Prior Infection by Colletotrichum spinaciae Lowers the Susceptibility to Infection by Powdery Mildew in Common Vetch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Pathogens
2.2. Growth Medium
2.3. Experiment Establishment, Management, and Harvest
2.4. Statistical Analysis
3. Results
3.1. Plant Fresh Weight and Dry Weight
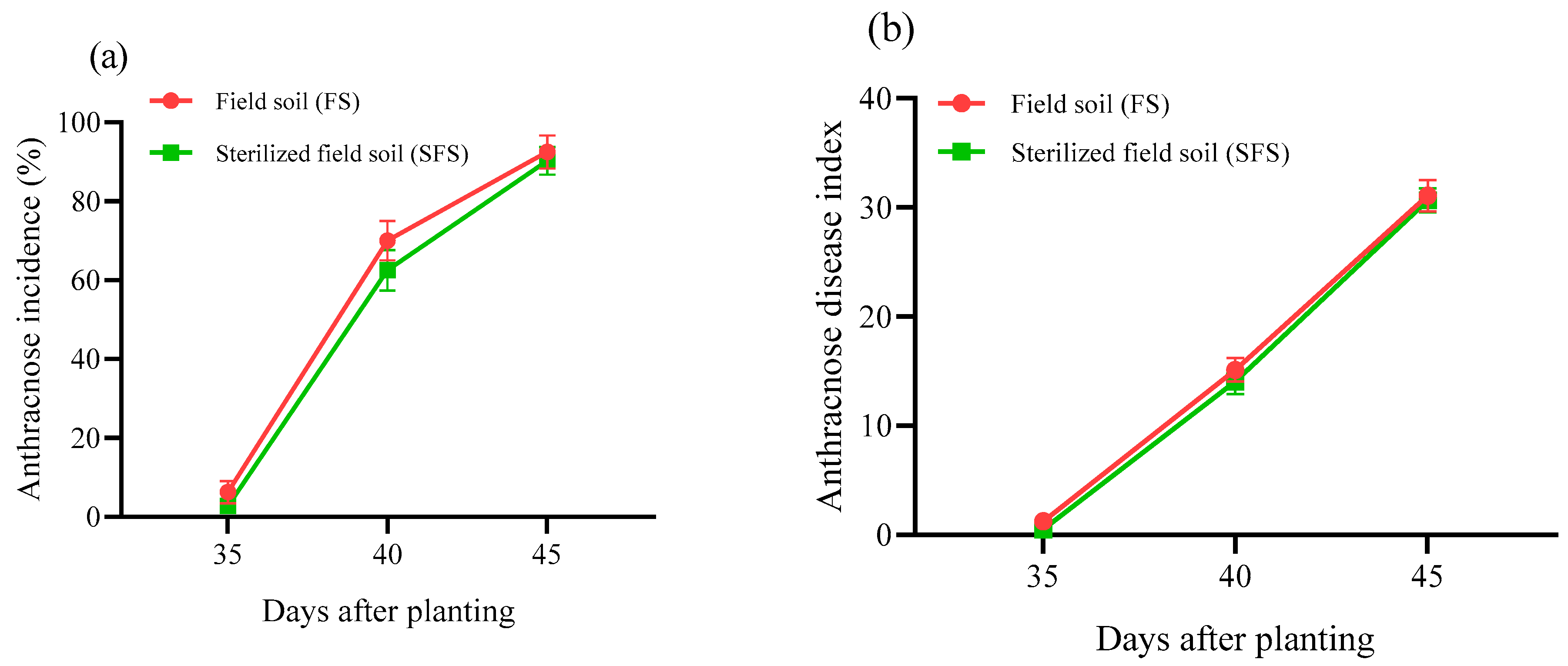
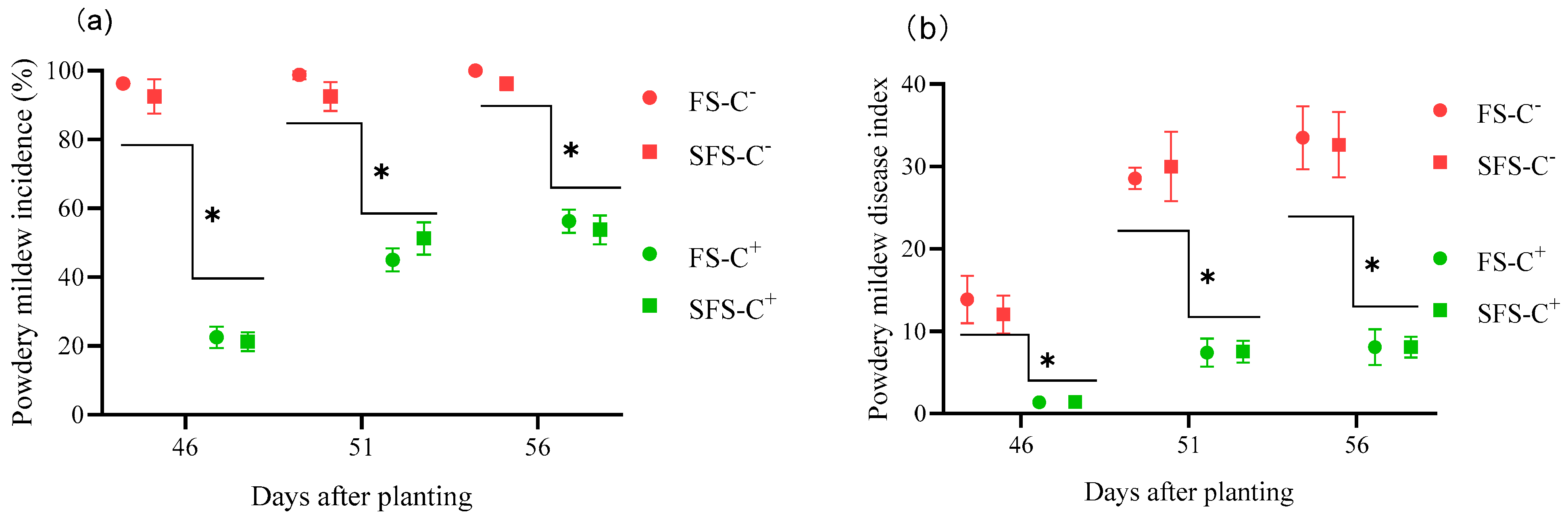
3.2. Disease Incidence and Disease Index
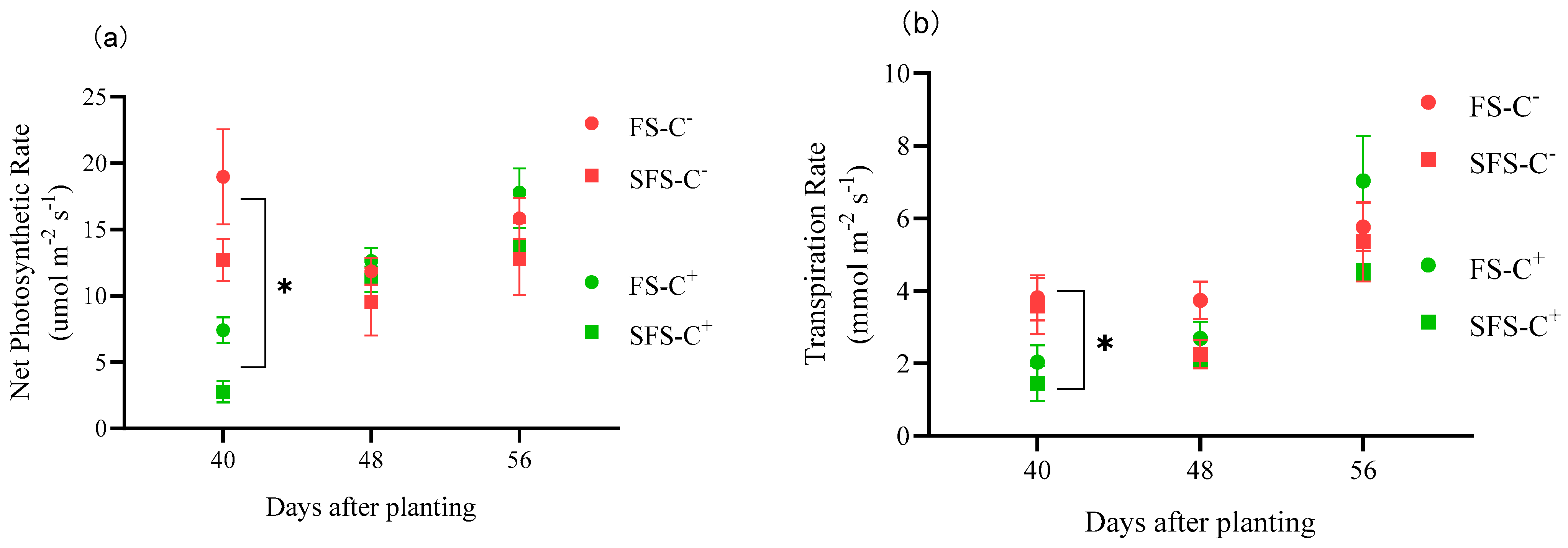
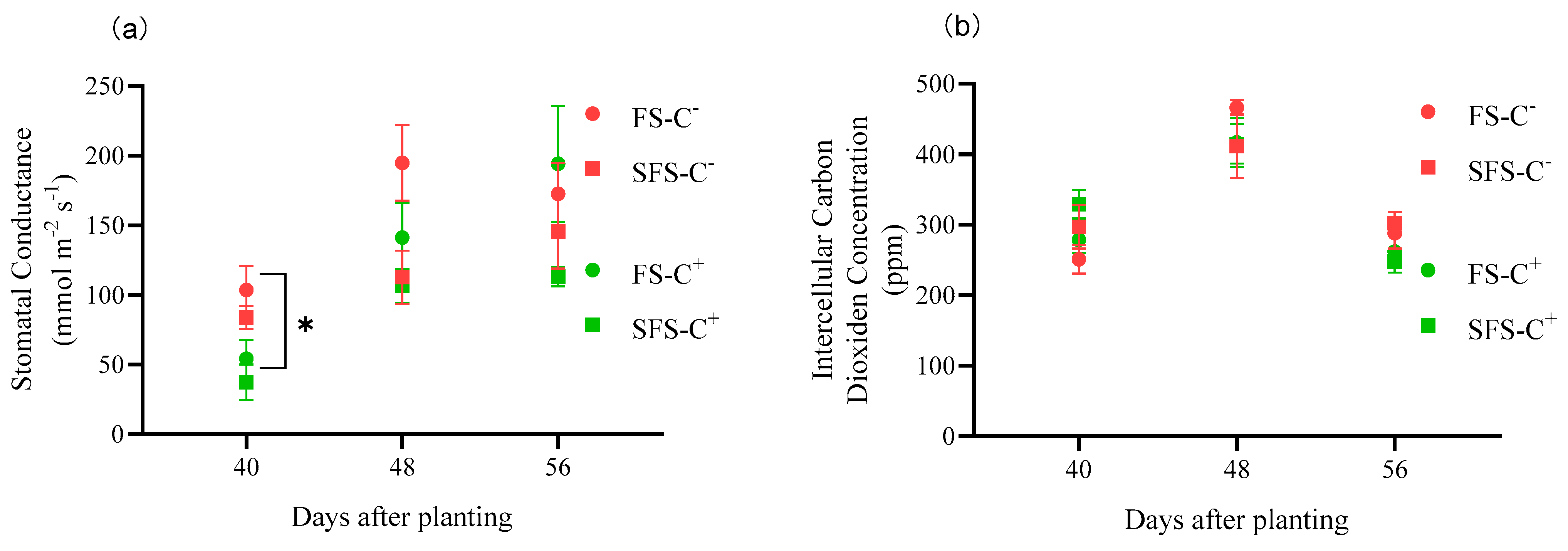
3.3. Photosynthetic and Physiological Indices
3.4. Enzyme Activities Related to Plant Defense
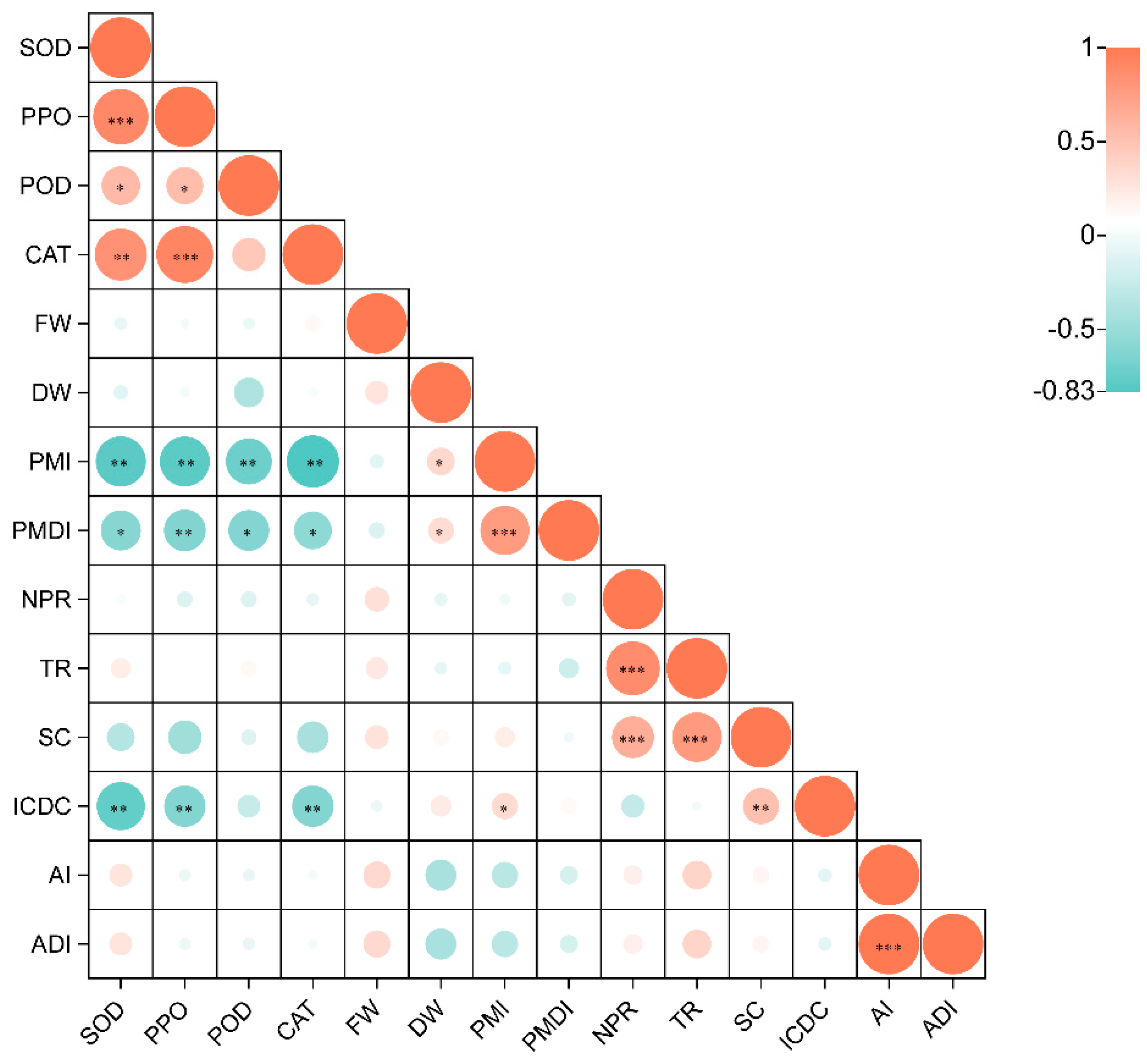
3.5. Correlation Analysis between the Level of Infection and the Photosynthetic Index, Biomass, and Defense Enzymes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nan, Z.B.; Abd El-Moneim, A.M.; Larbi, A.; Nie, B. Productivity of vetches (Vicia spp.) under alpine grassland conditions in China. Trop. Grassl. 2006, 40, 177–182. [Google Scholar]
- Beyaz, R. Impact of gamma irradiation pretreatment on the growth of common vetch (Vicia sativa L.) seedlings grown under salt and drought stress. Int. J. Radiat. Biol. 2020, 2, 96. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Shen, S.H.; Jahufer, M.Z.Z.; Dong, D.K.; Luo, D.; Zhou, Q.; Chai, X.T.; Luo, K.; Nan, Z.B.; Wang, Y.R.; et al. Effect of genotype and environment on agronomical characters of common vetch (Vicia sativa L.). Genet. Resour. Crop Evol. 2019, 66, 1587–1599. [Google Scholar] [CrossRef]
- Sharaiha, R.K.; Ziadat, F.M. Alternative cropping systems to control soil erosion in the arid to semi-arid areas of Jordan. Arid. Land Res. Manag. 2008, 22, 16–28. [Google Scholar] [CrossRef]
- Ab Rahman, S.F.S.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, Y.Z. Research advances on fungal diseases of Vicia sativa. Acta Prataculturae Sin. 2016, 25, 203–214. [Google Scholar]
- Wang, Q.; Ma, L.X.; Duan, T.Y.; Nan, Z.B. Research progress on non-fungal diseases of Vicia sativa. Pratacult. Sci. 2019, 36, 1578–1590. [Google Scholar]
- Dearness, J. New and noteworthy fungi-IV. Mycologia 1926, 18, 236–255. [Google Scholar] [CrossRef]
- Weimer, J.L. A new species of Colletotrichum on vetch. Phytopathology 1945, 35, 12. [Google Scholar]
- Horn, N.L. Acomparative study of two species of Colletotrichum on Vetch. Phytopathology 1952, 42, 670–674. [Google Scholar]
- Sawada, K. Descriptive catalogue of Taiwan (Formosan) fungi. Agric. Taiwan. Univ. 1959, 8, 268. [Google Scholar]
- Reed, P.J.; Dickens, J.S.W.; O’neill, T.M. Occurrence of anthracnose (Colletotrichum acutatum) on ornamental lupin in the United Kingdom. Plant Pathol. 1996, 45, 245–248. [Google Scholar] [CrossRef]
- Xu, S.; Li, Y.Z. First report of common vetch anthracnose caused by Colletotrichum lentis in China. Plant Dis. 2015, 99, 1859. [Google Scholar] [CrossRef]
- Wang, Q.; Duan, T.Y.; Nan, Z.B. First report of anthracnose caused by Colletotrichum spinaciae on Vicia sativa in China. Plant Dis. 2019, 103, 2138–2139. [Google Scholar] [CrossRef]
- Zhang, W. Sexual Genetic Characteristics of Colletotrichum structicola. Master’s Thesis, Northwest A&F University, Yangling, China, 2016. [Google Scholar]
- Williams, M.; Herman, M. Fighting for Their Lives: Plants and Pathogens. Plant Cell 2012, 24, 6. [Google Scholar]
- Ding, T.T.; Wang, X.Y.; Nie, B.; Zhang, W.Z.; Duan, T.Y. Effect of anthracnose on the growth, physiology, and biochemistry in Vicia sativa. Pratacult. Sci. 2019, 36, 2569–2579. [Google Scholar]
- Hu, H.; Tang, M.J.; Guo, H.W.; Zhang, G.Z.; Gu, Q.; Yuan, Y.W.; Sheng, L.; Zhou, H.K.; Liu, Z. Photosynthetic rate, chlorophyll fluorescence in anthracnose infected tea plants. Int. J. Agric. Biol. 2020, 24, 531–537. [Google Scholar]
- Li, Y.D.; Nan, Z.B.; Matthew, C.; Wang, Y.J.; Duan, T.Y. Arbuscular mycorrhizal fungus changes alfalfa (Medicago sativa) metabolites in response to leaf spot (Phoma medicaginis) infection, with subsequent effects on pea aphid (Acyrthosiphon pisum) behavior. New Phytol. 2023, 239, 286–300. [Google Scholar] [CrossRef]
- Glawe, D.A. The powdery mildews: A review of the world’s most familiar (yet poorly known) plant pathogens. Annu. Rev. Phytopathol. 2008, 46, 27–51. [Google Scholar] [CrossRef]
- Dreiseitl, A. A novel way to identify specific powdery mildew resistance genes in hybrid barley cultivars. Sci. Rep. 2020, 10, 18930. [Google Scholar] [CrossRef]
- Kang, Y.; Zhou, M.; Merry, A.; Barry, K. Mechanisms of powdery mildew resistance of wheat—A review of molecular breeding. Plant Pathol. 2020, 69, 601–617. [Google Scholar] [CrossRef]
- Liang, P. Powdery Mildew Adapts to a Specialized In Vivo Nutritional Lifestyle through the Contraction of Carbohydrate Metabolism Pathways and Diversification of Effector Molecules. Ph.D. Thesis, Hainan University, Haikou, China, 2018. [Google Scholar]
- Pearce, W.L.; VanSanford, D.A.; Hershman, D.E. Partial resistance to powdery mildew in soft red winter wheat. Plant Dis. 1996, 80, 1359–1362. [Google Scholar] [CrossRef]
- Pap, P.; Stojnic, S.; Nikolic, N.; Orlovic, S.; Markovic, M.; Vasic, V.; Stevanov, M. Impact of Erysiphe alphitoides (Griffon & Maubl.) U. Braun & S. Takam. on leaf physiological parameters in pedunculate oak (Quercus robur L.) saplings. Balt. For. 2014, 20, 2–9. [Google Scholar]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol. J. 2019, 35, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Haddoudi, I.; Mhadhbi, H.; Gargouri, M.; Barhoumi, F.; Romdhane, S.B.; Mrabet, M. Occurrence of fungal diseases in faba bean (Vicia faba L.) under salt and drought stress. Eur. J. Plant Pathol. 2021, 159, 385–398. [Google Scholar] [CrossRef]
- Ochola, D.; Ocimati, W.; Tinzaara, W.; Blomme, G.; Karamura, E.B. Effects of water stress on the development of banana Xanthomonas wilt disease. Plant Pathol. 2015, 64, 552–558. [Google Scholar] [CrossRef]
- Guilpart, N.; Roux, S.; Gary, C.; Metay, A. The trade-off between grape yield and grapevine susceptibility to powdery mildew and grey mould depends on inter-annual variations in water stress. Agric. For. Meteorol. 2017, 234, 203–211. [Google Scholar] [CrossRef]
- Harth, J.E.; Ferrari, M.J.; Tooker, J.F.; Stephenson, A.G. Zucchini yellow mosaic virus infection limits establishment and severity of powdery mildew in wild populations of Cucurbita pepo. Front. Plant Sci. 2018, 9, 792. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Tan, X.L.; Zhang, Z.F.; Zhu, J.Y.; Tian, H.G.; Liu, T.X. Infection of powdery mildew reduces the fitness of grain aphids (Sitobion avenae) through restricted nutrition and induced defense response in wheat. Front. Plant Sci. 2018, 9, 778. [Google Scholar] [CrossRef]
- Rohani, P.; Green, C.J.; Mantilla-Beniers, N.B.; Grenfell, B.T. Ecological interference between fatal diseases. Nature 2003, 422, 885–888. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Carrion, V.J.; Cordovez, V.; Tyc, O.; Etalo, D.W.; de Bruijn, I.; de Jager, V.C.L.; Medema, M.H.; Eberl, L.; Raaijmakers, J.M. Involvement of Burkholderiaceae and sulfurous volatiles in disease-suppressive soils. ISME J. 2018, 12, 2307–2321. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, J.M.; Mazzola, M. Soil immune responses soil microbiomes may be harnessed for plant health. Science 2016, 352, 1392–1393. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmolle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.X.; Cheng, H.H.; Li, J.Y.; Liu, Z.Y.; Peng, L.J. First report of Colletotrichum boninense causing anthracnose on goldthread (Coptis chinensis) in China. Plant Dis. 2020, 104, 1538–1539. [Google Scholar] [CrossRef]
- Li, H.S.; Sun, Q.; Zhao, S.J.; Zhang, W.H. Principle and Techniques of Botanic, Chemical and Physiological Experiments, 1st ed.; Senior Education Press: Beijing, China, 2000. [Google Scholar]
- Wang, P.; Luan, F.S.; Chen, K.N.; Dou, B.Q. Comparison of staining effects of four staining techniques for observation of Sphaerotheca fuliginea. Mycosystema 2008, 27, 673–678. [Google Scholar]
- Castro, G.L.S.; Silva Junior, D.D.; Bueno, A.C.S.O.; Silva, G.B. Anthracnose in acai palm leaves reduces leaf gas exchange and chlorophyll a fluorescence. Trop. Plant Pathol. 2017, 42, 13–20. [Google Scholar] [CrossRef]
- Lobato, A.K.S.; Goncalves-Vidigal, M.C.; Vidigal Filho, P.S.; Andrade, C.A.B.; Kvitschal, M.V.; Bonato, C.M. Relationships between leaf pigments and photosynthesis in common bean plants infected by anthracnose. N. Z. J. Crop Hortic. Sci. 2010, 38, 29–37. [Google Scholar] [CrossRef]
- Watanabe, M.; Kitaoka, S.; Eguchi, N.; Watanabe, Y.; Satomura, T.; Takagi, K.; Satoh, F.; Koike, T. Photosynthetic traits and growth of Quercus mongolica var. crispula sprouts attacked by powdery mildew under free-air CO2 enrichment. Eur. J. For. Res. 2014, 133, 725–733. [Google Scholar] [CrossRef]
- Moriondo, M.; Orlandini, S.; Giuntoli, A.; Bindi, M. The effect of downy and powdery mildew on grapevine (Vitis vinifera L.) leaf gas exchange. J. Phytopathol. 2005, 153, 350–357. [Google Scholar] [CrossRef]
- Lopes, D.B.; Berger, R.D. The effects of rust and anthracnose on the photosynthetic competence of diseased bean leaves. Phytopathology 2001, 91, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Pitsili, E.; Phukan, U.J.; Coll, N.S. Cell death in plant immunity. CSH Perspect. Biol. 2020, 12, a036483. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.L.; Hou, J.; Liu, L.P.; Liu, Z.Y.; Pan, Y.; Zhang, X.M.; Hu, T.J.; Liu, F.; Yu, Y. First report of anthracnose disease caused by Colletotrichum truncatum on the velvetleaf (Abutilon theophrasti) in China. Plant Dis. 2020, 104, 565. [Google Scholar] [CrossRef]
- Hu, J.L.; Zheng, M.Z.; Dang, S.Z.; Shi, M.; Zhang, J.; Li, Y.Z. Biocontrol potential of Bacillus amyloliquefaciens LYZ69 against anthracnose of alfalfa (Medicago sativa). Phytopathology 2021, 111, 1338–1348. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, T.; Xu, H.; Xu, G.; Qian, G.; Liu, F. Characterization of Lysobacter spp. strains and their potential use as biocontrol agents against pear anthracnose. Microbiol. Res. 2021, 242, 126624. [Google Scholar] [CrossRef]
- Zheng, X.R.; Zhang, M.J.; Shang, X.L.; Fang, S.Z.; Chen, F.M. Etiology of Cyclocarya paliurus anthracnose in Jiangsu Province, China. Front. Plant Sci. 2021, 11, 613499. [Google Scholar] [CrossRef]
- Huang, J.G.; Chi, M.Y.; Sun, X.M.; Qian, H.W.; Liang, W.X.; Zhou, X.F. First report of powdery mildew caused by Erysiphe alphitoides on Euonymus japonicas and its natural teleomorph stage in China. Plant Dis. 2017, 101, 387. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, B.Y.; Li, S.; Guo, A.H. Disease-weather relationships for wheat powdery mildew under climate change in China. J. Agric. Sci. 2017, 155, 1239–1252. [Google Scholar] [CrossRef]
- Chai, A.L.; Guo, W.T.; Kang, H.J.; Wu, C.L.; Li, B.J. First report of powdery mildew caused by Podosphaera xanthii on Momordica cochinchinensis in China. Plant Dis. 2018, 102, 2375. [Google Scholar] [CrossRef]
- Pei, D.L.; Zhang, Q.C.; Guo, Y.Y.; Wang, X.Y.; Yu, Z. First report of powdery mildew caused by Podosphaera fusca on Helianthus tuberosus in China. Plant Dis. 2021, 105, 709. [Google Scholar] [CrossRef]
- Fondevilla, S.; Rubiales, D. Powdery mildew control in pea. A review. Agron. Sustain. Dev. 2012, 32, 401–409. [Google Scholar] [CrossRef]
- Chen, B.H.; Guo, W.L.; Yang, H.L.; Li, Q.F.; Zhou, J.G.; Li, X.Z. Photosynthetic properties and biochemical metabolism of Cucurbita moschatagenotypes following infection with powdery mildew. J. Plant Pathol. 2020, 102, 1021–1027. [Google Scholar] [CrossRef]
- Behr, M.; Humbeck, K.; Hause, G.; Deising, H.B.; Wirsel, S.G.R. The hemibiotroph Colletotrichum graminicola locally induces photosynthetically active green islands but globally accelerates senescence on aging maize leaves. Mol. Plant Microbe 2010, 23, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Scandalios, J.G. Oxidative stress: Molecular perception and transduction of signals triggering antioxidant gene defenses. Braz. J. Med. Biol. Res. 2005, 38, 995–1014. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ullah, F.; Zhou, D.X.; Yi, M.; Zhao, Y. Mechanisms of ROS regulation of plant development and stress responses. Front. Plant Sci. 2019, 10, 800. [Google Scholar] [CrossRef]
- Padder, B.A.; Kamfwa, K.; Awale, H.E.; Kelly, J.D. Transcriptome profiling of the Phaseolus vulgaris—Colletotrichum lindemuthianum pathosystem. PLoS ONE 2016, 11, e0165823. [Google Scholar] [CrossRef]
- Utsumi, Y.; Tanaka, M.; Kurotani, A.; Yoshida, T.; Mochida, K.; Matsui, A.; Ishitani, M.; Sraphet, S.; Whankaew, S.; Asvarak, T.; et al. Cassava (Manihot esculenta) transcriptome analysis in response to infection by the fungus Colletotrichum gloeosporioides using an oligonucleotide-DNA microarray. J. Plant Res. 2016, 129, 711–726. [Google Scholar] [CrossRef]
- Mishra, R.; Nanda, S.; Rout, E.; Chand, S.K.; Mohanty, J.N.; Joshi, R.K. Differential expression of defense-related genes in chilli pepper infected with anthracnose pathogen Colletotrichum truncatum. Physiol. Mol. Plant Pathol. 2017, 97, 1–10. [Google Scholar] [CrossRef]
- Diniz, I.; Azinheira, H.; Figueiredo, A.; Gichuru, E.; Oliveira, H.; Guerra-Guimaraes, L.; Silva, M.C. Fungal penetration associated with recognition, signaling and defence-related genes and peroxidase activity during the resistance response of coffee to Colletotrichum kahawae. Physiol. Mol. Plant Pathol. 2019, 105, 119–127. [Google Scholar] [CrossRef]
- Moharam, M.H.A. Induction of defence-related biochemical changes in okra leaves to powdery mildew disease by several plant-derived agents. Arch. Phytopathol. Plant Prot. 2013, 46, 1667–1682. [Google Scholar] [CrossRef]
- Soliman, M.H.; El-Mohamedy, R.S.R. Induction of defense-related physiological and antioxidant enzyme response against powdery mildew disease in okra (Abelmoschus esculentus L.) plant by using chitosan and potassium salts. Mycobiology 2017, 45, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Hafez, Y.M.; El-Nagar, A.S.; Elzaawely, A.A.; Kamel, S.; Maswada, H.F. Biological control of Podosphaera xanthii the causal agent of squash powdery mildew disease by upregulation of defense-related enzymes. Egypt. J. Biol. Pest Control. 2018, 28, 57. [Google Scholar] [CrossRef]
- Sarhan, E.A.D.; Abd-Elsyed, M.H.F.; Ebrahiem, A.M.Y. Biological control of cucumber powdery mildew (Podosphaera xanthii) (Castagne) under greenhouse conditions. Egypt. J. Biol. Pest Control. 2020, 30, 65. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, Y.; Zhou, S. Comparative transcriptomic analyses of powdery mildew resistant and susceptible cultivated cucumber (Cucumis sativus L.) varieties to identify the genes involved in the resistance to Sphaerotheca fuliginea infection. Peer J. 2020, 8, e8250. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Pieterse, C.M.J.; de Jonge, R.; Berendsen, R.L. The Soil-Borne Legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhu, R.; Gao, F.; Duan, T. Prior Infection by Colletotrichum spinaciae Lowers the Susceptibility to Infection by Powdery Mildew in Common Vetch. Plants 2024, 13, 52. https://doi.org/10.3390/plants13010052
Li F, Zhu R, Gao F, Duan T. Prior Infection by Colletotrichum spinaciae Lowers the Susceptibility to Infection by Powdery Mildew in Common Vetch. Plants. 2024; 13(1):52. https://doi.org/10.3390/plants13010052
Chicago/Turabian StyleLi, Faxi, Rui Zhu, Feng Gao, and Tingyu Duan. 2024. "Prior Infection by Colletotrichum spinaciae Lowers the Susceptibility to Infection by Powdery Mildew in Common Vetch" Plants 13, no. 1: 52. https://doi.org/10.3390/plants13010052
APA StyleLi, F., Zhu, R., Gao, F., & Duan, T. (2024). Prior Infection by Colletotrichum spinaciae Lowers the Susceptibility to Infection by Powdery Mildew in Common Vetch. Plants, 13(1), 52. https://doi.org/10.3390/plants13010052







