Effect of Gibberellic Acid and Mechanical Scarification on the Germination and Seedling Stages of Chenopodium quinoa Willd. under Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Total Germination, Vigor Index, Mean Germination Time, and Germination Index
2.2. Fresh Weight, Dry Weight, Fresh Length, and Dry Length
3. Discussion
3.1. Effect of GA3
3.2. Effect of Salinity
3.3. Effect of Interaction between GA3 and Salinity
3.4. Effect of Interaction between MS and Salinity
4. Materials and Methods
4.1. Plant Material
4.2. Treatments
4.3. Seed Germination and Design of Experiments
4.4. Measured Parameters
- Total germination (TG) is measured according to the following formula [59].
- 2.
- Vigor index (VI) is calculated according to the following formula [60].
- 3.
- Mean germination time (MGT) is measured using the following formula [61].
- 4.
- Germination index (GI): The formula from [62] is used to measure the germination index (GI).
- 5.
- The length and weight of fresh and dry germinated seeds were determined as follows.Five days after germination, the length of the seedlings was measured using a millimeter ruler.
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mbarki, S.; Skalicky, M.; Vachova, P.; Hajihashemi, S.; Jouini, L.; Zivcak, M.; Tlustos, P.; Brestic, M.; Hejnak, V.; Zoghlami Khelil, A. Comparing Salt Tolerance at Seedling and Germination Stages in Local Populations of Medicago ciliaris L. to Medicago intertexta L. and Medicago scutellata L. Plants 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Amini, S.; Ghadiri, H.; Chen, C.; Marschner, P. Salt-Affected Soils, Reclamation, Carbon Dynamics, and Biochar: A Review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Yan, K.; He, W.; Bian, L.; Zhang, Z.; Tang, X.; An, M.; Li, L.; Han, G. Salt Adaptability in a Halophytic Soybean (Glycine Soja) Involves Photosystems Coordination. BMC Plant Biol. 2020, 20, 155. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAO: Salt-Affected Soils and Their Management Soils; FAO: Rome, Italy, 1988; Volume 39. [Google Scholar]
- Dakak, H.; Huang, J.; Zouahri, A.; Douaik, A.; Triantafilis, J. Mapping Soil Salinity in 3-dimensions Using an EM38 and EM4Soil Inversion Modelling at the Reconnaissance Scale in Central Morocco. Soil Use Manag. 2017, 33, 553–567. [Google Scholar] [CrossRef]
- Schilling, J.; Freier, K.P.; Hertig, E.; Scheffran, J. Climate Change, Vulnerability and Adaptation in North Africa with Focus on Morocco. Agric. Ecosyst. Environ. 2012, 156, 12–26. [Google Scholar] [CrossRef]
- Santos, C.A.D.; Silva, N.V.D.; Walter, L.S.; Silva, E.C.A.D.; Nogueira, R.J.M.C. Germinação de Duas Espécies Da Caatinga Sob Déficit Hídrico e Salinidade. Pesqui. Florest. Bras. 2016, 36, 219. [Google Scholar] [CrossRef]
- Pereira, M.; Martins, C.C.; Martins, D.; Silva, R.J.N. Estresse Hídrico Induzido Por Soluções de PEG e de NaCl Na Germinação de Sementes de Nabiça e Fedegoso. Biosci. J. 2014, 30, 687–696. [Google Scholar]
- El Hamdaoui, A.; Mechqoq, H.; El Yaagoubi, M.; Bouglad, A.; Hallouti, A.; El Mousadik, A.; El Aouad, N.; Ait Ben Aoumar, A.; Msanda, F. Effect of Pretreatment, Temperature, Gibberellin (GA3), Salt and Water Stress on Germination of Lavandula Mairei Humbert. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100314. [Google Scholar] [CrossRef]
- Dirik, H. Effet Du Stress Hydrique Osmotique Sur La Germination Des Graines Chez Les Provenancesde Cèdre Du Liban (Cedrus Libani A. Rich.) d’origine Turque. Ann. For. Sci. 2000, 57, 367–371. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef]
- Gholami, S.; Dehaghi, M.A.; Rezazadeh, A.; Naji, A.M. Seed Germination and Physiological Responses of Quinoa to Selenium Priming under Drought Stress. Bragantia 2022, 81, e0722. [Google Scholar] [CrossRef]
- Graf, B.L.; Rojo, L.E.; Delatorre-Herrera, J.; Poulev, A.; Calfio, C.; Raskin, I. Phytoecdysteroids and Flavonoid Glycosides among Chilean and Commercial Sources of Chenopodium quinoa: Variation and Correlation to Physico-chemical Characteristics. J. Sci. Food Agric. 2016, 96, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa Biodiversity and Sustainability for Food Security under Climate Change. A Review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Bazile, D.; Bertero, H.D.; Nieto, C. State of the Art Report on Quinoa around the World in 2013; FAO and CIRAD: Santiago, Chile, 2015. [Google Scholar]
- Bahrabadi, E.; Tavakkol Afshari, R.; Mahallati, M.N.; Seyyedi, S.M. Abscisic, Gibberellic, and Salicylic Acids Effects on Germination Indices of Corn under Salinity and Drought Stresses. J. Crop Improv. 2022, 36, 73–89. [Google Scholar] [CrossRef]
- Paiva, P.D.D.O.; Silva, D.P.C.D.; Silva, B.R.D.; Sousa, I.P.D.; Paiva, R.; Reis, M.V.D. How Scarification, GA3, and Graphene Oxide Influence the In Vitro Establishment and Development of Strelitzia. Plants 2023, 12, 2142. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Dara, B.L. Influence of Presoaking of Seeds with Gibberellin and Auxins on Growth and Yield Attributes of Wheat (Triticum aestivum L.) under High Salinity, Sodium Adsorption Ratio, and Boron Levels. Indian J. Agric. Sci. 1971, 41, 998–1003. [Google Scholar]
- Horváth, E.; Szalai, G.; Janda, T. Induction of Abiotic Stress Tolerance by Salicylic Acid Signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Misratia, K.; Islam, R.; Ismail, R.; Oad, C.; Hanafi, M.; Puteh, A. Interactive Effects of Gibberellic Acid (GA3) and Salt Stress on Growth and Ion Accumulation of Two Rice (Oryza sativa L.) Varieties Differing in Salt Tolerance. J. Food Agric. Environ. 2015, 13, 66–70. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zadeh, S.Y.; Ramin, A.A.; Baninasab, B. Effect of Gibberellic Acid, Stratification and Salinity on Seed Germination of Echinacea Purpurea Cv. Magnus. Herba Polonica 2015, 61, 13–22. [Google Scholar] [CrossRef]
- Padma, L.; Basvaraju, G.V.; Sarika, G.; Amrutha, N. Effect of Seed Treatments to Enhance Seed Quality of Papaya (Carica papaya L.) Cv. Surya. Glob. J. Biol. Agric. Health Sci. 2013, 2, 221–225. [Google Scholar]
- Macchia, M.; Angelini, L.G.; Ceccarini, L. Methods to Overcome Seed Dormancy in Echinacea Angustifolia DC. Sci. Hortic. 2001, 89, 317–324. [Google Scholar] [CrossRef]
- Khan, M.A.; Gul, B.; Weber, D.J. Improving Seed Germination of Salicornia Rubra (Chenopodiaceae) under Saline Conditions Using Germination-Regulating Chemicals. West. N. Am. Nat. 2002, 62, 101–105. [Google Scholar]
- Panuccio, M.R.; Jacobsen, S.E.; Akhtar, S.S.; Muscolo, A. Effect of Saline Water on Seed Germination and Early Seedling Growth of the Halophyte Quinoa. AoB PLANTS 2014, 6, plu047. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Wang, B.; Shi, L.; Li, Y.; Duo, L.; Zhang, W. Alleviation of Salt Stress-Induced Inhibition of Seed Germination in Cucumber (Cucumis sativus L.) by Ethylene and Glutamate. J. Plant Physiol. 2010, 167, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.A. Seed Priming to Alleviate Salinity Stress in Germinating Seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-H.; Park, K.-J.; Kim, B.-K.; Jeong, J.-W.; Kim, H.-J. Effect of Salinity Stress on Phenolic Compounds and Carotenoids in Buckwheat (Fagopyrum esculentum M.) Sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Effect of Salt Stress on Seed Germination and Seedling Growth of Three Salinity Plants. Pak. J. Biol. Sci. 2008, 11, 1268–1272. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Effect of Sucrose and Mannitol on the Accumulation of Health-Promoting Compounds and the Activity of Metabolic Enzymes in Broccoli Sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Causin, H.F.; Bordón, D.A.E.; Burrieza, H. Salinity Tolerance Mechanisms during Germination and Early Seedling Growth in Chenopodium Quinoa Wild. Genotypes with Different Sensitivity to Saline Stress. Environ. Exp. Bot. 2020, 172, 103995. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Eisa, S.S. Effect of Salinity on Composition, Viability, and Germination of Seeds of Chenopodium Quinoa Willd. Plant Soil 2008, 302, 79–90. [Google Scholar] [CrossRef]
- Kiani-Pouya, A.; Rasouli, F.; Bazihizina, N.; Zhang, H.; Hedrich, R.; Shabala, S. A Large-Scale Screening of Quinoa Accessions Reveals an Important Role of Epidermal Bladder Cells and Stomatal Patterning in Salinity Tolerance. Environ. Exp. Bot. 2019, 168, 103885. [Google Scholar] [CrossRef]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a Putative Calcium Sensor, Interacts with the Protein Kinase SOS2 to Protect Arabidopsis Shoots from Salt Stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [PubMed]
- Gul, B.; Ansari, R.; Flowers, T.J.; Khan, M.A. Germination Strategies of Halophyte Seeds under Salinity. Environ. Exp. Bot. 2013, 92, 4–18. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic Stress, the Field Environment and Stress Combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Pandey, P.; Ramegowda, V.; Senthil-Kumar, M. Shared and Unique Responses of Plants to Multiple Individual Stresses and Stress Combinations: Physiological and Molecular Mechanisms. Front. Plant Sci. 2015, 6, 723. [Google Scholar] [CrossRef]
- Jaramillo Roman, V.; Den Toom, L.A.; Castro Gamiz, C.; Van Der Pijl, N.; Visser, R.G.F.; Van Loo, E.N.; Van Der Linden, C.G. Differential Responses to Salt Stress in Ion Dynamics, Growth and Seed Yield of European Quinoa Varieties. Environ. Exp. Bot. 2020, 177, 104146. [Google Scholar] [CrossRef]
- Hariadi, Y.; Marandon, K.; Tian, Y.; Jacobsen, S.-E.; Shabala, S. Ionic and Osmotic Relations in Quinoa (Chenopodium Quinoa Willd.) Plants Grown at Various Salinity Levels. J. Exp. Bot. 2011, 62, 185–193. [Google Scholar] [CrossRef]
- Shabala, S. Learning from Halophytes: Physiological Basis and Strategies to Improve Abiotic Stress Tolerance in Crops. Ann. Bot. 2013, 112, 1209–1221. [Google Scholar] [CrossRef]
- Orsini, F.; Accorsi, M.; Gianquinto, G.; Dinelli, G.; Antognoni, F.; Carrasco, K.B.R.; Martinez, E.A.; Alnayef, M.; Marotti, I.; Bosi, S.; et al. Beyond the Ionic and Osmotic Response to Salinity in Chenopodium Quinoa: Functional Elements of Successful Halophytism. Funct. Plant Biol. 2011, 38, 818–831. [Google Scholar] [CrossRef]
- Terletskaya, N.V.; Erbay, M.; Zorbekova, A.N.; Prokofieva, M.Y.; Saidova, L.T.; Mamirova, A. Influence of Osmotic, Salt, and Combined Stress on Morphophysiological Parameters of Chenopodium Quinoa Photosynthetic Organs. Agriculture 2022, 13, 1. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.; Barrios-Masias, F.; Fuentes, F.; Murphy, K. Quinoa Abiotic Stress Responses: A Review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Carrasco, K.; Antognoni, F.; Coulibaly, A.K.; Lizardi, S.; Covarrubias, A.; Martínez, E.A.; Molina-Montenegro, M.A.; Biondi, S.; Zurita-Silva, A. Variation in Salinity Tolerance of Four Lowland Genotypes of Quinoa (Chenopodium quinoa Willd.) as Assessed by Growth, Physiological Traits, and Sodium Transporter Gene Expression. Plant Physiol. Biochem. 2011, 49, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, V.; Slabu, C.; Vitanescu, M.; Peres, C.; Cojocaru, A.; Covasa, M.; Mihalache, G. Tolerance of Three Quinoa Cultivars (Chenopodium quinoa Willd.) to Salinity and Alkalinity Stress During Germination Stage. Agronomy 2019, 9, 287. [Google Scholar] [CrossRef]
- Bejaoui, M. Intéractions Entre NaCl et Quelques Phytohormones Sur La Croissance Du Soja. J. Plant Physiol. 1985, 120, 95–110. [Google Scholar] [CrossRef]
- Kaur, S.; Gupta, A.K.; Kaur, N. Effect of GA3, kinetin and indole acetic acid on carbohydrate metabolism in chickpea seedlings germinating under water stress. Plant Growth Regul. 2000, 30, 61–70. [Google Scholar] [CrossRef]
- Chauhan, A.; AbuAmarah, B.A.; Kumar, A.; Verma, J.S.; Ghramh, H.A.; Khan, K.A.; Ansari, M.J. Influence of Gibberellic Acid and Different Salt Concentrations on Germination Percentage and Physiological Parameters of Oat Cultivars. Saudi J. Biol. Sci. 2019, 26, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Atia, A.; Debez, A.; Barhoumi, Z.; Smaoui, A.; Abdelly, C. ABA, GA3, and Nitrate May Control Seed Germination of Crithmum maritimum (Apiaceae) under Saline Conditions. Comptes Rendus Biol. 2009, 332, 704–710. [Google Scholar] [CrossRef]
- Gul, B.; Khan, M.A. Effect of Compatible Osmotica and Plant Growth Regulators in Alleviating Salinity Stress on the Seed Germination of Allenrolfea occidentalis. Pak. J. Bot. 2008, 40, 1957–1964. [Google Scholar]
- Daur, I. Effects of Hydro and Hormonal Priming on Quinoa (Chenopodium quinoa Willd.) Seed Germination under Salt and Drought Stress. Pak. J. Bot. 2018, 50, 1669–1673. [Google Scholar]
- Jarrar, H.; El-Keblawy, A.; Ghenai, C.; Abhilash, P.C.; Bundela, A.K.; Abideen, Z.; Sheteiwy, M.S. Seed Enhancement Technologies for Sustainable Dryland Restoration: Coating and Scarification. Sci. Total Environ. 2023, 904, 166150. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Dong, M.; Gutterman, Y. Factors Influencing Seed Dormancy and Germination in Sand, and Seedling Survival under Desiccation, of Psammochloa villosa (Poaceae), Inhabiting the Moving Sand Dunes of Ordos, China. Plant Soil 2004, 259, 231–241. [Google Scholar]
- Marcos Filho, J. Fisiologia de Sememntes de Plantas Cultivadas; Fealq: Piracicaba, Brazil, 2005; ISBN 978-85-7133-038-2. [Google Scholar]
- Song, J.; Feng, G.; Li, Z.K.; Chen, A.D.; Chen, X.M.; Zhang, F.S. Effects of Salinity and Scarifying Seed Coat on Ion Content of Embryos and Seed Germination for Suaeda physophora and Haloxylon ammodendron. Seed Sci. Technol. 2007, 35, 615–623. [Google Scholar] [CrossRef]
- Jacobsen, S.-E. The Scope for Adaptation of Quinoa in Northern Latitudes of Europe. J. Agron. Crop Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Cavalcante, J.A.; Almeida, A.S.; Nunes, C.A.; Serrão, A.F.A.; Konzen, L.H.; Suñé, A.S.; Tunes, L.V.M.D. Seed Morphobiometry, Morphology of Germination and Emergence of Quinoa Seeds ‘BRS Piabiru’. An. Acad. Bras. Ciênc. 2020, 92, e20181313. [Google Scholar] [CrossRef] [PubMed]
- ISTA International Rules for Seed Testing. Seed Science and Technology; ISTA: Wallisellen, Switzerland, 2020; Volume 2, ISSN 0251-0952. [Google Scholar]
- Kader, M.A.; Jutzi, S.C. Effects of Thermal and Salt Treatments during Imbibition on Germination and Seedling Growth of Sorghum at 42/19 °C. J. Agron. Crop Sci. 2004, 190, 35–38. [Google Scholar] [CrossRef]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proc. R. Soc. New South Wales 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Yang, N.; Guo, X.; Wu, Y.; Hu, X.; Ma, Y.; Zhang, Y.; Wang, H.; Tang, Z. The Inhibited Seed Germination by ABA and MeJA Is Associated with the Disturbance of Reserve Utilizations in Astragalus membranaceus. J. Plant Interact. 2018, 13, 388–397. [Google Scholar] [CrossRef]
- Nakagawa, J. Testes de Vigor Baseados No Desempenho Das Plântulas. In Vigor de Sementes: Conceitos e Testes; Associação Brasileira de Tradutores e Intérpretes: Londrina, Brazil, 1999; pp. 1–24. [Google Scholar]
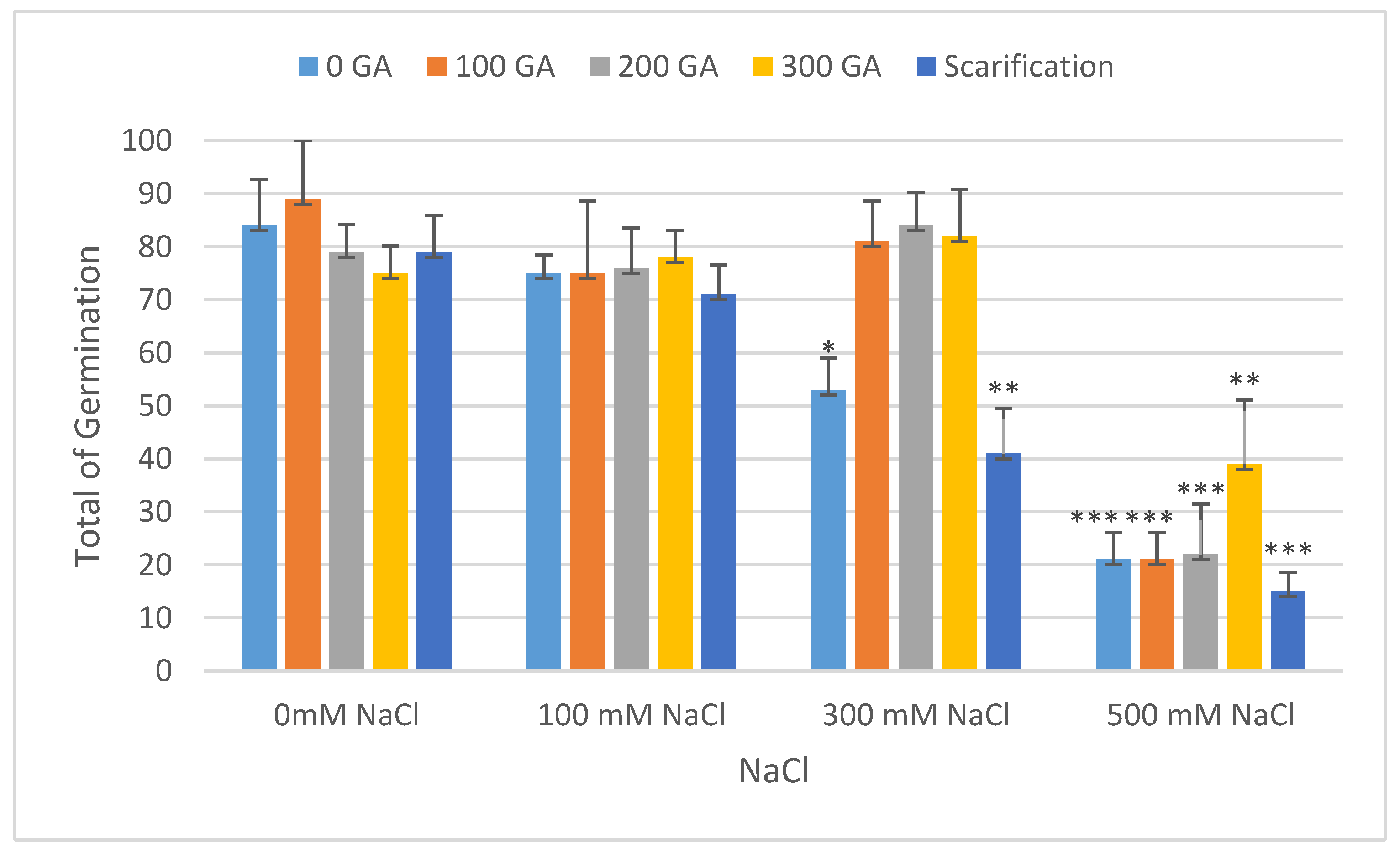


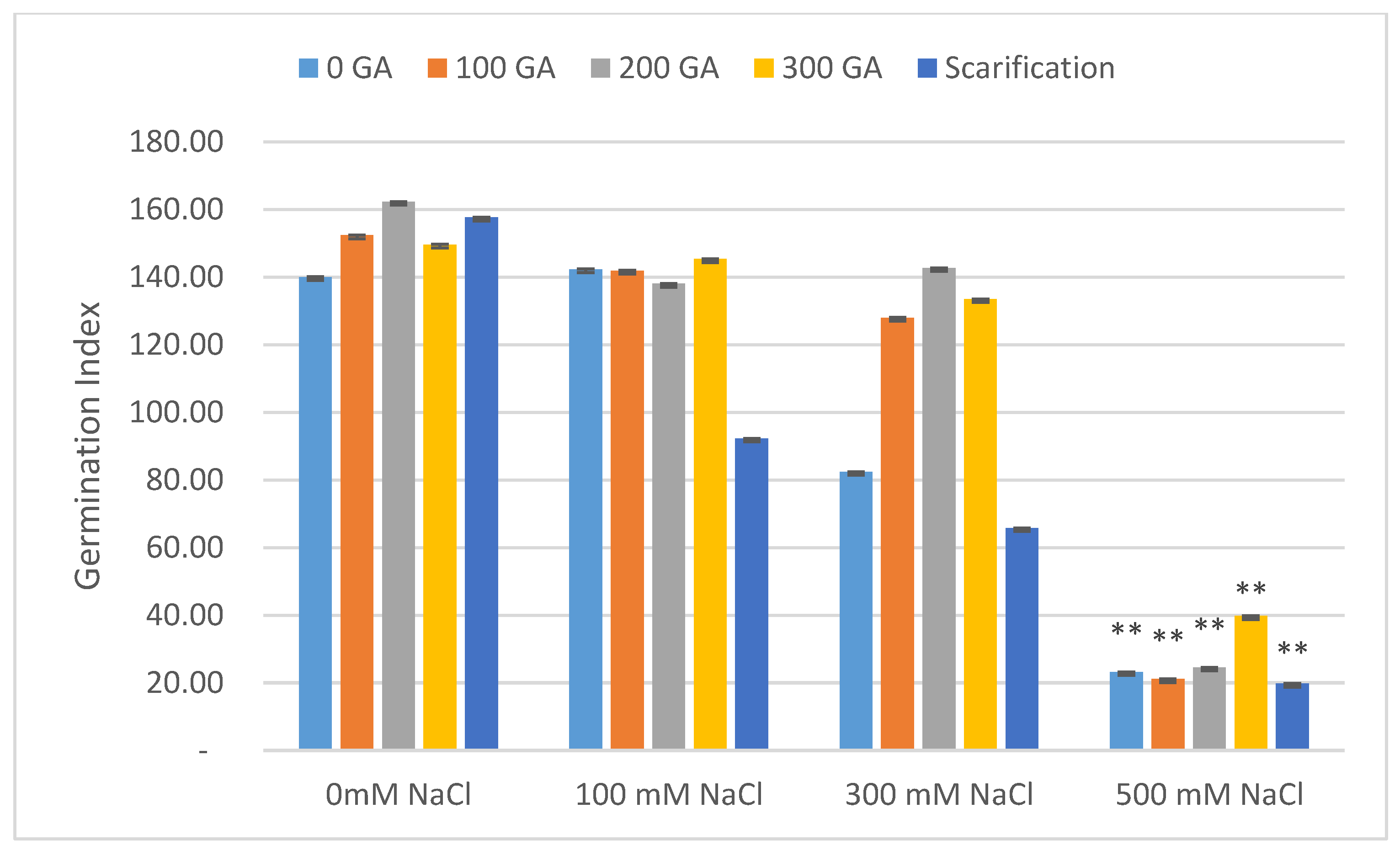
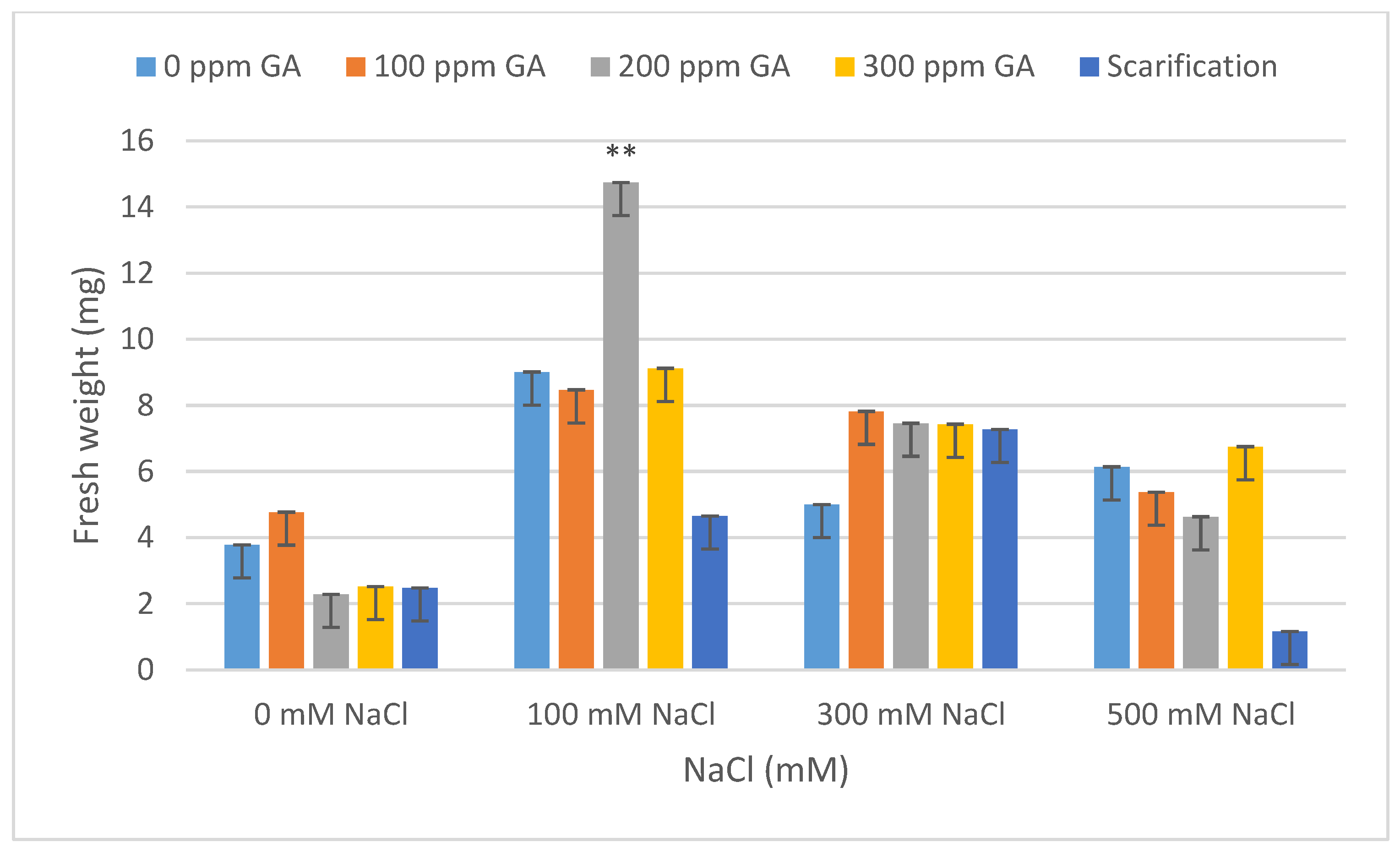
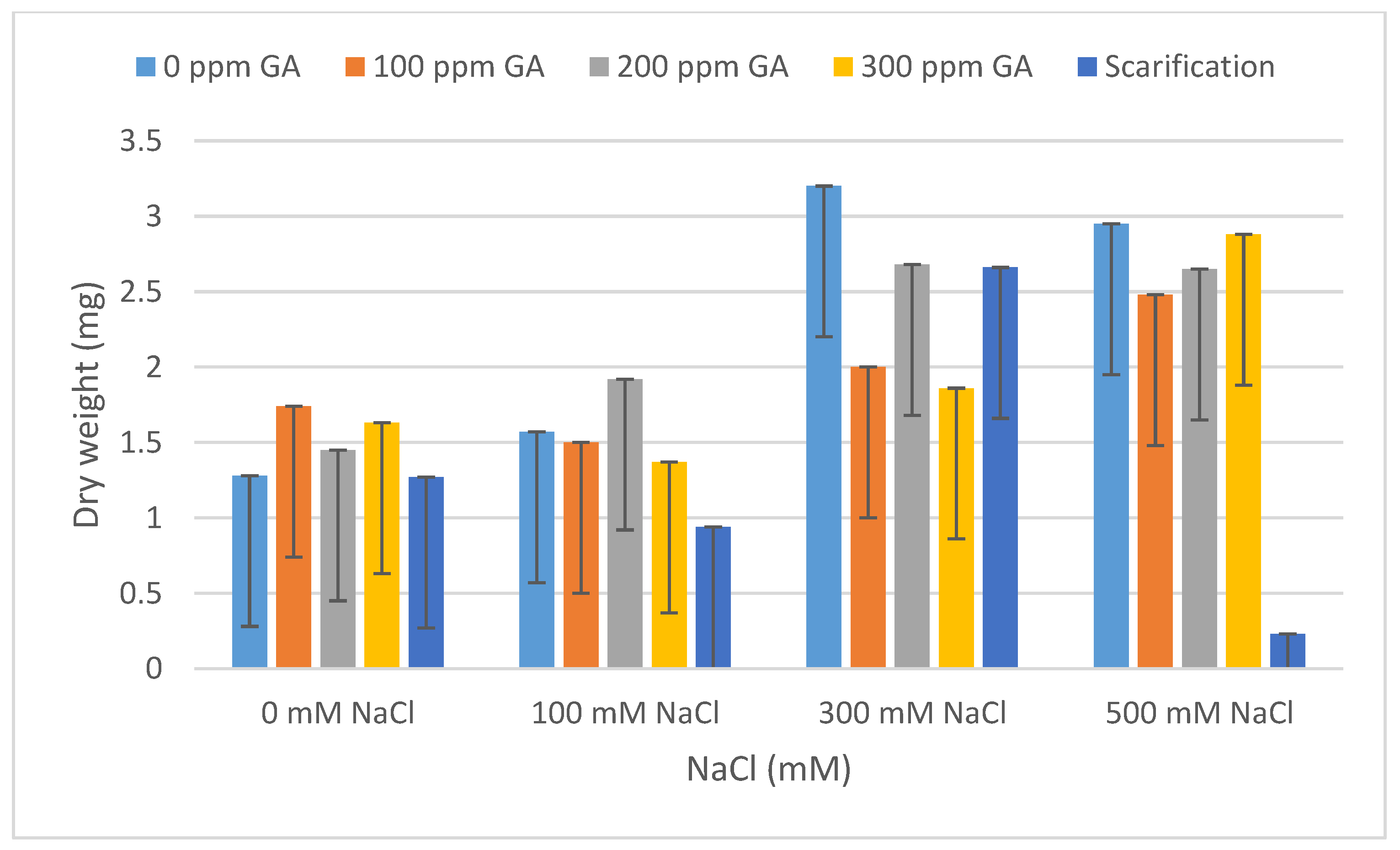

| Source of Variation | Df 1 | TG 2 | VI 3 | FW 4 | DW 5 | FL 6 | DL 7 | MGT 8 | GI 9 |
|---|---|---|---|---|---|---|---|---|---|
| Pretreatments (gibberellic acid+ scarification) | 4 | ns 13 | ** | ns | ns | ns | ns | ns | ns |
| Salinity | 3 | *** | *** | *** 12 | ns | ** 11 | * 10 | ns | *** |
| Pretreatments × salinity | 12 | ns | ns | ns | ns | ns | ns | ns | ns |
| Pretreatment | Salinity | Mean TG (%) | Increase | Decrease | Mean VI | Increase | Decrease |
|---|---|---|---|---|---|---|---|
| 0 GA | 0 NaCl (control) | 84.00 ± 8.66 | - | 1.13 ± 0.56 | - | - | |
| 100 NaCl | 75.33 ± 3.51 | - | 8.57 | 2.21 ± 0.32 ** | 1.08 | - | |
| 300 NaCl | 53.33 ± 6.02 * | - | 30.57 | 0.41 ± 0.07 * | - | 0.72 | |
| 500 NaCl | 21.33 ± 5.13 *** | - | 62.67 | 0.11 ± 0.04 ** | - | 1.02 | |
| 100 GA | 0 NaCl | 89.33 ± 11.01 | 5.33 | - | 1.72 ± 0.24 | 0.59 | - |
| 100 NaCl | 75.33 ± 13.45 | - | 8.67 | 2.71 ± 0.67 *** | 1.58 | - | |
| 300 NaCl | 81.33 ± 7.63 | - | 2.67 | 1.20 ± 0.08 | 0.07 | - | |
| 500 NaCl | 20.67 ± 5.13 *** | - | 63.33 | 0.10 ± 0.04 ** | - | 1.03 | |
| 200 GA | 0 NaCl | 79.67 ± 5.13 | - | 4.33 | 0.99 ± 0.19 | - | 0.14 |
| 100 NaCl | 76.00 ± 7.50 | - | 8.00 | 3.23 ± 0.10 *** | 2.10 | - | |
| 300 NaCl | 84.00 ± 6.24 | - | - | 1.02 ± 0.08 | - | 0.11 | |
| 500 NaCl | 22.00 ± 9.50 *** | - | 62.00 | 0.09 ± 0.04 ** | - | 1.04 | |
| 300 GA | 0 NaCl | 74.67 ± 5.13 | - | 9.33 | 0.65 ± 0.02 | - | 0.48 |
| 100 NaCl | 78.00 ± 5.00 | - | 6.00 | 2.76 ± 0.13 *** | 1.63 | - | |
| 300 NaCl | 82.00 ± 8.74 | - | 2.00 | 1.08 ± 0.10 | - | 0.05 | |
| 500 NaCl | 39.33 ± 12.12 ** | - | 44.67 | 0.21 ± 0.06 ** | - | 0.92 | |
| Scarification | 0 NaCl | 78.67 ± 6.93 | - | 5.33 | 0.79 ± 0.57 | - | 0.34 |
| 100 NaCl | 70.67 ± 5.57 | - | 13.33 | 1.72 ± 0.34 | 0.59 | - | |
| 300 NaCl | 41.33 ± 8.54 ** | - | 42.67 | 0.36 ± 0.09 * | - | 0.77 | |
| 500 NaCl | 15.33 ± 3.60 *** | - | 68.67 | 0.06 ± 0.03 ** | - | 1.07 |
| Pretreatment | Salinity | Mean MGT (Day) | Increase | Decrease | Mean GI | Increase | Decrease |
|---|---|---|---|---|---|---|---|
| 0 GA | 0 NaCl (control) | 3.26 ± 0.03 | - | - | 141.08 ± 7.07 | - | - |
| 100 NaCl | 3.17 ± 0.01 | - | 0.09 | 142.40 ± 1.41 | 1.32 | - | |
| 300 NaCl | 3.32 ± 0.06 | 0.06 | - | 82.44 ± 2.83 | - | 58.64 | |
| 500 NaCl | 3.64 ± 0.03 | 0.38 | - | 23.21 ± 4.24 ** | - | 117.87 | |
| 100 GA | 0 NaCl | 3.21 ± 0.01 | - | 0.05 | 152.42 ± 5.66 | 11.34 | - |
| 100 NaCl | 3.17 ± 0.01 | - | 0.09 | 141.96 ± 2.83 | - | 0.88 | |
| 300 NaCl | 3.32 ± 0.03 | 0.06 | - | 128.04 ± 4.24 | - | 13.04 | |
| 500 NaCl | 3.70 ± 0.03 | 0.44 | - | 21.13 ± 2.83 ** | - | 119.95 | |
| 200 GA | 0 NaCl | 3.09 ± 0.03 | - | 0.17 | 161.34 ± 8.49 | 20.26 | - |
| 100 NaCl | 3.20 ± 0.01 | - | 0.06 | 138.09 ± 4.24 | - | 2.99 | |
| 300 NaCl | 3.26 ± 0.03 | - | - | 142.74 ± 2.83 | 1.66 | - | |
| 500 NaCl | 3.65 ± 0.03 | 0.39 | - | 24.57 ± 4.24 ** | - | 116.51 | |
| 300 GA | 0 NaCl | 3.11 ± 0.06 | - | 0.15 | 149.71 ± 5.66 | 8.63 | - |
| 100 NaCl | 3.15 ± 0.03 | - | 0.11 | 145.42 ± 4.24 | 4.34 | - | |
| 300 NaCl | 3.30 ± 0.01 | 0.04 | - | 133.57 ± 4.24 | - | 7.51 | |
| 500 NaCl | 3.70 ± 0.03 | 0.44 | - | 39.81 ± 1.41 ** | - | 101.27 | |
| Scarification | 0 NaCl | 3.11 ± 0.01 | - | 0.15 | 157.73 ± 4.24 | 16.65 | - |
| 100 NaCl | 3.48 ± 0.03 | 0.22 | - | 92.30 ± 1.41 | - | 48.78 | |
| 300 NaCl | 3.32 ± 0.01 | 0.06 | - | 65.82 ± 2.83 | - | 75.26 | |
| 500 NaCl | 3.49 ± 0.03 | 0.23 | - | 19.79 ± 1.4 ** | - | 121.29 |
| Pretreatment | Salinity | Mean FW (mg) | Increase | Decrease | Mean DW (mg) | Increase | Decrease |
|---|---|---|---|---|---|---|---|
| 0 GA | 0 NaCl (control) | 3.78 ± 0.21 | - | - | 1.28 ± 0.01 | - | - |
| 100 NaCl | 9.01 ± 0.42 | 5.23 | - | 1.57 ± 0.16 | 0.29 | - | |
| 300 NaCl | 5.00 ± 0.67 | 1.22 | - | 3.20 ± 0.12 | 1.92 | - | |
| 500 NaCl | 6.14 ± 0.28 | 2.36 | - | 2.95 ± 0.01 | 1.67 | - | |
| 100 GA | 0 NaCl | 4.77 ± 0.17 | 0.99 | - | 1.74 ± 0.37 | 0.46 | - |
| 100 NaCl | 8.47 ± 0.49 | 4.69 | - | 1.50 ± 0.36 | 0.22 | - | |
| 300 NaCl | 7.82 ± 0.14 | 4.04 | - | 2.00 ± 0.45 | 0.72 | - | |
| 500 NaCl | 5.37 ± 0.59 | 1.59 | - | 2.48 ± 0.07 | 1.20 | - | |
| 200 GA | 0 NaCl | 2.28 ± 0.29 | - | 1.50 | 1.45 ± 0.04 | 0.17 | - |
| 100 NaCl | 14.74 ± 0.47 ** | 10.96 | - | 1.92 ± 0.04 | 0.64 | - | |
| 300 NaCl | 7.46 ± 0.48 | 3.68 | - | 2.68 ± 0.28 | 1.40 | - | |
| 500 NaCl | 4.63 ± 0.13 | 0.85 | - | 2.65 ± 0.18 | 1.37 | - | |
| 300 GA | 0 NaCl | 2.52 ± 0.79 | - | 1.26 | 1.63 ± 0.09 | 0.35 | - |
| 100 NaCl | 9.12 ± 0.12 | 5.34 | - | 1.37 ± 0.14 | 0.09 | - | |
| 300 NaCl | 7.43 ± 0.51 | 3.65 | - | 1.86 ± 0.18 | 0.58 | - | |
| 500 NaCl | 6.75 ± 0.39 | 2.97 | - | 2.88 ± 0.12 | 1.60 | - | |
| Scarification | 0 NaCl | 2.48 ± 0.48 | - | 1.30 | 1.27 ± 0.04 | - | 0.01 |
| 100 NaCl | 4.65 ± 0.34 | 0.87 | - | 0.94 ± 0.14 | - | 0.34 | |
| 300 NaCl | 7.27 ± 0.28 | 3.49 | - | 2.66 ± 0.30 | 1.38 | - | |
| 500 NaCl | 1.16 ± 0.29 | - | 2.62 | 0.23 ± 0.10 | - | 1.05 |
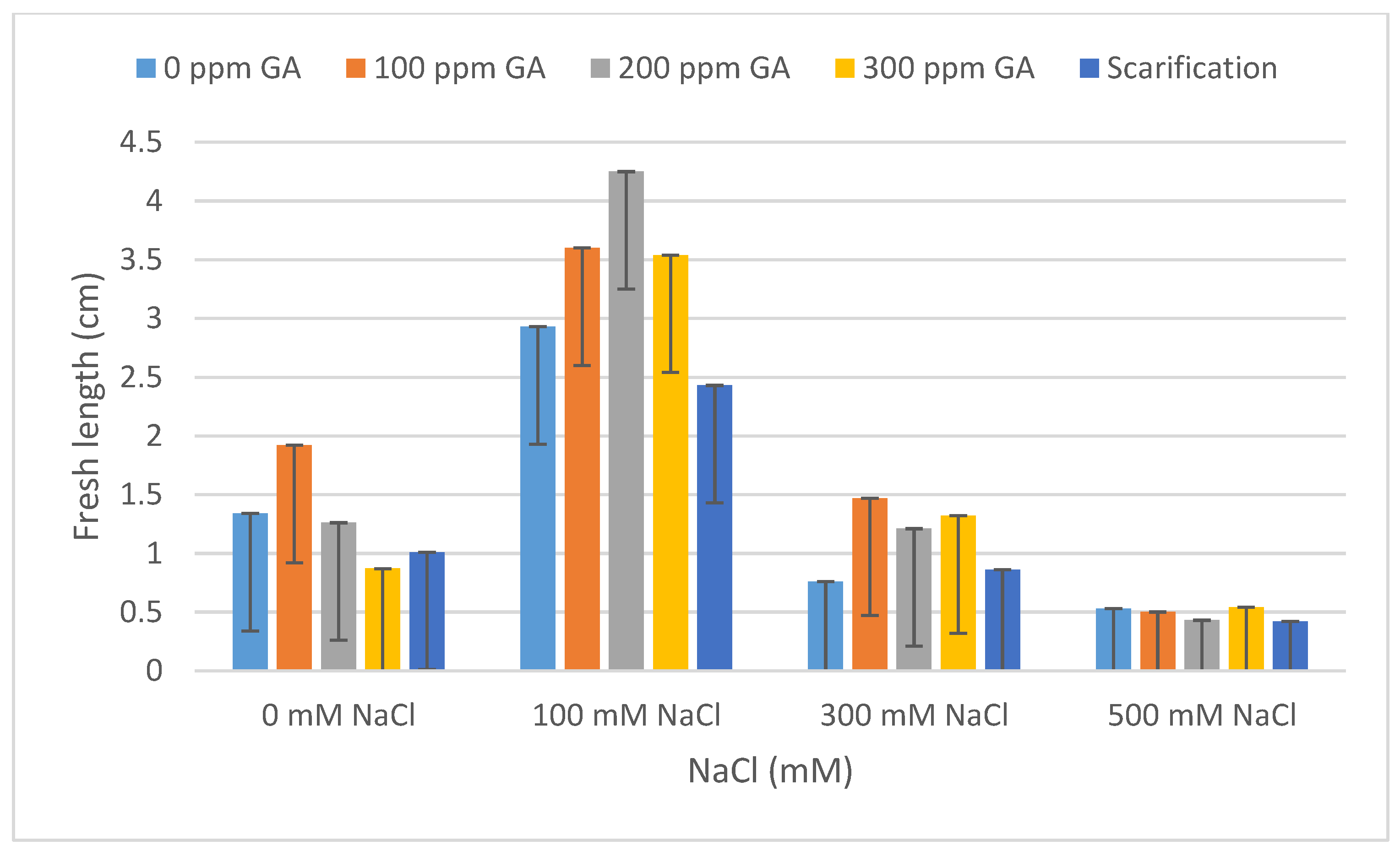
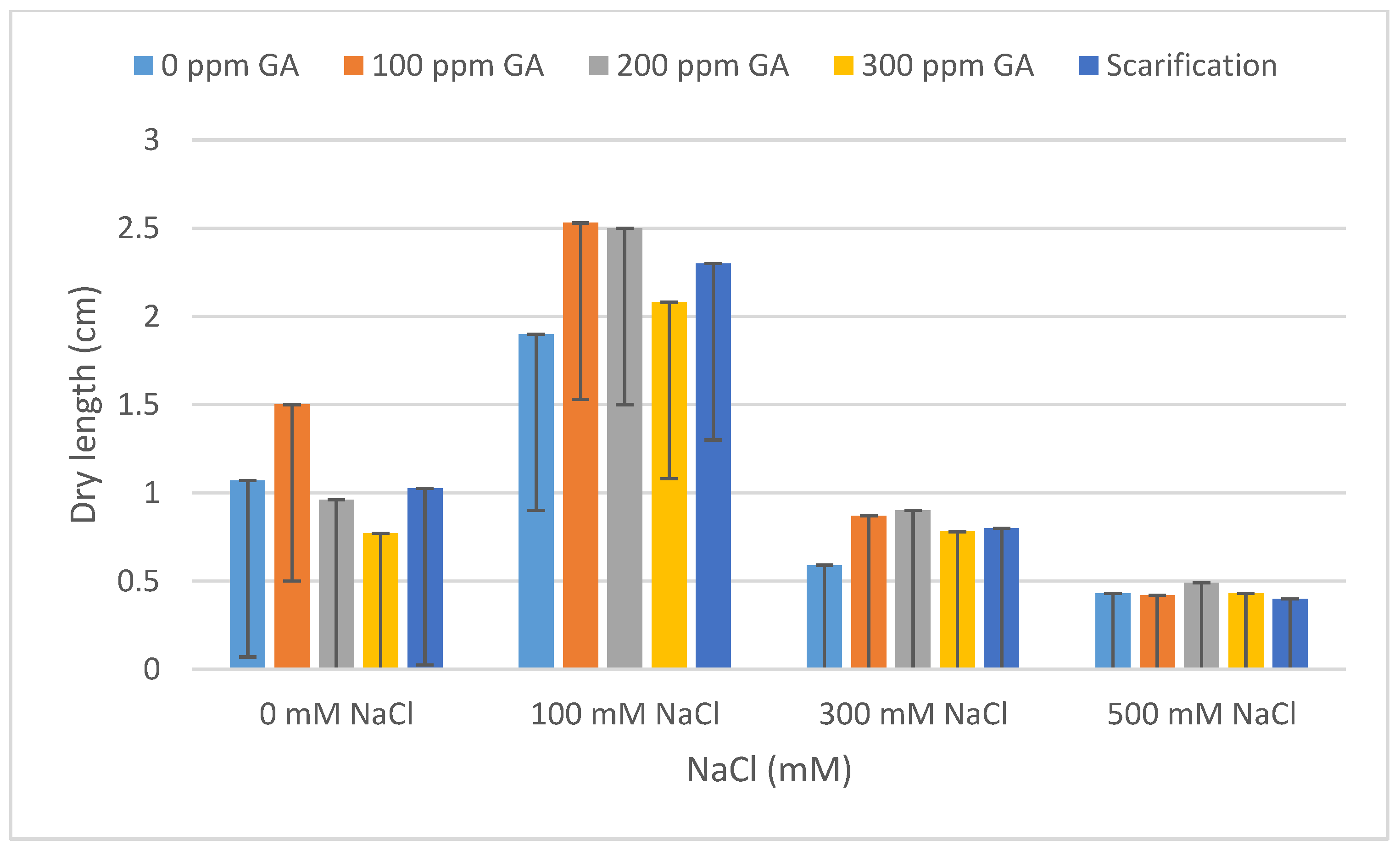
| Pretreatment | Salinity | Mean FL (cm) | Increase | Decrease | Mean DL (cm) | Increase | Decrease |
|---|---|---|---|---|---|---|---|
| 0 GA | 0 NaCl (control) | 1.34 ± 0.56 | - | - | 1.07 ± 0.13 | - | - |
| 100 NaCl | 2.93 ± 0.40 | 1.59 | - | 1.90 ± 0.02 | 0.83 | - | |
| 300 NaCl | 0.76 ± 0.04 | - | 0.58 | 0.59 ± 0.09 | - | 0.48 | |
| 500 NaCl | 0.53 ± 0.06 | - | 0.81 | 0.43 ± 0.04 | - | 0.64 | |
| 100 GA | 0 NaCl | 1.92 ± 0.04 | 0.58 | - | 1.51 ± 0.13 | 0.44 | - |
| 100 NaCl | 3.60 ± 0.20 | 2.26 | - | 2.53 ± 0.05 | 1.46 | - | |
| 300 NaCl | 1.47 ± 0.04 | 0.13 | - | 0.87 ± 0.09 | - | 0.20 | |
| 500 NaCl | 0.50 ± 0.10 | - | 0.84 | 0.42 ± 0.05 | - | 0.65 | |
| 200 GA | 0 NaCl | 1.27 ± 0.24 | - | 0.07 | 0.96 ± 0.1 | - | 0.11 |
| 100 NaCl | 4.25 ± 0.26 | 2.91 | - | 2.50 ± 0.13 | 1.43 | - | |
| 300 NaCl | 1.21 ± 0.15 | - | 0.13 | 0.90 ± 0.04 | - | 0.17 | |
| 500 NaCl | 0.43 ± 0.08 | - | 0.91 | 0.49 ± 0.08 | - | 0.58 | |
| 300 GA | 0 NaCl | 0.86 ± 0.05 | - | 0.48 | 0.77 ± 0.10 | - | 0.30 |
| 100 NaCl | 3.54 ± 0.10 | 2.20 | - | 2.08 ± 0.07 | 1.01 | - | |
| 300 NaCl | 1.32 ± 0.02 | - | 0.02 | 0.78 ± 0.03 | - | 0.29 | |
| 500 NaCl | 0.54 ± 0.04 | - | 0.80 | 0.43 ± 0.05 | - | 0.64 | |
| Scarification | 0 NaCl | 1.01 ± 0.55 | - | 0.33 | 1.02 ± 0.08 | - | 0.05 |
| 100 NaCl | 2.44 ± 0.26 | 1.10 | - | 2.30 ± 0.03 | 1.23 | - | |
| 300 NaCl | 0.86 ± 0.12 | - | 0.48 | 0.76 ± 0.02 | - | 0.31 | |
| 500 NaCl | 0.42 ± 0.04 | - | 0.92 | 0.40 ± 0.06 | - | 0.67 |
| Variables | Treatment | ||
|---|---|---|---|
| Pretreatments: GA3 (ppm) and MS | Salinity (NaCl) mM | ||
| Modalities | 0 GA3 100 GA3 200 GA3 300 GA3 MS | 0 | 0/0 100/0 200/0 300/0 MS/0 |
| 0 GA3 100 GA3 200 GA3 300 GA3 MS | 100 | 0/100 100/100 200/100 300/100 MS/100 | |
| 0 GA3 100 GA3 200 GA3 300 GA3 MS | 300 | 0/300 100/300 200/300 300/300 MS/300 | |
| 0 GA3 100 GA3 200 GA3 300 GA3 MS | 500 | 0/500 100/500 200/500 300/500 MS/500 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazih, A.; Baghour, M.; Maatougui, A.; Aboukhalid, K.; Chiboub, B.; Bazile, D. Effect of Gibberellic Acid and Mechanical Scarification on the Germination and Seedling Stages of Chenopodium quinoa Willd. under Salt Stress. Plants 2024, 13, 1330. https://doi.org/10.3390/plants13101330
Nazih A, Baghour M, Maatougui A, Aboukhalid K, Chiboub B, Bazile D. Effect of Gibberellic Acid and Mechanical Scarification on the Germination and Seedling Stages of Chenopodium quinoa Willd. under Salt Stress. Plants. 2024; 13(10):1330. https://doi.org/10.3390/plants13101330
Chicago/Turabian StyleNazih, Abderrahmane, Mourad Baghour, Abdesselam Maatougui, Kaoutar Aboukhalid, Basma Chiboub, and Didier Bazile. 2024. "Effect of Gibberellic Acid and Mechanical Scarification on the Germination and Seedling Stages of Chenopodium quinoa Willd. under Salt Stress" Plants 13, no. 10: 1330. https://doi.org/10.3390/plants13101330
APA StyleNazih, A., Baghour, M., Maatougui, A., Aboukhalid, K., Chiboub, B., & Bazile, D. (2024). Effect of Gibberellic Acid and Mechanical Scarification on the Germination and Seedling Stages of Chenopodium quinoa Willd. under Salt Stress. Plants, 13(10), 1330. https://doi.org/10.3390/plants13101330







