Antibacterial and Antibiofilm Potential of Ethanolic Extracts of Duguetia vallicola (Annonaceae) against in-Hospital Isolates of Pseudomonas aeruginosa
Abstract
1. Introduction
2. Results
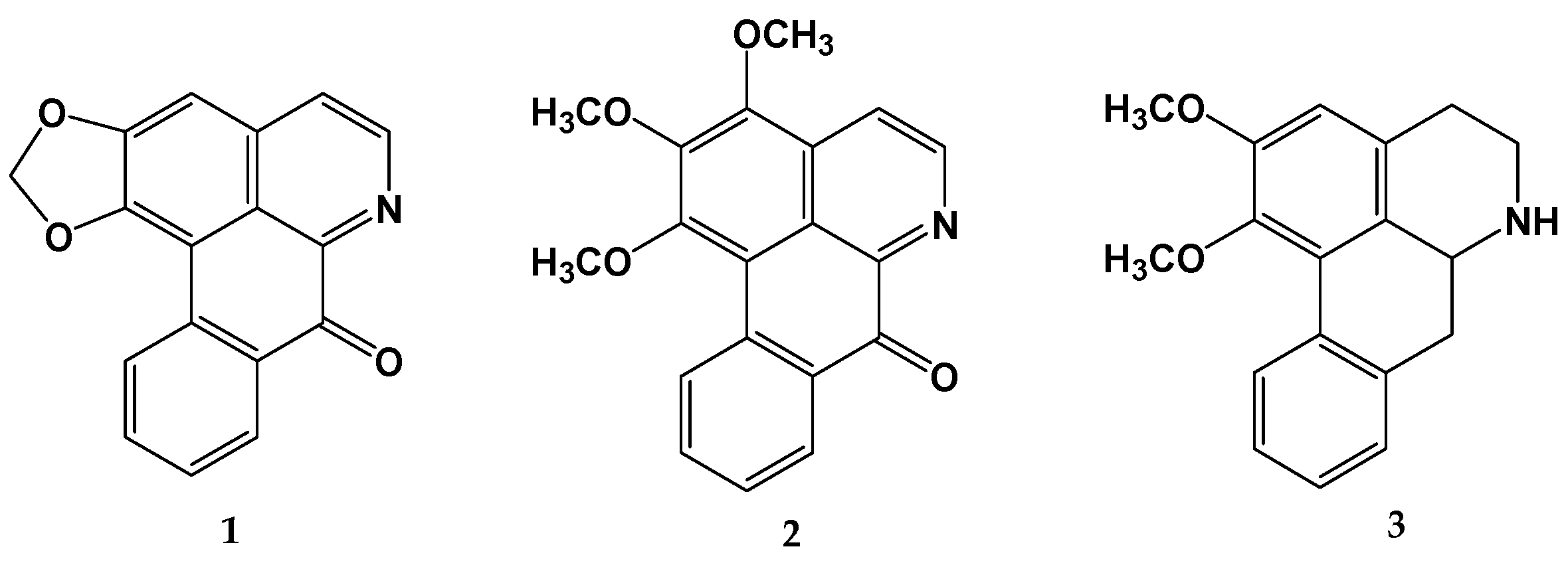
2.1. Identification of Alkaloids in the Wood Extract
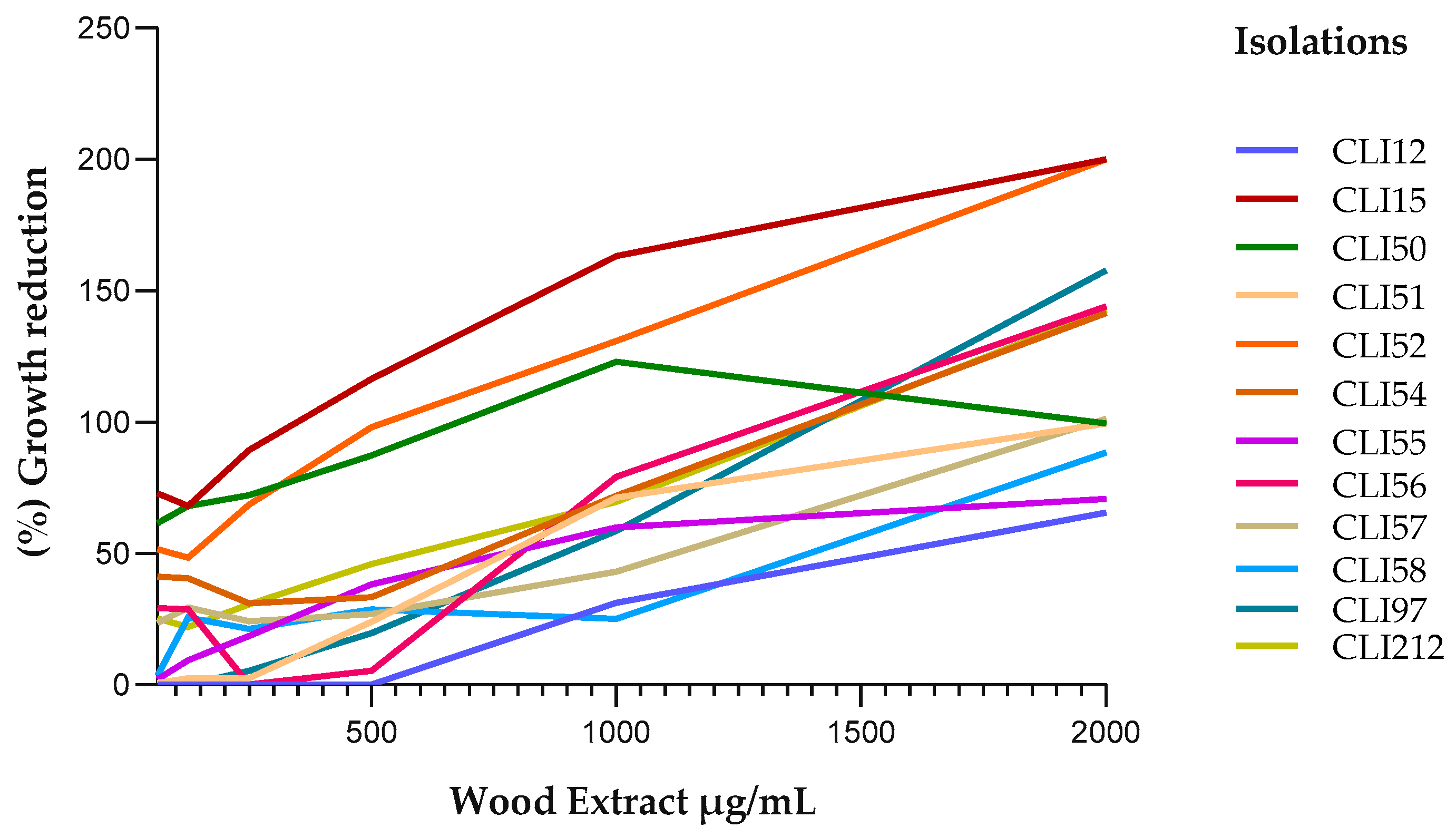
2.2. Antibacterial Susceptibility Testing
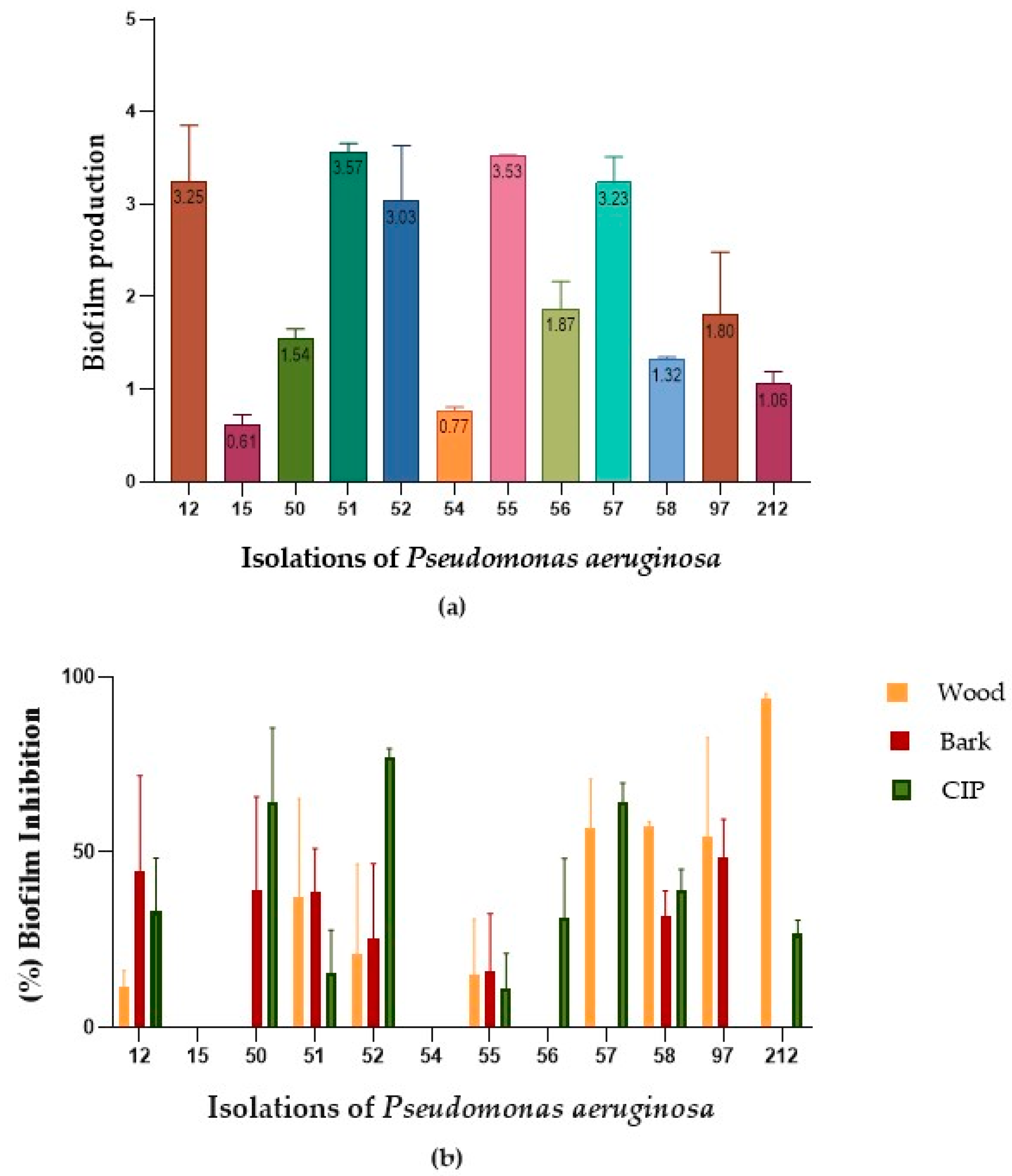
2.3. Biofilm Reduction
2.4. Effect of the Extracts on Cell Membrane Integrity
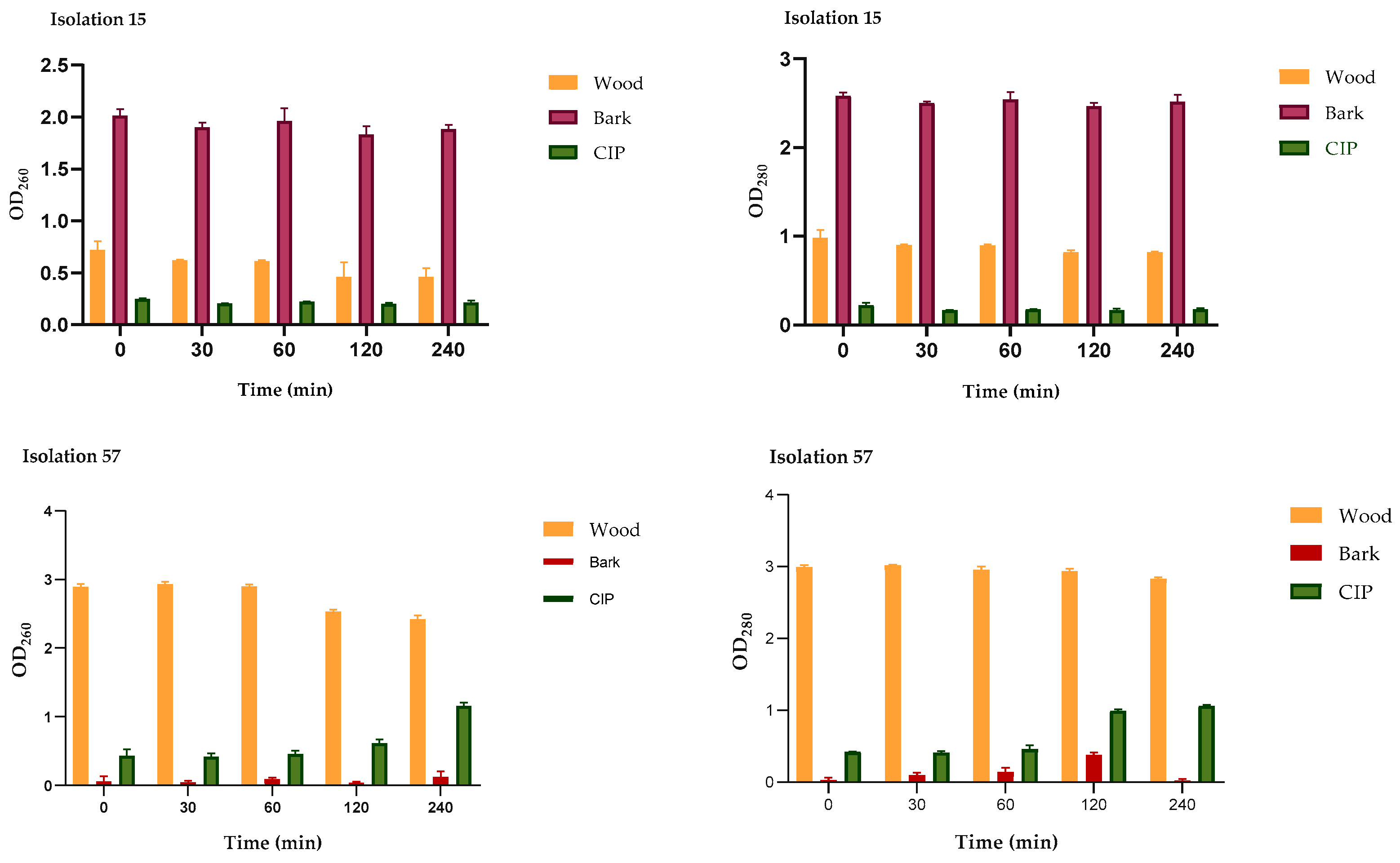
2.4.1. Leakage of Nucleic Acids and Proteins through the Cell Membrane
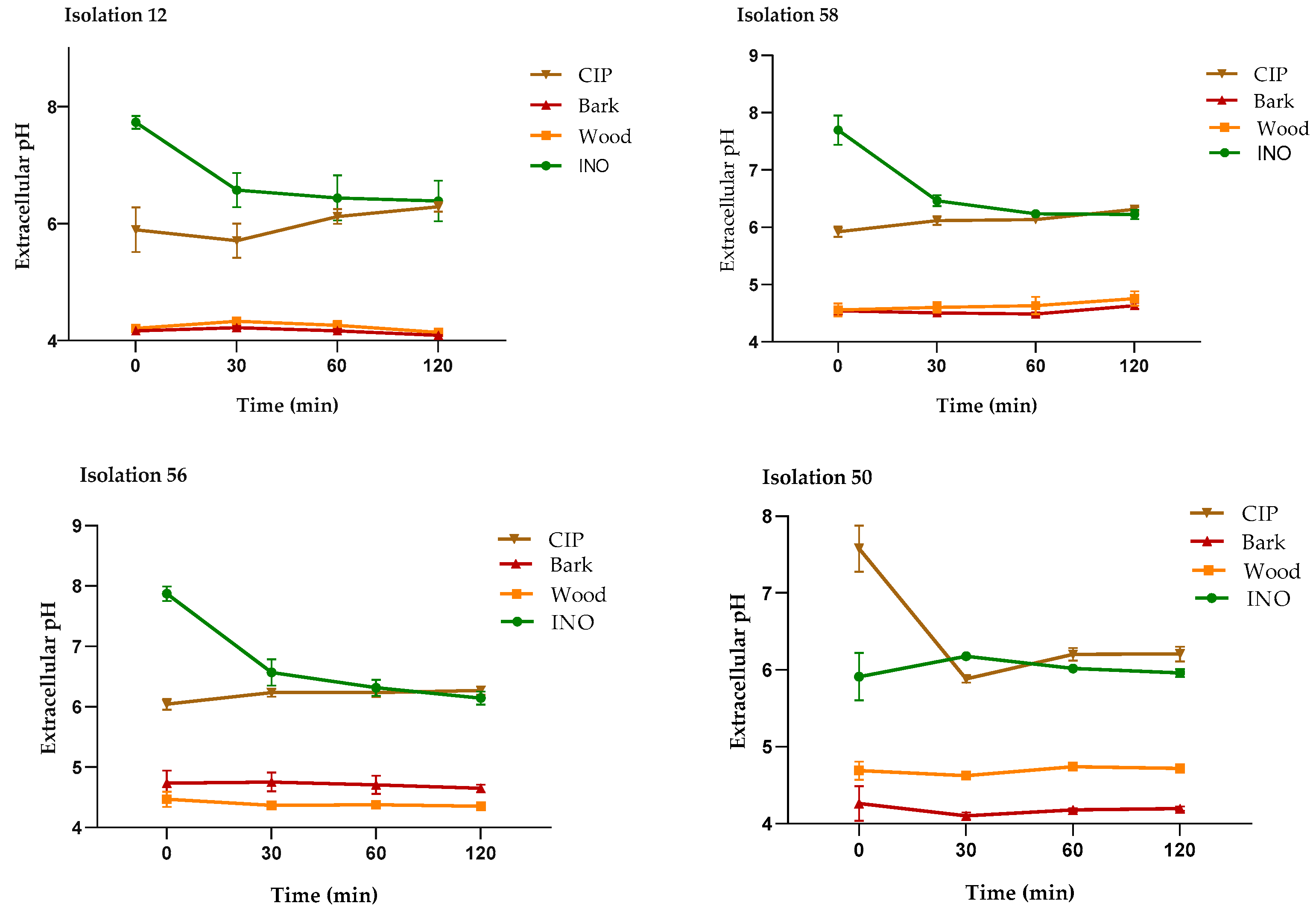
2.4.2. Measurement of the Extracellular pH
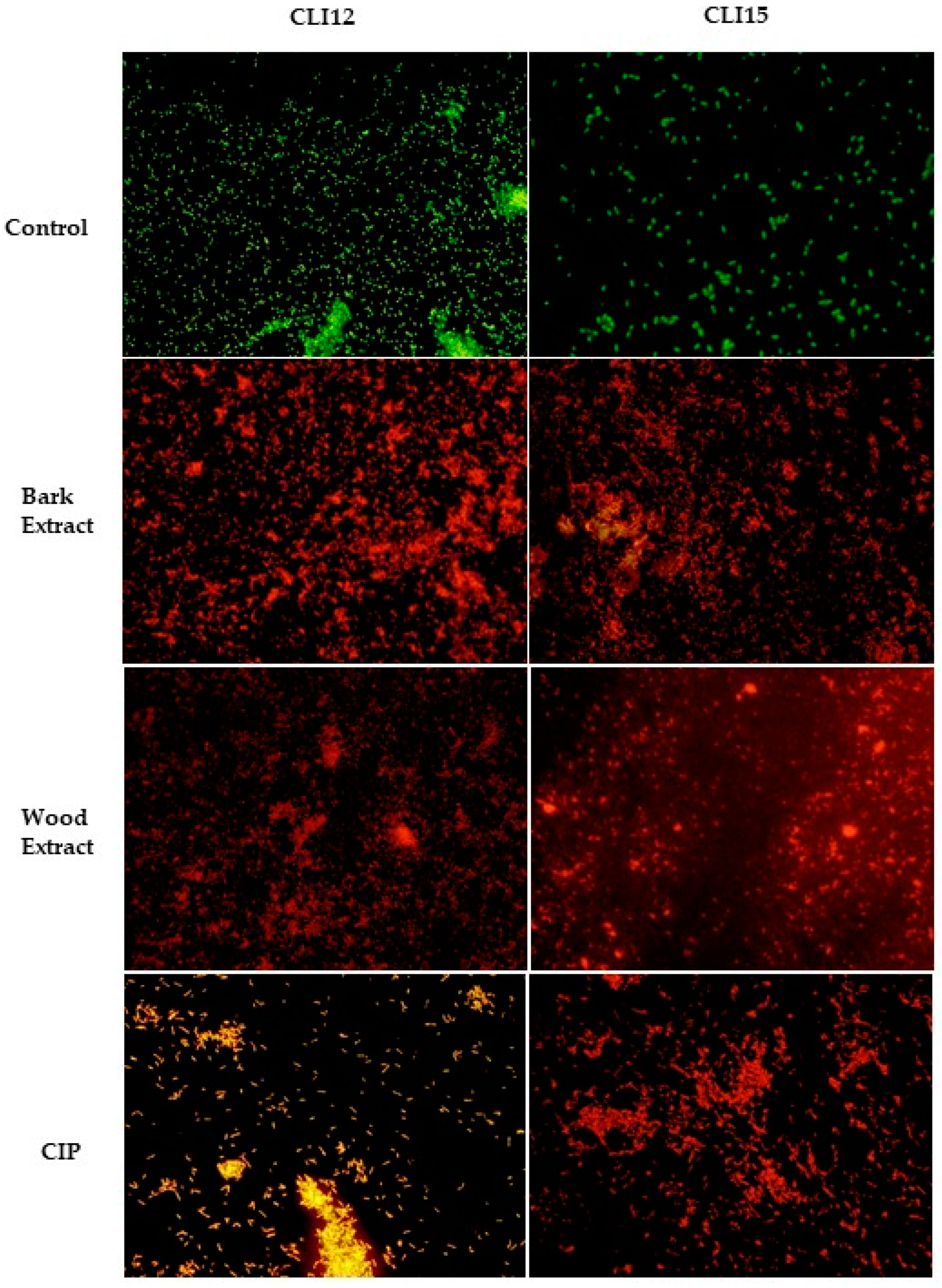
2.4.3. LIVE/DEAD Assays
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Obtaining the Extracts
4.3. Obtaining Alkaloids from the Wood Extract
4.4. Strains
4.5. Antibacterial Susceptibility Testing
4.6. Quantitative Assessment of Biofilm Formation
4.7. Effect of the Extracts on Cell Membrane Integrity
4.7.1. Leakage of Nucleic Acids and Proteins through the Cell Membrane
4.7.2. Measurement of Extracellular pH
4.7.3. LIVE/DEAD Assays
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diggle, S.P.; Whiteley, M. Microbe Profile: Pseudomonas aeruginosa: Opportunistic Pathogen and Lab Rat. Microbiology 2020, 166, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Callejas-Díaz, A.; Fernández-Pérez, C.; Ramos-Martínez, A.; Múñez-Rubio, E.; Sánchez-Romero, I.; Vargas Núñez, J.A. Impacto de La Bacteriemia Por Pseudomonas aeruginosa En Un Hospital de Tercer Nivel: Mortalidad y Factores Pronósticos. Med. Clin. 2019, 152, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Martín, I.; Sainz-Mejías, M.; McClean, S. Pseudomonas aeruginosa: An Audacious Pathogen with an Adaptable Arsenal of Virulence Factors. Int. J. Mol. Sci. 2021, 22, 3128. [Google Scholar] [CrossRef] [PubMed]
- Mielko, K.A.; Jabłoński, S.J.; Milczewska, J.; Sands, D.; Łukaszewicz, M.; Młynarz, P. Metabolomic Studies of Pseudomonas aeruginosa. World J. Microbiol. Biotechnol. 2019, 35, 178. [Google Scholar] [CrossRef]
- Camus, L.; Vandenesch, F.; Moreau, K. From Genotype to Phenotype: Adaptations of Pseudomonas aeruginosa to the Cystic Fibrosis Environment. Microb. Genom. 2021, 7, mgen000513. [Google Scholar] [CrossRef] [PubMed]
- Paz-Zarza, V.M.; Mangwani-Mordani, S.; Martínez-Maldonado, A.; Álvarez-Hernández, D.; Solano-Gálvez, S.G.; Vázquez-López, R. Pseudomonas aeruginosa: Patogenicidad y Resistencia Antimicrobiana En La Infección Urinaria. Patogenia 2019, 36, 180–189. [Google Scholar] [CrossRef]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.J.; Orange, N.; Dufour, A.; Cornelis, P. Structure, Function and Regulation of Pseudomonas aeruginosa Porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef] [PubMed]
- Huszczynski, S.M.; Lam, J.S.; Khursigara, C.M. The Role of Pseudomonas aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology. Pathogens 2020, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Pang, Z.; Li, H.; Yang, T.; Guo, L.; Liu, L.; Mei, J.; Song, X.; Xie, T.; Zhang, Y.; et al. CXCL4 Contributes to Host Defense against Acute Pseudomonas aeruginosa Lung Infection. PLoS ONE 2018, 13, e0205521. [Google Scholar] [CrossRef]
- Aman, S.; Mittal, D.; Shriwastav, S.; Tuli, H.S.; Chauhan, S.; Singh, P.; Sharma, S.; Saini, R.V.; Kaur, N.; Saini, A.K. Prevalence of Multidrug-Resistant Strains in Device Associated Nosocomial Infection and Their In Vitro Killing by Nanocomposites. Ann. Med. Surg. 2022, 78, 103687. [Google Scholar] [CrossRef]
- Sharma, G.; Rao, S.; Bansal, A.; Dang, S.; Gupta, S.; Gabrani, R. Pseudomonas aeruginosa Biofilm: Potential Therapeutic Targets. Biologicals 2014, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic Resistance in Pseudomonas aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Dickter, J.K. Nosocomial Infections: A History of Hospital-Acquired Infections. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Avato, P. Editorial to the Special Issue—“Natural Products and Drug Discovery”. Molecules 2020, 25, 1128. [Google Scholar] [CrossRef] [PubMed]
- Aylate, A.; Agize, M.; Ekero, D.; Kiros, A.; Ayledo, G.; Gendiche, K. In-Vitro and In-Vivo Antibacterial Activities of Croton macrostachyus Methanol Extract against E. coli and S. aureus. Adv. Anim. Vet. Sci. 2017, 5, 107–114. [Google Scholar]
- Lúcio, A.S.S.C.; Almeida, J.R.G.d.S.; da-Cunha, E.V.L.; Tavares, J.F.; Barbosa Filho, J.M. Alkaloids of the Annonaceae: Occurrence and a Compilation of Their Biological Activities. Alkaloids Chem. Biol. 2015, 74, 233–409. [Google Scholar] [CrossRef] [PubMed]
- Pérez, E.; Saez, J.; Blair, S.; Franck, X.; Figadere, B. Isoquinoline Alkaloids from Duguetia vallicola Stem Bark with Antiplasmodial Activity. Lett. Org. Chem. 2004, 1, 102–104. [Google Scholar] [CrossRef]
- Menezes, L.; D’Sousa Costa, C.; Rodrigues, A.C.; Santo, F.R.; Nepel, A.; Dutra, L.M.; Silva, F.; Soares, M.B.; Barison, A.; Costa, E.V.; et al. Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules 2016, 21, 890. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.S.; Machado, A.R.T.; Campos, V.A.C.; Oliveira, D.F.; Carvalho, G.A. Selection of Annonaceae Species for the Control of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Metabolic Profiling of Duguetia lanceolata Using Nuclear Magnetic Resonance Spectroscopy. J. Econ. Entomol. 2016, 109, 649–659. [Google Scholar] [CrossRef]
- Maia, D.S.; Lopes, C.F.; Saldanha, A.A.; Silva, N.L.; Sartori, Â.L.B.; Carollo, C.A.; Sobral, M.G.; Alves, S.N.; Silva, D.B.; de Siqueira, J.M. Larvicidal Effect from Different Annonaceae Species on Culex quinquefasciatus. Environ. Sci. Pollut. Res. 2020, 27, 36983–36993. [Google Scholar] [CrossRef]
- Fuentes, L.; Contreras, O.I.; Angulo, A.A. Evaluation of In Vitro Antioxidant Activity of Extracts from Duguetia vallicola J. F. Macbr.—Annonaceae. Rev. Fac. Ciencias Básicas 2021, 1, 63. [Google Scholar] [CrossRef]
- Pérez, E.G.; Cassels, B.K. Alkaloids from the Genus Duguetia. Alkaloids Chem. Biol. 2010, 68, 83–156. [Google Scholar]
- Da Silva, D.B.; Tulli, E.C.; Militão, G.C.; Costa-Lotufo, L.V.; Pessoa, C.; de Moraes, M.O.; Albuquerque, S.; de Siqueira, J.M. The Antitumoral, Trypanocidal and Antileishmanial Activities of Extract and Alkaloids Isolated from Duguetia furfuracea. Phytomedicine 2009, 16, 1059–1063. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, D.; Dai, Z.; Khan, A.; Wang, B.; Wei, X.; Sun, Y.; Zhao, Y.L.; Wang, Y.F.; Liu, Y.P.; et al. Antitumor Aporphine Alkaloids from Thalictrum wangii. Fitoterapia 2018, 128, 204–212. [Google Scholar] [CrossRef]
- Muhammad, I.; Dunbar, D.C.; Takamatsu, S.; Walker, L.A.; Clark, A.M. Antimalarial, Cytotoxic, and Antifungal Alkaloids from Duguetia hadrantha. J. Nat. Prod. 2001, 64, 559–562. [Google Scholar] [CrossRef]
- Hu, J.; Shi, X.; Chen, J.; Mao, X.; Zhu, L.; Yu, L.; Shi, J. Alkaloids from Toddalia asiatica and Their Cytotoxic, Antimicrobial and Antifungal Activities. Food Chem. 2014, 148, 437–444. [Google Scholar] [CrossRef]
- Almeida, M.C.; Resende, D.I.; da Costa, P.M.; Pinto, M.M.; Sousa, E. Tryptophan Derived Natural Marine Alkaloids and Synthetic Derivatives as Promising Antimicrobial Agents. Eur. J. Med. Chem. 2021, 209, 112945. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Zhou, H.; Peng-Deng; Zhang, B.Q.; Zhang, Z.J.; Wang, G.H.; Zhang, S.Y.; Wu, Z.R.; Wang, Y.R.; Liu, Y.Q. Antimicrobial Activity of Natural and Semi-Synthetic Carbazole Alkaloids. Eur. J. Med. Chem. 2023, 259, 115627. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.; Mukhamadiev, A.; Feng, J.; Gao, Y.; Zhuansun, X.; Han, R.; Chong, Y.; Jafari, S.M. Formulation Optimization and Characterization of Carvacrol-Loaded Nanoemulsions: In Vitro Antibacterial Activity/Mechanism and Safety Evaluation. Ind. Crops Prod. 2022, 181, 114816. [Google Scholar] [CrossRef]
- Arango, O.; Pérez, E.; Granados, H.; Rojano, B.; Sáez, J. Inhibition of Lipid Peroxidation and Free Radical Scavenging Capacity of Alkaloids Isolated from Two Annonaceae, Xylopia amazonica Cf. And Duguetia vallicola. Actual Biol. 2004, 26, 105–110. [Google Scholar] [CrossRef]
- Vieira Lemos, D.E.C.; Cavalcante-Silva, L.H.A.; De Almeida Lima, É.; Alves, A.F.; Carneiro Lúcio, A.S.; Barbosa-Filho, J.M.; Mascarenhas, S.R. Anti-Inflammatory Effect of Discretamine, a Protoberberine Alkaloid Isolated from Duguetia moricandiana. Nat. Prod. Commun. 2017, 12, 1595–1597. [Google Scholar] [CrossRef]
- do Santos, R.C.; de Souza, A.V.; Andrade-Silva, M.; Vera Cruz, K.C.; Leite Kassuya, C.A.; Lima Cardoso, C.A.; Vieira, M.D.C.; Nazari Formagio, A.S. Antioxidant, Anti-Rheumatic and Anti-Inflammatory Investigation of Extract and Dicentrinone from Duguetia furfuracea (A. St.-Hil.) Benth. & Hook. F. J. Ethnopharmacol. 2018, 211, 9–16. [Google Scholar] [CrossRef]
- Saldanha, A.A.; Vieira, L.; Ribeiro, R.I.d.A.; Thomé, R.G.; dos Santos, H.B.; Silva, D.B.; Carollo, C.A.; de Oliveira, F.M.; Lopes, D.d.O.; de Siqueira, J.M.; et al. Chemical Composition and Evaluation of the Anti-Inflammatory and Antinociceptive Activities of Duguetia furfuracea Essential Oil: Effect on Edema, Leukocyte Recruitment, Tumor Necrosis Factor Alpha Production, INOS Expression, and Adenosinergic and Opioid. J. Ethnopharmacol. 2019, 231, 325–336. [Google Scholar] [CrossRef]
- Pérez, E.; Saez, J.; Cassels, B. A Convenient, Renewable Source of the Anxiolytic Proaporphine Alkaloid Glaziovine: Duguetia vallicola Leaves. J. Chil. Chem. Soc. 2005, 50, 553–557. [Google Scholar] [CrossRef]
- Contreras, O.; Angulo, A.; Santafé, G. Mechanism of Antifungal Action of Monoterpene Isoespintanol against Clinical Isolates of Candida tropicalis. Molecules 2022, 27, 5808. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef]
- Jangra, V.; Sharma, N.; Chhillar, A.K. Therapeutic Approaches for Combating Pseudomonas aeruginosa Infections. Microbes Infect. 2022, 24, 104950. [Google Scholar] [CrossRef]
- Horcajada, J.P.; Montero, M.; Oliver, A.; Sorlí, L.; Luque, S.; Gómez-Zorrilla, S.; Benito, N.; Grau, S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin. Microbiol. Rev. 2019, 32, e00031-19. [Google Scholar] [CrossRef]
- Sousa, O.V.; Del-Vechio-Vieira, G.; Alves, M.S.; Araújo, A.A.; Pinto, M.A.; Amaral, M.P.; Rodarte, M.P.; Kaplan, M.A. Chemical Composition and Biological Activities of the Essential Oils from Duguetia lanceolata St. Hil. Barks. Molecules 2012, 17, 11056–11066. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.C.; Nogueira, M.L.; de Oliveira, F.P.; Costa, E.V.; Bezerra, D.P. Essential Oils of Duguetia Species A. St. Hill (Annonaceae): Chemical Diversity and Pharmacological Potential. Biomolecules 2022, 12, 615. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Cushnie, B.; Lamb, A.J. Alkaloids: An Overview of Their Antibacterial, Antibiotic-Enhancing and Antivirulence Activities. Int. J. Antimicrob. Agents 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Dahmer, J.; do Carmo, G.; Mostardeiro, M.A.; Neto, A.T.; da Silva, U.F.; Dalcol, I.I.; Morel, A.F. Antibacterial Activity of Discaria americana Gillies Ex Hook (Rhamnaceae). J. Ethnopharmacol. 2019, 239, 111635. [Google Scholar] [CrossRef]
- Gao, W.; He, Y.; Li, F.; Chai, C.; Zhang, J.; Guo, J.; Chen, C.; Wang, J.; Zhu, H.; Hu, Z.; et al. Antibacterial Activity against Drug-Resistant Microbial Pathogens of Cytochalasan Alkaloids from the Arthropod-Associated Fungus Chaetomium globosum TW1-1. Bioorg. Chem. 2019, 83, 98–104. [Google Scholar] [CrossRef]
- Plazas, E.; Avila, M.M.; Muñoz, D.R.; Cuca, S.L. Natural Isoquinoline Alkaloids: Pharmacological Features and Multi-Target Potential for Complex Diseases. Pharmacol. Res. 2022, 177, 106126. [Google Scholar] [CrossRef]
- Pässler, U.; Knölker, H.J. The Pyrrolo[2,1-a]Isoquinoline Alkaloids. Alkaloids Chem. Biol. 2011, 70, 79–151. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. New Trends in the Practical Use of Isoquinoline Alkaloids as Potential Drugs Applicated in Infectious and Non-Infectious Diseases. Biomed. Pharmacother. 2023, 168, 115704. [Google Scholar] [CrossRef]
- Nair, J.J.; Wilhelm, A.; Bonnet, S.L.; van Staden, J. Antibacterial Constituents of the Plant Family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2017, 27, 4943–4951. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.K.; Yeung, A.T.Y.; Hancock, R.E.W. Antibiotic Resistance in Pseudomonas aeruginosa Biofilms: Towards the Development of Novel Anti-Biofilm Therapies. J. Biotechnol. 2014, 191, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Tolker-Nielsen, T. Pattern Formation in Pseudomonas aeruginosa Biofilms. Curr. Opin. Microbiol. 2008, 11, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Aswathanarayan, J.B.; Vittal, R.R. Inhibition of Biofilm Formation and Quorum Sensing Mediated Phenotypes by Berberine in: Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018, 8, 36133–36141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, X.; Zhu, Z.; Tang, T.; Dai, K.; Sadovskaya, I.; Flahaut, S.; Jabbouri, S. Effect of Berberine on Staphylococcus epidermidis Biofilm Formation. Int. J. Antimicrob. Agents 2009, 34, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, W.; Liu, M.; Zhang, J.; Yang, M.; Wang, T.; Qian, W. In Vitro Anti-Biofilm Efficacy of Sanguinarine against Carbapenem-Resistant Serratia marcescens. Biofouling 2021, 37, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Yang, M.; Li, X.; Sun, Z.; Li, Y.; Wang, X.; Wang, T. Anti-Microbial and Anti-Biofilm Activities of Combined Chelerythrine-Sanguinarine and Mode of Action against Candida albicans and Cryptococcus neoformans In Vitro. Colloids Surf. B Biointerfaces 2020, 191, 111003. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32. [Google Scholar]
- Quave, C.L.; Plano, L.R.W.; Pantuso, T.; Bennett, B.C. Effects of Extracts from Italian Medicinal Plants on Planktonic Growth, Biofilm Formation and Adherence of Methicillin-Resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef]
- Rossi, C.; Serio, A.; Chaves-López, C.; Anniballi, F.; Auricchio, B.; Goffredo, E.; Cenci-Goga, B.T.; Lista, F.; Fillo, S.; Paparella, A. Biofilm Formation, Pigment Production and Motility in Pseudomonas Spp. Isolated from the Dairy Industry. Food Control 2018, 86, 241–248. [Google Scholar] [CrossRef]
- Yousefpour, Z.; Davarzani, F.; Owlia, P. Evaluating of the Effects of Sub-MIC Concentrations of Gentamicin on Biofilm Formation in Clinical Isolates of Pseudomonas aeruginosa. Iran. J. Pathol. 2021, 16, 403–410. [Google Scholar]
- Donadu, M.G.; Peralta-ruiz, Y.; Usai, D.; Maggio, F.; Molina-hernandez, J.B.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A.; et al. Colombian Essential Oil of Ruta graveolens against Nosocomial Antifungal Resistant Candida Strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]

| P. aeruginosa | Wood Extract | Bark Extract |
|---|---|---|
| MIC90 | MIC90 | |
| CLI 12 | 3800 | 6300 |
| CLI 15 | 180 | 1460 |
| CLI 50 | 500 | 2200 |
| CLI 51 | 3000 | 1600 |
| CLI 52 | 490 | 73 |
| CLI 54 | 1270 | 300 |
| CLI 55 | 3120 | 2000 |
| CLI 56 | 1480 | 392 |
| CLI 57 | 2600 | 62.5 |
| CLI 58 | 2790 | 794 |
| CLI 97 | 1560 | 250 |
| CLI 212 | 1430 | 100 |
| P. aeruginosa | Wood | Bark | CIP |
|---|---|---|---|
| CLI12 | 11.51 | 44.79 | 33.03 |
| CLI15 | 0.00 | 0.00 | 0.00 |
| CLI50 | 0.00 | 38.97 | 64.09 |
| CLI51 | 37.09 | 38.85 | 15.49 |
| CLI52 | 20.91 | 25.51 | 77.19 |
| CLI54 | 0.00 | 0.00 | 0.00 |
| CLI55 | 15.20 | 16.07 | 11.28 |
| CLI56 | 0.00 | 0.00 | 31.41 |
| CLI57 | 56.79 | 0.00 | 64.19 |
| CLI58 | 57.58 | 31.80 | 39.07 |
| CLI97 | 54.31 | 48.46 | 0.00 |
| CLI212 | 93.74 | 0.00 | 26.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras-Martínez, O.I.; Sierra-Quiroz, D.; Angulo-Ortíz, A. Antibacterial and Antibiofilm Potential of Ethanolic Extracts of Duguetia vallicola (Annonaceae) against in-Hospital Isolates of Pseudomonas aeruginosa. Plants 2024, 13, 1412. https://doi.org/10.3390/plants13101412
Contreras-Martínez OI, Sierra-Quiroz D, Angulo-Ortíz A. Antibacterial and Antibiofilm Potential of Ethanolic Extracts of Duguetia vallicola (Annonaceae) against in-Hospital Isolates of Pseudomonas aeruginosa. Plants. 2024; 13(10):1412. https://doi.org/10.3390/plants13101412
Chicago/Turabian StyleContreras-Martínez, Orfa Inés, Daniela Sierra-Quiroz, and Alberto Angulo-Ortíz. 2024. "Antibacterial and Antibiofilm Potential of Ethanolic Extracts of Duguetia vallicola (Annonaceae) against in-Hospital Isolates of Pseudomonas aeruginosa" Plants 13, no. 10: 1412. https://doi.org/10.3390/plants13101412
APA StyleContreras-Martínez, O. I., Sierra-Quiroz, D., & Angulo-Ortíz, A. (2024). Antibacterial and Antibiofilm Potential of Ethanolic Extracts of Duguetia vallicola (Annonaceae) against in-Hospital Isolates of Pseudomonas aeruginosa. Plants, 13(10), 1412. https://doi.org/10.3390/plants13101412






