Multicontamination Toxicity Evaluation in the Model Plant Lactuca sativa L.
Abstract
:1. Introduction
2. Results
2.1. Accumulation of Toxic Elements in Lettuce
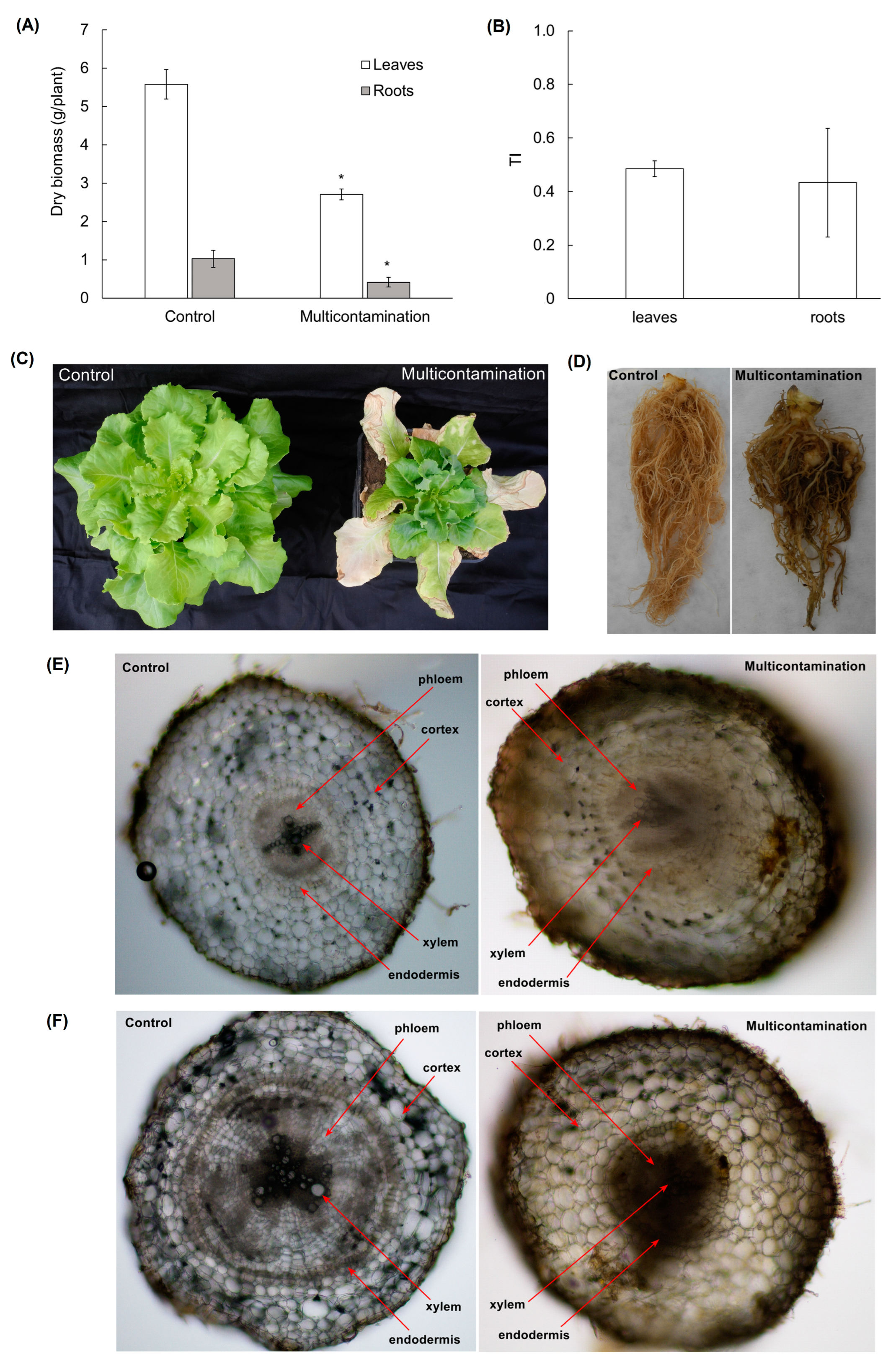
2.2. Biomass Production, Morphology, and Root Anatomy of Lettuce
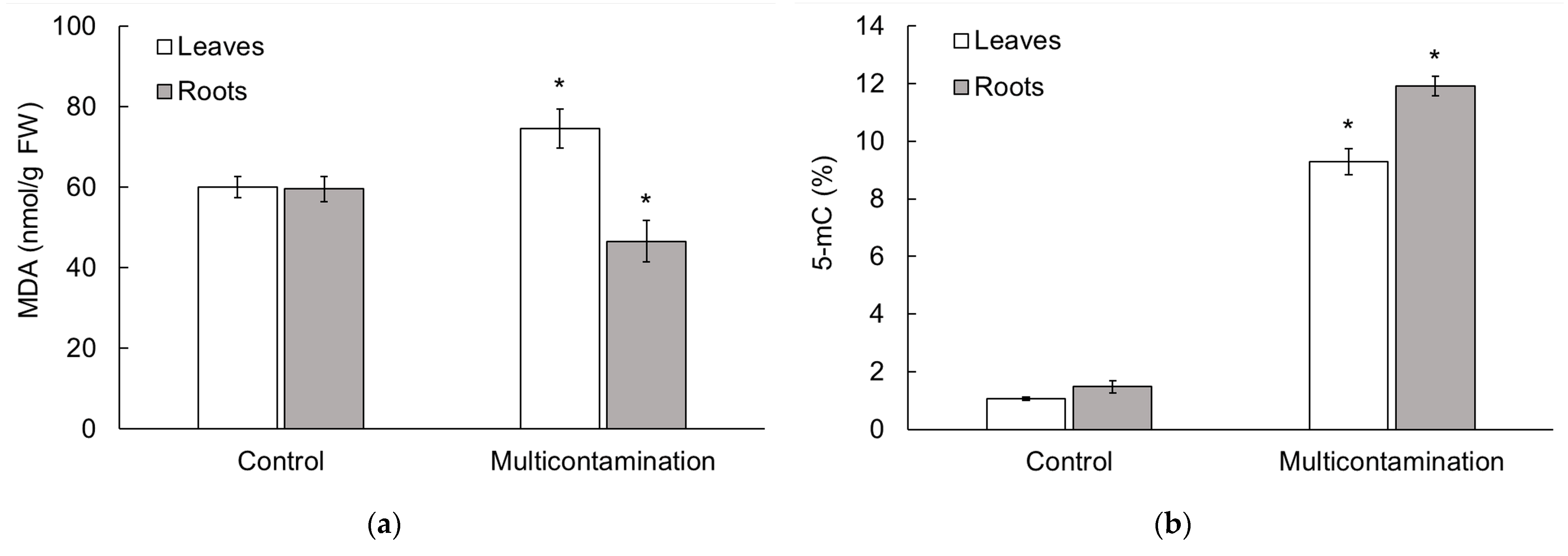
2.3. Malondialdehyde and 5-Methylcytosine Content in Lettuce
2.4. Free Amino Acid Content in Lettuce
2.5. Photosynthetic Parameters and Pigments of Lettuce
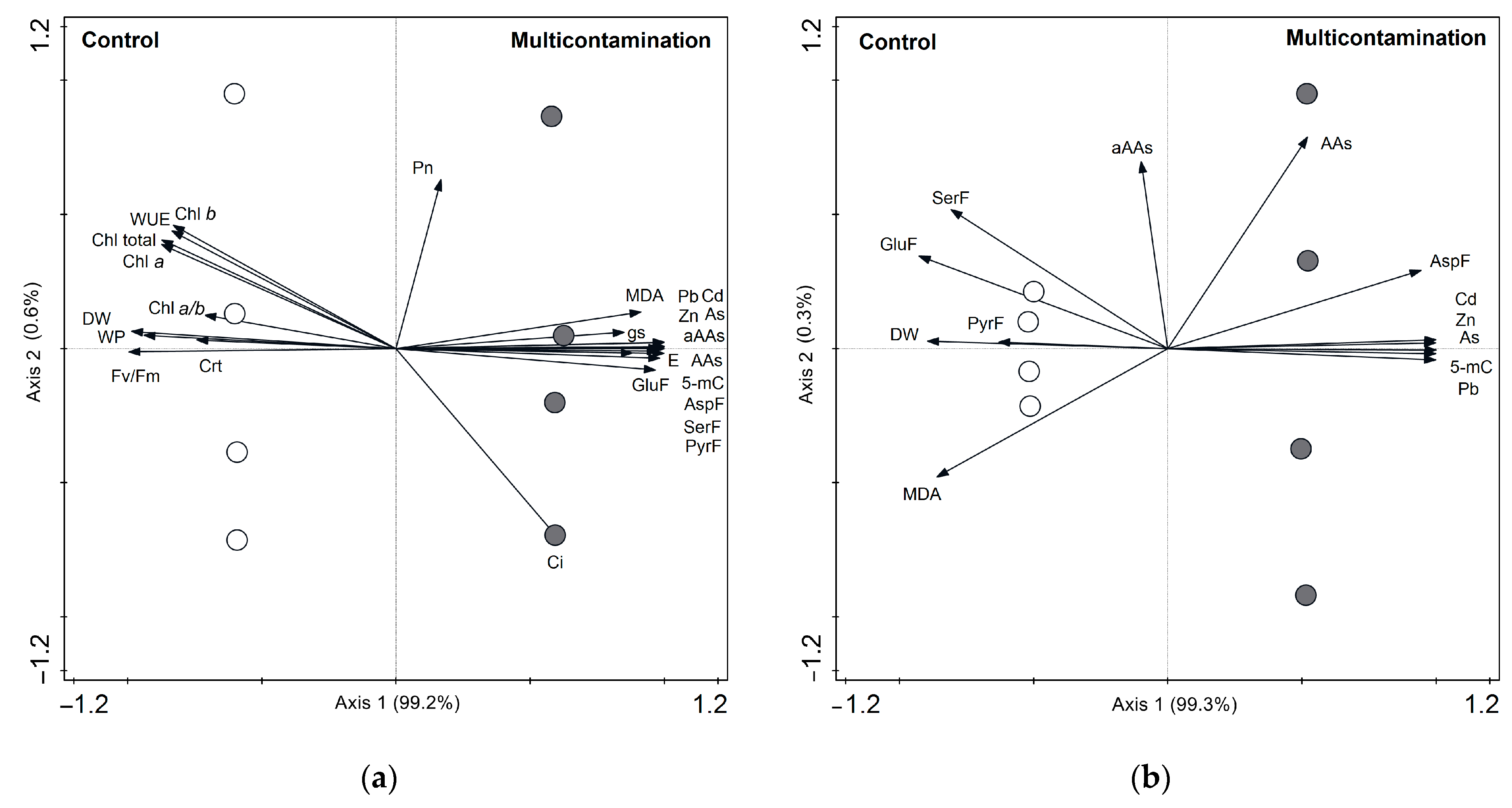
2.6. Relationship between Toxic Elements and Physiological and Metabolic Parameters in Lettuce
3. Discussion
3.1. Impact of Toxic Element Multicontamination on Growth and Morpho-Anatomical Changes in Lettuce
3.2. Impact of Toxic Element Multicontamination on the Metabolic Response of Lettuce
3.3. Impact of Toxic Element Multicontamination on Photosynthesis and Water Potential of Lettuce
4. Materials and Methods
4.1. Plants and Soil
4.2. Microscopic Observation
4.3. Toxic Element Determination
4.4. Factors Calculation
4.5. Malondialdehyde and 5-Methylcytosine Determination
4.6. Free Amino Acid Determination
4.7. Photosynthetic Parameters and Pigment Determination
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaněk, A.; Ettler, V.; Grygar, T.; Borůvka, L.; Šebek, O.; Drábek, O. Combined chemical and mineralogical evidence for heavy metal binding in mining- and smelting-affected alluvial soils. Pedosphere 2008, 18, 464–478. [Google Scholar] [CrossRef]
- Kebonye, N.M.; Eze, P.N.; John, K.; Agyeman, P.C.; Němeček, K.; Borůvka, L. An in-depth human health risk assessment of potentially toxic elements in highly polluted riverine soils, Příbram (Czech Republic). Environ. Geochem. Health 2021, 44, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, D.; Zemanová, V.; Pavlík, M. Health risk and quality assessment of vegetables cultivated on soils from a heavily polluted old mining area. Toxics 2023, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation—A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Jibril, S.A.; Hassan, S.A.; Ishak, C.F.; Megat Wahab, P.E. Cadmium toxicity affects phytochemicals and nutrient elements composition of lettuce (Lactuca sativa L.). Adv. Agric. 2017, 2017, 1236830. [Google Scholar] [CrossRef]
- Pietrelli, L.; Menegoni, P.; Papetti, P. Bioaccumulation of heavy metals by herbaceous species grown in urban and rural sites. Water Air Soil Pollut. 2022, 233, 141. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A Comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2022, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, V.B.; Zhukovskaya, N.V. Effect of heavy metals on root growth and the use of roots as test objects. Russ. J. Plant Physiol. 2021, 68, S1–S25. [Google Scholar] [CrossRef]
- Nazir, A.; Rafique, F.; Ahmed, K.; Khan, S.A.; Khan, N.; Akbar, M.; Zafar, M. Evaluation of heavy metals effects on morpho-anatomical alterations of wheat (Triticum aestivum L.) seedlings. Microsc. Res. Tech. 2021, 84, 2517–2529. [Google Scholar] [CrossRef]
- Dey, U.; Mondal, N.K. Ultrastructural deformation of plant cell under heavy metal stress in Gram seedlings. Cogent Environ. Sci. 2016, 2, 1196472. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An overview of heavy metal challenge in plants: From roots to shoots. Metallomics 2013, 5, 1117–1132. [Google Scholar] [CrossRef] [PubMed]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Bidar, G.; Pelfrêne, A.; Schwartz, C.; Waterlot, C.; Sahmer, K.; Marot, F.; Douay, F. Urban kitchen gardens: Effect of the soil contamination and parameters on the trace element accumulation in vegetables—A review. Sci. Total Environ. 2020, 738, 139569. [Google Scholar] [CrossRef] [PubMed]
- Mustățea, G.; Belc, N.; Ungureanu, E.L.; Lăcătușu, R.; Petre, J.; Pruteanu, A. Heavy metals contamination of the soil—Water—Vegetables chain in the Ilfov region. E3S Web Conf. 2019, 112, 03030. [Google Scholar] [CrossRef]
- Trovato, M.; Funck, D.; Forlani, G.; Okumoto, S.; Amir, R. Editorial: Amino acids in plants: Regulation and functions in development and stress defense. Front. Plant Sci. 2021, 12, 772810. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; He, E.; Qiu, H.; Van Gestel, C.A.M.; Romero-Freire, A.; Zhao, L.; Xu, X.; Cao, X. Interactions of arsenic, copper, and zinc in soil-plant system: Partition, uptake and phytotoxicity. Sci. Total Environ. 2020, 745, 140926. [Google Scholar] [CrossRef]
- Ackova, D.G. Heavy metals and their general toxicity on plants. Plant Sci. Today 2018, 5, 15–19. [Google Scholar] [CrossRef]
- Ejaz, U.; Khan, S.M.; Khalid, N.; Ahmad, Z.; Jehangir, S.; Rizvi, Z.F.; Lho, L.H.; Han, H.; Raposo, A. Detoxifying the heavy metals: A multipronged study of tolerance strategies against heavy metals toxicity in plants. Front. Plant Sci. 2023, 14, 1154571. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, W.-T.; Liu, L.; Gu, J.F.; Wang, W.-L.; Zou, J.-L.; Tian, T.; Peng, P.-Q.; Liao, B.-H. Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 289. [Google Scholar] [CrossRef]
- Xiao, W.; Ye, X.Z.; Zhang, Q.; Chen, D.; Hu, J.; Gao, N. Evaluation of cadmium transfer from soil to leafy vegetables: Influencing factors, transfer models, and indication of soil threshold contents. Ecotox. Environ. Saf. 2018, 164, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Li, M.-Y.; Yan, C.-A.; Tian, W.; Deng, Z.-H.; Wang, Z.-X.; Xu, W.-M.; Tuo, Y.-F.; Xiang, P. Refining health risk assessment of heavy metals in vegetables from high geochemical background areas: Role of bioaccessibility and cytotoxicity. Process SAF Environ. 2022, 159, 345–353. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Yao, Z.-L.; Ren, Y.-B.; Wang, L.-Q.; Ou, X.-B. Arsenic accumulation and physiological response of three leafy vegetable varieties to as stress. Int. J. Environ. Res. Public Health 2022, 19, 2501. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, Z.; Li, X.; Wang, H.; Wang, H.; Chen, W. A comprehensive assessment of heavy metal(loid) contamination in leafy vegetables grown in two mining areas in Yunnan, China-a focus on bioaccumulation of cadmium in Malabar spinach. Environ. Sci. Pollut. Res. Int. 2022, 30, 14959–14974. [Google Scholar] [CrossRef] [PubMed]
- Tajdar-Oranj, B.; Javanmardi, F.; Parastouei, K.; Taghdir, M.; Fathi, M.; Abbaszadeh, S. Health Risk assessment of lead, cadmium, and arsenic in leafy vegetables in Tehran, Iran: The concentration data study. Biol. Trace Elem. Res. 2024, 202, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Zorrig, W.; Cornu, J.Y.; Maisonneuve, B.; Rouached, A.; Sarrobert, C.; Shahzad, Z.; Berthomieu, P. Genetic analysis of cadmium accumulation in lettuce (Lactuca sativa). Plant Physiol. Biochem. 2019, 136, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Capelo, A.; Santos, C.; Loureiro, S.; Pedrosa, M.A. Phytotoxicity of lead on Lactuca sativa: Effects on growth, mineral nutrition, phytosynthetic activity and oxidant metabolism. Fresenius Environ. Bull. 2012, 21, 450–459. [Google Scholar]
- Kongtawee, K.; Ketrot, D.; Wisawapipat, W.; Tawornpruek, S. Assessing critical level of lead in soils for leafy vegetables. Water Air Soil Pollut. 2022, 233, 459. [Google Scholar] [CrossRef]
- Commission Regulation (EU). Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance). Off. J. Eur. Union. 2023, 119, 103–157. [Google Scholar]
- Akhter, M.F.; Omelon, C.; Gordon, R.A.; Moser, D.; Macfie, S.M. Localization and chemical speciation of cadmium in the roots of barley and lettuce. Environ. Exp. Bot. 2014, 100, 10–19. [Google Scholar] [CrossRef]
- Pérez-Figueroa, C.E.; Salazar-Moreno, R.; Rodríguez, E.F.; Cruz, I.L.L.L.; Schmidt, U.; Danneh, D. Heavy metals accumulation in lettuce and cherry tomatoes cultivated in cities. Pol. J. Environ. Stud. 2023, 32, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Musilová, J.; Franková, H.; Lidiková, J.; Chlpík, J.; Vollmannová, A.; Árvay, J.; Harangozo, L.; Urminská, J.; Tóth, T. Impact of old environmental burden in the Spiš region (Slovakia) on soil and home-grown vegetable contamination, and health effects of heavy metals. Sci. Rep. 2022, 12, 16371. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Dai, H.; Pan, S. Health risk assessment of heavy metal exposure through vegetable consumption around a phosphorus chemical plant in the Kaiyang karst area, southwestern China. Environ. Sci. Pollut. Res. 2023, 30, 35617–35634. [Google Scholar] [CrossRef] [PubMed]
- Sanjosé, I.; Navarro-Roldán, F.; Montero, Y.; Ramírez-Acosta, S.; Jiménez-Nieva, F.J.; Infante-Izquierdo, M.D.; Polo-Ávila, A.; Muñoz-Rodríguez, A.F. The bioconcentration and the translocation of heavy metals in recently consumed Salicornia ramosissima J. woods in highly contaminated estuary marshes and its food risk. Diversity 2022, 14, 452. [Google Scholar] [CrossRef]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; El Mzibri, M.; Bogusz, D.; Smouni, A. Effect of lead on root growth. Front. Plant Sci. 2013, 4, 175. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Mitra, M.; Agarwal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in response to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef]
- Berni, R.; Luyckx, M.; Xu, X.; Legay, S.; Sergeanty, K.; Hausman, J.F.; Lutts, S.; Cai, G.; Guirriero, G. Reactive oxygen species and heavy metal stress in plants: Impact on the cell wall and secondary metabolism. Environ. Exp. Bot. 2019, 161, 98–106. [Google Scholar] [CrossRef]
- Gill, R.A.; Kanwar, M.K.; dos Reis, A.R.; Ali, B. Editorial: Heavy metal toxicity in plants: Recent insights on physiological and molecular aspects. Front. Plant Sci. 2022, 12, 830682. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Charles, F.; Vidal, V.; Laurent, S.; Klopp, C.; Lauri, F.; Sallanon, H.; Roux, D. Transcriptomic view of detached lettuce leaves during storage: A crosstalk between wounding, dehydration and senescence. Postharvest Biol. Technol. 2019, 152, 73–88. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Age-associated alterations in the somatic mutation and DNA methylation levels in plants. Plant Biol. 2016, 18, 185–196. [Google Scholar] [CrossRef]
- Ikkonen, E.; Kaznina, N. Physiological responses of lettuce (Lactuca sativa L.) to soil contamination with Pb. Horticulturae 2022, 8, 951. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Z.Q.; Chen, D.; Liu, L.; Hamid, Y.; Zhang, S.J.; Shan, A.Q.; Kang, K.J.; Feng, Y.; Yang, X.E. Combined cadmium and fluorine inhibit lettuce growth through reducing root elongation, photosynthesis, and nutrient absorption. Environ. Sci. Pollut. Res. Int. 2022, 29, 91255–91267. [Google Scholar] [CrossRef] [PubMed]
- Janku, M.; Luhova, L.; Petrivalsky, M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- de Dios Alché, J. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019, 23, 101136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and utilization of malondialdehyde in exotic pine under drought stress using near-infrared spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Liu, W.; Zhang, Y.; Zhou, C.; Shao, M. Morphological and physiological stress responses of lettuce to different intensities of continuous light. Front. Plant Sci. 2019, 6, 1440. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Li, X.; Li, H.; Peng, X.; Zhang, R.; Tang, W.; Dong, Y.; Tang, Y. Intercropping affects the physiology and cadmium absorption of pakchoi, lettuce, and radish seedlings. Environ. Sci. Pollut. Res. 2023, 30, 4744–4753. [Google Scholar] [CrossRef]
- da Cunha Neto, A.R.; Carvalho, M.; Morais, G.M.M.; Guaraldo, M.M.D.S.; dos Santos, H.O.; Pereira, W.V.S.; Barbosa, S. Changes in chromosome complement and germination of lettuce (Lactuca sativa L.) exposed to heavy metal stress. Water Air Soil Pollut. 2023, 234, 243. [Google Scholar] [CrossRef]
- Gao, T.; Wang, H.; Li, C.; Zuo, M.; Wang, X.; Liu, Y.; Yang, Y.; Xu, D.; Liu, Y.; Fang, X. Effects of heavy metal stress on physiology, hydraulics, and anatomy of three desert plants in the Jinchang mining area, China. Int. J. Environ. Res. Public Health 2022, 19, 15873. [Google Scholar] [CrossRef] [PubMed]
- Knieper, M.; Viehhauser, A.; Dietz, K.J. Oxylipins and reactive carbonyls as regulators of the plant redox and reactive oxygen species network under stress. Antioxidants 2023, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska-Gnatowska, E.; Polkowska-Kowalczyk, L.; Szczegielniak, J.; Barciszewska, M.; Barciszewski, J.; Muszyńska, G. Is DNA methylation modulated by wounding-induced oxidative burstin maize? Plant. Physiol. Biochem. 2014, 82, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mohapatra, T. Dynamics of DNA methylation and its functions in plant growth and development. Front. Plant Sci. 2021, 12, 596236. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, R.; Xu, Y.; Zhang, C.; Niu, Q.; Lang, Z. DNA cytosine methylation dynamics and functional roles in horticultural crops. Hortic. Res. 2023, 10, uhad170. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yang, Z.; Liu, L.; Duan, L. DNA methylation in plant responses and adaption to abiotic stresses. Int. J. Mol. Sci. 2022, 23, 6910. [Google Scholar] [CrossRef] [PubMed]
- Chmielowska-Bąk, J.; Searle, I.R.; Wakai, T.N.; Arasimowicz-Jelonek, M. The role of epigenetic and epitranscriptomic modifications in plants exposed to nonessential metals. Front. Plant Sci. 2023, 14, 1278185. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.D.; Sun, J.W.; Huang, L.Y.; Chen, N.; Wang, Q.W. Effects of cadmium stress on DNA methylation in soybean. Biotechnol. Biotechnol. Eq. 2021, 35, 1696–1705. [Google Scholar] [CrossRef]
- Tang, M.J.; Xu, L.A.; Wang, Y.; Dong, J.H.; Zhang, X.L.; Wang, K.; Ying, J.L.; Li, C.; Liu, L.W. Melatonin-induced DNA demethylation of metal transporters and antioxidant genes alleviates lead stress in radish plants. Hortic. Res. 2021, 8, 124. [Google Scholar] [CrossRef]
- Lhotská, M.; Zemanová, V.; Pavlík, M.; Pavlíková, D.; Hnilička, F.; Popov, M. Leaf fitness and stress response after the application of contaminated soil dust particulate matter. Sci. Rep. 2022, 12, 10046. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, H.; Guo, Q.; Zhang, Z.; Zhao, J.; Yang, D. Effects of cadmium stress on growth and amino acid metabolism in two Compositae plants. Ecotoxicol. Environ. Saf. 2018, 158, 300–308. [Google Scholar] [CrossRef]
- Zhu, G.; Cheng, D.; Wang, X.; Guo, Q.; Zhang, Q.; Zhang, J.; Tu, Q.; Li, W. Free amino acids, carbon and nitrogen isotopic compositions responses to cadmium stress in two castor (Ricinus communis L.) species. Plant Physiol. Biochem. 2022, 184, 40–46. [Google Scholar] [CrossRef]
- Kumar, A.; Dwivedi, S.; Singh, R.P.; Chakrabarty, D.; Mallick, S.; Trivedi, P.K.; Adhikari, B.; Tripathi, R.D. Evaluation of amino acid profile in contrasting arsenic accumulating rice genotypes under arsenic stress. Biol. Plant. 2014, 58, 733–742. [Google Scholar] [CrossRef]
- Campos, N.V.; Araújo, T.O.; Arcanjo-Silva, S.; Freitas-Silva, L.; Azevedo, A.A.; Nunes-Nesi, A. Arsenic hyperaccumulation induces metabolic reprogramming in Pityrogramma calomelanos to reduce oxidative stress. Physiol. Plant. 2016, 157, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Okunev, R.V. Free amino acid accumulation in soil and tomato plants (Solanum lycopersicum L.) associated with arsenic stress. Water Air Soil Pollut. 2019, 230, 253. [Google Scholar] [CrossRef]
- Zemanova, V.; Popov, M.; Pavlíková, D.; Kotrba, P.; Hnilička, F.; Česká, J.; Pavlík, M. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol. 2020, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Zemanová, V.; Pavlíková, D.; Hnilička, F.; Pavlík, M. Arsenic toxicity-induced physiological and metabolic changes in the shoots of Pteris cretica and Spinacia oleracea. Plants 2021, 10, 2009. [Google Scholar] [CrossRef]
- Dguimi, H.M.; Nasraoui, H.A.; Alzahrani, F.O. Cadmium effect on growth, ammonium assimilation, and amino acids levels in roots of Arabidopsis thaliana. Euro-Mediterr. J. Environ. Integrat. 2023, 8, 161–165. [Google Scholar] [CrossRef]
- Hildebrandt, T.; Nunes-Nesi, A.; Araujo, W.; Braun, H.-P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, A.; Dubey, A.K.; Ranjan, R.; Pandey, A.; Kumari, B.; Singh, G.; Mandotra, S.; Chauhan, P.S.; Srikrishna, S.; et al. GABA mediated reduction of arsenite toxicity in rice seedling through modulation of fatty acids, stress responsive amino acids and polyamines biosynthesis. Ecotoxicol. Environ. Saf. 2019, 173, 15–27. [Google Scholar] [CrossRef]
- Praveen, A.; Pandey, A.; Gupta, M. Protective role of nitric oxide on nitrogen-thiol metabolism and amino acids profiling during arsenic exposure in Oryza sativa L. Ecotoxicology 2020, 29, 825–836. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, D.; Liu, Q. Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Lea, P.J.; Sodek, L.; Parry, M.A.; Shewry, P.R.; Halford, N.G. Asparagine in plants. Ann. Appl. Biol. 2007, 150, 1–26. [Google Scholar] [CrossRef]
- González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on salt tolerance of two endemic Limonium species from Spain. Metabolites 2019, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Okumoto, S.; Funck, D.; Trovato, M.; Forlani, G. Editorial: Amino acids of the glutamate family: Functions beyond primary metabolism. Front. Plant Sci. 2016, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Kirma, M.; Araujo, W.L.; Fernie, A.R.; Galili, G. The multifaceted role of aspartate-family amino acids in plant metabolism. J. Exp. Bot. 2012, 63, 4995–5001. [Google Scholar] [CrossRef]
- Planchet, E.; Limami, A.M. Amino acid synthesis under abiotic stress. In Amino Acids in Higher Plants; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2015; pp. 262–276. [Google Scholar] [CrossRef]
- Ros, R.; Muñoz-Bertomeu, J.; Krueger, S. Serine in plants: Biosynthesis, metabolism, and functions. Trends Plant Sci. 2014, 19, 564–569. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.; Zhang, L.; Li, L.; Liu, S.; Yu, J.; You, L.; Zhou, D.; Xia, C.; Zhao, J.; et al. Metabolic profiling of cadmium-induced effects in one pioneer intertidal halophyte Suaeda salsa by NMR-based metabolomics. Ecotoxicology 2011, 20, 1422–1431. [Google Scholar] [CrossRef]
- Ingle, R.A. Histidine biosynthesis. Arabidopsis Book 2011, 9, e0141. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Suravajhala, R.; Rajasheker, G.; Marka, N.; Shridhar, K.K.; Dhulala, D.; Scinthia, K.P.; Divya, K.; Doma, M.; Edupuganti, S.; et al. Lysine, lysine-rich, serine, and serine-rich proteins: Link between metabolism, development, and abiotic stress tolerance and the role of ncRNAs in their regulation. Front. Plant Sci. 2020, 11, 546213. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 2006, 57, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Azevedo Neto, A.D.; Prisco, J.T.; Gomes-Filho, E. Changes in soluble amino-N, soluble proteins and free amino acids in leaves and roots of salt-stressed maize genotypes. J. Plant Interact. 2009, 4, 137–144. [Google Scholar] [CrossRef]
- Kang, T.; Wu, H.D.; Lu, B.Y.; Luo, X.J.; Gong, C.M.; Bai, J. Low concentrations of glycine inhibit photorespiration and enhance the net rate of photosynthesis in Caragana Korshinskii. Photosynthetica 2018, 56, 512–519. [Google Scholar] [CrossRef]
- Cochavi, A.; Cohen, I.H.; Rachmilevitch, S. The role of different root orders in nutrient uptake. Environ. Exp. Bot. 2020, 179, 104212. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2002, 51, 1531–1542. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Holm, P.B. Transcriptome analysis of senescence in the flag leaf of wheat (Triticum aestivum L.). Plant Biotechnol. J. 2007, 5, 192–206. [Google Scholar] [CrossRef]
- Chen, X.J.; Tao, H.F.; Wu, Y.Z.; Xu, X.M. Effects of cadmium on metabolism of photosynthetic pigment and photosynthetic system in Lactuca sativa L. revealed by physiological and proteomics analysis. Sci. Hortic. 2022, 305, 111371. [Google Scholar] [CrossRef]
- Tuba, Z.; Lichtenthaler, H.; Csintalan, Z.; Nagy, Z.; Szente, K. Loss of chlorophylls, cessation of photosynthesis CO2 assimilation and respiration in the poikilochlorophyllous plant Xerophyta scabrida during desiccation. Physiol. Plant 1996, 96, 383–388. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Kotakis, C.; Kyzeridou, A.; Manetas, Y. Photosynthetic electron flow during leaf senescence: Evidence for a preferential maintenance of photosystem I activity and increased cyclic electron flow. Photosynthetica 2014, 52, 413–420. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Sobejano-Paz, V.; Mo, X.; Liu, S.; Mikkelsen, T.N.; He, L.; Jin, H.; García, M. Heat dissipation from photosynthesis contributes to maize thermoregulation under suboptimal temperature conditions. bioRxiv 2023. bioRxiv:2023.01.27.525868. [Google Scholar] [CrossRef]
- Firmansyah; Argosubekti, N. A review of heat stress signaling in plants. IOP Conf. Ser. Earth Environ. Sci. 2020, 484, 012041. [Google Scholar] [CrossRef]
- Rastgoo, L.; Alemzadeh, A. Biochemical responses of Gouan (Aeluropus littoralis) to heavy metals stress. Aust. J. Crop Sci. 2011, 5, 375–383. [Google Scholar]
- Chandra, R.; Kang, H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci. Technol. 2016, 12, 55–61. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, X.; Zhang, J.; Li, J.; Niu, T.; Tang, C.; Wang, C.; Xie, J. Zinc oxide nanoparticles improve lettuce (Lactuca sativa L.) plant tolerance to cadmium by stimulating antioxidant defense, enhancing lignin content and reducing the metal accumulation and translocation. Front. Plant Sci. 2022, 13, 1015745. [Google Scholar] [CrossRef]
- Felemban, A.; Braguy, J.; Zurbriggen, M.D.; Al-Babili, S. Apocarotenoids involved in plant development and stress response. Front. Plant Sci. 2019, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Rao, S.; Zhou, X.; Li, L. Plant carotenoids: Recent advances and future perspectives. Mol. Hortic. 2022, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Czech Ministry of the Environment. Public Notice No. 153/2016 for the Management of Soil Protection; Czech Ministry of the Environment: Prague, Czech Republic, 2016. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta BBA Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]

| TEs | Control (mg/kg DW) | BCF | Multicontamination (mg/kg DW) | BCF |
|---|---|---|---|---|
| As—leaves | ND | - | 7.95 ± 0.72 | 0.03 |
| Cd—leaves | 0.92 ± 0.04 | 2.30 | 19.08 ± 0.44 * | 0.51 |
| Pb—leaves | ND | - | 18.16 ± 3.44 | 0.01 |
| Zn—leaves | 22.76 ± 0.73 | 0.27 | 669.37 ± 9.50 * | 0.19 |
| As—roots | ND | - | 8.56 ± 0.05 | 0.03 |
| Cd—roots | 0.96 ± 0.09 | 2.39 | 27.38 ± 3.14 *# | 0.73 |
| Pb—roots | ND | - | 28.60 ± 0.74 # | 0.01 |
| Zn—roots | 56.63 ± 1.65 # | 0.66 | 688.74 ± 55.07 * | 0.20 |
| Parameter | Control | Multicontamination |
|---|---|---|
| WP (MPa) | −1.39 ± 0.06 | −1.95 ± 0.15 * |
| Pn (μmol CO2/m2/s) | 8.20 ± 1.93 | 8.81 ± 2.03 |
| E (mmol H2O/m2/s) | 1.51 ± 0.18 | 2.66 ± 0.45 * |
| gs (mol H2O/m2/s) | 0.11 ± 0.03 | 0.21 ± 0.04 * |
| Ci (μmol CO2/mol) | 266.29 ± 20.96 | 296.60 ± 25.92 * |
| WUE (μmol CO2/mmol H2O) 1 | 5.39 ± 0.83 | 3.35 ± 0.72 * |
| Fv/Fm 2 | 0.78 ± 0.002 | 0.75 ± 0.002 * |
| Chl a (mg/m2) | 156.66 ± 17.71 | 112.90 ± 10.16 * |
| Chl b (mg/m2) | 47.59 ± 4.36 | 37.94 ± 3.28 * |
| Chl a/Chl b | 3.32 ± 0.23 | 3.02 ± 0.08 * |
| Chltotal (mg/m2) | 204.26 ± 21.27 | 150.84 ± 13.30 * |
| Crt (mg/m2) | 36.83 ± 3.51 | 30.79 ± 2.75 * |
| Parameter | Control | Multicontamination |
|---|---|---|
| Locality | Suchdol (50°8′8″ N, 14°22′43″ E) | Litavka (49°43′ N, 14°0′ E) |
| Soil type | Haplic chernozem | Gleyic fluvisol |
| Soil texture | Silt loam | Sandy loam |
| pHH2O | 7.1 ± 0.1 | 5.4 ± 0.1 |
| CEC (mmol(+)/kg) 1 | 230.1 ± 5.0 | 109.0 ± 31.9 |
| Corganic (%) | 1.8 ± 0.3 | 3.6 ± 0.4 |
| DOC (mg/kg) 2 | 153.0 ± 3.4 | 317.3 ± 19.3 |
| Aspseudo-total (mg/kg) | 18.1 ± 1.0 | 283.9 ± 7.7 |
| Cdpseudo-total (mg/kg) | 0.4 ± 0.01 | 37.4 ± 1.1 |
| Pbpseudo-total (mg/kg) | 32.1 ± 0.7 | 2361.2 ± 32.4 |
| Znpseudo-total (mg/kg) | 85.5 ± 2.5 | 3496.6 ± 60.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zemanová, V.; Lhotská, M.; Novák, M.; Hnilička, F.; Popov, M.; Pavlíková, D. Multicontamination Toxicity Evaluation in the Model Plant Lactuca sativa L. Plants 2024, 13, 1356. https://doi.org/10.3390/plants13101356
Zemanová V, Lhotská M, Novák M, Hnilička F, Popov M, Pavlíková D. Multicontamination Toxicity Evaluation in the Model Plant Lactuca sativa L. Plants. 2024; 13(10):1356. https://doi.org/10.3390/plants13101356
Chicago/Turabian StyleZemanová, Veronika, Marie Lhotská, Milan Novák, František Hnilička, Marek Popov, and Daniela Pavlíková. 2024. "Multicontamination Toxicity Evaluation in the Model Plant Lactuca sativa L." Plants 13, no. 10: 1356. https://doi.org/10.3390/plants13101356
APA StyleZemanová, V., Lhotská, M., Novák, M., Hnilička, F., Popov, M., & Pavlíková, D. (2024). Multicontamination Toxicity Evaluation in the Model Plant Lactuca sativa L. Plants, 13(10), 1356. https://doi.org/10.3390/plants13101356







