Chitosan-GSNO Nanoparticles and Silicon Priming Enhance the Germination and Seedling Growth of Soybean (Glycine max L.)
Abstract
:1. Introduction
2. Results
2.1. Analysis of Variance (ANOVA) of Germination and Seedling Growth
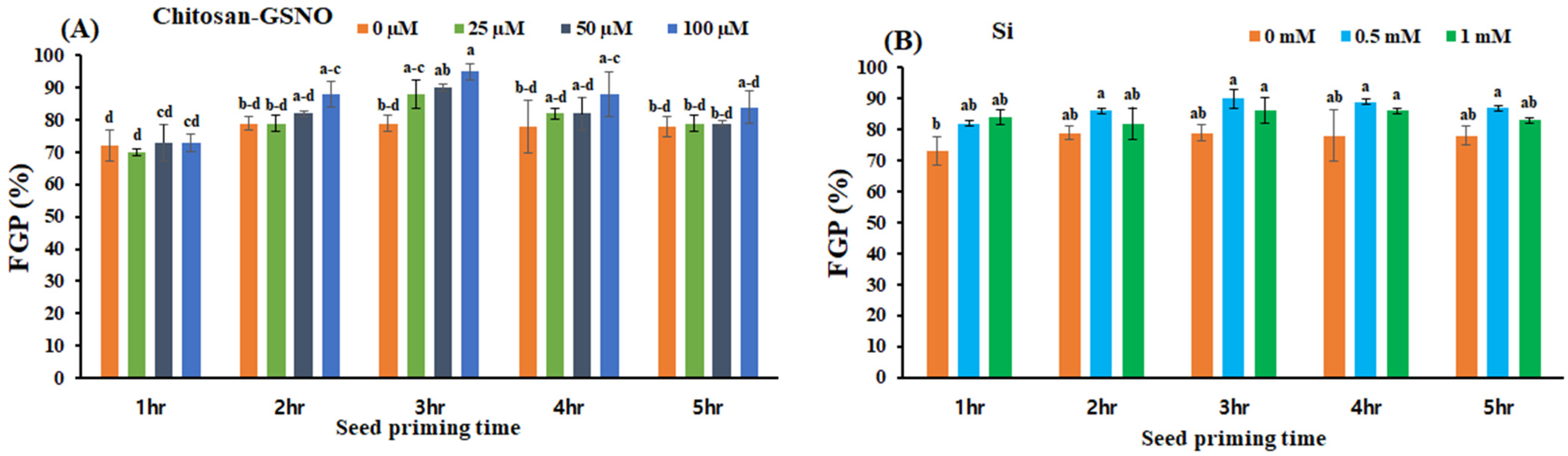
2.2. Effects of Chitosan-GSNO Nanoparticles and Si on the FGP
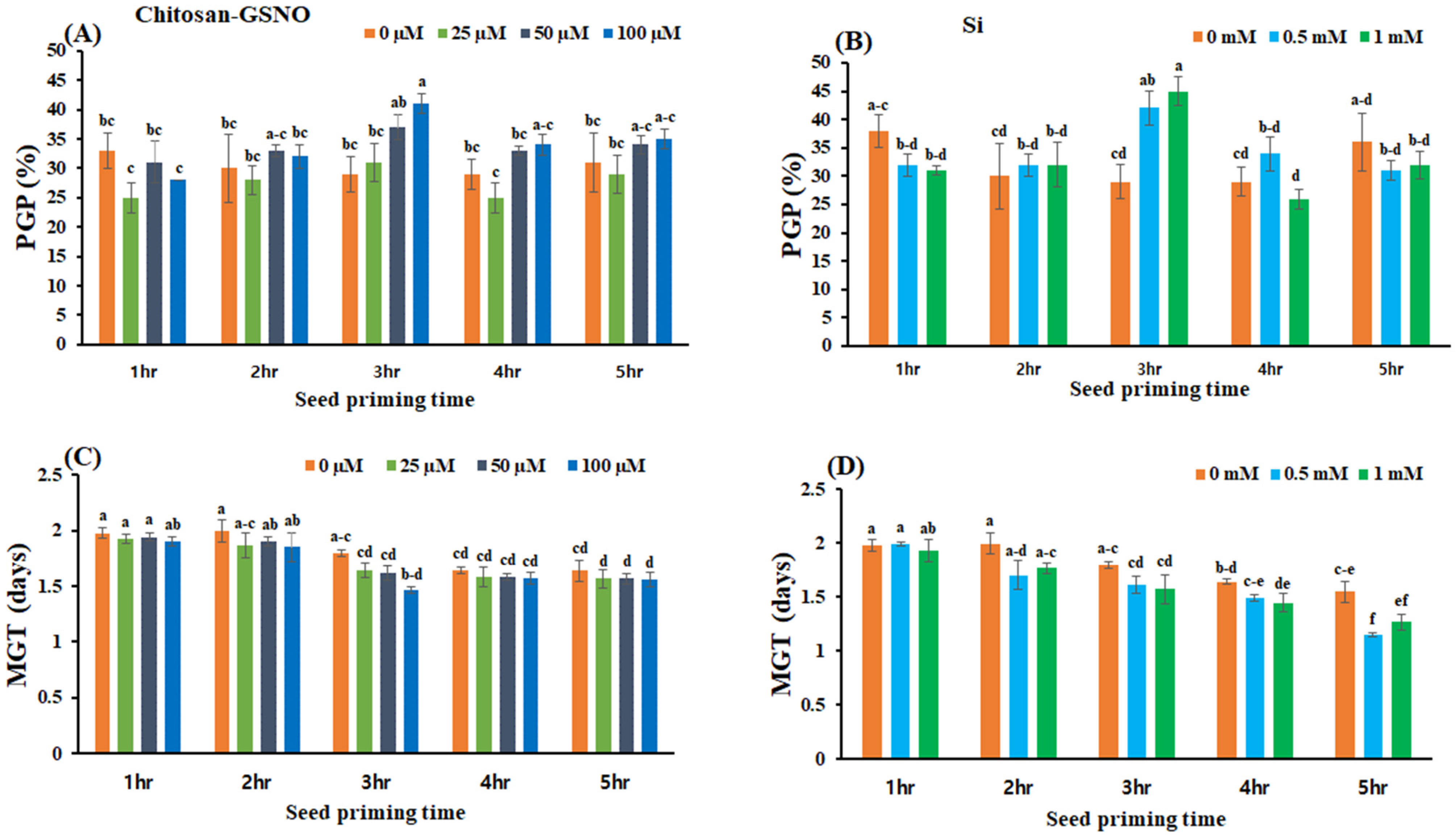
2.3. Influence of Chitosan-GSNO Nanoparticles and Si on the PGP and MGT
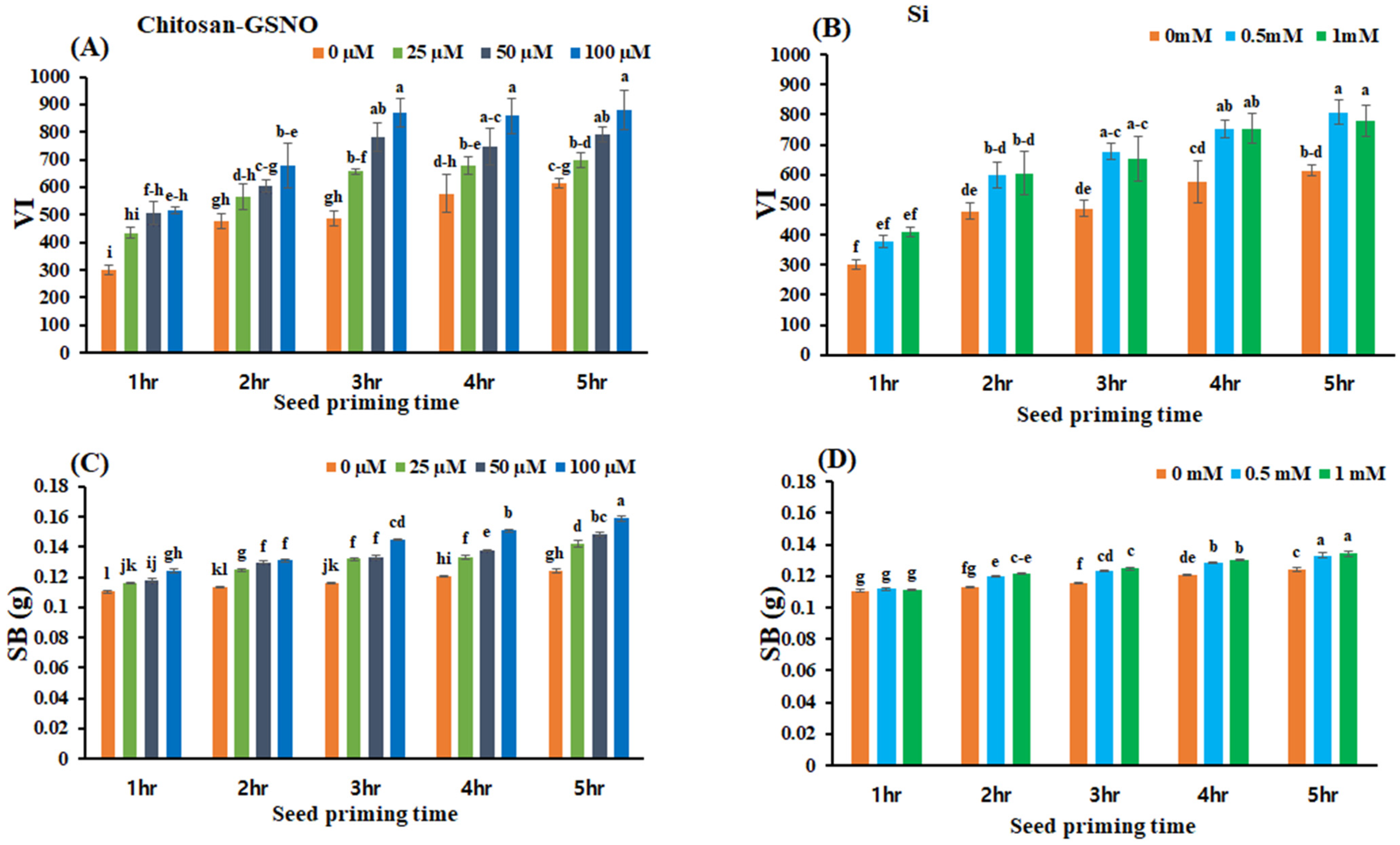
2.4. Effects of Chitosan-GSNO Nanoparticles and Si on the VI and SB
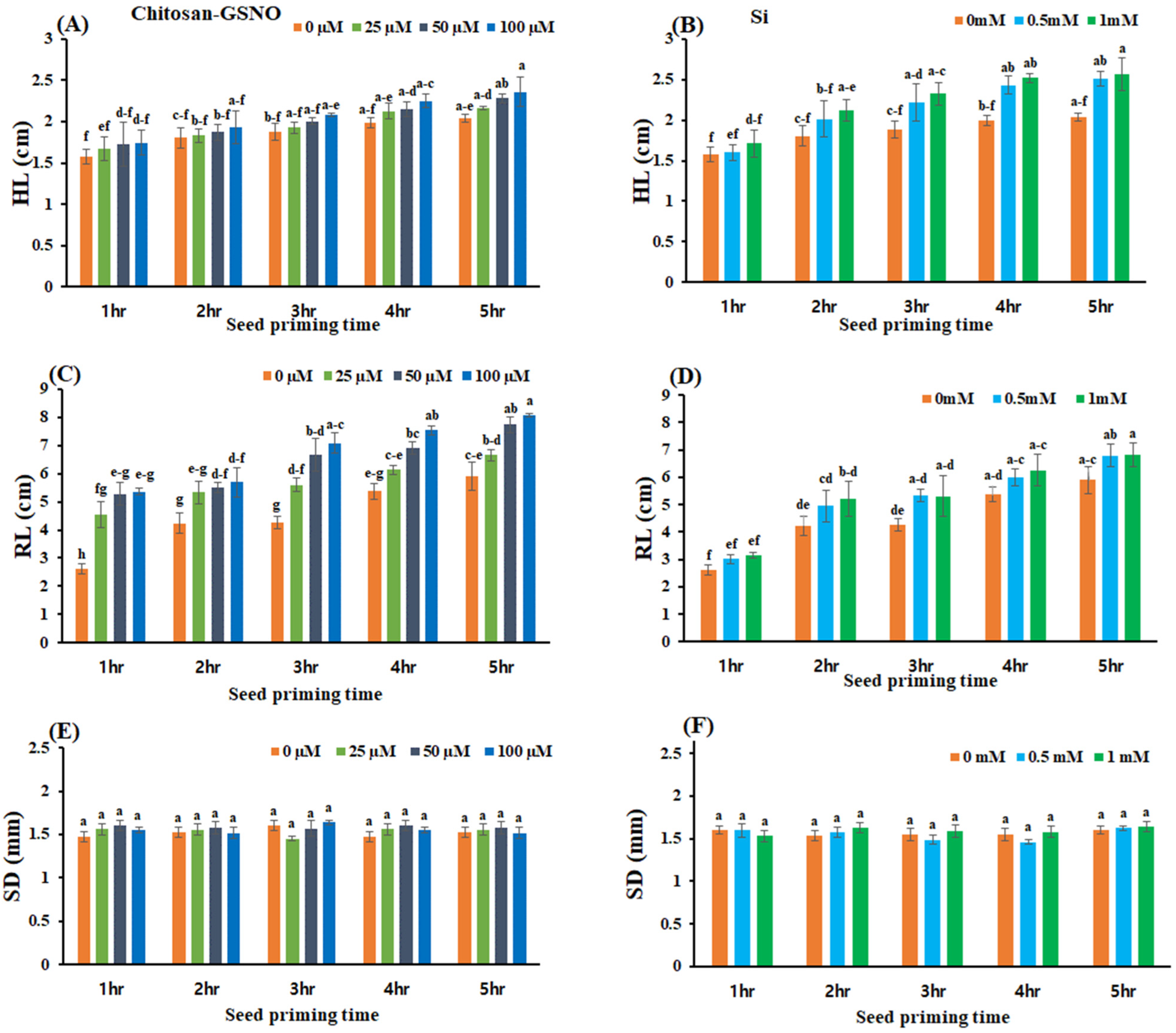
2.5. Impact of Chitosan-GSNO Nanoparticles and Si on HL, RL, and SD
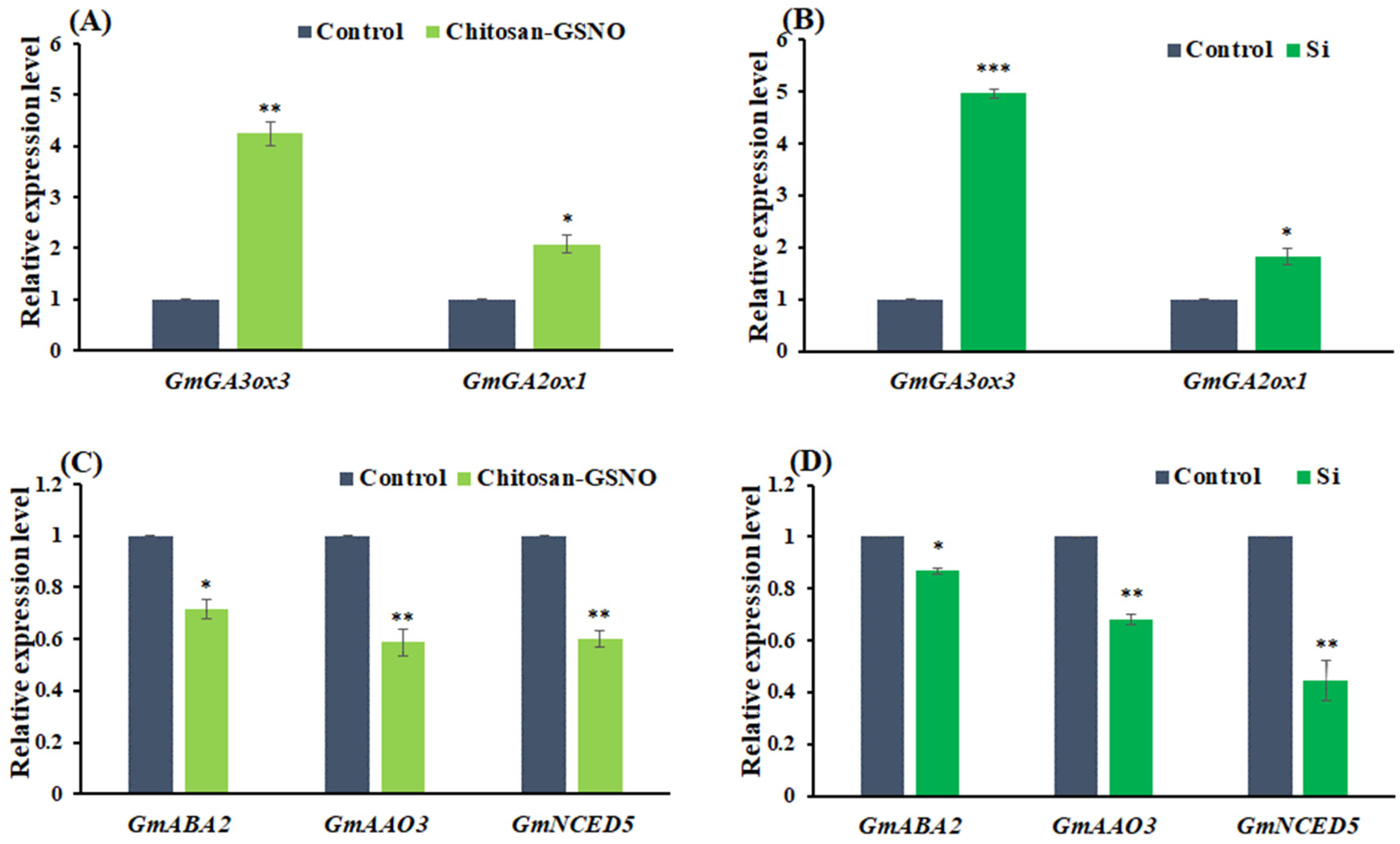
2.6. GA- and ABA-Pathway-Related Gene Expression in Soybean Seeds
2.7. Impact of Chitosan-GSNO Nanoparticle and Si Priming on Endogenous Bioactive GA4 and ABA
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Treatments, and Experimental Design
4.2. Seed Priming, Germination Testing, Growth Conditions, and Data Collection
4.3. Determination of Germination Parameters
4.4. Determination of Morphological Parameters
4.5. qRT-PCR Analysis of Gene Expression
4.6. Quantification of GA4 in Soybean Seeds
4.7. Quantification of ABA in Soybean Seeds
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Zhang, M.; Feng, F.; Tian, Z. Toward a “green revolution” for soybean. Mol. Plant. 2020, 13, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Kamal, G.M.; Nadeem, F.; Shabir, G. Variations of quality characteristics among oils of different soybean varieties. J. King Saud Univ. Sci. 2016, 28, 332–338. [Google Scholar] [CrossRef]
- Hamza, M.; Basit, A.W.; Shehzadi, I.; Tufail, U.; Hassan, A.; Hussain, T.; Siddique, M.U.; Hayat, H.M. Global Impact of Soybean Production: A Review. Asian J. Biochem. Genet. Mol. Biol. 2024, 16, 12–20. [Google Scholar] [CrossRef]
- Pagano, M.C.; Miransari, M. The importance of soybean production worldwide. In Abiotic and Biotic Stresses in Soybean Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–26. [Google Scholar]
- Johnson, K.V.H.; Krishna, T.P.A.; Dash, M.; Thiyageshwari, S.; Ceasar, S.A.; Selvi, D. Food and nutritional security: Innovative approaches for improving micronutrient use efficiency in soybean (Glycine max (L.) Merrill) under hostile soils. J. Soil Sci. Plant Nutr. 2023, 23, 56–70. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed germination and vigor: Ensuring crop sustainability in a changing climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The functions of the endosperm during seed germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Tsindi, A.; Kawuki, R.; Tukamuhabwa, P. Adaptation and stability of vegetable soybean genotypes in Uganda. Afr. Crop Sci. J. 2019, 27, 267–280. [Google Scholar] [CrossRef]
- Devika, O.S.; Singh, S.; Sarkar, D.; Barnwal, P.; Suman, J.; Rakshit, A. Seed priming: A potential supplement in integrated resource management under fragile intensive ecosystems. Front. Sustain. Food Syst. 2021, 5, 654001. [Google Scholar] [CrossRef]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Singapore, 2019; pp. 1–10. [Google Scholar]
- Singh, V.K.; Singh, R.; Tripathi, S.; Devi, R.S.; Srivastava, P.; Singh, P.; Kumar, A.; Bhadouria, R. Seed priming: State of the art and new perspectives in the era of climate change. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–170. [Google Scholar]
- El-Mougy, N.S.; Abdel-Kader, M.M. Long-term activity of bio-priming seed treatment for biological control of faba bean root rot pathogens. Australas. Plant Pathol. 2008, 37, 464–471. [Google Scholar] [CrossRef]
- Griffo, A.; Bosco, N.; Pagano, A.; Balestrazzi, A.; Macovei, A. Noninvasive Methods to Detect Reactive Oxygen Species as a Proxy of Seed Quality. Antioxidants 2023, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, S.; Łoziński, M.; Marczewski, K.; Kozak, M.; Schmidtke, K. Influence of priming on germination, development, and yield of soybean varieties. Open Agric. 2020, 5, 930–935. [Google Scholar] [CrossRef]
- Gou, T.; Chen, X.; Han, R.; Liu, J.; Zhu, Y.; Gong, H. Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiol. Plant. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Methela, N.J.; Pande, A.; Islam, M.S.; Rahim, W.; Hussain, A.; Lee, D.-S.; Mun, B.-G.; Maria Joseph Raj, N.P.; Kim, S.-J.; Kim, Y. Chitosan-GSNO nanoparticles: A positive modulator of drought stress tolerance in soybean. BMC Plant Biol. 2023, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.K.; Ma, J.F.; Bélanger, R.R. Role of silicon in plants. Front. Plant Sci. 2017, 8, 1858. [Google Scholar] [CrossRef] [PubMed]
- Tayade, R.; Ghimire, A.; Khan, W.; Lay, L.; Attipoe, J.Q.; Kim, Y. Silicon as a smart fertilizer for sustainability and crop improvement. Biomolecules 2022, 12, 1027. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, Y.; Duan, L.; Li, Z.; Eneji, A.E.; Li, J. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.K.; Song, X.-P.; Li, D.-M.; Singh, M.; Rajput, V.D.; Malviya, M.K.; Minkina, T.; Singh, R.K.; Singh, P.; Li, Y.-R. Interactive role of silicon and plant–rhizobacteria mitigating abiotic stresses: A new approach for sustainable agriculture and climate change. Plants 2020, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Shamshiripour, M.; Motesharezadeh, B.; Rahmani, H.A.; Alikhani, H.A.; Etesami, H. Optimal concentrations of silicon enhance the growth of soybean (Glycine Max L.) cultivars by improving nodulation, root system architecture, and soil biological properties. Silicon 2022, 14, 5333–5345. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Lamsaadi, N.; Lebreton, S.; Mouradi, M.; Cabassa, C.; Carol, P.; Savoure, A.; Farissi, M. Silicon improves seed germination and seedling growth and alleviates salt stress in Medicago sativa L. by regulating seed reserve mobilization and antioxidant system defense. Biologia 2023, 78, 1961–1977. [Google Scholar] [CrossRef]
- Rahbari, K.; Madandoust, M. The Effect of Seed Moisture Content of Hybrid Maize at Harvest Time on Seed Germination Traits and Antioxidant Enzymes Activity under Simulated Environmental Stresses with Silicon Foliar Application. Silicon 2024, 1–11. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Ceritoglu, M.; Erman, M.; Çığ, F.; Ceritoglu, F.; Uçar, Ö.; Soysal, S.; El Sabagh, A. Enhancement of Root System Architecture, Seedling Growth, and Germination in Lentil under Salinity Stress by Seed Priming with Silicon and Salicylic Acid. Pol. J. Environ. Stud. 2023, 32, 4481. [Google Scholar] [CrossRef] [PubMed]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and its application in agriculture in context of molecular weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Vijayan, S.; Nair, S.J.; Jisha, M.S. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int. J. Biol. Macromol. 2019, 124, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Joshi, A.; Kumari, S.; Kumaraswamy, R.V.; Saharan, V. Preparation of Cu-chitosan nanoparticle and its effect on growth and enzyme activity during seed germination in maize. J. Pharmacogn. Phytochem. 2017, 6, 669–673. [Google Scholar]
- Abdel-Aziz, H. Effect of priming with chitosan nanoparticles on germination, seedling growth and antioxidant enzymes of broad beans. Catrina Int. J. Environ. Sci. 2019, 18, 81–86. [Google Scholar] [CrossRef]
- Shu, K.; Liu, X.-d.; Xie, Q.; He, Z.-h. Two faces of one seed: Hormonal regulation of dormancy and germination. Mol. Plant. 2016, 9, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Waqas, M.; Korres, N.E.; Khan, M.D.; Nizami, A.-S.; Deeba, F.; Ali, I.; Hussain, H. Advances in the concept and methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Singapore, 2019; pp. 11–41. [Google Scholar]
- Salehzade, H.; Shishvan, M.I.; Ghiyasi, M.; Forouzin, F.; Siyahjani, A.A. Effect of seed priming on germination and seedling growth of wheat (Triticum aestivum L.). Res. J. Biol. Sci 2009, 4, 629–631. [Google Scholar]
- Dezfuli, P.M.; Sharif-Zadeh, F.; Janmohammadi, M. Influence of priming techniques on seed germination behavior of maize inbred lines (Zea mays L.). ARPN J. Agric. Biol. Sci. 2008, 3, 22–25. [Google Scholar]
- Al-Salhy, S.J.; Rasheed, A.A. Effect of mungbean seed priming methods and duration on seed germination and seedling vigour. Plant Arch. 2020, 20, 27–31. [Google Scholar]
- Ajouri, A.; Asgedom, H.; Becker, M. Seed priming enhances germination and seedling growth of barley under conditions of P and Zn deficiency. J. Plant Nutr. Soil Sci. 2004, 167, 630–636. [Google Scholar] [CrossRef]
- Eskandari, H.; Alizadeh-Amraie, A. Improvement of lentil germination performance under salt and drought conditions using seed priming treatments. Seed Sci. Technol. 2014, 42, 87–91. [Google Scholar] [CrossRef]
- Alam, A.U.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-um, S.; Datta, A. Seed priming enhances germination and morphological, physio-biochemical, and yield traits of cucumber under water-deficit stress. J. Soil Sci. Plant Nutr. 2023, 23, 3961–3978. [Google Scholar] [CrossRef]
- Pandey, A.; Khan, M.K.; Hamurcu, M.; Athar, T.; Yerlikaya, B.A.; Yerlikaya, S.; Kavas, M.; Rustagi, A.; Zargar, S.M.; Sofi, P.A. Role of exogenous nitric oxide in protecting plants against abiotic stresses. Agronomy 2023, 13, 1201. [Google Scholar] [CrossRef]
- Rahim, W.; Khan, M.; Al Azzawi, T.N.I.; Pande, A.; Methela, N.J.; Ali, S.; Imran, M.; Lee, D.-S.; Lee, G.-M.; Mun, B.-G. Exogenously applied sodium nitroprusside mitigates lead toxicity in rice by regulating antioxidants and metal stress-related transcripts. Int. J. Mol. Sci. 2022, 23, 9729. [Google Scholar] [CrossRef] [PubMed]
- Methela, N.J.; Islam, M.S.; Lee, D.-S.; Yun, B.-W.; Mun, B.-G. S-nitrosoglutathione (GSNO)-mediated lead detoxification in soybean through the regulation of ROS and metal-related transcripts. Int. J. Mol. Sci. 2023, 24, 9901. [Google Scholar] [CrossRef] [PubMed]
- Agbodjato, N.A.; Noumavo, P.A.; Adjanohoun, A.; Agbessi, L.; Baba-Moussa, L. Synergistic effects of plant growth promoting rhizobacteria and chitosan on in vitro seeds germination, greenhouse growth, and nutrient uptake of maize (Zea mays L.). Biotechnol. Res. Int. 2016, 2016, 7830182. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, G.; Roopa, K.S.; Prashanth, G.N.; Shekar Shetty, H. Chitosan enhances disease resistance in pearl millet against downy mildew caused by Sclerospora graminicola and defence-related enzyme activation. Pest Manag. Sci. 2008, 64, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Nakasato, D.Y.; Pereira, A.E.S.; Oliveira, J.L.; Oliveira, H.C.; Fraceto, L.F. Evaluation of the effects of polymeric chitosan/tripolyphosphate and solid lipid nanoparticles on germination of Zea mays, Brassica rapa and Pisum sativum. Ecotoxicol. Environ. Saf. 2017, 142, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, J.; Miao, X.; Lin, X.; Liu, W.; Ren, H. Effects of exogenous silicon on maize seed germination and seedling growth. Sci. Rep. 2021, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Biju, S.; Fuentes, S.; Gupta, D. Silicon improves seed germination and alleviates drought stress in lentil crops by regulating osmolytes, hydrolytic enzymes and antioxidant defense system. Plant Physiol. Biochem. 2017, 119, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Sohn, E.Y.; Hamayun, M.; Yoon, J.Y.; Lee, I.J. Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agrofor. Syst. 2010, 80, 333–340. [Google Scholar] [CrossRef]
- Farooq, M.; Cheema, Z.A.; Wahid, A. Seed priming with boron improves growth and yield of fine grain aromatic rice. Plant Growth Regul. 2012, 68, 189–201. [Google Scholar]
- Varier, A.; Vari, A.K.; Dadlani, M. The subcellular basis of seed priming. Curr. Sci. 2010, 99, 450–456. [Google Scholar]
- Di Girolamo, G.; Barbanti, L. Treatment conditions and biochemical processes influencing seed priming effectiveness. Ital. J. Agron. 2012, 7, e25. [Google Scholar] [CrossRef]
- Sharma, A.D.; Rathore, S.V.S.; Srinivasan, K.; Tyagi, R.K. Comparison of various seed priming methods for seed germination, seedling vigour and fruit yield in okra (Abelmoschus esculentus L. Moench). Sci. Hortic. 2014, 165, 75–81. [Google Scholar] [CrossRef]
- Lange, T. Molecular biology of gibberellin synthesis. Planta 1998, 204, 409–419. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Mitchum, M.G.; Yamaguchi, S.; Hanada, A.; Kuwahara, A.; Yoshioka, Y.; Kato, T.; Tabata, S.; Kamiya, Y.; Sun, T.p. Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant J. 2006, 45, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, C.; Adams, E.; Bhattacharya, A.; Page, A.F.; Anthony, P.; Kourmpetli, S.; Power, J.B.; Lowe, K.C.; Thomas, S.G.; Hedden, P. Over-expression of a gibberellin 2-oxidase gene from Phaseolus coccineus L. enhances gibberellin inactivation and induces dwarfism in Solanum species. Plant Cell Rep. 2008, 27, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, D.M.; Wickramarathna, A.D.; Ozga, J.A.; Kurepin, L.V.; Jin, A.L.; Good, A.G.; Pharis, R.P. Gibberellin 3-oxidase gene expression patterns influence gibberellin biosynthesis, growth, and development in pea. Plant Physiol. 2013, 163, 929–945. [Google Scholar] [CrossRef] [PubMed]
- Ritonga, F.N.; Zhou, D.; Zhang, Y.; Song, R.; Li, C.; Li, J.; Gao, J. The roles of gibberellins in regulating leaf development. Plants 2023, 12, 1243. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Gampala, S.S.L.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14, S15–S45. [Google Scholar] [CrossRef]
- Cutler, A.J.; Krochko, J.E. Formation and breakdown of ABA. Trends Plant Sci. 1999, 4, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Khan, Z.; Zhu, L.; Li, Y.; Wu, H. Impact of chitosan and chitosan-based metallic nanoparticles on the regulation of plant hormones. In Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–356. [Google Scholar]
- Pereira, A.E.S.; Sandoval-Herrera, I.E.; Zavala-Betancourt, S.A.; Oliveira, H.C.; Ledezma-Pérez, A.S.; Romero, J.; Fraceto, L.F. γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: Characterization and evaluation of biological activity. Carbohydr. Polym. 2017, 157, 1862–1873. [Google Scholar] [CrossRef]
- Lang, D.Y.; Fei, P.X.; Cao, G.Y.; Jia, X.X.; Li, Y.T.; Zhang, X.H. Silicon promotes seedling growth and alters endogenous IAA, GA3 and ABA concentrations in Glycyrrhiza uralensis under 100 mM NaCl stress. J. Hortic. Sci. Biotechnol. 2019, 94, 87–93. [Google Scholar] [CrossRef]
- Rao, N.K. Manual of Seed Handling in Genebanks; Bioversity International: Rome, Italy, 2006. [Google Scholar]
- Raja, K.; Sowmya, R.; Sudhagar, R.; Moorthy, P.S.; Govindaraju, K.; Subramanian, K.S. Biogenic ZnO and Cu nanoparticles to improve seed germination quality in blackgram (Vigna mungo). Mater. Lett. 2019, 235, 164–167. [Google Scholar] [CrossRef]
- Ali, L.G.; Nulit, R.; Ibrahim, M.H.; Yien, C.Y.S. Efficacy of KNO3, SiO2 and SA priming for improving emergence, seedling growth and antioxidant enzymes of rice (Oryza sativa), under drought. Sci. Rep. 2021, 11, 3864. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Anosheh, H.P.; Sadeghi, H.; Emam, Y. Chemical priming with urea and KNO 3 enhances maize hybrids (Zea mays L.) seed viability under abiotic stress. J. Crop Sci. Biotechnol. 2011, 14, 289–295. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Ziegler, V.; Schwanz Goebel, J.T.; Hoffmann, J.F.; Carvalho, I.R.; Chaves, F.C.; de Oliveira, M. Changes in phenolic acid and isoflavone contents during soybean drying and storage. J. Agric. Food Chem. 2019, 67, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; Constantin, J.; Schoving, C.; Maury, P.; Debaeke, P.; Aubertot, J.-N.; Dürr, C. Analysis of soybean germination, emergence, and prediction of a possible northward establishment of the crop under climate change. Eur. J. Agron. 2020, 113, 125972. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Hamayun, M.; Kamran, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Assessment of endophytic fungi cultural filtrate on soybean seed germination. Afr. J. Biotechnol. 2012, 11, 15135–15143. [Google Scholar]
- Ranal, M.A.; Santana, D.G.d. How and why to measure the germination process? Rev. Bras. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Roh, M.; Bentz, J.-A.; Wang, P.; Li, E.; Koshioka, M. Maturity and temperature stratification affect the germination of Styrax japonicus seeds. J. Hortic. Sci. Biotechnol. 2004, 79, 645–651. [Google Scholar] [CrossRef]
- Fang, L.; Ma, Z.; Wang, Q.; Nian, H.; Ma, Q.; Huang, Q.; Mu, Y. Plant growth and photosynthetic characteristics of soybean seedlings under different LED lighting quality conditions. J. Plant Growth Regul. 2021, 40, 668–678. [Google Scholar] [CrossRef]
- Pradeep, P. Seed quality parameters (Germination percentage and seedling vigor index) of rabi sorghum seeds influenced by rice weevil infestation. MOJ Toxicol. 2018, 4, 391–396. [Google Scholar] [CrossRef]
- Lee, I.-J.; Foster, K.R.; Morgan, P.W. Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol. 1998, 116, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Rose, P.A.; Abrams, G.D.; Taylor, D.C.; Abrams, S.R.; Cutler, A.J. (+)-Abscisic acid metabolism, 3-ketoacyl-coenzyme A synthase gene expression, and very-long-chain monounsaturated fatty acid biosynthesis in Brassica napus embryos. Plant Physiol. 1998, 117, 979–987. [Google Scholar] [CrossRef] [PubMed]



| Chitosan-GSNO | |||||||||
| Source | Df | FGP | PGP | MGT | VI | SB | RL | HL | SD |
| Time | 4 | 6.515 | 2.528 | 26.292 | 24.228 | 226.5 | 30.31 | 9.673 | 0.27 |
| <0.001 *** | 0.0464 * | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | 0.76 ns | ||
| Conc. | 3 | 3.039 | 2.436 | 2.686 | 25.342 | 305.7 | 35.265 | 2.762 | 0.292 |
| 0.03581 * | 0.04502 * | 0.05 * | <0.001 *** | <0.001 *** | <0.001 *** | 0.0484 * | 0.83 ns | ||
| Time × Conc. | 12 | 0.32 | 0.991 | 0.244 | 0.507 | 9 | 0.756 | 0.716 | 0.896 |
| 0.9828 ns | 0.4678 ns | 0.9949 ns | 0.902 ns | <0.001 *** | 0.691 ns | 0.6381 ns | 0.58 ns | ||
| Si | |||||||||
| Source | Df | FGP | PGP | MGT | VI | SB | RL | HL | SD |
| Time | 4 | 4.375 | 2.687 | 22.598 | 24.893 | 150.48 | 23.256 | 10.552 | 0.804 |
| 0.0029 ** | 0.0431 * | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | <0.001 *** | 0.53 ns | ||
| Conc. | 2 | 7.89 | 2.316 | 7.899 | 14.732 | 74.58 | 4.621 | 8.165 | 0.496 |
| 0.00115 ** | 0.0496 * | 0.00115 ** | <0.001 *** | <0.001 *** | 0.015 * | <0.001 *** | 0.60 ns | ||
| Time × Conc. | 8 | 0.225 | 2.084 | 0.733 | 0.265 | 3.71 | 0.503 | 0.406 | 0.427 |
| 0.9844 ns | 0.0575 ns | 0.6616 ns | 0.973 ns | <0.002 ** | 0.8513 ns | 0.9111 ns | 0.89 ns | ||
| Primers | Sequence (Forward 5′-3′) | Sequence (Reverse 5′-3′) |
|---|---|---|
| GmGA3ox3 | CTCGCATCTCTTCCTTCTTCC | AATCCAACATCAGCCACATCAG |
| GmABA2 | CATAGTCAACAATGCTGGAATCTC | ACCTAAGGCACTTGCTACAC |
| GmAAO3 | AACTGAAGAAGACACCAACAAG | CTACGCAAGCACCACAAC |
| GmGA2ox1 | TGTGAGCTTCTTGATCTGGTG | GGGAGGGTATTGATTGATCCT |
| GmNCED5 | TACTTGTGCATAGCGGAACC | GCACAAAAGCCATCACGTAC |
| GmActin7 | GCAAGAACTCGAGACTGCAA | CCAGCAGCTTCCATTCCAAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steven, S.; Islam, M.S.; Ghimire, A.; Methela, N.J.; Kwon, E.-H.; Yun, B.-W.; Lee, I.-J.; Kim, S.-H.; Kim, Y. Chitosan-GSNO Nanoparticles and Silicon Priming Enhance the Germination and Seedling Growth of Soybean (Glycine max L.). Plants 2024, 13, 1290. https://doi.org/10.3390/plants13101290
Steven S, Islam MS, Ghimire A, Methela NJ, Kwon E-H, Yun B-W, Lee I-J, Kim S-H, Kim Y. Chitosan-GSNO Nanoparticles and Silicon Priming Enhance the Germination and Seedling Growth of Soybean (Glycine max L.). Plants. 2024; 13(10):1290. https://doi.org/10.3390/plants13101290
Chicago/Turabian StyleSteven, Senabulya, Mohammad Shafiqul Islam, Amit Ghimire, Nusrat Jahan Methela, Eun-Hae Kwon, Byung-Wook Yun, In-Jung Lee, Seong-Hoon Kim, and Yoonha Kim. 2024. "Chitosan-GSNO Nanoparticles and Silicon Priming Enhance the Germination and Seedling Growth of Soybean (Glycine max L.)" Plants 13, no. 10: 1290. https://doi.org/10.3390/plants13101290
APA StyleSteven, S., Islam, M. S., Ghimire, A., Methela, N. J., Kwon, E. -H., Yun, B. -W., Lee, I. -J., Kim, S. -H., & Kim, Y. (2024). Chitosan-GSNO Nanoparticles and Silicon Priming Enhance the Germination and Seedling Growth of Soybean (Glycine max L.). Plants, 13(10), 1290. https://doi.org/10.3390/plants13101290









