Cadmium-Tolerant Bacterium Strain Cdb8-1 Contributed to the Remediation of Cadmium Pollution through Increasing the Growth and Cadmium Uptake of Chinese Milk Vetch (Astragalus sinicus L.) in Cadmium-Polluted Soils
Abstract
:1. Introduction
2. Results
2.1. Isolation and Identification of Cd-Tolerant Bacteria
2.2. Effects of Cdb8-1 Inoculation on Chinese Milk Vetch Growth in Cd-Contaminated Soil
2.3. Effects of Cdb8-1 on the Photosynthetic Pigment Contents of Chinese Milk Vetch under Cd-Contaminated Soil
2.4. Effects of Cdb8-1 Inoculation on the Antioxidant Enzyme System and Contents of H2O2 and MDA of Chinese Milk Vetch under Cd-Contaminated Soil
2.5. Effects of Cdb8-1 on Cd Uptake by Chinese Milk Vetch under Cd-Contaminated Soil
2.6. Effects of Cdb8-1 on Soil Physiochemical Properties by Chinese Milk Vetch under Cd-Contaminated Soil
2.7. Redundant Analysis of TCd, ACd and Physicochemical Factors Respectively
3. Materials and Methods
3.1. Materials
3.2. Bacterial 16S rRNA Sequencing and Phylogenetic Analysis
3.3. Pot Experiments
3.4. Methods
3.5. Data Analysis
4. Discussion
4.1. Isolation and Characterization of Cd-Tolerant Bacteria
4.2. Cd-Tolerant Bacteria Cdb8-1 Promote Chinese Milk Vetch Growth in Cd-Contaminated Soil
4.3. Effects of Cdb8-1 on the Chlorophyll Content of Chinese Milk Vetch under Cd-Contaminated Soil
4.4. Effects of Cdb8-1 on the Antioxidant Defence System and Oxidative Damage of Chinese Milk Vetch under Cd-Contaminated Soil
4.5. Effects of Cdb8-1 Inoculation on Cd Uptake by Chinese Milk Vetch and Soil Physio-Chemical Properties
4.6. Effects of Cdb8-1 on Physical and Chemical Properties of the Soil by Chinese Milk Vetch in Cd-Contaminated Soil
4.7. RDA Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Environmental Implication
References
- Nicholson, F.A.; Smith, S.R.; Alloway, B.J.; Carlton-Smith, C.; Chambers, B.J. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ. 2003, 311, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Stefanowicz, A.M.; Woch, M.W.; Kapusta, P. Inconspicuous waste heaps left by historical Zn-Pb mining are hot spots of soil contamination. Geoderma 2014, 235–236, 1–8. [Google Scholar] [CrossRef]
- Kim, S.W.; Chae, Y.; Moon, J.; Kim, D.; Cui, R.; An, G.; Jeong, S.W.; An, Y.J. In Situ Evaluation of Crop Productivity and Bioaccumulation of Heavy Metals in Paddy Soils after Remediation of Metal-Contaminated Soils. J. Agric. Food Chem. 2017, 65, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, G.; Ge, X.; Xu, G.; Guan, Y. Novel insights into heavy metal pollution of farmland based on reactive heavy metals (RHMs): Pollution characteristics, predictive models, and quantitative source apportionment. J. Hazard. Mater. 2018, 360, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Saez, J.M.; Costa, J.S.D.; Colin, V.L.; Fuentes, M.S.; Cuozzo, S.A.; Benimeli, C.S.; Polti, M.A.; Amoroso, M.J. Actinobacteria: Current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 2017, 166, 41–62. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef]
- Ali, W.; Mao, K.; Zhang, H.; Junaid, M.; Xu, N.; Rasool, A.; Feng, X.; Yang, Z. Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J. Hazard. Mater. 2020, 397, 122720. [Google Scholar] [CrossRef]
- Mori, M.; Kotaki, K.; Gunji, F.; Kubo, N.; Kobayashi, S.; Ito, T.; Itabashi, H. Suppression of cadmium uptake in rice using fermented bark as a soil amendment. Chemosphere 2016, 148, 487–494. [Google Scholar] [CrossRef]
- Yun, S.W.; Park, C.G.; Jeon, J.H.; Darnault, C.J.G.; Baveye, P.C.; Yu, C. Dissolution behavior of As and Cd in submerged paddy soil after treatment with stabilizing agents. Geoderma 2016, 270, 10–20. [Google Scholar] [CrossRef]
- Wan, Y.; Camara, A.Y.; Yu, Y.; Wang, Q.; Guo, T.; Zhu, L.; Li, H. Cadmium dynamics in soil pore water and uptake by rice: Influences of soil-applied selenite with different water managements. Environ. Pollut. 2018, 240, 523–533. [Google Scholar] [CrossRef]
- Xu, X.; Xia, L.; Zhu, W.; Zhang, Z.; Huang, Q.; Chen, W. Role of Penicillium chrysogenum XJ-1 in the detoxification and bioremediation of cadmium. Front. Microbiol. 2015, 6, 1422. [Google Scholar] [CrossRef] [PubMed]
- Danh, L.T.; Truong, P.; Mammucari, R.; Tran, T.; Foster, N. Vetiver grass, Vetiveria zizanioides: A choice plant for phytoremediation of heavy metals and organic wastes. Int. J. Phytoremediation 2009, 11, 664–691. [Google Scholar] [CrossRef] [PubMed]
- Helmisaari, H.-S.; Salemaa, M.; Derome, J.; Kiikkilä, O.; Uhlig, C.; Nieminen, T.M. Remediation of Heavy Metal-Contaminated Forest Soil Using Recycled Organic Matter and Native Woody Plants. J. Environ. Qual. 2007, 36, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Volesky, B.; Holan, Z.R. Biosorption of Heavy Metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Heavy metal accumulation by bacteria and other microorganisms. Experientia 1990, 46, 834–840. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Babalola, O.O. Microbial and Plant-Assisted Bioremediation of Heavy Metal Polluted Environments: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef]
- Suksabye, P.; Pimthong, A.; Dhurakit, P.; Mekvichitsaeng, P.; Thiravetyan, P. Effect of biochars and microorganisms on cadmium accumulation in rice grains grown in Cd-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 962–973. [Google Scholar] [CrossRef]
- Siripornadulsil, S.; Siripornadulsil, W. Cadmium-tolerant bacteria reduce the uptake of cadmium in rice: Potential for microbial bioremediation. Ecotoxicol. Environ. Saf. 2013, 94, 94–103. [Google Scholar] [CrossRef]
- Ma, Q.; Li, J.; Aamer, M.; Huang, G. Effect of Chinese milk vetch (Astragalus Sinicus L.) and rice straw incorporated in paddy soil on greenhouse gas emission and soil properties. Agronomy 2020, 10, 717. [Google Scholar] [CrossRef]
- Sumi, A.; Sugata, S.; Yahiro, I.; Odawara, M. Effect of fertilizer and fixed nitrogen on the water use efficiency of genge (Astragalus sinicus L.). Plant Prod. Sci. 2015, 18, 104–108. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, H.; Li, S.; Zhang, Z.; Liao, Y.; Lu, Y.; Zhou, G.; Gao, S.; Nie, J.; Cao, W. Co-utilizing milk vetch, rice straw, and lime reduces the Cd accumulation of rice grain in two paddy soils in south China. Sci. Total Environ. 2022, 806, 150622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Deng, Y.; Fu, S.; Xu, M.; Zhu, P.; Liang, Y.; Yin, H.; Jiang, L.; Bai, L.; Liu, X.; et al. Reduction mechanism of Cd accumulation in rice grain by Chinese milk vetch residue: Insight into microbial community. Ecotoxicol. Environ. Saf. 2020, 202, 110908. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, X.X.; Lang, M.; Zhang, T.L. Effects of amendments on the fraction transform of heavy metals in soil contaminated by copper and cadmium. Zhongguo Huanjing Kexue/China Environ. Sci. 2012, 32, 1241–1249. [Google Scholar]
- Qin, J.; Long, J.; Peng, P.; Huang, J.; Tang, S.; Hou, H. Regrow Napier grass–Chinese milk vetch relay intercropping system: A cleaner production strategy in Cd-contaminated farmland. J. Clean. Prod. 2022, 339, 130724. [Google Scholar] [CrossRef]
- Zheng, S.; Liao, Y.; Xu, C.; Wang, Y.; Zhang, Q.; Zhu, Q.; Zhu, H.; Sun, Y.; Zhou, Y.; Zhong, D.; et al. Milk vetch returning reduces rice grain Cd concentration in paddy fields: Roles of iron plaque and soil reducing-bacteria. Chemosphere 2022, 308, 136158. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, Z.; Bai, Z.; Hou, H.; Yuan, Y.; Guo, A.; Li, Y. Effects of mycorrhizae and water conditions on perennial ryegrass growth in rare earth tailings. RSC Adv. 2019, 9, 10881–10888. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, L.; Cheng, G.; Shu, X.; Wang, N.; Zhang, F.; Zhuang, W.; Wang, Z. Physiological and molecular analysis reveals the differences of photosynthesis between colored and green leaf poplars. Int. J. Mol. Sci. 2021, 22, 8982. [Google Scholar] [CrossRef]
- Zong, X.J.; Li, D.P.; Gu, L.K.; Li, D.Q.; Liu, L.X.; Hu, X.L. Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta 2009, 229, 485–495. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, M.; Zhang, H.; Xiong, H.; Liu, P.; Ali, J.; Li, J.; Li, Z. OsMIOX, a myo-inositol oxygenase gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Plant Sci. 2012, 196, 143–151. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, D.X.; Lei, Q.; Chen, H.; Wang, L.; Li, Q.; Gao, Y.; Kong, J. Melatonin and its potential biological functions in the fruits of sweet cherry. J. Pineal Res. 2013, 55, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Hong, S.Y.; Jo, S.H.; Woo, J.C.; Lee, S.; Park, C.M. Molecular and functional characterization of cold-responsive C-repeat binding factors from Brachypodium distachyon. BMC Plant Biol. 2014, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, X.; Yin, S.; Kong, X.; Zhou, S.; Xu, Y.; Luo, Y.; Wang, W. The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol. Biochem. 2014, 84, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.A.; Alfaro, M.A.; Jarvis, S.C.; Gregory, P.J. Factors affecting potassium leaching in different soils. Soil. Use Manag. 2004, 20, 182–189. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Wang, Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of soil physicochemical properties and enzyme activities to long-term reclamation of coastal saline soil, Eastern China. Sci. Total Environ. 2017, 607–608, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.Y.; Yan, Y.J.; Yu, Z.Q.; Zhang, L.; Cheng, Y.Y.; Shi, W.L. Modification-bioremediation of copper, lead, and cadmium-contaminated soil by combined ryegrass (Lolium multiflorum Lam.) and Pseudomonas aeruginosa treatment. Environ. Sci. Pollut. Res. 2020, 27, 37668–37676. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Song, L.; Xu, X.; Zheng, Y.; Hong, W.; Li, X.; Ai, Y.; Wang, Y.; Zhang, Z.; Chen, H.; Huang, Y.; et al. Dynamic mechanisms of cadmium accumulation and detoxification by Lolium perenne grown in soil inoculated with the cadmium-tolerant bacterium strain Cdq4-2. Sci. Total Environ. 2023, 873, 162314. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, L.; He, X.; Chen, X.; Cui, Z. Long-term optimization of crop yield while concurrently improving soil quality. Land. Degrad. Dev. 2019, 30, 897–909. [Google Scholar] [CrossRef]
- Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere 2018, 194, 327–339. [Google Scholar] [CrossRef]
- Haider, F.U.; Liqun, C.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Wenjun, M.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.Q.; Yan, X.W.; Wei, G.H.; Zhang, J.H.; Fang, L.C. Rhizobium inoculation enhances copper tolerance by affecting copper uptake and regulating the ascorbate-glutathione cycle and phytochelatin biosynthesis-related gene expression in Medicago sativa seedlings. Ecotoxicol. Environ. Saf. 2018, 162, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Jeyasundar, P.G.S.A.; Ali, A.; Azeem, M.; Li, Y.; Guo, D.; Sikdar, A.; Abdelrahman, H.; Kwon, E.; Antoniadis, V.; Mani, V.M.; et al. Green remediation of toxic metals contaminated mining soil using bacterial consortium and Brassica juncea. Environ. Pollut. 2021, 277, 116789. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 5855. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, Z.; Gu, D.; Li, D.; Tao, Y.; Zhang, D.; Su, L.; Ao, Y. Characterization of cadmium-resistant rhizobacteria and their promotion effects on Brassica napus growth and cadmium uptake. J. Basic. Microbiol. 2019, 59, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Bashir, A.; Rizwan, M.; Rehman, M.Z.U.; Zubair, M.; Riaz, M.; Qayyum, M.F.; Alharby, H.F.; Bamagoos, A.A.; Ali, S. Application of co-composted farm manure and biochar increased the wheat growth and decreased cadmium accumulation in plants under different water regimes. Chemosphere 2020, 246, 125809. [Google Scholar] [CrossRef]
- Mishra, P.K.; Bisht, S.C.; Mishra, S.; Selvakumar, G.; Bisht, J.K.; Gupta, H.S. Coinoculation of Rhizobium leguminosarum-PR1 with a cold tolerant Pseudomonas sp. improves iron acquisition, nutrient uptake and growth of field pea (Pisum sativum L.). J. Plant Nutr. 2012, 35, 243–256. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. BioMetals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Ke, T.; Guo, G.; Liu, J.; Zhang, C.; Tao, Y.; Wang, P.; Xu, Y.; Chen, L. Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ. Pollut. 2021, 271, 116314. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rehman, M.Z.U.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Kong, Z.; Mohamad, O.A.; Deng, Z.; Liu, X.; Glick, B.R.; Wei, G. Rhizobial symbiosis effect on the growth, metal uptake, and antioxidant responses of Medicago lupulina under copper stress. Environ. Sci. Pollut. Res. 2015, 22, 12479–12489. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Xi, J.; Ke, J.; Wang, Y.; Chen, X.; Zhang, Z.; Lin, Y. Deciphering soil amendments and actinomycetes for remediation of cadmium (Cd) contaminated farmland. Ecotoxicol. Environ. Saf. 2023, 249, 114388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Y.; Wang, P.; Zhang, H.; Ali, E.F.; Li, R.; Shaheen, S.M.; Zhang, Z. Lactic acid bacteria promoted soil quality and enhanced phytoextraction of Cd and Zn by mustard: A trial for bioengineering of toxic metal contaminated mining soils. Environ. Res. 2023, 216, 114646. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, G.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Zhu, L.; Li, T.; Wang, C.; Zhang, X.; Xu, L.; Xu, R.; Zhao, Z. The effects of dark septate endophyte (DSE) inoculation on tomato seedlings under Zn and Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 35232–35241. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Song, Y.; Kai, J.; Yang, X.; Ji, P. Evaluation of EROD and CYP3A4 activities in earthworm Eisenia fetida as biomarkers for soil heavy metal contamination. J. Hazard. Mater. 2012, 243, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, J.; Yue, F.; Yan, X.; Wang, F.; Bloszies, S.; Wang, Y. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere 2018, 194, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Liu, L.; Yan, D.; He, Y. Physiological stress responses, mineral element uptake and phytoremediation potential of Morus alba L. in cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109973. [Google Scholar] [CrossRef]
- Llimós, M.; Bistué, M.; Marcelino, J.; Poschenrieder, C.; Martos, S. A native Zn-solubilising bacterium from mine soil promotes plant growth and facilitates phytoremediation. J. Soils Sediments 2021, 21, 2301–2314. [Google Scholar] [CrossRef]
- Sharma, P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: An update. Bioresour. Technol. 2021, 328, 124835. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.Y.; Zhang, X.Z.; Luo, H.J.; Fu, J.M. Toxic effect of NaCl on ion metabolism, antioxidative enzymes and gene expression of perennial ryegrass. Ecotoxicol. Environ. Saf. 2011, 74, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.F.; Rehman, M.Z.U.; Ali, S.; Rizwan, M.; Naeem, A.; Maqsood, M.A.; Khalid, H.; Rinklebe, J.; Ok, Y.S. Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 2017, 174, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, C.; Wang, J.; Zhou, C.; Feng, H.; Mahajan, M.D.; Han, X. Influence and interaction of iron and cadmium on photosynthesis and antioxidative enzymes in two rice cultivars. Chemosphere 2017, 171, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, X.; Wang, X.; Tang, Y.; Zhang, H.; Yuan, Z.; Zhou, J.; Han, Y.; Li, T. Bioremediation of a saline-alkali soil polluted with Zn using ryegrass associated with Fusarium incarnatum. Environ. Pollut. 2022, 312, 119929. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Tam, N.F.Y. Differences in lead tolerance between Kandelia obovata and Acanthus ilicifolius seedlings under varying treatment times. Aquat. Toxicol. 2013, 126, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Lima, I.M.; Klasson, K.T.; Chang, S.; Wartelle, L.H.; Rodgers, J.E. Immobilization of heavy metal ions (CuII, CdII, NiII, and PbII) by broiler litter-derived biochars in water and soil. J. Agric. Food Chem. 2010, 58, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, M.M.; van Gestel, C.A.M. Sorption and pH determine the long-term partitioning of cadmium in natural soils. Environ. Sci. Pollut. Res. 2016, 23, 18492–18501. [Google Scholar] [CrossRef]
- Yin, Z.; Song, L.; Lin, Z.; Hui, K.; Wang, Q.; Song, H.; Xuan, L.; Wang, Z.; Gao, W. Granular activated carbon-supported titanium dioxide nanoparticles as an amendment for amending copper-contaminated sediments: Effect on the pH in sediments and enzymatic activities. Ecotoxicol. Environ. Saf. 2020, 206, 111325. [Google Scholar] [CrossRef]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 2007, 69, 220–228. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, S.; Liu, D.; Yu, Z.; Zhang, L.; Cui, J.; Xie, K.; Li, T.; Fu, C. Mobility and potential risk of sediment-associated heavy metal fractions under continuous drought-rewetting cycles. Sci. Total Environ. 2018, 625, 79–86. [Google Scholar] [CrossRef]
- Shi, G.L.; Lu, H.Y.; Liu, J.Z.; Lou, L.Q.; Tang, X.J.; Wu, Y.H.; Ma, H.X. Periphyton growth reduces cadmium but enhances arsenic accumulation in rice (Oryza sativa) seedlings from contaminated soil. Plant Soil. 2017, 421, 137–146. [Google Scholar] [CrossRef]
- Zeng, H.Y.; Chen, L.H.; Yang, Y.; Deng, X.; Zhou, X.H.; Zeng, Q.R. Basal and Foliar Treatment using an Organic Fertilizer Amendment Lowers Cadmium Availability in Soil and Cadmium Uptake by Rice on Field Micro-Plot Experiment Planted in Contaminated Acidic Paddy Soil. Soil. Sediment. Contam. 2019, 28, 1–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Liu, T.; Liu, B.; Huang, D.; Zhu, Q.; Xu, C. The influence of liming on cadmium accumulation in rice grains via iron-reducing bacteria. Sci. Total Environ. 2018, 645, 109–118. [Google Scholar] [CrossRef] [PubMed]
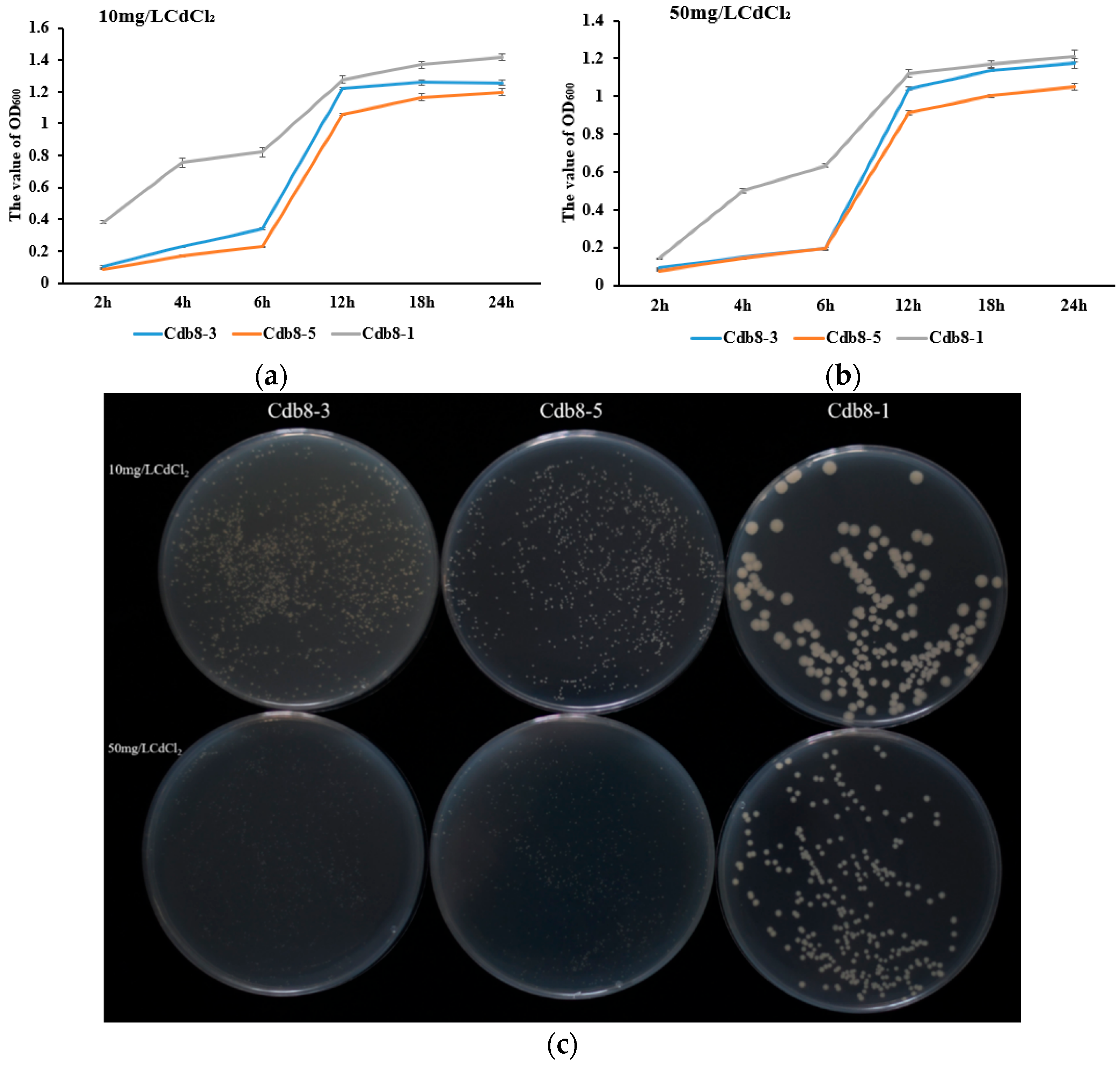
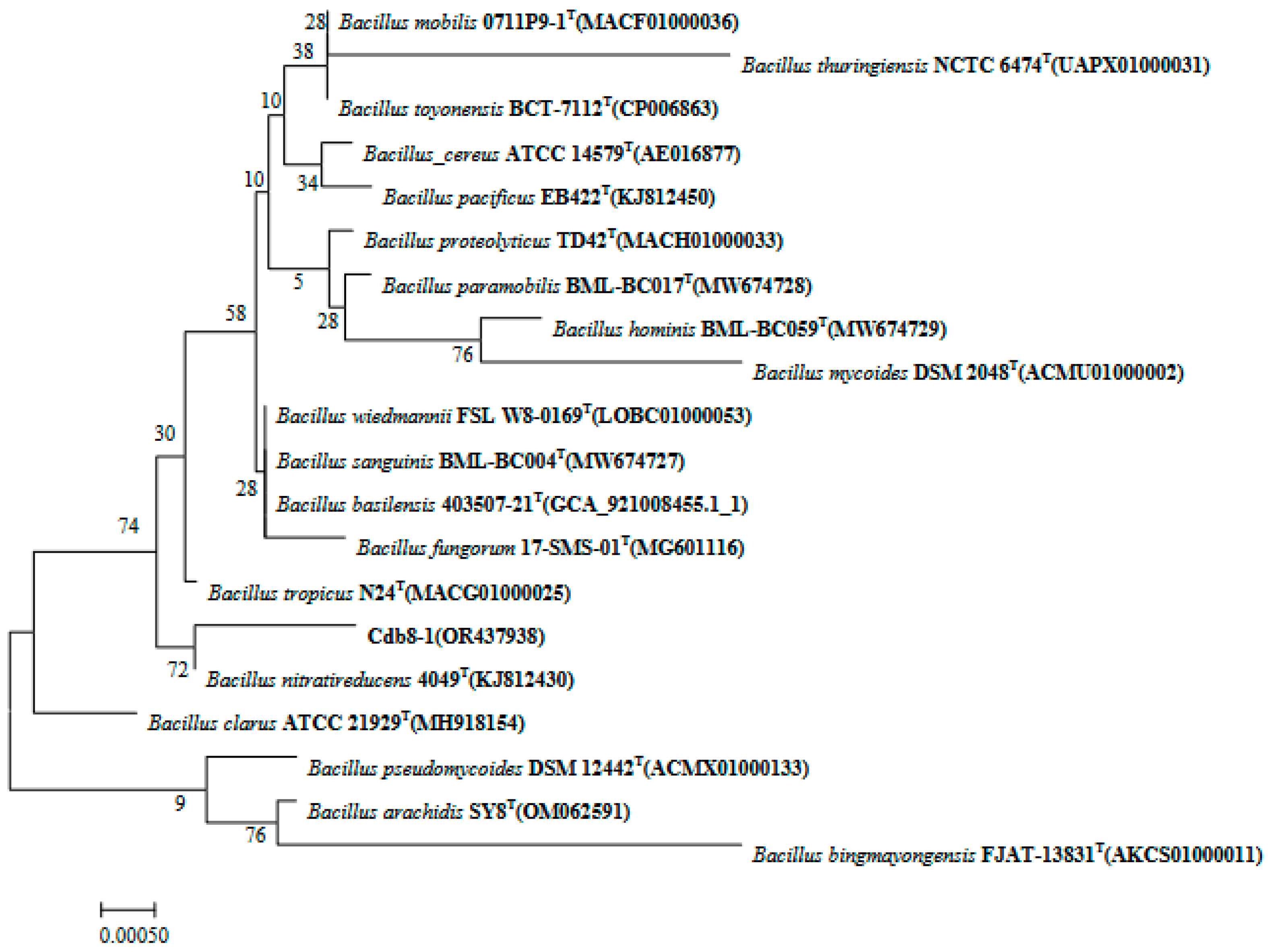
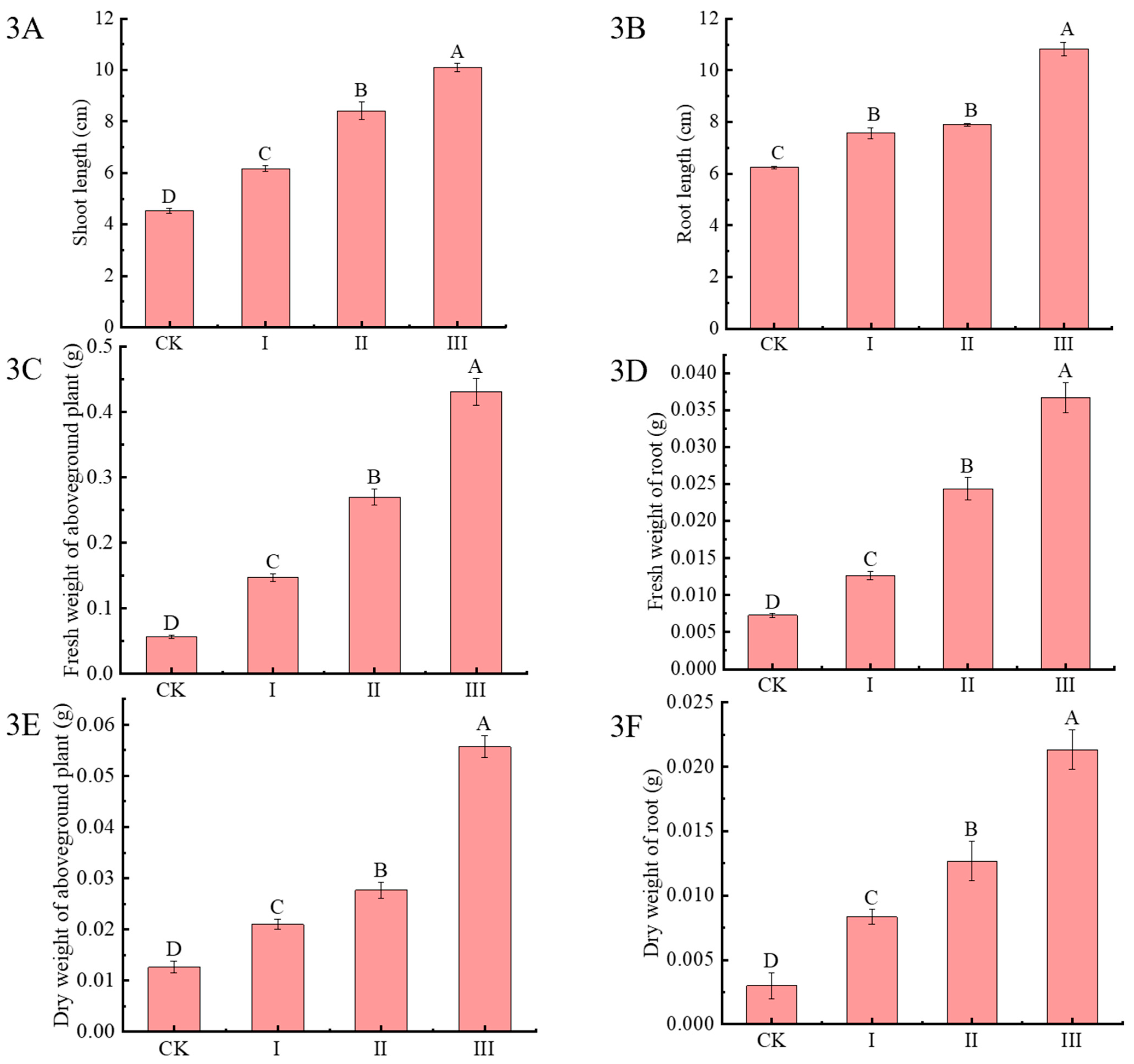


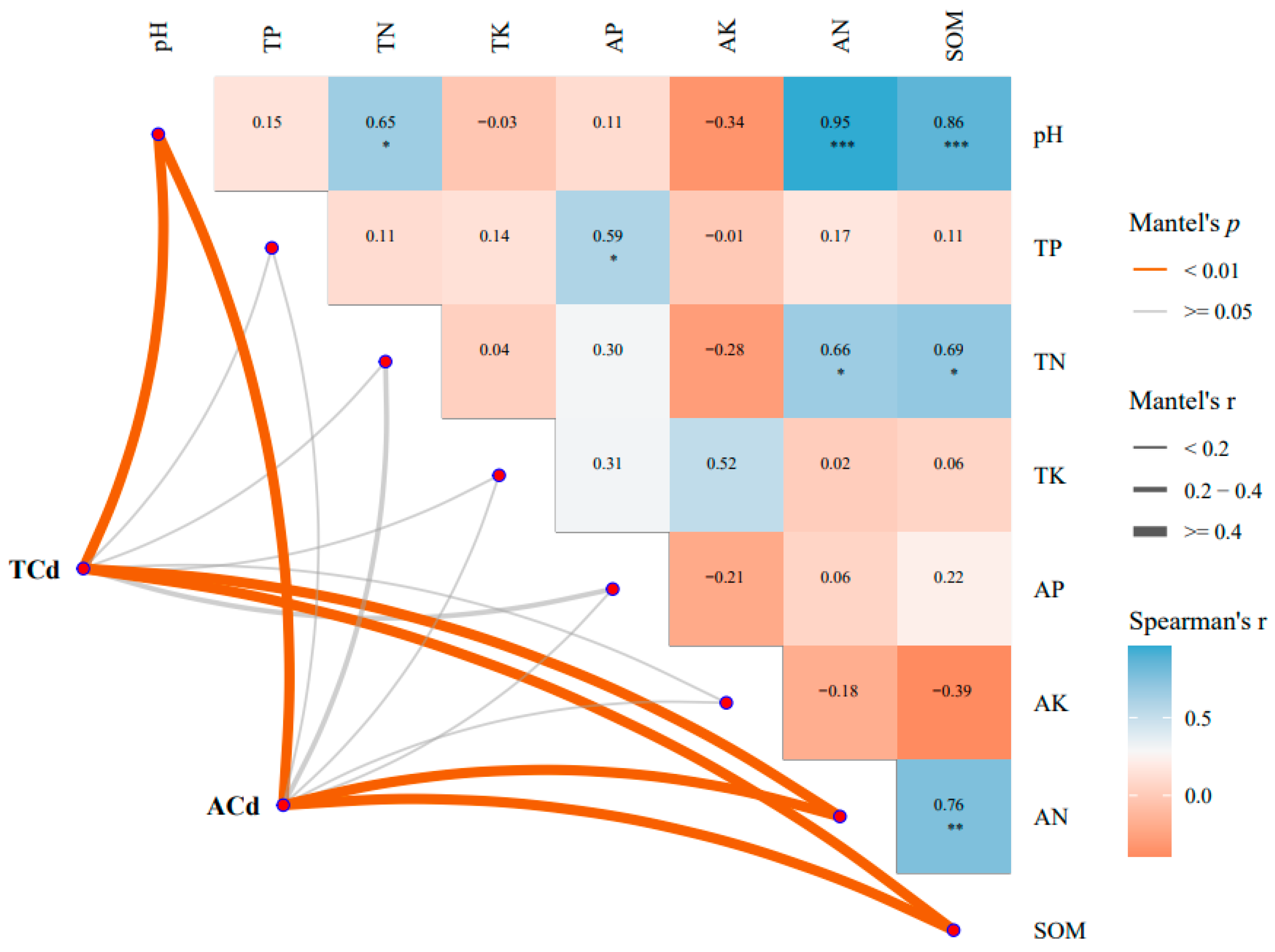
| Treatment | Cd Content in Soil (mg/kg) | Cd Content in the Aboveground Parts (mg/kg) | Cd Content in the Underground Parts (mg/kg) | Cd Content in the Whole Plant (mg/kg) |
|---|---|---|---|---|
| CK | 1.65 ± 0.03 A | 0.31 ± 0.01 A | 1.92 ± 0.12 C | 2.23 ± 0.12 B |
| I | 1.44 ± 0.02 B | 0.29 ± 0.02 A | 2.87 ± 0.15 B | 3.16 ± 0.16 A |
| II | 1.35 ± 0.04 C | 0.26 ± 0.03 A | 3.18 ± 0.21 AB | 3.44 ± 0.18 A |
| III | 1.23 ± 0.02 D | 0.17 ± 0.01 B | 3.34 ± 0.05 A | 3.52 ± 0.04 A |
| Treatment | Bioconcentration Factor (BCF) | Translocation Factor (TF) | ||
|---|---|---|---|---|
| Aboveground Parts | Underground Parts | Whole Plant | ||
| CK | 0.19 ± 0.01 A | 1.17 ± 0.05 D | 1.35 ± 0.05 D | 0.16 ± 0.01 A |
| I | 0.20 ± 0.02 A | 2.00 ± 0.13 C | 2.20 ± 0.14 C | 0.10 ± 0.00 B |
| II | 0.19 ± 0.02 A | 2.35 ± 0.14 B | 2.54 ± 0.13 B | 0.08 ± 0.01 B |
| II | 0.14 ± 0.01 B | 2.72 ± 0.08 A | 2.86 ± 0.07 A | 0.05 ± 0.00 C |
| PH | TP (g/kg) | TN (g/kg) | TK (g/kg) | AP (mg/kg) | AK (mg/kg) | AN (mg/kg) | SOM (g/kg) | ACd (mg/kg) | TCd (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|
| CK | 8.01 ± 0.02 B | 0.61 ± 0.06 AB | 1.24 ± 0.08 A | 11.91 ± 0.21 A | 19.20 ± 2.13 A | 243.00 ± 14.27 A | 115.77 ± 4.58 C | 18.72 ± 0.53 B | 0.65 ± 0.04 A | 1.65 ± 0.03 A |
| I | 8.07 ± 0.03 B | 0.60 ± 0.02 AB | 1.20 ± 0.10 A | 12.15 ± 0.61 A | 18.28 ± 0.73 A | 242.67 ± 14.70 A | 122.42 ± 3.36 BC | 19.05 ± 0.14 B | 0.58 ± 0.01 B | 1.44 ± 0.02 B |
| II | 8.17 ± 0.03 A | 0.53 ± 0.01 B | 1.30 ± 0.07 A | 11.89 ± 0.17 A | 18.02 ± 0.30 A | 228.52 ± 14.58 A | 134.26 ± 5.73 B | 20.24 ± 0.27 A | 0.55 ± 0.01 BC | 1.35 ± 0.04 C |
| III | 8.23 ± 0.03 A | 0.67 ± 0.06 A | 1.33 ± 0.01 A | 11.76 ± 0.36 A | 19.31 ± 0.43 A | 228.48 ± 14.46 A | 153.76 ± 7.40 A | 20.49 ± 0.41 A | 0.50 ± 0.01 C | 1.23 ± 0.02 D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Sun, M.; Wang, Y.; Yan, T.; Li, Y.; Wu, X.; Wang, Y.; Zhuang, W. Cadmium-Tolerant Bacterium Strain Cdb8-1 Contributed to the Remediation of Cadmium Pollution through Increasing the Growth and Cadmium Uptake of Chinese Milk Vetch (Astragalus sinicus L.) in Cadmium-Polluted Soils. Plants 2024, 13, 76. https://doi.org/10.3390/plants13010076
Wang B, Sun M, Wang Y, Yan T, Li Y, Wu X, Wang Y, Zhuang W. Cadmium-Tolerant Bacterium Strain Cdb8-1 Contributed to the Remediation of Cadmium Pollution through Increasing the Growth and Cadmium Uptake of Chinese Milk Vetch (Astragalus sinicus L.) in Cadmium-Polluted Soils. Plants. 2024; 13(1):76. https://doi.org/10.3390/plants13010076
Chicago/Turabian StyleWang, Bo, Minghui Sun, Yuekai Wang, Tengyue Yan, Yuhang Li, Xinxin Wu, Youbao Wang, and Weibing Zhuang. 2024. "Cadmium-Tolerant Bacterium Strain Cdb8-1 Contributed to the Remediation of Cadmium Pollution through Increasing the Growth and Cadmium Uptake of Chinese Milk Vetch (Astragalus sinicus L.) in Cadmium-Polluted Soils" Plants 13, no. 1: 76. https://doi.org/10.3390/plants13010076





