Improving Crop Yield through Increasing Carbon Gain and Reducing Carbon Loss
Abstract
:1. Introduction
2. Improving Photosynthesis by Optimizing Rubisco
2.1. Modification of Rubisco Chaperones
2.2. Alteration in Rubisco Subunits
2.3. Manipulation of Rubisco through Rubisco Activase
3. Introduction of a CO2 Concentrating Mechanism (CCM) for Enhancing Photosynthesis
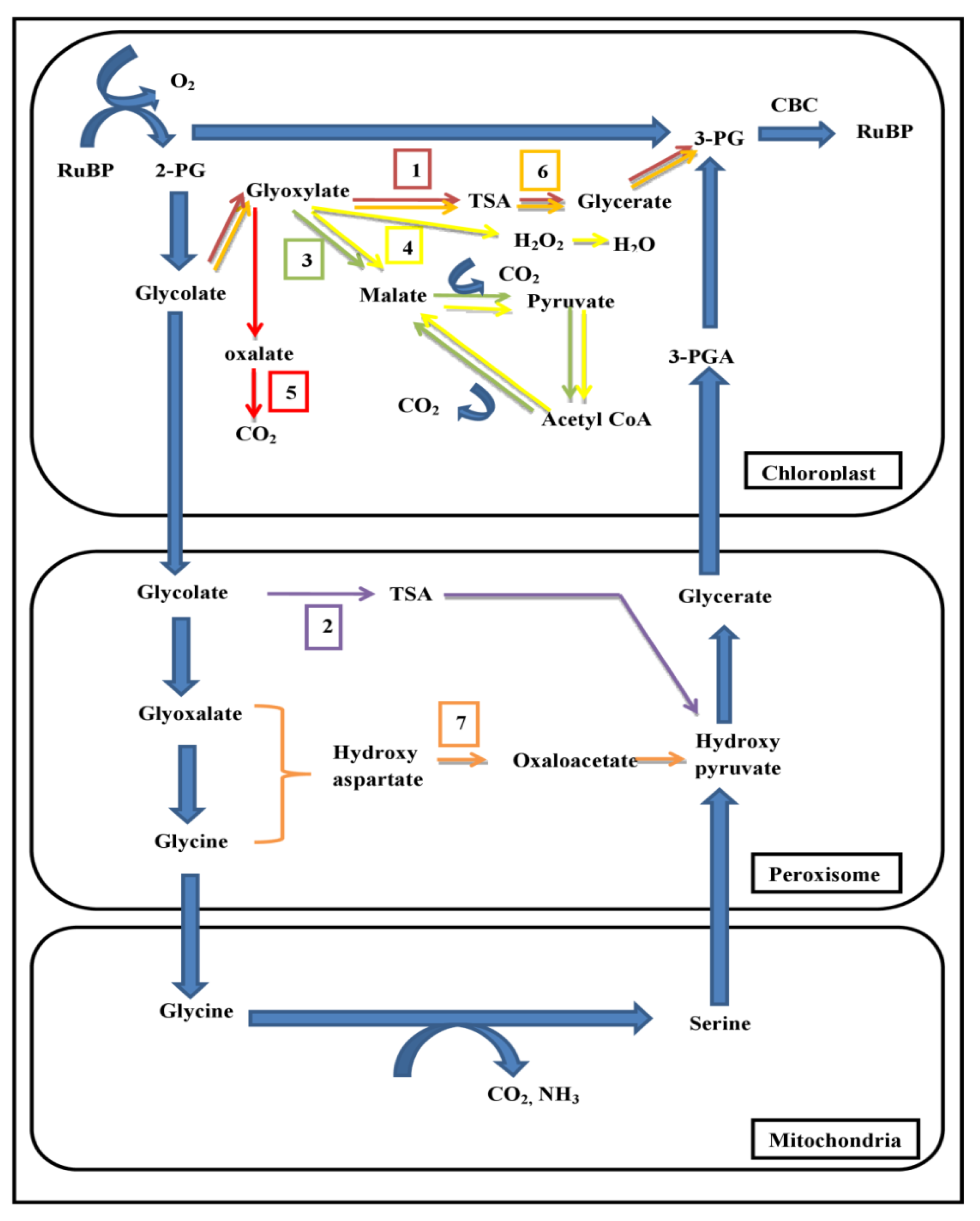
4. Utilization of Photorespiratory Bypasses for Improving Photosynthetic Efficiency
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Department of Economic and Social Affairs Population Division, United Nations. World Population Prospects 2022; United Nations: New York, NY, USA, 2022. [Google Scholar]
- Yuan, S.; Nie, L.; Wang, F.; Huang, J.; Peng, S. Agronomic performance of inbred and hybrid rice cultivars under simplified and reduced-input practices. Field Crop. Res. 2017, 210, 129–135. [Google Scholar] [CrossRef]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed]
- John, D.A.; Babu, G.R. Lessons from the aftermaths of green revolution on food system and health. Front. Sustain. Food Syst. 2021, 5, 644559. [Google Scholar] [CrossRef]
- Shivanna, K. Climate change and its impact on biodiversity and human welfare. Proc. Indian Natl. Sci. Acad. 2022, 88, 160–171. [Google Scholar] [CrossRef]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.N.; van Aalst, M.; Tosens, T.; Niinemets, Ü.; Stich, B.; Morosinotto, T.; Alboresi, A.; Erb, T.; Gómez-Coronado, P.A.; Tolleter, D. Improving photosynthetic efficiency toward food security: Strategies, advances, and perspectives. Mol. Plant 2023, 16, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Orr, D.J.; Pereira, A.M.; da Fonseca Pereira, P.; Pereira-Lima, Í.A.; Zsögön, A.; Araújo, W.L. Engineering photosynthesis: Progress and perspectives. F1000Research 2017, 6, PMC5658708. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Chen, G.; Yang, Y.; Zhang, Z.; Lu, T. Strategies for manipulating Rubisco and creating photorespiratory bypass to boost C3 photosynthesis: Prospects on modern crop improvement. Plant Cell Environ. 2023, 46, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Peterhansel, C.; Maurino, V.G. Photorespiration redesigned. Plant Physiol. 2011, 155, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Miyao, M. Molecular evolution and genetic engineering of C4 photosynthetic enzymes. J. Exp. Bot. 2003, 54, 179–189. [Google Scholar] [CrossRef]
- Sales, C.R.; Wang, Y.; Evers, J.B.; Kromdijk, J. Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. J. Exp. Bot. 2021, 72, 5942–5960. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.; Holtum, J.A. Facultative crassulacean acid metabolism (CAM) plants: Powerful tools for unravelling the functional elements of CAM photosynthesis. J. Exp. Bot. 2014, 65, 3425–3441. [Google Scholar] [CrossRef] [PubMed]
- Long, S. Photosynthesis: The final frontier. Resour. Mag. 2014, 21, 16. [Google Scholar] [CrossRef]
- Ellis, R.J. Tackling unintelligent design. Nature 2010, 463, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.M.; Houtz, R.L.; Alonso, H. Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiol. 2011, 155, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Andersson, I. Catalysis and regulation in Rubisco. J. Exp. Bot. 2008, 59, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Sharwood, R.E. Rubisco, the imperfect winner: It’s all about the base. J. Exp. Bot. 2023, 74, 562–580. [Google Scholar] [CrossRef] [PubMed]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and metabolic maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J. Molecular chaperones: The plant connection. Science 1990, 250, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Koumoto, Y.; Shimada, T.; Kondo, M.; Hara-Nishimura, I.; Nishimura, M. Chloroplasts have a novel Cpn10 in addition to Cpn20 as co-chaperonins in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 29688–29694. [Google Scholar] [CrossRef] [PubMed]
- Trösch, R.; Mühlhaus, T.; Schroda, M.; Willmund, F. ATP-dependent molecular chaperones in plastids—More complex than expected. Biochim. Biophys. Acta (BBA)—Bioenerg. 2015, 1847, 872–888. [Google Scholar] [CrossRef]
- Vitlin Gruber, A.; Feiz, L. Rubisco assembly in the chloroplast. Front. Mol. Biosci. 2018, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Kromdijk, J.; Bardal, V.; Stern, D.B. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat. Plants 2018, 4, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Hauser, T.; Bhat, J.Y.; Miličić, G.; Wendler, P.; Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Structure and mechanism of the Rubisco-assembly chaperone Raf1. Nat. Struct. Mol. Biol. 2015, 22, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Hotto, A.M.; Salesse-Smith, C.; Lin, M.; Busch, F.A.; Simpson, I.; Stern, D.B. Rubisco production in maize mesophyll cells through ectopic expression of subunits and chaperones. J. Exp. Bot. 2021, 72, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Aigner, H.; Wilson, R.; Bracher, A.; Calisse, L.; Bhat, J.; Hartl, F.; Hayer-Hartl, M. Plant RuBisCo assembly in E. coli with five chloroplast chaperones including BSD2. Science 2017, 358, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.M.; Birch, R.; Kelso, C.; Beck, J.L.; Kapralov, M.V. Improving recombinant Rubisco biogenesis, plant photosynthesis and growth by co-expressing its ancillary RAF1 chaperone. Proc. Natl. Acad. Sci. USA 2015, 112, 3564–3569. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ohkubo, M.; Hatakeyama, H.; Ohashi, K.; Yoshizawa, R.; Kojima, S.; Hayakawa, T.; Yamaya, T.; Mae, T.; Makino, A. Increased Rubisco content in transgenic rice transformed with the “sense” rbcS gene. Plant Cell Physiol. 2007, 48, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Fukayama, H.; Ueguchi, C.; Nishikawa, K.; Katoh, N.; Ishikawa, C.; Masumoto, C.; Hatanaka, T.; Misoo, S. Overexpression of Rubisco activase decreases the photosynthetic CO2 assimilation rate by reducing Rubisco content in rice leaves. Plant Cell Physiol. 2012, 53, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Kurek, I.; Chang, T.K.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 3230–3241. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Sakoda, K.; Fukayama, H.; Kondo, E.; Suzuki, Y.; Makino, A.; Terashima, I.; Yamori, W. Overexpression of both Rubisco and Rubisco activase rescues rice photosynthesis and biomass under heat stress. Plant Cell Environ. 2021, 44, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Kandoi, D.; Mohanty, S.; Govindjee; Tripathy, B.C. Towards efficient photosynthesis: Overexpression of Zea mays phosphoenolpyruvate carboxylase in Arabidopsis thaliana. Photosynth. Res. 2016, 130, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, Z.; Deng, D.; Chao, M.; Gao, Q.; Wang, Y.; Yang, Z.; Bian, Y.; Hao, D.; Xu, C. Characterization of RuBisCo activase genes in maize: An α-isoform gene functions alongside a β-isoform gene. Plant Physiol. 2014, 164, 2096–2106. [Google Scholar] [CrossRef] [PubMed]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawson, T.; Parry, M.A.; Raines, C.A. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160384. [Google Scholar] [CrossRef] [PubMed]
- Boran, S.; Limin, W.; Xiuling, L.; Zhen, Y.; Huawei, X.; Chenghua, Z.; Haiyan, T.; Cui, C.; Liu, E.; Jianjun, Z. Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Mol. Plant 2019, 12, 199–214. [Google Scholar]
- Feng, Y.; Wu, H.; Liu, H.; He, Y.; Yin, Z. Effects of OsRCA Overexpression on Rubisco Activation State and Photosynthesis in Maize. Plants 2023, 12, 1614. [Google Scholar] [CrossRef] [PubMed]
- Nölke, G.; Houdelet, M.; Kreuzaler, F.; Peterhänsel, C.; Schillberg, S. The expression of a recombinant glycolate dehydrogenase polyprotein in potato (Solanum tuberosum) plastids strongly enhances photosynthesis and tuber yield. Plant Biotechnol. J. 2014, 12, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.; Lobo, A.K.; Carmo-Silva, E. Regulation of Rubisco activity in crops. New Phytol. 2024, 241, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Catherall, E.; Díaz-Ramos, A.; Greiff, G.R.; Azinas, S.; Gunn, L.; McCormick, A.J. The small subunit of Rubisco and its potential as an engineering target. J. Exp. Bot. 2023, 74, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Shiomi, K.; Yamamoto, A.; Taketani, Y.; Kobayashi, N.; Yoshizawa, T.; Tanaka, S.-i.; Yoshikawa, H.; Endo, M.; Fukayama, H. Hybrid Rubisco with complete replacement of rice Rubisco small subunits by sorghum counterparts confers C4 plant-like high catalytic activity. Mol. Plant 2020, 13, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Sharwood, R.E.; von Caemmerer, S.; Maliga, P.; Whitney, S.M. The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth. Plant Physiol. 2008, 146, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Whitney, S.M.; Andrews, T.J. Plastome-encoded bacterial ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) supports photosynthesis and growth in tobacco. Proc. Natl. Acad. Sci. USA 2001, 98, 14738–14743. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Riaz, S.; Davey, P.; Zhao, Z.; Sun, Y.; Dykes, G.F.; Zhou, F.; Hartwell, J.; Lawson, T.; Nixon, P.J. Producing fast and active Rubisco in tobacco to enhance photosynthesis. Plant Cell 2023, 35, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Gunn, L.H.; Martin Avila, E.; Birch, R.; Whitney, S.M. The dependency of red Rubisco on its cognate activase for enhancing plant photosynthesis and growth. Proc. Natl. Acad. Sci. USA 2020, 117, 25890–25896. [Google Scholar] [CrossRef] [PubMed]
- Slattery, R.A.; Ort, D.R. Photosynthesis: Photosynthetic efficiency improvement. Encycl. Biol. Chem. III 2021, 2, 256–267. [Google Scholar]
- Masumoto, C.; Fukayama, H.; Hatanaka, T.; Uchida, N. Photosynthetic characteristics of antisense transgenic rice expressing reduced levels of Rubisco activase. Plant Prod. Sci. 2012, 15, 174–182. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Salvucci, M.E. The activity of Rubisco’s molecular chaperone, Rubisco activase, in leaf extracts. Photosynth. Res. 2011, 108, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Sharwood, R.E.; Sonawane, B.V.; Ghannoum, O.; Whitney, S.M. Improved analysis of C4 and C3 photosynthesis via refined in vitro assays of their carbon fixation biochemistry. J. Exp. Bot. 2016, 67, 3137–3148. [Google Scholar] [CrossRef] [PubMed]
- Degen, G.E.; Worrall, D.; Carmo-Silva, E. An isoleucine residue acts as a thermal and regulatory switch in wheat Rubisco activase. Plant J. 2020, 103, 742–751. [Google Scholar] [CrossRef]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 2012, 71, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Scafaro, A.P.; Atwell, B.J.; Muylaert, S.; Reusel, B.V.; Ruiz, G.A.; Rie, J.V.; Gallé, A. A thermotolerant variant of Rubisco activase from a wild relative improves growth and seed yield in rice under heat stress. Front. Plant Sci. 2018, 9, 355020. [Google Scholar] [CrossRef] [PubMed]
- Salvucci, M.E.; Crafts-Brandner, S.J. Mechanism for deactivation of Rubisco under moderate heat stress. Physiol. Plant. 2004, 122, 513–519. [Google Scholar] [CrossRef]
- Sage, R. Photorespiratory compensation: A driver for biological diversity. Plant Biol. 2013, 15, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Shivhare, D.; Mueller-Cajar, O. In vitro characterization of thermostable CAM Rubisco activase reveals a Rubisco interacting surface loop. Plant Physiol. 2017, 174, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Chinnusamy, V.; Pareek, A. Photosynthesis: Diving deep into the process in the era of climate change. Plant Physiol. Rep. 2022, 27, 539–542. [Google Scholar] [CrossRef]
- Sedelnikova, O.V.; Hughes, T.E.; Langdale, J.A. Understanding the genetic basis of C4 Kranz anatomy with a view to engineering C3 crops. Annu. Rev. Genet. 2018, 52, 249–270. [Google Scholar] [CrossRef]
- Peng, W.; Khoshravesh, R.; Karki, S.; Tapia, R.; Balahadia, C.; Bandyopadhyay, A.; Quick, W.; Furbank, R.; Sage, T.; Langdale, J. Re-creation of a key step in the evolutionary switch from C3 to C4 leaf anatomy. Curr. Biol. 2017, 27, 3278–3287. [Google Scholar]
- Li, X.; Wang, P.; Li, J.; Wei, S.; Yan, Y.; Yang, J.; Zhao, M.; Langdale, J.A.; Zhou, W. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun. Biol. 2020, 3, 151. [Google Scholar] [CrossRef] [PubMed]
- Edwards, E.J. Evolutionary trajectories, accessibility and other metaphors: The case of C4 and CAM photosynthesis. New Phytol. 2019, 223, 1742–1755. [Google Scholar] [CrossRef] [PubMed]
- Rae, B.D.; Long, B.M.; Förster, B.; Nguyen, N.D.; Velanis, C.N.; Atkinson, N.; Hee, W.Y.; Mukherjee, B.; Price, G.D.; McCormick, A.J. Progress and challenges of engineering a biophysical CO2-concentrating mechanism into higher plants. J. Exp. Bot. 2017, 68, 3717–3737. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Barrera Zambrano, V.A.; Ceusters, J.; Shorrock, K. The photosynthetic plasticity of crassulacean acid metabolism: An evolutionary innovation for sustainable productivity in a changing world. New Phytol. 2011, 191, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stessman, D.J.; Spalding, M.H. The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: How Chlamydomonas works against the gradient. Plant J. 2015, 82, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Fei, C.; Wilson, A.T.; Mangan, N.M.; Wingreen, N.S.; Jonikas, M.C. Modelling the pyrenoid-based CO2-concentrating mechanism provides insights into its operating principles and a roadmap for its engineering into crops. Nat. Plants 2022, 8, 583–595. [Google Scholar] [CrossRef]
- Kono, A.; Spalding, M.H. LCI1, a Chlamydomonas reinhardtii plasma membrane protein, functions in active CO2 uptake under low CO2. Plant J. 2020, 102, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Van, K.; Spalding, M.H. Periplasmic carbonic anhydrase structural gene (Cah1) mutant in Chlamydomonas reinhardtii. Plant Physiol. 1999, 120, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Burlacot, A.; Dao, O.; Auroy, P.; Cuiné, S.; Li-Beisson, Y.; Peltier, G. Alternative photosynthesis pathways drive the algal CO2-concentrating mechanism. Nature 2022, 605, 366–371. [Google Scholar] [CrossRef]
- Atkinson, N.; Leitão, N.; Orr, D.J.; Meyer, M.T.; Carmo-Silva, E.; Griffiths, H.; Smith, A.M.; McCormick, A.J. Rubisco small subunits from the unicellular green alga Chlamydomonas complement Rubisco-deficient mutants of Arabidopsis. New Phytol. 2017, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.; Díaz-Ramos, A.; Mao, Y.; Pukacz, K.R.; Fei, C.; McCormick, A.J. New horizons for building pyrenoid-based CO2-concentrating mechanisms in plants to improve yields. Plant Physiol. 2022, 190, 1609–1627. [Google Scholar] [CrossRef] [PubMed]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Espie, G.S.; Kimber, M.S. Carboxysomes: Cyanobacterial RubisCO comes in small packages. Photosynth. Res. 2011, 109, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Rae, B.D.; Long, B.M.; Whitehead, L.F.; Förster, B.; Badger, M.R.; Price, G.D. Cyanobacterial carboxysomes: Microcompartments that facilitate CO2 fixation. J. Mol. Microbiol. Biotechnol. 2013, 23, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Price, G.D.; Pengelly, J.J.; Forster, B.; Du, J.; Whitney, S.M.; von Caemmerer, S.; Badger, M.R.; Howitt, S.M.; Evans, J.R. The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J. Exp. Bot. 2013, 64, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Huffine, C.A.; Zhao, R.; Tang, Y.J.; Cameron, J.C. Role of carboxysomes in cyanobacterial CO2 assimilation: CO2 concentrating mechanisms and metabolon implications. Environ. Microbiol. 2023, 25, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Occhialini, A.; Lin, M.T.; Andralojc, P.J.; Hanson, M.R.; Parry, M.A. Transgenic tobacco plants with improved cyanobacterial Rubisco expression but no extra assembly factors grow at near wild-type rates if provided with elevated CO2. Plant J. 2016, 85, 148–160. [Google Scholar] [CrossRef] [PubMed]
- South, P.F.; Cavanagh, A.P.; Lopez-Calcagno, P.E.; Raines, C.A.; Ort, D.R. Optimizing photorespiration for improved crop productivity. J. Integr. Plant Biol. 2018, 60, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Borden, J.S.; Savage, D.F. New discoveries expand possibilities for carboxysome engineering. Curr. Opin. Microbiol. 2021, 61, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, W.; Teng, P.K.; Afonso, B.; Niederholtmeyer, H.; Grob, P.; Silver, P.A.; Savage, D.F. Modularity of a carbon-fixing protein organelle. Proc. Natl. Acad. Sci. USA 2012, 109, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Vasieva, O.; Sun, Y.; Faulkner, M.; Dykes, G.F.; Zhao, Z.; Liu, L.-N. Roles of RbcX in carboxysome biosynthesis in the cyanobacterium Synechococcus elongatus PCC7942. Plant Physiol. 2019, 179, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, J.J.; Flamholz, A.I.; Blikstad, C.; Dugan, E.J.; Laughlin, T.G.; Oltrogge, L.M.; Chen, A.W.; Wetmore, K.; Diamond, S.; Wang, J.Y. DABs are inorganic carbon pumps found throughout prokaryotic phyla. Nat. Microbiol. 2019, 4, 2204–2215. [Google Scholar] [CrossRef] [PubMed]
- Long, B.M.; Hee, W.Y.; Sharwood, R.E.; Rae, B.D.; Kaines, S.; Lim, Y.-L.; Nguyen, N.D.; Massey, B.; Bala, S.; von Caemmerer, S. Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts. Nat. Commun. 2018, 9, 3570. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Bloom, A. Photorespiration: The futile cycle? Plants 2021, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Kebeish, R.; Niessen, M.; Thiruveedhi, K.; Bari, R.; Hirsch, H.-J.; Rosenkranz, R.; Stäbler, N.; Schönfeld, B.; Kreuzaler, F.; Peterhänsel, C. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat. Biotechnol. 2007, 25, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.d.F.; Madgwick, P.J.; Powers, S.J.; Keys, A.J.; Lea, P.J.; Parry, M.A. An engineered pathway for glyoxylate metabolism in tobacco plants aimed to avoid the release of ammonia in photorespiration. BMC Biotechnol. 2011, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Fahnenstich, H.; Von Caemmerer, S.; Engqvist, M.K.; Weber, A.P.; Flügge, U.-I.; Maurino, V.G. Transgenic introduction of a glycolate oxidative cycle into A. thaliana chloroplasts leads to growth improvement. Front. Plant Sci. 2012, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Pick, T.R.; Bräutigam, A.; Schulz, M.A.; Obata, T.; Fernie, A.R.; Weber, A.P. PLGG1, a plastidic glycolate glycerate transporter, is required for photorespiration and defines a unique class of metabolite transporters. Proc. Natl. Acad. Sci. USA 2013, 110, 3185–3190. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, C.; Li, Q.; Chen, Z.; Sun, S.; Wang, X. Spatiotemporal resolved leaf angle establishment improves rice grain yield via controlling population density. iScience 2020, 23, 101489. [Google Scholar] [CrossRef]
- Wang, L.-M.; Shen, B.-R.; Li, B.-D.; Zhang, C.-L.; Lin, M.; Tong, P.-P.; Cui, L.-L.; Zhang, Z.-S.; Peng, X.-X. A synthetic photorespiratory shortcut enhances photosynthesis to boost biomass and grain yield in rice. Mol. Plant 2020, 13, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Schada von Borzyskowski, L.; Severi, F.; Krüger, K.; Hermann, L.; Gilardet, A.; Sippel, F.; Pommerenke, B.; Claus, P.; Cortina, N.S.; Glatter, T. Marine Proteobacteria metabolize glycolate via the β-hydroxyaspartate cycle. Nature 2019, 575, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Roell, M.-S.; Schada von Borzyskowski, L.; Westhoff, P.; Plett, A.; Paczia, N.; Claus, P.; Schlueter, U.; Erb, T.J.; Weber, A.P. A synthetic C4 shuttle via the β-hydroxyaspartate cycle in C3 plants. Proc. Natl. Acad. Sci. USA 2021, 118, e2022307118. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, K.; Kromdijk, J.; Salesse-Smith, C.E.; Smith, C.; Driever, S.M.; Long, S.P. Is chloroplast size optimal for photosynthetic efficiency? New Phytol. 2023, 239, 2197–2211. [Google Scholar] [CrossRef] [PubMed]
| Crop | Approach | Impact | Reference |
|---|---|---|---|
| Tobacco | Overexpression of Arabidopsis Rubisco chaperone RAF1 and subunit in Tobacco | Improvement in Rubisco content and activity was found along with more photosynthetic rate | Whitney et al. [28] |
| Rice | Increasing the expression of Rubisco subunit | Enhances the Rubisco content as well as higher photosynthetic rate | Suzuki et al. [29] |
| Maize | Overexpression of Rubisco subunit | 23% Decrease in Rubisco activation state due to Rubisco activase limitation | Salesse-Smith et al. [24] |
| Rice | Overexpression of Rubisco activase | Decrease in Rubisco content as well as photosynthetic rate | Fukayama et al. [30] |
| Arabidopsis | Increasing the thermal stability of Rubisco activase | Increases the photosynthetic rate | Kurek et al. [31] |
| Rice | Overexpression of both Rubisco and Rubisco activase under heat stress | Increases photosynthetic rate and yield | Qu et al. [32] |
| Arabidopsis | Overexpressing C4 specific Phosphoenolpyruvate carboxylase | Reduced Photorespiration | Kandoi et al. [33] |
| Maize | Characterization of Rubisco activase isoforms (α-form and β-form) | Enhances the photosynthetic rate | Yin et al. [34] |
| Wheat | Increasing Seduheptulose—1,7—Bis Phosphatase (SBPase) activity by overexpression | Enhanced photosynthetic rate, biomass and yield | Driever et al. [35] |
| Rice | Introduction of GOC photorespiratory bypass | Increase in photosynthetic rate and nitrogen content with reduction in photorespiration | BoRan et al. [36] |
| Maize | Rubisco Activase Overexpression | Improved Rubisco content, activity and photosynthetic rate. | Feng et al. [37] |
| Potato | Genetic modification to express Glycolate Dehydrogenase (GDH) | Increased CO2 assimilation rate and biomass accumulation | Nölke et al. [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthick, P.V.; Senthil, A.; Djanaguiraman, M.; Anitha, K.; Kuttimani, R.; Boominathan, P.; Karthikeyan, R.; Raveendran, M. Improving Crop Yield through Increasing Carbon Gain and Reducing Carbon Loss. Plants 2024, 13, 1317. https://doi.org/10.3390/plants13101317
Karthick PV, Senthil A, Djanaguiraman M, Anitha K, Kuttimani R, Boominathan P, Karthikeyan R, Raveendran M. Improving Crop Yield through Increasing Carbon Gain and Reducing Carbon Loss. Plants. 2024; 13(10):1317. https://doi.org/10.3390/plants13101317
Chicago/Turabian StyleKarthick, Palanivelu Vikram, Alagarswamy Senthil, Maduraimuthu Djanaguiraman, Kuppusamy Anitha, Ramalingam Kuttimani, Parasuraman Boominathan, Ramasamy Karthikeyan, and Muthurajan Raveendran. 2024. "Improving Crop Yield through Increasing Carbon Gain and Reducing Carbon Loss" Plants 13, no. 10: 1317. https://doi.org/10.3390/plants13101317







