Plant Biostimulants Enhance Tomato Resilience to Salinity Stress: Insights from Two Greek Landraces
Abstract
1. Introduction
2. Results
2.1. Yield Parameters
2.2. Fruit Quality
2.3. Macro- and Micronutrients in Leaves
2.4. Macro- and Micronutrients in Fruit
2.5. PCA (Principal Component Analysis)
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Growth Conditions
4.3. Nutrient Solution Formula
4.4. Application of Biostimulants
4.5. Total Yield and Yield Components
4.6. Quality Traits
4.7. Leaf and Fruit Sampling for Nutrient Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, A.; Bano, A.; Khan, N. Climate Change and Salinity Effects on Crops and Chemical Communication between Plants and Plant Growth-Promoting Microorganisms under Stress. Front. Sustain. Food Syst. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J. Improving Crop Salt Tolerance. J. Exp. Bot. 2004, 55, 307–319. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil Salinity and Sodicity in Drylands: A Review of Causes, Effects, Monitoring, and Restoration Measures. Front. Environ. Sci. 2021, 9, 1–16. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2022. Leveraging Automation in Agriculture for Transforming Agrifood Systems; FAO: Rome, Italy, 2022. [Google Scholar]
- Chele, K.H.; Tinte, M.M.; Piater, L.A.; Dubery, I.A.; Tugizimana, F. Soil Salinity, a Serious Environmental Issue and Plant Responses: A Metabolomics Perspective. Metabolites 2021, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Saddique, M.; Kausar, A.; Iqra, I.; Akhter, N.; Mujahid, N.; Parveen, A.; Zaman, Q.; Hussain, S. Amino Acids Application Alleviated Salinity Stress in Spinach (Spinacia oleracea L.) by Improving Oxidative Defense, Osmolyte Accumulation, and Nutrient Balance. Turkish J. Agric. For. 2022, 46, 875–887. [Google Scholar] [CrossRef]
- Singh, A. Soil Salinity: A Global Threat to Sustainable Development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Raza, A.; Tabassum, J.; Fakhar, A.Z.; Sharif, R.; Chen, H.; Zhang, C.; Ju, L.; Fotopoulos, V.; Siddique, K.H.M.; Singh, R.K.; et al. Smart Reprograming of Plants against Salinity Stress Using Modern Biotechnological Tools. Crit. Rev. Biotechnol. 2023, 43, 1035–1062. [Google Scholar] [CrossRef]
- Magán, J.J.; Gallardo, M.; Thompson, R.B.; Thompson, P.L. Effects of Salinity on Fruit Yield and Quality of Tomato Grown in Soil-Less Culture in Greenhouses in Mediterranean Climatic Conditions. Agric. Water Manag. 2008, 95, 1041–1055. [Google Scholar] [CrossRef]
- Nishimura, T.; Cha-um, S.; Takagaki, M.; Ohyama, K.; Kirdmanee, C. Survival Percentage, Photosynthetic Abilities and Growth Characters of Two Indica Rice (Oryza sativa L. spp. Indica) Cultivars in Response to Iso-Osmotic Stress. Span. J. Agric. Res. 2011, 9, 262. [Google Scholar] [CrossRef]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of Salinity Stress on Crop Plants: Improving Salt Tolerance through Genetic and Molecular Dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef]
- Filippou, P.; Zarza, X.; Antoniou, C.; Obata, T.; Villarroel, C.A.; Ganopoulos, I.; Harokopos, V.; Gohari, G.; Aidinis, V.; Madesis, P.; et al. Systems Biology Reveals Key Tissue-Specific Metabolic and Transcriptional Signatures Involved in the Response of Medicago truncatula Plant Genotypes to Salt Stress. Comput. Struct. Biotechnol. J. 2021, 19, 2133–2147. [Google Scholar] [CrossRef]
- Gama, P.B.S.; Inanaga, S.; Tanaka, K.; Nakazawa, R. Physiological Response of Common Bean (Phaseolus vulgaris L.) Seedlings to Salinity Stress. Afr. J. Biotechnol. 2007, 6, 079–088. [Google Scholar]
- Nouck, A.E.; Taffouo, V.D.; Tsoata, E.; Dibong, D.S.; Nguemezi, S.T.; Gouado, I.; Youmbi, E. Growth, Biochemical Constituents, Micronutrient Uptake and Yield Response of Six Tomato (Lycopersicum esculentum L.) Cultivars Grown under Salinity Stress. J. Agron. 2016, 15, 58–67. [Google Scholar] [CrossRef]
- Win, K.T.; Tanaka, F.; Okazaki, K.; Ohwaki, Y. The ACC Deaminase Expressing Endophyte Pseudomonas spp. Enhances NaCl Stress Tolerance by Reducing Stress-Related Ethylene Production, Resulting in Improved Growth, Photosynthetic Performance, and Ionic Balance in Tomato Plants. Plant Physiol. Biochem. 2018, 127, 599–607. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Ali Raza, M.; Singh, K.; Anwar Hossain, M.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 1–20. [Google Scholar] [CrossRef]
- Mishra, A.K.; Das, R.; George Kerry, R.; Biswal, B.; Sinha, T.; Sharma, S.; Arora, P.; Kumar, M. Promising Management Strategies to Improve Crop Sustainability and to Amend Soil Salinity. Front. Environ. Sci. 2023, 10, 1–19. [Google Scholar] [CrossRef]
- Martínez-Lorente, S.E.; Martí-Guillén, J.M.; Pedreño, M.Á.; Almagro, L.; Sabater-Jara, A.B. Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress. Antioxidants 2024, 13, 318. [Google Scholar] [CrossRef]
- Chabili, A.; Minaoui, F.; Hakkoum, Z.; Douma, M.; Meddich, A.; Loudiki, M. A Comprehensive Review of Microalgae and Cyanobacteria-Based Biostimulants for Agriculture Uses. Plants 2024, 13, 159. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A Sufficiently Effective Tool for Sustainable Agriculture in the Era of Climate Change? Faisal. Plant Physiol. Biochem. 2024, 211, 108699. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, M.; Fusco, G.M.; Woodrow, P.; Carillo, P.; Rouphael, Y. Unravelling the Nexus of Plant Response to Non-Microbial Biostimulants under Stress Conditions. Plant Stress 2024, 11, 100421. [Google Scholar] [CrossRef]
- Bisht, A.; Chhabra, R. Biostimulants: Paving Way towards Sustainable Agriculture and Food Security. Theor. Exp. Plant Physiol. 2024, 36, 139–163. [Google Scholar] [CrossRef]
- Kisvarga, S.; Farkas, D.; Boronkay, G.; Neményi, A.; Orlóci, L. Effects of Biostimulants in Horticulture, with Emphasis on Ornamental Plant Production. Agronomy 2022, 12, 1043. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 1043. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Joel, J.M.; Puthur, J.T. Biostimulants: The Futuristic Sustainable Approach for Alleviating Crop Productivity and Abiotic Stress Tolerance. J. Plant Growth Regul. 2024, 43, 659–674. [Google Scholar] [CrossRef]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant Biostimulants: Importance of the Quality and Yield of Horticultural Crops and the Improvement of Plant Tolerance to Abiotic Stress-a Review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant Growth-Promoting Rhizobacteria Act as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, G.; Ertani, A.; Bulgari, R.; Franzoni, G.; Nicola, S.; Ferrante, A. Biostimulants and Plant Response under Adverse Environmental Conditions: A Functional Interplay. In Plant Performance Under Environmental Stress; Husen, A., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino Acids Biostimulants and Protein Hydrolysates in Agricultural Sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Nanda, S.; Kumar, G.; Hussain, S. Utilization of Seaweed-Based Biostimulants in Improving Plant and Soil Health: Current Updates and Future Prospective. Int. J. Environ. Sci. Technol. 2022, 19, 12839–12852. [Google Scholar] [CrossRef]
- Nkhata, W.; Shimelis, H.; Melis, R.; Chirwa, R.; Mzengeza, T.; Mathew, I.; Shayanowako, A. Combining Ability Analysis of Common Bean (Phaseolus vulgaris L) Genotypes for Resistance to Bean Fly (Ophiomyia spp.), and Grain Yield and Component Traits. Euphytica 2021, 217, 93. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed Extracts as Biostimulants in Horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- San-Martín-Hernández, C.; Gómez-Merino, F.C.; Rivera-Vargas, G.; Saucedo-Veloz, C.; Vaquera-Huerta, H.; Trejo-Téllez, L.I. Tomato Fruit Quality between Clusters Is Differentially Affected by Nitrogen and Potassium Supply. Rev. Fitotec. Mex. 2022, 45, 183–192. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants Research in Some Horticultural Plant Species—A Review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Villa e Vila, V.; Rezende, R.; Marques, P.A.A.; Wenneck, G.S.; de Nocchi, R.C.F.; de Terassi, D.S.; Andrean, A.F.B.A.; Matumoto-Pintro, P.T. Seaweed Extract of Ascophyllum nodosum Applied in Tomato Crop as a Biostimulant for Improving Growth, Yield and Soil Fertility in Subtropical Condition. J. Appl. Phycol. 2023, 35, 2531–2541. [Google Scholar] [CrossRef]
- Bonomelli, C.; Celis, V.; Lombardi, G.; Mártiz, J. Salt Stress Effects on Avocado (Persea americana Mill.) Plants with and without Seaweed Extract (Ascophyllum nodosum) Application. Agronomy 2018, 8, 64. [Google Scholar] [CrossRef]
- Bahmani Jafarlou, M.; Pilehvar, B.; Modaresi, M.; Mohammadi, M. Seaweed Liquid Extract as an Alternative Biostimulant for the Amelioration of Salt-Stress Effects in Calotropis procera (Aiton) W.T. J. Plant Growth Regul. 2023, 42, 449–464. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Colla, G.; Lucini, L. Plant Biostimulants from Seaweeds or Vegetal Proteins Enhance the Salinity Tolerance in Greenhouse Lettuce by Modulating Plant Metabolism in a Distinctive Manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Rana, V.S.; Sharma, V.; Sharma, S.; Rana, N.; Kumar, V.; Sharma, U.; Almutairi, K.F.; Avila-Quezada, G.D.; Abd_Allah, E.F.; Gudeta, K. Seaweed Extract as a Biostimulant Agent to Enhance the Fruit Growth, Yield, and Quality of Kiwifruit. Horticulturae 2023, 9, 432. [Google Scholar] [CrossRef]
- EU. Regulation of the European Parliament and of the Council Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 2019, 114. [Google Scholar]
- Singh, R.; Kaur, S.; Bhullar, S.S.; Singh, H.; Sharma, L.K. Bacterial Biostimulants for Climate Smart Agriculture Practices: Mode of Action, Effect on Plant Growth and Roadmap for Commercial Products. J. Sustain. Agric. Environ. 2024, 3, e12085. [Google Scholar] [CrossRef]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial Amelioration of Crop Salinity Stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-Based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef]
- Consentino, B.B.; Sabatino, L.; Vultaggio, L.; Rotino, G.L.; La Placa, G.G.; D’Anna, F.; Leto, C.; Iacuzzi, N.; De Pasquale, C. Grafting Eggplant onto Underutilized Solanum Species and Biostimulatory Action of Azospirillum brasilense Modulate Growth, Yield, NUE and Nutritional and Functional Traits. Horticulturae 2022, 8, 722. [Google Scholar] [CrossRef]
- Consentino, B.B.; Aprile, S.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Alibrandi, P.; Sabatino, L. Application of PGPB Combined with Variable N Doses Affects Growth, Yield-Related Traits, N-Fertilizer Efficiency and Nutritional Status of Lettuce Grown under Controlled Condition. Agronomy 2022, 12, 236. [Google Scholar] [CrossRef]
- Savvas, D.; Giannothanasis, E.; Ntanasi, T.; Karavidas, I.; Ntatsi, G. State of the Art and New Technologies to Recycle the Fertigation Effluents in Closed Soilless Cropping Systems Aiming to Maximise Water and Nutrient Use Efficiency in Greenhouse Crops. Agron. J. 2024, 14, 61. [Google Scholar] [CrossRef]
- Granada, C.E.; Passaglia, L.M.P.; de Souza, E.M.; Sperotto, R.A. Is Phosphate Solubilization the Forgotten Child of Plant Growth-Promoting Rhizobacteria? Front. Microbiol. 2018, 9, 2016–2019. [Google Scholar] [CrossRef] [PubMed]
- Shailendra Singh, G.G. Plant Growth Promoting Rhizobacteria (PGPR): Current and Future Prospects for Development of Sustainable Agriculture. J. Microb. Biochem. Technol. 2015, 07, 96–102. [Google Scholar] [CrossRef]
- Shin, R. Potassium Sensing, Signaling, and Transport: Toward Improved Potassium Use Efficiency in Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128113080. [Google Scholar]
- Olaniyan, F.T.; Alori, E.T.; Adekiya, A.O.; Ayorinde, B.B.; Daramola, F.Y.; Osemwegie, O.O.; Babalola, O.O. The Use of Soil Microbial Potassium Solubilizers in Potassium Nutrient Availability in Soil and Its Dynamics. Ann. Microbiol. 2022, 72, 1–12. [Google Scholar] [CrossRef]
- Gray, E.J.; Smith, D.L. Intracellular and Extracellular PGPR: Commonalities and Distinctions in the Plant-Bacterium Signaling Processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Barbieri, P.; Galli, E. Effect on Wheat Root Development of Inoculation with an Azospirillum brasilense Mutant with Altered Indole-3-Acetic Acid Production. Res. Microbiol. 1993, 144, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B. Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–448. [Google Scholar] [CrossRef]
- Aasfar, A.; Bargaz, A.; Yaakoubi, K.; Hilali, A.; Bennis, I.; Zeroual, Y.; Meftah Kadmiri, I. Nitrogen Fixing Azotobacter Species as Potential Soil Biological Enhancers for Crop Nutrition and Yield Stability. Front. Microbiol. 2021, 12, 628379. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.A.; Chai, X.; Ibrahim, A.; Mondal, S.; Férachou, D.; Ropagnol, X.; Ozaki, T. Intense Terahertz Radiation and Their Applications. J. Opt. 2016, 18, 093004. [Google Scholar] [CrossRef]
- Velmourougane, K.; Prasanna, R.; Chawla, G.; Nain, L.; Kumar, A.; Saxena, A.K. Trichoderma–Azotobacter Biofilm Inoculation Improves Soil Nutrient Availability and Plant Growth in Wheat and Cotton. J. Basic Microbiol. 2019, 59, 632–644. [Google Scholar] [CrossRef]
- Kizilkaya, R. Nitrogen Fixation Capacity of Azotobacter spp. Strains Isolated from Soils in Different Ecosystems and Relationship between Them and the Microbiological Properties of Soils. J. Environ. Biol. 2009, 30, 73–82. [Google Scholar]
- Hindersah, R.; Halimatusy, A.; Joy, B.; Setiawati, M.R.; Herdiyantoro, D. Retarded Growth of Lowland Rice in Saline Soil Inoculated With Nitrogen-Fixer Azotobacter. J. Agroekoteknologi 2020, 12, 99. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- FAOSTAT. FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 March 2024).
- Singh, J.; Sastry, E.V.D.; Singh, V. Effect of Salinity on Tomato (Lycopersicon esculentum Mill.) during Seed Germination Stage. Physiol. Mol. Biol. Plants 2012, 18, 45–50. [Google Scholar] [CrossRef]
- Willumsen, J.; Petersen, K.K.; Kaack, K. Yield and Blossom-End Rot of Tomato as Affected by Salinity and Cation Activity Ratios in the Root Zone. J. Hortic. Sci. Biotechnol. 1996, 71, 81–98. [Google Scholar] [CrossRef]
- Yang, H.; Du, T.; Mao, X.; Ding, R.; Shukla, M.K. A Comprehensive Method of Evaluating the Impact of Drought and Salt Stress on Tomato Growth and Fruit Quality Based on EPIC Growth Model. Agric. Water Manag. 2019, 213, 116–127. [Google Scholar] [CrossRef]
- Alshami, A.K.; El-Shafei, A.; Al-Omran, A.M.; Alghamdi, A.G.; Louki, I.; Alkhasha, A. Responses of Tomato Crop and Water Productivity to Deficit Irrigation Strategies and Salinity Stress in Greenhouse. Agronomy 2023, 13, 3016. [Google Scholar] [CrossRef]
- Athinodorou, F.; Foukas, P.; Tsaniklidis, G.; Kotsiras, A.; Chrysargyris, A.; Delis, C.; Kyratzis, A.C.; Tzortzakis, N.; Nikoloudakis, N. Morphological Diversity, Genetic Characterization, and Phytochemical Assessment of the Cypriot Tomato Germplasm. Plants 2021, 10, 1698. [Google Scholar] [CrossRef]
- Tagiakas, R.I.; Avdikos, I.D.; Goula, A.; Koutis, K.; Nianiou-Obeidat, I.; Mavromatis, A.G. Characterization and Evaluation of Greek Tomato Landraces for Productivity and Fruit Quality Traits Related to Sustainable Low-Input Farming Systems. Front. Plant Sci. 2022, 13, 994530. [Google Scholar] [CrossRef]
- Caramante, M.; Rouphael, Y.; Corrado, G. Genetic Diversity among and within Tomato (Solanum lycopersicum L.) Landraces Grown in Southern Italy. Genet. Resour. Crop Evol. 2024, 71, 157–166. [Google Scholar] [CrossRef]
- Thanopoulos, R.; Negri, V.; Pinheiro de Carvalho, M.A.A.; Petrova, S.; Chatzigeorgiou, T.; Terzopoulos, P.; Ralli, P.; Suso, M.J.; Bebeli, P.J. Landrace Legislation in the World: Status and Perspectives with Emphasis in EU System; Springer: Dordrecht, The Netherlands, 2024; Volume 71, ISBN 1072202301. [Google Scholar]
- Karanikolas, P.; Bebeli, P.J.; Thanopoulos, R. Farm Economic Sustainability and Agrobiodiversity: Identifying Viable Farming Alternatives during the Economic Crisis in Greece. J. Environ. Econ. Policy 2018, 7, 69–84. [Google Scholar] [CrossRef]
- Ntanasi, T.; Karavidas, I.; Zioviris, G.; Ziogas, I.; Karaolani, M.; Fortis, D.; Conesa, M.; Schubert, A.; Savvas, D.; Ntatsi, G. Assessment of Growth, Yield, and Nutrient Uptake of Mediterranean Tomato Landraces in Response to Salinity Stress. Plants 2023, 12, 3551. [Google Scholar] [CrossRef]
- Lazaridi, E.; Kapazoglou, A.; Gerakari, M.; Kleftogianni, K.; Passa, K.; Sarri, E.; Papasotiropoulos, V.; Tani, E.; Bebeli, P.J. Crop Landraces and Indigenous Varieties: A Valuable Source of Genes for Plant Breeding. Plants 2024, 13, 758. [Google Scholar] [CrossRef]
- Enthoven, L.; Van den Broeck, G. Local Food Systems: Reviewing Two Decades of Research. Agric. Syst. 2021, 193, 103226. [Google Scholar] [CrossRef]
- Rodrigues, M.; Baptistella, J.L.C.; Horz, D.C.; Bortolato, L.M.; Mazzafera, P. Organic Plant Biostimulants and Fruit Quality-a Review. Agronomy 2020, 10, 988. [Google Scholar] [CrossRef]
- Rouphael, Y.; Corrado, G.; Colla, G.; De Pascale, S.; Dell’aversana, E.; D’amelia, L.I.; Fusco, G.M.; Carillo, P. Biostimulation as a Means for Optimizing Fruit Phytochemical Content and Functional Quality of Tomato Landraces of the San Marzano Area. Foods 2021, 10, 926. [Google Scholar] [CrossRef]
- Roșca, M.; Mihalache, G.; Stoleru, V. Tomato Responses to Salinity Stress: From Morphological Traits to Genetic Changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef]
- Gedeon, S.; Ioannou, A.; Balestrini, R.; Fotopoulos, V.; Antoniou, C. Application of Biostimulants in Tomato Plants (Solanum lycopersicum) to Enhance Plant Growth and Salt Stress Tolerance. Plants 2022, 11, 3082. [Google Scholar] [CrossRef]
- Caro, M.; Cruz, V.; Cuartero, J.; Estañ, M.T.; Bolarin, M.C. Salinity Tolerance of Normal-Fruited and Cherry Tomato Cultivars. Plant Soil 1991, 136, 249–255. [Google Scholar] [CrossRef]
- Eltez, R.; Tüzel, Y.; Gül, A.; Tüzel, I.; Duyar, H. Effects of Different EC Levels of Nutrient Solution on Greenhouse Tomato Growing. Acta Hortic. 2002, 573, 443–448. [Google Scholar] [CrossRef]
- Psarras, G.; Bertaki, M.; Chartzoulakis, K. Response of Greenhouse Tomato to Salt Stress and K+ Supplement. Plant Biosyst. 2008, 142, 149–153. [Google Scholar] [CrossRef]
- Aa, H. Effect of Irrigation with Different Levels of Saline Water Type on Husk Tomato Productivity. Adv. Plants Agric. Res. 2017, 6, 114–120. [Google Scholar] [CrossRef]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The Effect of Ascophyllum nodosum Extract on the Growth, Yield and Fruit Quality of Tomato Grown under Tropical Conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Subramaniyan, L.; Veerasamy, R.; Prabhakaran, J.; Selvaraj, A.; Algarswamy, S.; Karuppasami, K.M.; Thangavel, K.; Nalliappan, S. Biostimulation Effects of Seaweed Extract (Ascophyllum nodosum) on Phytomorpho-Physiological, Yield, and Quality Traits of Tomato (Solanum lycopersicum L.). Horticulturae 2023, 9, 348. [Google Scholar] [CrossRef]
- Rajendran, R.; Jagmohan, S.; Jayaraj, P.; Ali, O.; Ramsubhag, A.; Jayaraman, J. Effects of Ascophyllum nodosum Extract on Sweet Pepper Plants as an Organic Biostimulant in Grow Box Home Garden Conditions. J. Appl. Phycol. 2022, 34, 647–657. [Google Scholar] [CrossRef]
- Rashad, Y.M.; El-Sharkawy, H.H.A.; Elazab, N.T. Ascophyllum nodosum Extract and Mycorrhizal Colonization Synergistically Trigger Immune Responses in Pea Plants against Rhizoctonia Root Rot, and Enhance Plant Growth and Productivity. J. Fungi 2022, 8, 268. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A. Ascophyllum nodosum and Silicon-Based Biostimulants Differentially Affect the Physiology and Growth of Watermelon Transplants under Abiotic Stress Factors: The Case of Salinity. Plants 2023, 12, 433. [Google Scholar] [CrossRef]
- Dell’aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum Based Extracts Counteract Salinity Stress in Tomato by Remodeling Leaf Nitrogen Metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Stergiou, P.; Xanthou, M.-Z.; Kakabouki, I.; Vlachakis, D.; et al. Evaluation of Plant Growth Promoting Bacteria Strains on Growth, Yield and Quality of Industrial Tomato. Microorganisms 2021, 9, 2099. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Di Stasio, E.; Cirillo, V.; Silletti, S.; Ventorino, V.; Pepe, O.; Raimondi, G.; Maggio, A. Root Inoculation with Azotobacter Chroococcum 76A Enhances Tomato Plants Adaptation to Salt Stress under Low N Conditions. BMC Plant Biol. 2018, 18, 205. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Marra, R.; Vitale, S.; Pironti, A.; Fiorentino, N.; Mori, M. Yield and Quality of Processing Tomato as Improved by Biostimulants Based on Trichoderma Sp. and Ascophyllum nodosum and Biodegradable Mulching Films. Agronomy 2023, 13, 901. [Google Scholar] [CrossRef]
- Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Egea, I.; Massaretto, I.L.; Morales, B.; Purgatto, E.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Traditional Tomato Varieties Improve Fruit Quality without Affecting Fruit Yield under Moderate Salt Stress. Front. Plant Sci. 2020, 11, 587754. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Rozhon, W. The Effect of Salinity on Fruit Quality and Yield of Cherry Tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, S.; Dai, Y.; Zhang, Z.; Senge, M. Combined Treatment of Salinity Stress and Fruit Thinning Effect on Tomato. Front. Nutr. 2022, 9, 857977. [Google Scholar] [CrossRef]
- Krauss, S.; Schnitzler, W.H.; Grassmann, J.; Woitke, M. The Influence of Different Electrical Conductivity Values in a Simplified Recirculating Soilless System on Inner and Outer Fruit Quality Characteristics of Tomato. J. Agric. Food Chem. 2006, 54, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.S.; Yasuor, H.; Ben-Gal, A.; Yermiyahu, U.; Saranga, Y.; Elbaum, R. Salinity Induced Fruit Hypodermis Thickening Alters the Texture of Tomato (Solanum lycopersicum Mill) Fruits. Sci. Hortic. 2015, 192, 244–249. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-Based Algal Extracts Act as Enhancers of Growth, Fruit Quality, and Adaptation to Stress in Salinized Tomato Plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Hanson, B.R.; Grattan, S.R.; Fulton, A. Irrigation and Drainage Specialist Plant-Water Relations Specialist. Agric. Salin. Drain. 2006, 159. Available online: https://hos.ifas.ufl.edu/media/hosifasufledu/documents/pdf/in-service-training/ist30688/IST30688---24.pdf (accessed on 22 April 2024).
- Babu, M.A.; Singh, D.; Gothandam, K.M. The Effect of Salinity on Growth, Hormones and Mineral Elements in Leaf and Fruit of Tomato Cultivar PKM1. J. Anim. Plant Sci. 2012, 22, 159–164. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Jung, J.-Y.; Shin, R.; Schachtman, D.P. Ethylene Mediates Response and Tolerance to Potassium Deprivation in Arabidopsis. Plant Cell 2009, 21, 607–621. [Google Scholar] [CrossRef]
- Rayirath, P.; Benkel, B.; Mark Hodges, D.; Allan-Wojtas, P.; MacKinnon, S.; Critchley, A.T.; Prithiviraj, B. Lipophilic Components of the Brown Seaweed, Ascophyllum nodosum, Enhance Freezing Tolerance in Arabidopsis thaliana. Planta 2009, 230, 135–147. [Google Scholar] [CrossRef]
- Kumari, R.; Kaur, I.; Bhatnagar, A.K. Effect of Aqueous Extract of Sargassum Johnstonii Setchell & Gardner on Growth, Yield and Quality of Lycopersicon esculentum Mill. J. Appl. Phycol. 2011, 23, 623–633. [Google Scholar] [CrossRef]
- Zodape, S.T.; Gupta, A.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar Application of Seaweed Sap as Biostimulant for Enhancement of Yield and Quality of Tomato (Lycopersicon esculentum Mill.). Proc. ASME Des. Eng. Tech. Conf. 2010, 4, 265–273. [Google Scholar] [CrossRef]
- Suarez, D.L.; Celis, N.; Ferreira, J.F.S.; Reynolds, T.; Sandhu, D. Linking Genetic Determinants with Salinity Tolerance and Ion Relationships in Eggplant, Tomato and Pepper. Sci. Rep. 2021, 11, 16298. [Google Scholar] [CrossRef]
- Adams, P. Nutritional Control in Hydroponics. In Hydroponic Production of Vegetables and Ornamental; Savvas, D., Passam, H.C., Eds.; Embryo Publications: Athens, Greece, 2002; pp. 211–261. [Google Scholar]
- Sonneveld, C.; Voogt, W. Plant Nutritions of Greenhouse Crop; Springer: Dordrecht, The Netherlands, 2009; ISBN 9788578110796. [Google Scholar]
- Farruggia, D.; Tortorici, N.; Iacuzzi, N.; Alaimo, F.; Leto, C.; Tuttolomondo, T. Biostimulants Improve Plant Performance of Rosemary Growth in Agricultural Organic System. Agronomy 2024, 14, 158. [Google Scholar] [CrossRef]
- Gazoulis, I.; Kanatas, P.; Antonopoulos, N.; Kokkini, M.; Tsekoura, A.; Demirtzoglou, T.; Travlos, I. The Integrated Effects of Biostimulant Application, Mechanical Weed Control, and Herbicide Application on Weed Growth and Maize (Zea mays L.) Yield. Agronomy 2023, 13, 2614. [Google Scholar] [CrossRef]
- Ntanasi, T.; Ntatsi, G.; Karavidas, I.; Ziogas, I.; Karaolani, M.; Fortis, D.; Zioviris, G.; Fotopoulos, V.; Schubert, A.; Guillaume, M.; et al. Impact of Salinity Stress on Fruit Quality of Different Mediterranean Cherry-Type Tomato Landraces. Acta Hortic. 2023, 1372, 301–307. [Google Scholar] [CrossRef]
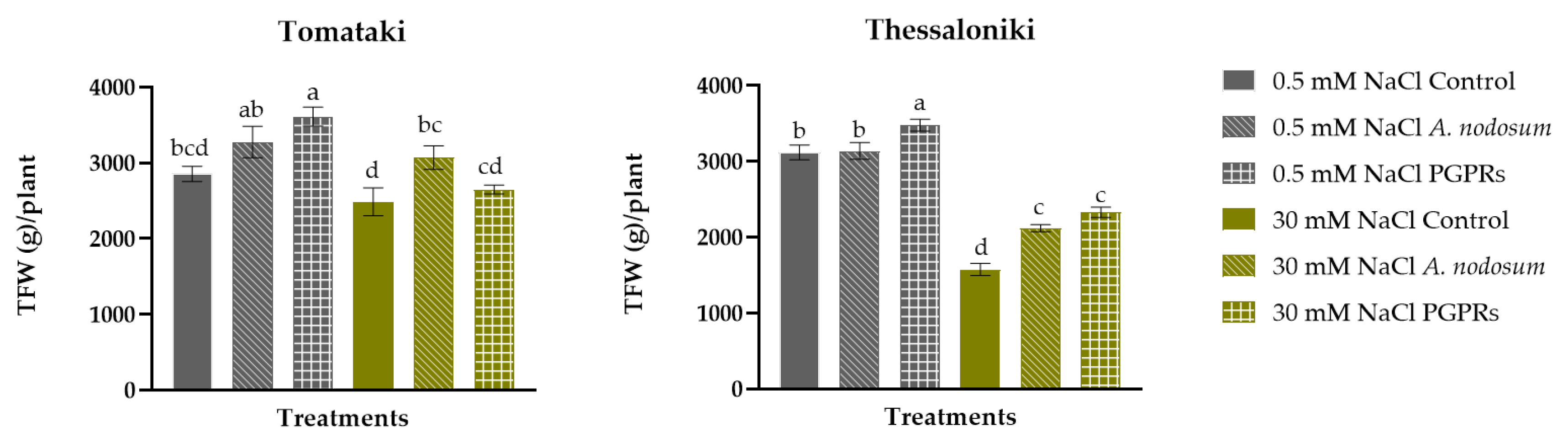
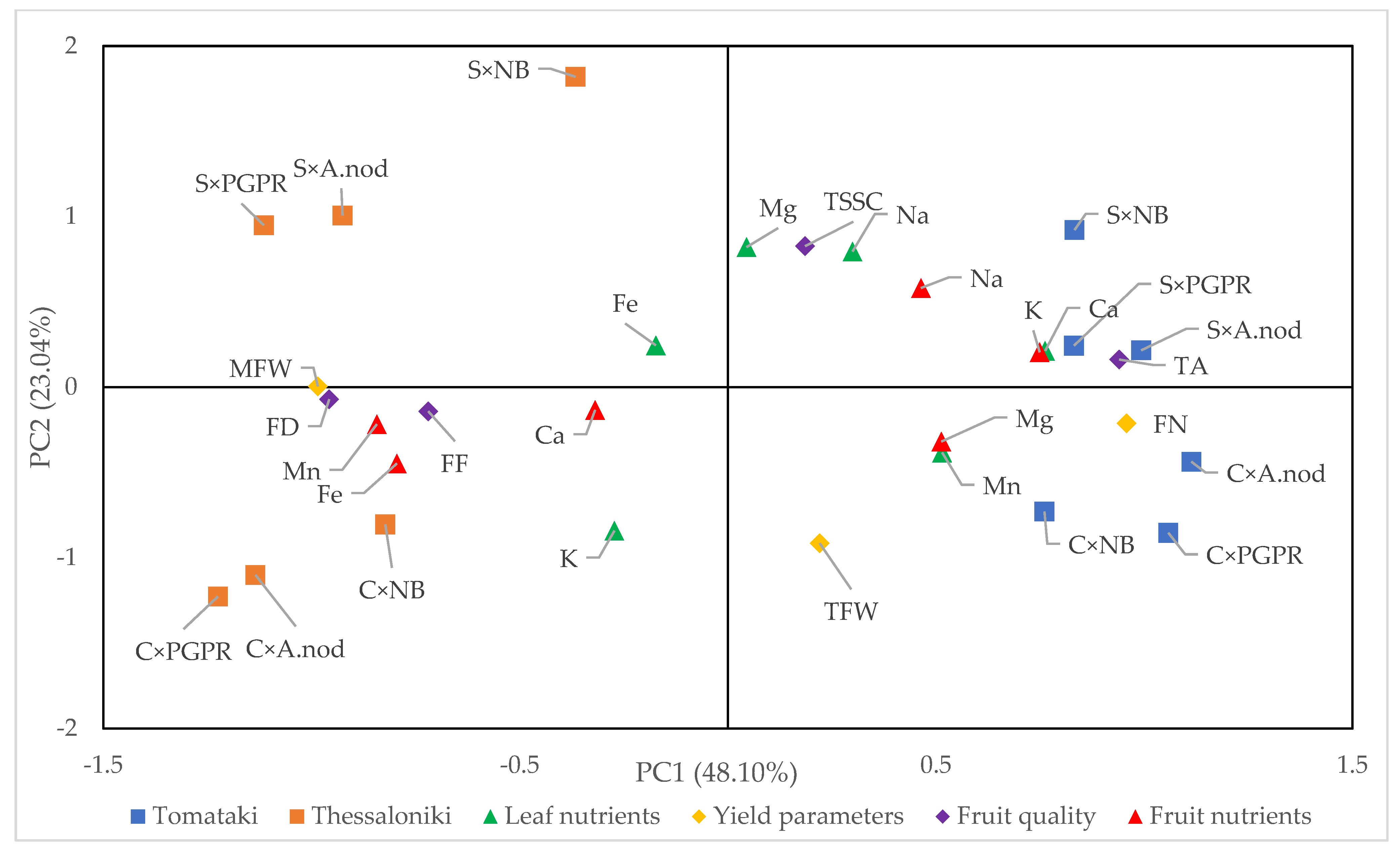
| Greek Landraces | |||||||
|---|---|---|---|---|---|---|---|
| ‘Tomataki’ | ‘Thessaloniki’ | ||||||
| Salinity Stress | PB | FN (no plant−1) | MFW (g) | FD (mm) | FN (no plant−1) | MFW (g) | FD (mm) |
| Main effects (Salinity stress) | |||||||
| 0.5 mM NaCl | 69.36 | 46.91 a | 54.41 a | 19.69 a | 165.12 a | 77.43 a | |
| 30 mM NaCl | 67.14 | 40.75 b | 49.43 b | 13.00 b | 152.75 b | 73.86 b | |
| Main effects (Biostimulant) | |||||||
| Control | 62.25 b | 43.02 | 50.39 b | 15.38 b | 149.67 b | 75.46 | |
| A. nodosum | 70.25 a | 45.14 | 52.72 a | 16.13 ab | 162.13 a | 75.15 | |
| PGPRs | 72.25 a | 43.33 | 52.28 ab | 17.54 a | 165.01 a | 76.75 | |
| Interaction | |||||||
| 0.5 mM NaCl | Control | 61.08 | 46.93 a | 53.30 ab | 19.00 | 164.46 a | 79.10 |
| A. nodosum | 72.17 | 45.40 a | 53.95 ab | 19.75 | 159.24 a | 76.37 | |
| PGPRs | 74.83 | 48.41 a | 56.05 a | 20.33 | 171.66 a | 77.25 | |
| 30 mM NaCl | Control | 63.42 | 39.11 b | 47.28 d | 11.75 | 134.87 b | 71.52 |
| A. nodosum | 68.33 | 44.89 a | 51.63 bc | 12.50 | 165.01 a | 73.45 | |
| PGPRs | 69.67 | 38.25 b | 49.13 cd | 14.75 | 158.36 a | 76.25 | |
| Significance | |||||||
| Salinity Stress | NS | *** | *** | *** | ** | ** | |
| PB | * | NS | * | * | * | NS | |
| Salinity Stress × PB | NS | * | * | NS | ** | NS | |
| Greek Landraces | |||||||
|---|---|---|---|---|---|---|---|
| ‘Tomataki’ | ‘Thessaloniki’ | ||||||
| Salinity Stress | PB | TSSC (°Brix) | TA (g Citric Acid per 100 g Juice) | FF (kg/cm2) | TSSC (°Brix) | TA (g Citric Acid per 100 g Juice) | FF (kg/cm2) |
| Main effects (salinity stress) | |||||||
| 0.5 mM NaCl | 4.60 b | 0.59 b | 1.02 | 4.45 b | 0.34 b | 1.36 a | |
| 30 mM NaCl | 4.89 a | 0.69 a | 1.02 | 5.00 a | 0.41 a | 1.18 b | |
| Main effects (biostimulant) | |||||||
| Control | 4.58 b | 0.64 | 1.01 b | 5.06 a | 0.40 | 1.32 | |
| A. nodosum | 4.92 a | 0.66 | 1.10 a | 4.68 b | 0.36 | 1.29 | |
| PGPRs | 4.67 b | 0.62 | 0.96 b | 4.51 b | 0.39 | 1.23 | |
| Interaction | |||||||
| 0.5 mM NaCl | Control | 4.23 c | 0.59 | 0.94 c | 4.69 | 0.35 | 1.36 |
| A. nodosum | 4.91 ab | 0.60 | 1.26 a | 4.49 | 0.34 | 1.38 | |
| PGPRs | 4.60 b | 0.59 | 0.91 c | 4.21 | 0.33 | 1.35 | |
| 30 mM NaCl | Control | 5.00 a | 0.68 | 1.09 b | 5.37 | 0.45 | 1.27 |
| A. nodosum | 4.93 ab | 0.76 | 0.97 c | 4.81 | 0.37 | 1.18 | |
| PGPRs | 4.76 ab | 0.64 | 1.00 bc | 4.82 | 0.42 | 1.12 | |
| Significance | |||||||
| Salinity Stress | ** | *** | NS | *** | *** | *** | |
| PB | * | NS | *** | ** | NS | NS | |
| Salinity Stress × PB | ** | NS | *** | NS | NS | NS | |
| Greek Landraces | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Tomataki’ | ‘Thessaloniki’ | ||||||||||||
| Salinity Stress | PB | K | Na | Ca | Mg | Fe | Mn | K | Na | Ca | Mg | Fe | Mn |
| Main effects (salinity stress) | |||||||||||||
| 0.5 mM NaCl | 29.00 a | 1.35 b | 36.52 | 5.21 | 62.65 a | 238.14 a | 30.50 a | 0.54 b | 22.06 | 4.44 b | 51.26 b | 142.67 | |
| 30 mM NaCl | 22.33 b | 7.97 a | 33.41 | 5.03 | 46.12 b | 137.33 b | 24.17 b | 5.39 a | 30.53 | 6.05 a | 66.33 a | 150.55 | |
| Main effects (biostimulant) | |||||||||||||
| Control | 24.75 | 5.30 a | 34.39 | 5.16 | 54.61 | 165.80 | 26.50 b | 3.21 a | 24.37 b | 5.45 | 56.40 | 146.66 | |
| A. nodosum | 25.75 | 4.60 b | 36.31 | 5.19 | 51.44 | 195.11 | 28.87 a | 2.50 b | 28.72 a | 5.12 | 57.58 | 144.42 | |
| PGPRs | 26.50 | 4.08 b | 33.92 | 5.01 | 56.09 | 193.25 | 26.63 b | 3.19 a | 26.41 ab | 5.13 | 62.11 | 148.20 | |
| Interaction | |||||||||||||
| 0.5 mM NaCl | Control | 29.75 | 1.30 d | 38.57 | 4.97 | 65.58 | 239.51 | 30.25 a | 0.52 c | 19.05 | 4.56 | 44.86 c | 133.22 |
| A. nodosum | 27.75 | 1.30 d | 35.38 | 5.29 | 63.56 | 237.16 | 30.50 a | 0.55 c | 25.59 | 4.10 | 49.06 bc | 145.67 | |
| PGPRs | 29.50 | 1.45 d | 36.13 | 5.32 | 59.56 | 238.10 | 30.75 a | 0.56 c | 22.42 | 4.57 | 59.32 ab | 149.87 | |
| 30 mM NaCl | Control | 19.75 | 9.30 a | 31.26 | 5.31 | 46.39 | 110.53 | 22.75 c | 5.90 a | 29.69 | 6.34 | 67.95 a | 160.11 |
| A. nodosum | 23.75 | 7.90 b | 37.24 | 5.08 | 39.33 | 153.06 | 27.25 b | 4.45 b | 31.85 | 6.14 | 66.09 a | 143.17 | |
| PGPRs | 23.50 | 6.70 c | 31.72 | 4.69 | 52.63 | 148.40 | 22.50 c | 5.83 a | 30.39 | 5.68 | 64.91 a | 146.53 | |
| Significance | |||||||||||||
| Salinity Stress | *** | *** | NS | NS | *** | *** | *** | *** | *** | *** | *** | NS | |
| PB | NS | *** | NS | NS | NS | NS | * | *** | * | NS | NS | NS | |
| Salinity Stress × PB | NS | *** | NS | NS | NS | NS | * | *** | NS | NS | * | NS | |
| Greek Landraces | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Tomataki’ | ‘Thessaloniki’ | ||||||||||||
| Salinity Stress | Biostimulant | K | Na | Ca | Mg | Fe | Mn | K | Na | Ca | Mg | Fe | Mn |
| Main effects (salinity stress) | |||||||||||||
| 0.5 mM NaCl | 39.83 | 0.44 b | 0.15 a | 1.12 | 26.81 a | 10.56 | 34.07 | 0.31 b | 0.15 | 1.03 | 35.79 a | 12.08 | |
| 30 mM NaCl | 43.08 | 1.48 a | 0.12 b | 1.10 | 21.83 b | 10.12 | 36.57 | 0.76 a | 0.15 | 1.02 | 29.70 b | 11.77 | |
| Main effects (biostimulant) | |||||||||||||
| Control | 41.75 | 0.95 | 0.13 | 1.09 | 24.74 | 10.26 | 34.33 | 0.53 ab | 0.16 | 0.96 b | 32.38 | 10.76 b | |
| A. nodosum | 41.25 | 0.97 | 0.15 | 1.13 | 23.13 | 10.51 | 36.25 | 0.63 a | 0.15 | 1.05 ab | 33.03 | 12.91 a | |
| PGPRs | 41.38 | 0.97 | 0.13 | 1.11 | 25.10 | 10.24 | 35.88 | 0.46 b | 0.14 | 1.10 a | 32.04 | 12.54 a | |
| Interaction | |||||||||||||
| 0.5 mM NaCl | Control | 39.00 | 0.43 | 0.15 | 1.08 | 29.66 a | 10.92 | 33.67 | 0.28 | 0.14 | 0.96 | 34.53 | 11.18 |
| A. nodosum | 37.25 | 0.46 | 0.16 | 1.12 | 23.40 bc | 10.43 | 34.50 | 0.41 | 0.15 | 1.05 | 37.71 | 12.52 | |
| PGPRs | 43.25 | 0.45 | 0.14 | 1.15 | 27.39 ab | 10.33 | 34.25 | 0.28 | 0.14 | 1.12 | 35.78 | 12.99 | |
| 30 mM NaCl | Control | 44.50 | 1.47 | 0.10 | 1.10 | 19.82 c | 9.61 | 35.00 | 0.79 | 0.16 | 0.97 | 30.77 | 10.34 |
| A. nodosum | 45.25 | 1.48 | 0.13 | 1.13 | 22.86 bc | 10.60 | 38.00 | 0.85 | 0.14 | 1.04 | 28.36 | 13.22 | |
| PGPRs | 39.50 | 1.50 | 0.12 | 1.07 | 22.81 bc | 10.15 | 37.50 | 0.65 | 0.15 | 1.08 | 29.05 | 12.10 | |
| Significance | |||||||||||||
| Salinity Stress | NS | *** | *** | NS | ** | NS | NS | *** | NS | NS | ** | NS | |
| PB | NS | NS | NS | NS | NS | NS | NS | ** | NS | * | NS | ** | |
| Salinity Stress × PB | NS | NS | NS | NS | * | NS | NS | NS | NS | NS | NS | NS | |
| Nutrient | Starter Solution (11 April 2022) | Vegetative Stage (13 April 2022) | Reproductive Stage 1 (19 May 2022) | Reproductive Stage 2 (1 June 2022) | Unit |
|---|---|---|---|---|---|
| NO3− | 16.76 | 14.70 | 14.04 | 14.79 | mM |
| K+ | 7.50 | 8.88 | 8.53 | 8.70 | mM |
| Ca2+ | 9.40 | 5.36 | 5.39 | 5.49 | mM |
| Mg2+ | 4.25 | 2.51 | 2.34 | 2.38 | mM |
| SO42− | 7.19 | 3.59 | 3.80 | 3.42 | mM |
| H2PO4− | 1.20 | 1.42 | 1.45 | 1.45 | mM |
| NH4+ | 1.34 | 1.28 | 1.91 | 1.46 | mM |
| Fe | 15.00 | 20.00 | 19.19 | 19.19 | μM |
| Mn++ | 10.00 | 10.00 | 9.86 | 9.86 | μM |
| Zn++ | 5.00 | 6.50 | 6.54 | 6.54 | μM |
| B | 30.00 | 35.00 | 36.93 | 36.93 | μM |
| Cu++ | 0.75 | 0.80 | 1.00 | 1.00 | μM |
| Mo | 0.50 | 0.50 | 0.90 | 0.90 | μΜ |
| Cl− | 4.00 | 2.80 | 3.00 | 3.00 | μΜ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntanasi, T.; Karavidas, I.; Spyrou, G.P.; Giannothanasis, E.; Aliferis, K.A.; Saitanis, C.; Fotopoulos, V.; Sabatino, L.; Savvas, D.; Ntatsi, G. Plant Biostimulants Enhance Tomato Resilience to Salinity Stress: Insights from Two Greek Landraces. Plants 2024, 13, 1404. https://doi.org/10.3390/plants13101404
Ntanasi T, Karavidas I, Spyrou GP, Giannothanasis E, Aliferis KA, Saitanis C, Fotopoulos V, Sabatino L, Savvas D, Ntatsi G. Plant Biostimulants Enhance Tomato Resilience to Salinity Stress: Insights from Two Greek Landraces. Plants. 2024; 13(10):1404. https://doi.org/10.3390/plants13101404
Chicago/Turabian StyleNtanasi, Theodora, Ioannis Karavidas, George P. Spyrou, Evangelos Giannothanasis, Konstantinos A. Aliferis, Costas Saitanis, Vasileios Fotopoulos, Leo Sabatino, Dimitrios Savvas, and Georgia Ntatsi. 2024. "Plant Biostimulants Enhance Tomato Resilience to Salinity Stress: Insights from Two Greek Landraces" Plants 13, no. 10: 1404. https://doi.org/10.3390/plants13101404
APA StyleNtanasi, T., Karavidas, I., Spyrou, G. P., Giannothanasis, E., Aliferis, K. A., Saitanis, C., Fotopoulos, V., Sabatino, L., Savvas, D., & Ntatsi, G. (2024). Plant Biostimulants Enhance Tomato Resilience to Salinity Stress: Insights from Two Greek Landraces. Plants, 13(10), 1404. https://doi.org/10.3390/plants13101404











