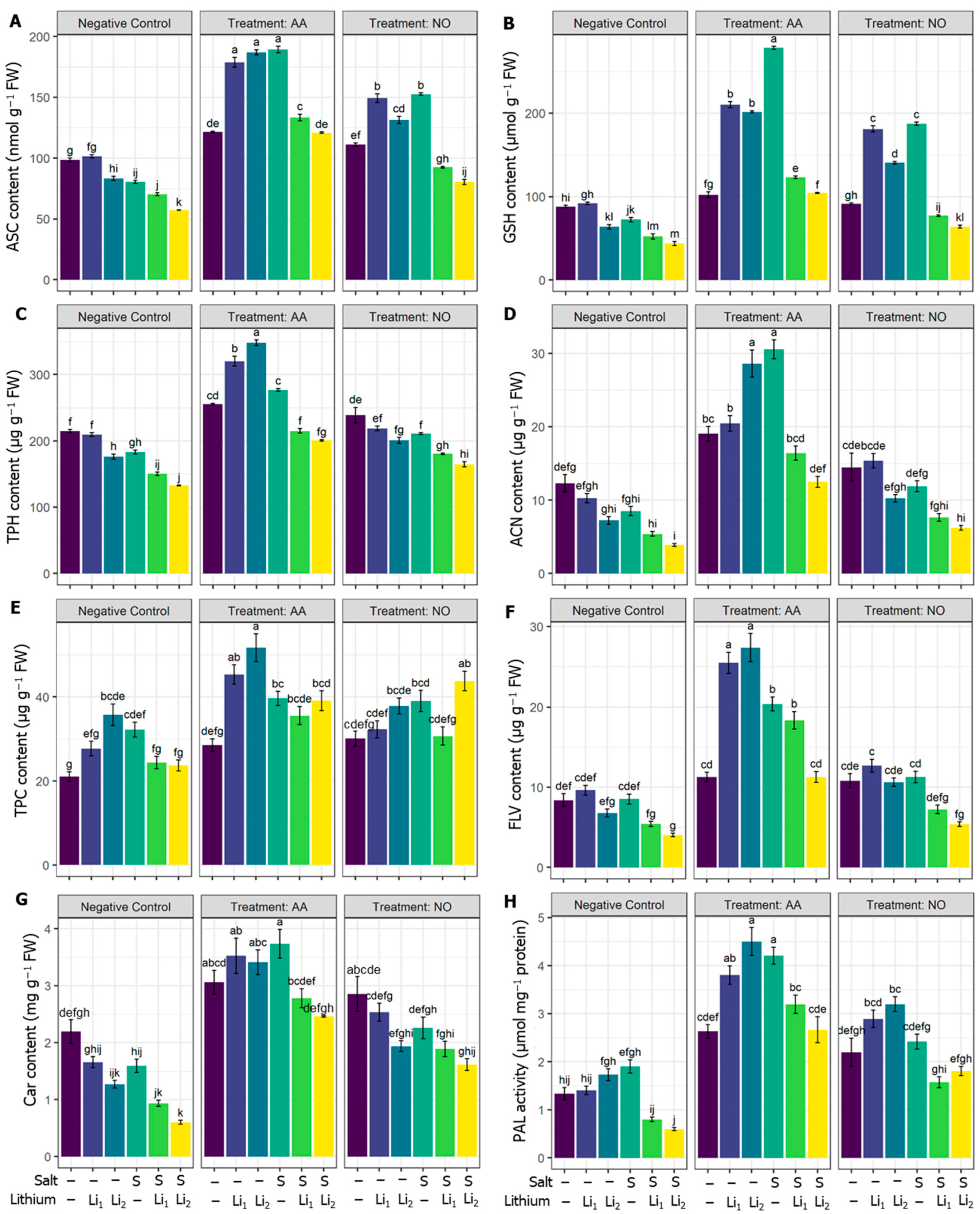
2.3. Effects of AA or NO on Non-Enzymatic Antioxidants and Secondary Metabolites
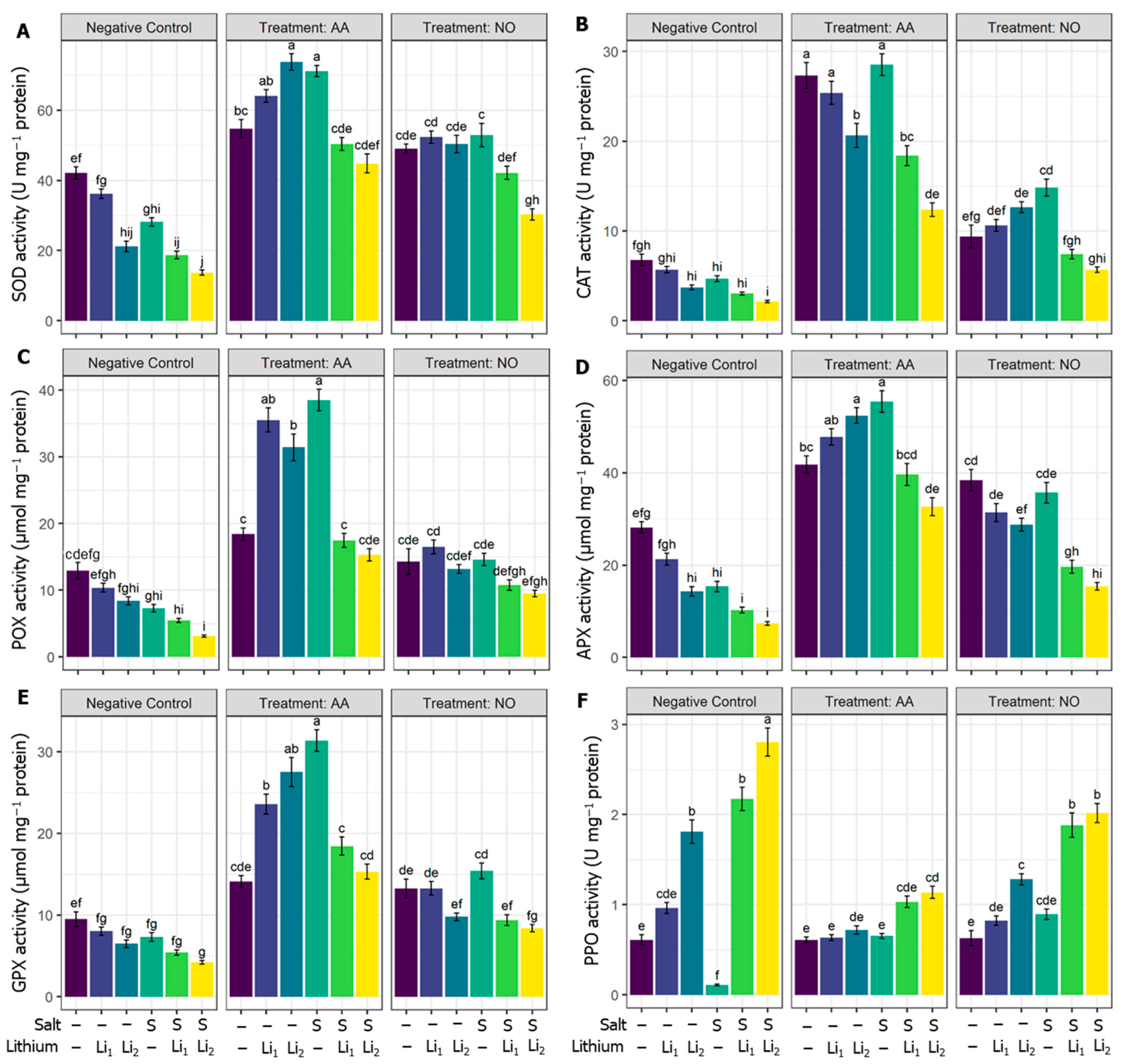
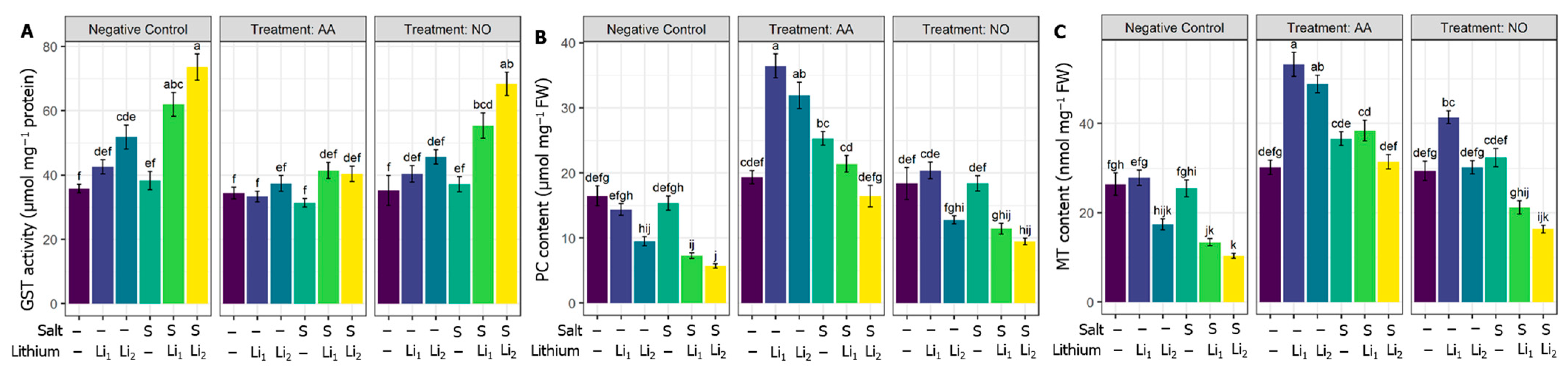
2.4. Effects of AA or NO on Enzymatic Antioxidants
2.5. Effects of AA or NO on Trehalose and Proline Contents, and Na+ and K+ Homeostasis
2.6. Effects of AA or NO on Li-Chelation-Related Parameters
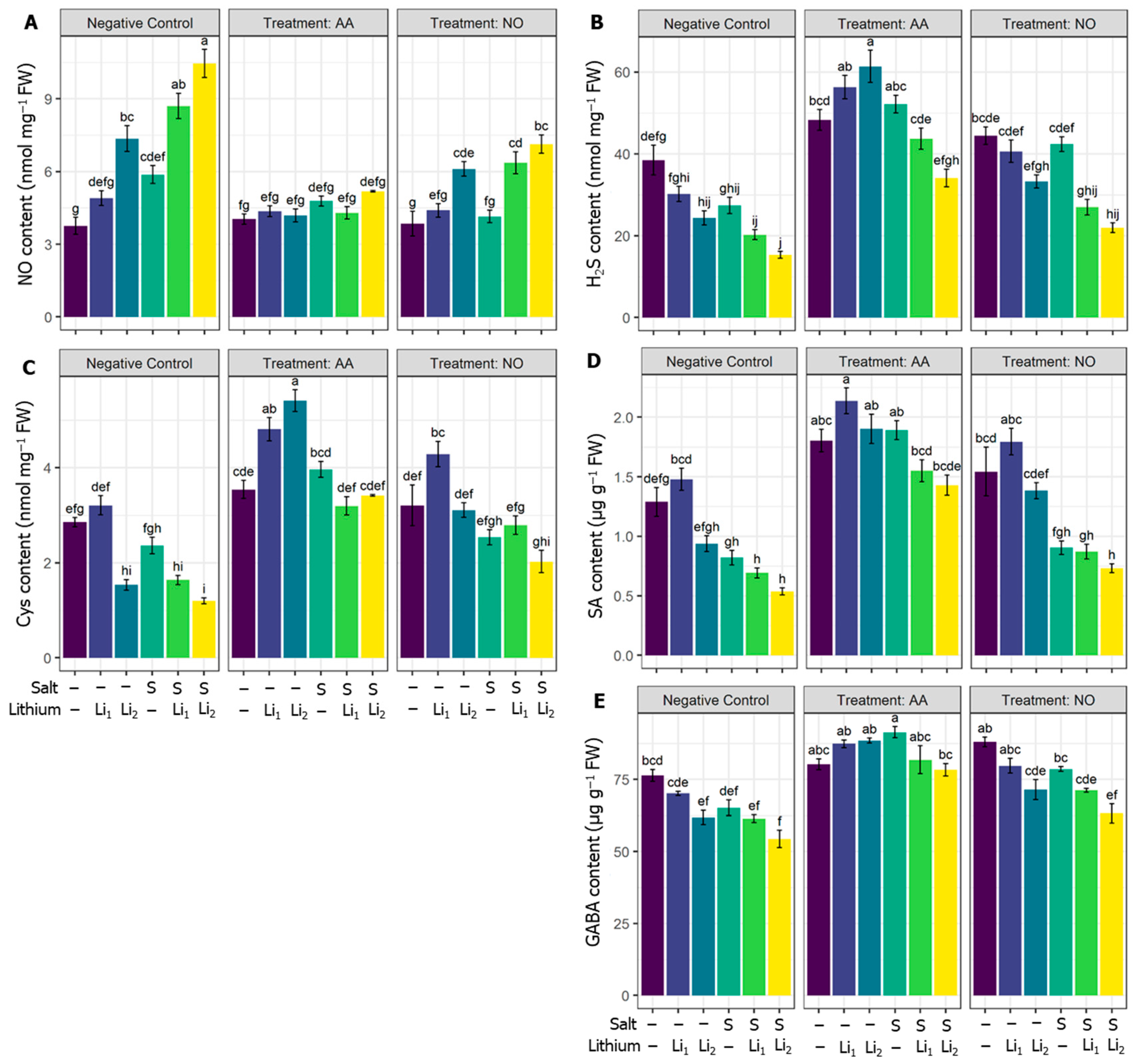
2.7. Effects of AA or NO on Endogenous NO, H2S, Cys, GABA, and SA Contents
2.8. Heat map and PCA Analyses
3. Material and Methods
3.1. Growth Condition and Experimental Setup
3.2. Growth Criteria, Relative Water Content, and Pigment Content
3.3. Quantification of ROS, Electrolyte Leakage, Methylglyoxal, Lipoxygenase, and Lipid Peroxidation
3.4. Measurement of Salicylic Acid, δ-Amino Butyric Acid, Proline, Trehalose, Na+, and K+ Contents
3.5. Non-Enzymatic Antioxidants, Antioxidant Enzymes and Other Enzyme Activity Measurements
3.6. Determination of Nitric Oxide (NO) and Hydrogen Sulfide (H2S)
3.7. Determination of Phytochelatins, Cysteine, and Metallothioneins
3.8. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Li, G.; Yang, J.; Huang, X.; Ji, Q.; Liu, Z.; Ke, W.; Hou, H. Effect of Salt Stress on Growth, Physiological Parameters, and Ionic Concentration of Water Dropwort (Oenanthe javanica) Cultivars. Front. Plant Sci. 2021, 12, 660409. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Map of Salt-Affected Soils 2021; FAO: Rome, Italy, 2021. [Google Scholar]
- Shabbir, R.; Singhal, R.K.; Mishra, U.N.; Chauhan, J.; Javed, T.; Hussain, S.; Kumar, S.; Anuragi, H.; Lal, D.; Chen, P. Combined Abiotic Stresses: Challenges and Potential for Crop Improvement. Agronomy 2022, 12, 2795. [Google Scholar] [CrossRef]
- Zhang, H.; Sonnewald, U. Differences and Commonalities of Plant Responses to Single and Combined Stresses. Plant J. 2017, 90, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Hoque, T.S.; Sohag, A.A.M.; Kordrostami, M.; Hossain, M.A.; Islam, M.S.; Burritt, D.J.; Hossain, M.A. The Effect of Exposure to a Combination of Stressors on Rice Productivity and Grain Yields. In Rice Research for Quality Improvement: Genomics and Genetic Engineering: Volume 1: Breeding Techniques and Abiotic Stress Tolerance; Roychoudhury, A., Ed.; Springer: Singapore, 2020; pp. 675–727. ISBN 978-981-15-4120-9. [Google Scholar]
- Dawood, M.F.; Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Latef, A.A.H.A. Hydrogen Sulfide Priming Can Enhance the Tolerance of Artichoke Seedlings to Individual and Combined Saline-Alkaline and Aniline Stresses. Plant Physiol. Biochem. 2021, 159, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Kuloğlu, S.S.; Yalçin, E.; Çavuşoğlu, K.; Acar, A. Dose-Dependent Toxicity Profile and Genotoxicity Mechanism of Lithium Carbonate. Sci. Rep. 2022, 12, 13504. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000; ISBN 0-429-19112-X. [Google Scholar]
- Shahzad, B.; Tanveer, M.; Hassan, W.; Shah, A.N.; Anjum, S.A.; Cheema, S.A.; Ali, I. Lithium Toxicity in Plants: Reasons, Mechanisms and Remediation Possibilities—A Review. Plant Physiol. Biochem. 2016, 107, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Mukherjee, A.B. Trace Elements from Soil to Human; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 3-540-32713-4. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef] [PubMed]
- Bano, C.; Amist, N.; Singh, N.B. Morphological and Anatomical Modifications of Plants for Environmental Stresses. In Molecular Plant Abiotic Stress; Wiley: Hoboken, NJ, USA, 2019; pp. 29–44. ISBN 978-1-119-46366-5. [Google Scholar]
- Muhammad, I.; Shalmani, A.; Ali, M.; Yang, Q.H.; Ahmad, H.; Li, F.B. Mechanisms Regulating the Dynamics of Photosynthesis Under Abiotic Stresses. Front. Plant Sci. 2020, 11, 615942. [Google Scholar] [CrossRef]
- Brini, F.; Masmoudi, K. Ion Transporters and Abiotic Stress Tolerance in Plants. ISRN Mol. Biol. 2012, 2012, 927436. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, K.K.; Chaudhary, N.; Gupta, S.; Sahu, M.; Tejaswini, B.; Sarkar, S. Salt Stress Resilience in Plants Mediated through Osmolyte Accumulation and Its Crosstalk Mechanism with Phytohormones. Front. Plant Sci. 2022, 13, 1006617. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.; Srivastava, S. Morphological, Physiological and Molecular Markers for Salt-Stressed Plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Mostofa, M.G.; Rahman, M.A.; Islam, M.R.; Keya, S.S.; Das, A.K.; Miah, M.G.; Kawser, A.Q.M.R.; Ahsan, S.M.; Hashem, A.; et al. Acetic Acid: A Cost-Effective Agent for Mitigation of Seawater-Induced Salt Toxicity in Mung Bean. Sci. Rep. 2019, 9, 15186. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.M.; Allen, D.J. Biostimulant Potential of Acetic Acid Under Drought Stress Is Confounded by pH-Dependent Root Growth Inhibition. Front. Plant Sci. 2020, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Afrin, S.; Khan, M.K.; Hannan, M.A.; Skalicky, M.; Mortuza, M.G.; Brestic, M.; Hossain, M.A.; Murata, Y. Insights into Nitric Oxide-Mediated Water Balance, Antioxidant Defence and Mineral Homeostasis in Rice (Oryza sativa L.) under Chilling Stress. Nitric Oxide 2020, 100–101, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ragaey, M.M.; Sadak, M.S.; Dawood, M.F.; Mousa, N.H.; Hanafy, R.S.; Latef, A.A.H.A. Role of Signaling Molecules Sodium Nitroprusside and Arginine in Alleviating Salt-Induced Oxidative Stress in Wheat. Plants 2022, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, D.; Suzuki, Y.; Yokoo, T.; Katoh, E.; Teruya, M.; Muramatsu, M.; Ma, J.F.; Yoshida, Y.; Isaji, S.; Ogo, Y.; et al. Acetic-Acid-Induced Jasmonate Signaling in Root Enhances Drought Avoidance in Rice. Sci. Rep. 2021, 11, 6280. [Google Scholar] [CrossRef]
- Rasheed, S.; Bashir, K.; Kim, J.-M.; Ando, M.; Tanaka, M.; Seki, M. The Modulation of Acetic Acid Pathway Genes in Arabidopsis Improves Survival under Drought Stress. Sci. Rep. 2018, 8, 7831. [Google Scholar] [CrossRef]
- Cronan, J.E., Jr.; Laporte, D. Tricarboxylic Acid Cycle and Glyoxylate Bypass. EcoSal Plus 2005, 1, 10–1128. [Google Scholar] [CrossRef]
- Mirfattahi, Z.; Eshghi, S. Acetic Acid Alleviates Salinity Damage and Improves Fruit Yield in Strawberry by Mediating Hormones and Antioxidant Activity. Erwerbs-Obstbau 2023, 65, 1403–1412. [Google Scholar] [CrossRef]
- Utsumi, Y.; Utsumi, C.; Tanaka, M.; Ha, C.V.; Takahashi, S.; Matsui, A.; Matsunaga, T.M.; Matsunaga, S.; Kanno, Y.; Seo, M.; et al. Acetic Acid Treatment Enhances Drought Avoidance in Cassava (Manihot esculenta Crantz). Front. Plant Sci. 2019, 10, 521. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Ohri, P. Say “NO” to Plant Stresses: Unravelling the Role of Nitric Oxide under Abiotic and Biotic Stress. Nitric Oxide 2022, 130, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Tao, J.; An, J.; Song, X.; Sheteiwy, M.S.; Holford, P.; Hu, J.; Jośko, I.; Guan, Y. Nitric Oxide and Brassinosteroids Enhance Chromium Stress Tolerance in Glycine max L. (Merr.) by Modulating Antioxidative Defense and Glyoxalase Systems. Environ. Sci. Pollut. Res. 2023, 30, 51638–51653. [Google Scholar] [CrossRef] [PubMed]
- Sardar, H.; Khalid, Z.; Ahsan, M.; Naz, S.; Nawaz, A.; Ahmad, R.; Razzaq, K.; Wabaidur, S.M.; Jacquard, C.; Širić, I.; et al. Enhancement of Salinity Stress Tolerance in Lettuce (Lactuca sativa L.) via Foliar Application of Nitric Oxide. Plants 2023, 12, 1115. [Google Scholar] [CrossRef] [PubMed]
- Mfarrej, M.; Wang, X.; Hamzah Saleem, M.; Hussain, I.; Rasheed, R.; Arslan Ashraf, M.; Iqbal, M.; Sohaib Chattha, M.; Nasser Alyemeni, M. Hydrogen Sulphide and Nitric Oxide Mitigate the Negative Impacts of Waterlogging Stress on Wheat (Triticum aestivum L.). Plant Biol. 2022, 24, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Gharelo, R.; Noparvar, P.M. Molecular Response of Canola to Salt Stress: Insights on Tolerance Mechanisms. PeerJ 2018, 6, e4822. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, Q.; Zheng, J.; You, J.; Yang, G.; Leng, S. Salt Stress Decreases Seed Yield and Postpones Growth Process of Canola (Brassica napus L.) by Changing Nitrogen and Carbon Characters. Sci. Rep. 2022, 12, 17884. [Google Scholar] [CrossRef]
- Romeh, A.A. Potential Risks from the Accumulation of Heavy Metals in Canola Plants. Environ. Sci. Pollut. Res. 2021, 28, 52529–52546. [Google Scholar] [CrossRef]
- Delil, A.D.; Köleli, N.; Dağhan, H.; Bahçeci, G. Recovery of Heavy Metals from Canola (Brassica napus) and Soybean (Glycine max) Biomasses Using Electrochemical Process. Environ. Technol. Innov. 2020, 17, 100559. [Google Scholar] [CrossRef]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Mohammed, A.R. Effects of Ultraviolet-B Radiation on Cotton (Gossypium hirsutum L.) Morphology and Anatomy. Ann. Bot. 2003, 91, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-UI-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.S.U.; Polash, M.A.S.; Hossain, M.T.; Sohel, M.A.T.; Brestic, M.; et al. Differential Response of Sugar Beet to Long-Term Mild to Severe Salinity in a Soil–Pot Culture. Agriculture 2019, 9, 223. [Google Scholar] [CrossRef]
- Geng, B.; Huang, D.; Zhu, S. Regulation of Hydrogen Sulfide Metabolism by Nitric Oxide Inhibitors and the Quality of Peaches during Cold Storage. Antioxidants 2019, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- González, L.; González-Vilar, M. Determination of Relative Water Content. In Handbook of Plant Ecophysiology Techniques; Springer: Berlin/Heidelberg, Germany, 2001; pp. 207–212. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Halliwell, B. Generation of Hydrogen Peroxide, Superoxide and Hydroxyl Radicals during the Oxidation of Dihydroxyfumaric Acid by Peroxidase. Biochem. J. 1977, 163, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Choudhuri, M. Implications of Water Stress-induced Changes in the Levels of Endogenous Ascorbic Acid and Hydrogen Peroxide in Vigna Seedlings. Physiol. Plant. 1983, 58, 166–170. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The Deoxyribose Method: A Simple “Test-Tube” Assay for Determination of Rate Constants for Reactions of Hydroxyl Radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Rao, K.M.; Sresty, T. Antioxidative Parameters in the Seedlings of Pigeonpea (Cajanus cajan (L.) Millspaugh) in Response to Zn and Ni Stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Gilbert, R.P.; Brandt, R.B. Spectrophotometric Determination of Methyl Glyoxal with 2, 4-Dinitrophenylhydrazine. Anal. Chem. 1975, 47, 2418–2422. [Google Scholar] [CrossRef]
- Minguez-Mosquera, M.; Jaren-Galan, M.; Garrido-Fernandez, J. Lipoxygenase Activity during Pepper Ripening and Processing of Paprika. Phytochemistry 1993, 32, 1103–1108. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Araújo, S.A.M.; Lima, J.P.M.S.; Viégas, R.A. Roots and Leaves Display Contrasting Osmotic Adjustment Mechanisms in Response to NaCl-Salinity in Atriplex nummularia. Environ. Exp. Bot. 2009, 66, 1–8. [Google Scholar] [CrossRef]
- Warrier, R.; Paul, M.; Vineetha, M. Estimation of Salicylic Acid in Eucalyptus Leaves Using Spectrophotometric Methods. Genet. Plant Physiol. 2013, 3, 90–97. [Google Scholar]
- Zhang, Q.; Xiang, J.; Zhang, L.; Zhu, X.; Evers, J.; van der Werf, W.; Duan, L. Optimizing Soaking and Germination Conditions to Improve Gamma-Aminobutyric Acid Content in Japonica and Indica Germinated Brown Rice. J. Funct. Foods 2014, 10, 283–291. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Li, Z.-G.; Luo, L.-J.; Zhu, L.-P. Involvement of Trehalose in Hydrogen Sulfide Donor Sodium Hydrosulfide-Induced the Acquisition of Heat Tolerance in Maize (Zea mays L.) Seedlings. Bot. Stud. 2014, 55, 20. [Google Scholar] [CrossRef] [PubMed]
- Tahjib-Ul-Arif, M.; Sayed, M.A.; Islam, M.M.; Siddiqui, M.N.; Begum, S.; Hossain, M.A. Screening of Rice Landraces (Oryza sativa L.) for Seedling Stage Salinity Tolerance Using Morpho-Physiological and Molecular Markers. Acta Physiol. Plant. 2018, 40, 70. [Google Scholar] [CrossRef]
- Williams, V.; Twine, S. Flame Photometric Method for Sodium, Potassium and Calcium. Mod. Methods Plant Anal. 1960, 5, 3–5. [Google Scholar]
- Jagota, S.; Dani, H. A New Colorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Kivçak, B.; Mert, T. Quantitative Determination of α-Tocopherol in Arbutus Unedo by TLC-Densitometry and Colorimetry. Fitoterapia 2001, 72, 656–661. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Kofalvi, S.; Nassuth, A. Influence of Wheat Streak Mosaic Virus Infection on Phenylpropanoid Metabolism and the Accumulation of Phenolics and Lignin in Wheat. Physiol. Mol. Plant Pathol. 1995, 47, 365–377. [Google Scholar] [CrossRef]
- Krizek, D.T.; Kramer, G.F.; Upadhyaya, A.; Mirecki, R.M. UV-B Response of Cucumber Seedlings Grown under Metal Halide and High Pressure Sodium/Deluxe Lamps. Physiol. Plant. 1993, 88, 350–358. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant Activity of a Flavonoid-Rich Extract of Hypericum perforatum L. in Vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 0076-6879. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Kumar, K.; Khan, P. Peroxidase and Polyphenol Oxidase in Excised Ragi (Eleusine corocana Cv PR 202) Leaves during Senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 114–120. ISBN 0076-6879. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Havir, E.A.; Hanson, K.R. L-Phenylalanine Ammonia-Lyase (Maize and Potato). Evidence That the Enzyme Is Composed of Four Subunits. Biochemistry 1973, 12, 1583–1591. [Google Scholar] [CrossRef]
- Hu, X.; Neill, S.J.; Cai, W.; Tang, Z. Nitric Oxide Mediates Elicitor-Induced Saponin Synthesis in Cell Cultures of Panax Ginseng. Funct. Plant Biol. 2003, 30, 901–907. [Google Scholar] [CrossRef]
- Nashef, A.S.; Osuga, D.T.; Lee, H.S.; Ahmed, A.I.; Whitaker, J.R.; Feeney, R.E. Effects of Alkali on Proteins. Disulfides and Their Products. J. Agric. Food Chem. 1977, 25, 245–251. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Polyamine and Nitric Oxide Crosstalk: Antagonistic Effects on Cadmium Toxicity in Mung Bean Plants through Upregulating the Metal Detoxification, Antioxidant Defense and Methylglyoxal Detoxification Systems. Ecotoxicol. Environ. Saf. 2016, 126, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, R.J.; Hidalgo, L.M.E.; Neaman, A.; Gaete, O.H. Use of Molecular Biomarkers in Eisenia foetida to Assess Copper Toxicity in Agricultural Soils Affected by Mining Activities. J. Soil Sci. Plant Nutr. 2011, 11, 57–70. [Google Scholar]
- Gaitonde, M. A Spectrophotometric Method for the Direct Determination of Cysteine in the Presence of Other Naturally Occurring Amino Acids. Biochem. J. 1967, 104, 627. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ulhassan, Z.; Qi, W.; Lu, H.; AbdElgawad, H.; Minkina, T.; Sushkova, S.; Rajput, V.D.; El-Keblawy, A.; Jośko, I.; et al. Association of Jasmonic Acid Priming with Multiple Defense Mechanisms in Wheat Plants under High Salt Stress. Front. Plant Sci. 2022, 13, 886862. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Egert, A.; Süssenbacher, I.; Kräutler, B.; Bartels, D.; Peters, S.; Hörtensteiner, S. Water Deficit Induces Chlorophyll Degradation via the “PAO/Phyllobilin” Pathway in Leaves of Homoio-(Craterostigma pumilum) and Poikilochlorophyllous (Xerophyta viscosa) Resurrection Plants. Plant Cell Environ. 2014, 37, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.; Baenziger, P.S.; Börner, A. Drought Stress Tolerance in Wheat and Barley: Advances in Physiology, Breeding and Genetics Research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Ghosh, U.; Islam, M.; Siddiqui, M.; Cao, X.; Khan, M. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and Plant Stress Responses: Friend or Foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.; Xu, Y.; Limwachiranon, J.; Li, D.; Ban, Z.; Luo, Z. Effect of Exogenous Nitro Oxide on Chilling Tolerance, Polyamine, Proline, and γ-Aminobutyric Acid in Bamboo Shoots (Phyllostachys praecox f. Prevernalis). J. Agric. Food Chem. 2017, 65, 5607–5613. [Google Scholar] [CrossRef]
- Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging Roles of γ Aminobutyric Acid (GABA) Gated Channels in Plant Stress Tolerance. Plants 2021, 10, 2178. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA Signalling Modulates Plant Growth by Directly Regulating the Activity of Plant-Specific Anion Transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Tyerman, S.D.; Gilliham, M.; Xu, B. γ-Aminobutyric Acid (GABA) Signalling in Plants. Cell. Mol. Life Sci. 2017, 74, 1577–1603. [Google Scholar] [CrossRef] [PubMed]
- Baetz, U.; Eisenach, C.; Tohge, T.; Martinoia, E.; De Angeli, A. Vacuolar Chloride Fluxes Impact Ion Content and Distribution during Early Salinity Stress. Plant Physiol. 2016, 172, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Wu, Q.; Chen, J.; Shabala, L.; Mithöfer, A.; Wang, H.; Qu, M.; Yu, M.; Cui, J.; Shabala, S. GABA Operates Upstream of H+-ATPase and Improves Salinity Tolerance in Arabidopsis by Enabling Cytosolic K+ Retention and Na+ Exclusion. J. Exp. Bot. 2019, 70, 6349–6361. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Isayenkov, S.V. The Role of the γ-Aminobutyric Acid (GABA) in Plant Salt Stress Tolerance. Horticulturae 2023, 9, 230. [Google Scholar] [CrossRef]
- Tiwari, S.; Lata, C. Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview. Front. Plant Sci. 2018, 9, 452. [Google Scholar] [CrossRef] [PubMed]
- Amin, I.; Rasool, S.; Mir, M.A.; Wani, W.; Masoodi, K.Z.; Ahmad, P. Ion Homeostasis for Salinity Tolerance in Plants: A Molecular Approach. Physiol. Plant 2021, 171, 578–594. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Xing, J.; Li, H.; Miao, J.; Xu, B. Acetic Acid Mitigated Salt Stress by Alleviating Ionic and Oxidative Damages and Regulating Hormone Metabolism in Perennial Ryegrass (Lolium perenne L.). Grass Res. 2021, 1, 3. [Google Scholar] [CrossRef]
- Assaha, D.; Mekawy, A.; Liu, L.; Noori, M.; Kokulan, K.; Ueda, A.; Nagaoka, T.; Saneoka, H. Na+ Retention in the Root Is a Key Adaptive Mechanism to Low and High Salinity in the Glycophyte, Talinum paniculatum (Jacq.) Gaertn. (Portulacaceae). J. Agron. Crop Sci. 2017, 203, 56–67. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Q.; Wu, F.; Dong, X.; He, J.; Pei, Z.; Zheng, H. Nitric Oxide Enhances Salt Secretion and Na+ Sequestration in a Mangrove Plant, Avicennia marina, through Increasing the Expression of H+-ATPase and Na+/H+ Antiporter under High Salinity. Tree Physiol. 2010, 30, 1570–1585. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiong, D.-Y.; Wang, W.-H.; Hu, W.-J.; Simon, M.; Xiao, Q.; Chen, J.; Liu, T.-W.; Liu, X.; Zheng, H.-L. Nitric Oxide Mediates Root K+/Na+ Balance in a Mangrove Plant, Kandelia obovata, by Enhancing the Expression of AKT1-Type K+ Channel and Na+/H+ Antiporter under High Salinity. PLoS ONE 2013, 8, e71543. [Google Scholar] [CrossRef] [PubMed]
- Pospíšil, P. Production of Reactive Oxygen Species by Photosystem II as a Response to Light and Temperature Stress. Front. Plant Sci. 2016, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Hu, Q.; Cui, H.; Ma, C.; Li, Y.; Yang, C.; Wang, K.; Sun, Y. Lipidomic Metabolism Associated with Acetic Acid Priming-Induced Salt Tolerance in Carex rigescens. Plant Physiol. Biochem. 2021, 167, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Wu, X.; Zhong, Y. Exogenously Applied Nitric Oxide Enhances the Drought Tolerance in Hulless Barley. Plant Prod. Sci. 2015, 18, 52–56. [Google Scholar] [CrossRef]
- Nabaei, M.; Amooaghaie, R. Nitric Oxide Is Involved in the Regulation of Melatonin-Induced Antioxidant Responses in Catharanthus roseus Roots under Cadmium Stress. Botany 2019, 97, 681–690. [Google Scholar] [CrossRef]
- Xu, J.; Liu, T.; Qu, F.; Jin, X.; Huang, N.; Wang, J.; Hu, X. Nitric Oxide Mediates γ-Aminobutyric Acid-Enhanced Muskmelon Tolerance to Salinity–Alkalinity Stress Conditions. Sci. Hortic. 2021, 286, 110229. [Google Scholar] [CrossRef]
- Khator, K.; Saxena, I.; Shekhawat, G.S. Nitric Oxide Induced Cd Tolerance and Phytoremediation Potential of B. juncea by the Modulation of Antioxidant Defense System and ROS Detoxification. Biometals 2021, 34, 15–32. [Google Scholar] [CrossRef]
- Sami, F.; Siddiqui, H.; Alam, P.; Hayat, S. Nitric Oxide Mitigates the Salt-Induced Oxidative Damage in Mustard by Upregulating the Activity of Various Enzymes. J. Plant Growth Regul. 2021, 40, 2409–2432. [Google Scholar] [CrossRef]
- Valderrama, R.; Corpas, F.J.; Carreras, A.; Fernández-Ocaña, A.; Chaki, M.; Luque, F.; Gómez-Rodríguez, M.V.; Colmenero-Varea, P.; Del Río, L.A.; Barroso, J.B. Nitrosative Stress in Plants. FEBS Lett. 2007, 581, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J. Nitric Oxide and Hydrogen Sulfide in Higher Plants under Physiological and Stress Conditions. Antioxidants 2019, 8, 457. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Yang, X.; Zhu, X.; Wei, J.; Li, W.; Wang, H.; Xu, Y.; Yang, Z.; Xu, C.; Li, P. Primary Root Response to Combined Drought and Heat Stress Is Regulated via Salicylic Acid Metabolism in Maize. BMC Plant Biol. 2022, 22, 417. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic Acid-Induced Abiotic Stress Tolerance and Underlying Mechanisms in Plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Lindermayr, C.; Saalbach, G.; Bahnweg, G.; Durner, J. Differential Inhibition of Arabidopsis Methionine Adenosyltransferases by Protein S-Nitrosylation. J. Biol. Chem. 2006, 281, 4285–4291. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Vargas, M.A.; González-Gordo, S.; Palma, J.M.; Corpas, F.J. H2S in Horticultural Plants: Endogenous Detection by an Electrochemical Sensor, Emission by a Gas Detector, and Its Correlation with L-Cysteine Desulfhydrase (LCD) Activity. Int. J. Mol. Sci. 2022, 23, 5648. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.-H.; Wu, F.-H.; He, E.-M.; Liu, X.; Shangguan, Z.-P.; Zheng, H.-L. Hydrogen Sulfide Enhances Salt Tolerance through Nitric Oxide-Mediated Maintenance of Ion Homeostasis in Barley Seedling Roots. Sci. Rep. 2015, 5, 12516. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M. Hydrogen Sulfide: A Novel Component in Arabidopsis Peroxisomes Which Triggers Catalase Inhibition. J. Integr. Plant Biol. 2019, 61, 871–883. [Google Scholar] [CrossRef]
- Singh, V.P.; Tripathi, D.K.; Fotopoulos, V. Hydrogen Sulfide and Nitric Oxide Signal Integration and Plant Development under Stressed/Non-Stressed Conditions. Physiol. Plant. 2020, 168, 239–240. [Google Scholar] [CrossRef]
- Dawood, M.F.; Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Abdel Latef, A.A.H. Fluoride Mitigates Aluminum-Toxicity in Barley: Morpho-Physiological Responses and Biochemical Mechanisms. BMC Plant Biol. 2022, 22, 287. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, M.; Zhang, X.; Chen, S. Genome-Wide Analysis of the Metallothionein Gene Family in Cassava Reveals Its Role in Response to Physiological Stress through the Regulation of Reactive Oxygen Species. BMC Plant Biol. 2023, 23, 227. [Google Scholar] [CrossRef]
- Alvi, A.F.; Iqbal, N.; Albaqami, M.; Khan, N.A. The Emerging Key Role of Reactive Sulfur Species in Abiotic Stress Tolerance in Plants. Physiol. Plant. 2023, 175, e13945. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawood, M.F.A.; Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Abdel Latef, A.A.H. Role of Acetic Acid and Nitric Oxide against Salinity and Lithium Stress in Canola (Brassica napus L.). Plants 2024, 13, 51. https://doi.org/10.3390/plants13010051
Dawood MFA, Tahjib-Ul-Arif M, Sohag AAM, Abdel Latef AAH. Role of Acetic Acid and Nitric Oxide against Salinity and Lithium Stress in Canola (Brassica napus L.). Plants. 2024; 13(1):51. https://doi.org/10.3390/plants13010051
Chicago/Turabian StyleDawood, Mona F. A., Md. Tahjib-Ul-Arif, Abdullah Al Mamun Sohag, and Arafat Abdel Hamed Abdel Latef. 2024. "Role of Acetic Acid and Nitric Oxide against Salinity and Lithium Stress in Canola (Brassica napus L.)" Plants 13, no. 1: 51. https://doi.org/10.3390/plants13010051
APA StyleDawood, M. F. A., Tahjib-Ul-Arif, M., Sohag, A. A. M., & Abdel Latef, A. A. H. (2024). Role of Acetic Acid and Nitric Oxide against Salinity and Lithium Stress in Canola (Brassica napus L.). Plants, 13(1), 51. https://doi.org/10.3390/plants13010051









