Analysis of Quantitative Phytochemical Content and Antioxidant Activity of Leaf, Stem, and Bark of Gymnosporia senegalensis (Lam.) Loes.
Abstract
:1. Introduction
2. Results
2.1. Determination of Quantitative Phytochemical Assays
2.1.1. Total Phenol Content (TPC)
2.1.2. Total Flavonoid Content (TFC)
2.1.3. Total Flavonol Content
2.1.4. Total Tannin Content
2.1.5. Total Saponin Content
2.2. Evaluation of Antioxidant Activity
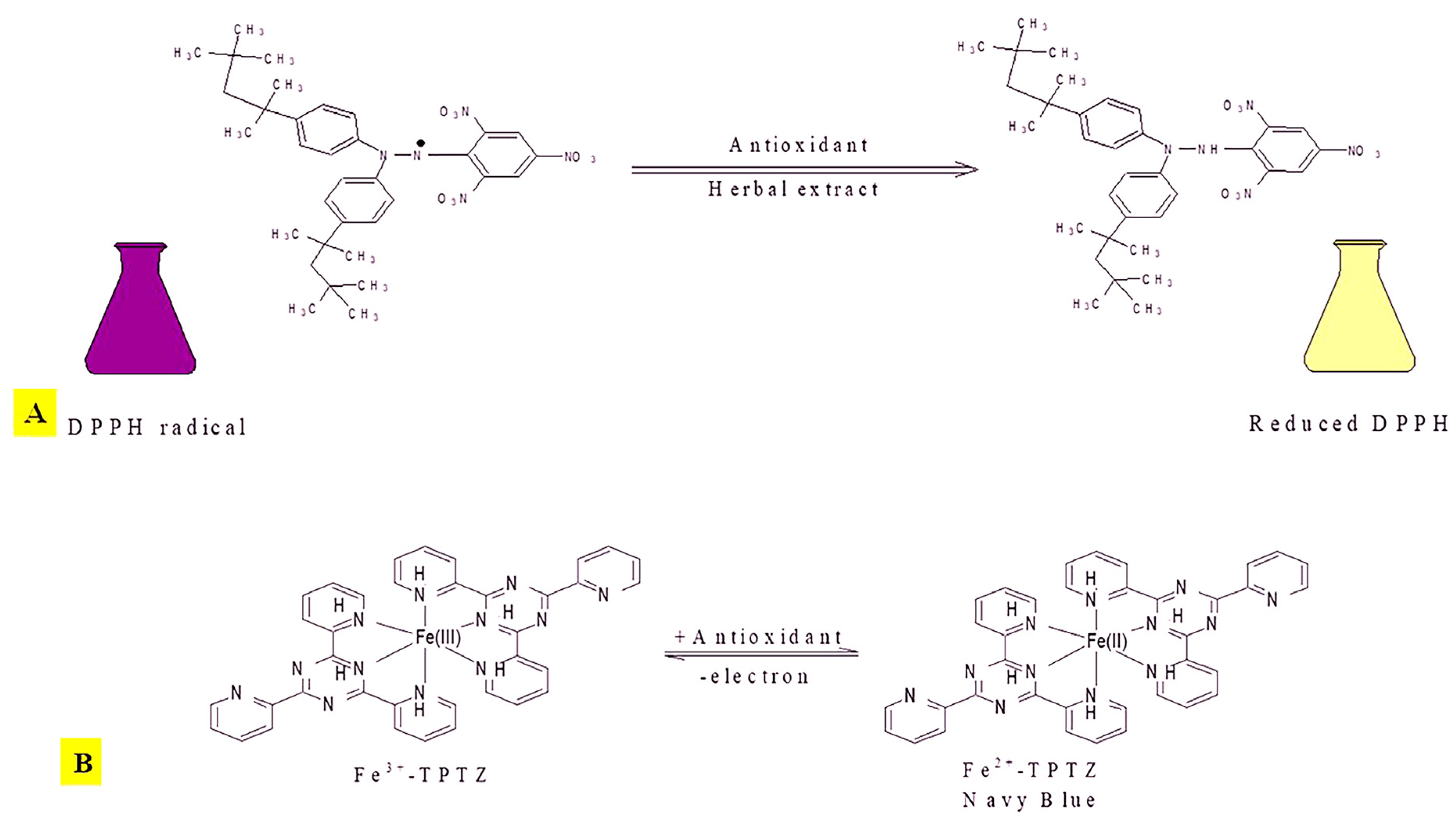
2.2.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.2.2. Hydrogen Peroxide Scavenging Assay
2.2.3. Superoxide Anion Radical Scavenging Assay
2.2.4. Metal Chelating Ferrous Ions Assay
2.2.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.2.6. Total Antioxidant Activity (TAC)
3. Discussion
3.1. Quantitative Estimation
3.1.1. Total Phenol Content
3.1.2. Total Flavonoid and Flavonols Content
3.1.3. Total Tannin Content
3.1.4. Total Saponin Content
3.1.5. DPPH
3.1.6. Hydrogen Peroxide Scavenging Assay
3.1.7. Superoxide Anion Scavenging Assay
3.1.8. Metal Chelating Assay
3.1.9. FRAP
3.1.10. TAC
4. Materials and Methods
4.1. Literature Search
4.2. Collection of Plant Materials
4.3. Chemicals, Reagents, and Extract Preparation
4.4. Quantitative Phytochemical Assays
4.4.1. Total Phenol Content
4.4.2. Total Flavonoid Content
4.4.3. Total Flavonol Content
4.4.4. Total Tannin Content
4.4.5. Total Saponin Content
4.5. Evaluation of In Vitro Antioxidant Activity
4.5.1. 2,2-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
4.5.2. Scavenging Activity Hydrogen Peroxide (H2O2)
4.5.3. Superoxide Anion Radical Scavenging Activity
4.5.4. Ability to Chelate Metal-Ferrous Ions Activity
4.5.5. Determination of Ferric Reducing Antioxidant Power (FRAP) Assay
4.5.6. Total Antioxidant Capacity
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kinnula, V.L.; Crapo, J.D. Superoxide dismutases in malignant cells and human tumors. Free Radic. Biol. Med. 2004, 36, 718–744. [Google Scholar] [CrossRef]
- Bartsch, H.; Nair, J. Ultrasensitive and specific detection method for exocyclic DNA adducts markers for lipid peroxidation and oxidative stress. Toxicology 2000, 153, 105–114. [Google Scholar] [CrossRef]
- Bartosz, G. Reactive oxygen species: Destroyers or messengers. Biochem. Pharmacol. 2009, 77, 1303–1315. [Google Scholar] [CrossRef]
- Safer, A.M.; AI-Nughamish, A.J. Hepatotoxicity induced by the anti-oxidant food additive, butylated hydroxytoluene (BHT), in rats: An electron microscopical study. Histol. Histopathol. 1999, 14, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I. Reactive oxygen species (ROS): An introduction. In Reactive Oxygen Species in Plants; Sachdev, S., Akhtar, S., Ansari, M.I., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Gu, R.; Wang, Y.; Long, B.; Kennelly, E.; Wu, S.; Liu, B. Prospecting for bioactive constituents from traditional medicinal plants through ethnobotanical approaches. Biol. Pharm. Bull. 2014, 37, 903–915. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of tenuazonic acid, alternariol and alternariol monomethyl ether produced by Alternaria species phytopathogenic on plants. Sci. Rep. 2017, 7, 8777. [Google Scholar] [CrossRef]
- Cirla, A.; Mann, J. Combretastatins: From natural products to drug discovery. Nat. Prod. Rep. 2003, 20, 558–564. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Rocha, J.; Barros, A.I. Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep. 2021, 11, 10041. [Google Scholar] [CrossRef] [PubMed]
- Christova-Bagdassarian, V.L.; Bagdassarian, K.S.; Atanassova, M.S.; Ahmad, M.A. Comparative analysis of total phenolic and total flavonoid contents, rutin, tannins and antioxidant capacity in Apiaceae and Lamiaceae families. Indian Hortic. J. 2014, 4, 131–140. [Google Scholar]
- Martin, R.E.; Postiglione, A.E.; Muday, G.K. Reactive oxygen species function as signaling molecules in controlling plant development and hormonal responses. Curr. Opin. Plant Biol. 2022, 69, 102293. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, A.E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef]
- Chaudhary, P.; Meena, M.; Janmeda, P. Microscopic characterization, TLC fingerprinting and optimization of total lipid content from Euphorbia neriifolia (L.) using response surface methodology. Microsc. Res. Tech. 2023, 87, 565–590. [Google Scholar] [CrossRef]
- Jain, D.; Janmeda, P. Morphology, anatomy, and histochemistry of leaves, stem, and bark of Gymnosporia senegalensis (Lam.) Loes. Lett. Appl. NanoBiosci. 2023, 12, 33. [Google Scholar] [CrossRef]
- Jordaan, M.; Van wyk, A.E. Systematic studies in sub-family Celastroideae (Celastraceae) in southern Africa: Reinstatement of the genus Gymnospnria. S. Afr. J Bot. 1999, 65, 177–181. [Google Scholar]
- Burkil, H.M. The Useful Plants of West Tropical Africa; Royal Botanic Gradens Kew, The University of Chicago Press: Richmond upon Thames, UK, 2004; p. 869. [Google Scholar]
- Maydell, H.V. Trees and Shrubs of the Sahel. Their Characteristics and Uses [Deutsche Gesells Chaft Furtechnischezu Sammenarbeit]; Backhuys Publishers: Zuid-Holland, The Netherlands; Cornell University: Ithaca, NY, USA, 1990; p. 525. (In Germany) [Google Scholar]
- Makgatho, M.E.; Nxumalo, W.; Raphoko, L.A. Anti-mycobacterial, oxidative, proliferative and inflammatory activities of dichloromethane leaf extracts of Gymnosporia senegalensis (Lam.) Loes. S. Afr. J Bot. 2018, 114, 217–222. [Google Scholar] [CrossRef]
- Ndako, M.; Jigam, A.A.; Kabiru, A.Y.; Umar, S.I.; Lawal, B. Polar extracts from Gymnosporia senegalensis (syn Maytenus senegalensis) root bark, its effects on nociception, edema, and malarial infection. Phytomed. Plus 2021, 1, 100113. [Google Scholar] [CrossRef]
- Tatsimo, J.S.N.; Toume, K.; Nagata, T.; Havyarimana, L.; Fujii, T.; Komatsu, K. Monoglycerol ester, galloyl glucoside and phenolic derivatives from Gymnosporia senegalensis leaves. Biochem. Syst. Ecol. 2019, 83, 33–38. [Google Scholar] [CrossRef]
- Da Silva, G.; Taniça, M.; Rocha, J.; Serrano, R.; Gommes, E.T.; Sepodes, B.; Silva, O. In vivo anti-inflammatory effect and toxicological screening of Maytenus heterophylla and Maytenus senegalensis extracts. Hum. Exp. Toxicol. 2010, 30, 693–700. [Google Scholar] [CrossRef]
- Ahmed, A.S.; McGaw, L.J.; Eloff, J.N. Evaluation of pharmacological activities, cytotoxicity and phenolic composition of four Maytenus species used in sourthern African medicine to treat intestinal and diarrhoeal diseases. BMC Complement Altern. Med. 2013, 13, 100–115. [Google Scholar] [CrossRef]
- Lall, N.; Meyer, J.J.M. In vitro inhibition of drug-resistant strains of Mycobacterium tuberculosis by ethnobotanically selected South African plants. J. Ethnopharmacol. 1999, 66, 347–354. [Google Scholar] [CrossRef]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.B.; Grace, O.M.; Matsabisa, M.S.; Bhagwadin, N.; Smith, P.J.; Folb, P.I. In vitro antiplasmodial activity of nedicinal plants native or natulised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef]
- Sosa, S.; Morelli, C.F.; Tubaro, A.; Cairoli, P.; Speranza, G.; Manitto, P. Anti-inflammatory activity of Maytenus senegalensis root extracts and maytenoic acid. Phytomedicine 2007, 14, 109–114. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Murugan, R.; Parimelazhagan, T. Comparative evaluation of different extraction methods for antioxidant and antiinflammatory properties from Osbeckia parvifolia Arn.—An in vitro approach. J. King Saud Univ. Sci. 2014, 26, 267–275. [Google Scholar] [CrossRef]
- Sonigra, P.; Meena, M. Metabolic profile, bioactivities, and variations in the chemical constituents of essential oils of the Ferula genus (Apiaceae). Front. Pharmacol. 2021, 11, 608649. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of antioxidants: Mechanisms and pharmaceutical applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; Pham-Huy, H.; He, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Kumar, S.; Chandra, R.; Behera, L.; Sudhir, I.; Meena, M.; Singh, S.; Keswani, C. Microbial consortium mediated acceleration of the defense response in potato against Alternaria solani through prodigious inflation in phenylpropanoid derivatives and redox homeostasis. Heliyon 2023, 9, e22148. [Google Scholar] [CrossRef]
- Mohanty, I.R.; Gupta, S.K.; Arya, D.S.; Mohanty, N.; Deshmukh, Y. Medicinal herbs can play significant role in attenuation of ischemia and reperfusion injury. J. Homeop. Ayurv. Med. 2013, 2, 1–11. [Google Scholar] [CrossRef]
- Arora, J.; Kanthaliya, B.; Joshi, A.; Meena, M.; Meena, S.; Siddiqui, M.H.; Alamri, S.; Devkota, H.P. Evaluation of total isoflavones in chickpea (Cicer arietinum L.) sprouts germinated under precursors (p-coumaric acid and L-phenylalanine) supplementation. Plants 2023, 12, 2823. [Google Scholar] [CrossRef]
- Zafar, M.; Naeem-ul-Hassan, N.; Ahmed, M.; KaimKhani, Z.A. Altered kidney morphology and enzymes in streptozotocin-induced diabetic rats. Int. J. Morphol. 2009, 27, 783–790. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Swapnil, P.; Dubey, M.K.; Patel, C.B.; Upadhyay, R.S. Effect on lycopene, β-carotene, ascorbic acid and phenolic content in tomato fruits infected by Alternaria alternata and its toxins (TeA, AOH and AME). Arch. Phytopathol. Plant Protect. 2017, 50, 317–329. [Google Scholar] [CrossRef]
- Zehra, A.; Dubey, M.K.; Meena, M.; Aamir, M.; Ahirwal, L.; Upadhyay, R.S. Improvement of lycopene, ascorbic acid and total phenol content of postharvest tomato fruits by exogenous application of salicylic acid and methyl jasmonate. Food Pharma Int. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Mohan, P.R.; Savithramma, N. Estimation of total phenol and tannin content present in the leaf, bark and fruits of an endemic semievergreen tree species Terminalia pallida Brandis. Pharma Inn. 2019, 8, 518–522. [Google Scholar]
- Choudhary, R.; Rajput, V.D.; Ghodake, G.; Ahmad, F.; Meena, M.; ul Rehman, R.; Prasad, R.; Sharma, R.K.; Singh, R.; Seth, C.S. Comprehensive journey from past to present to future about seed priming with hydrogen peroxide and hydrogen sulfide in relation to drought, temperature, UV and ozone stresses—A review. Plant Soil 2024, 494, 1–23. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Sharififar, F.; Nudeh-dehghn, G.; Mirtajaldini, M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2008, 112, 885–888. [Google Scholar] [CrossRef]
- Chandha, S.; Dave, R. In vitro models for antioxidant activity evaluation and some medicinal plants possessing antioxidant properties: An overview. Afr. J. Micro. Res. 2009, 3, 981–996. [Google Scholar]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Phenolic compounds and antioxidant activities of Rumex hastatus D. Don. leaves. J. Med. Plants Res. 2011, 5, 2755–2765. [Google Scholar]
- Feeny, P. Seasonal changes in oak leaf tannins and nutrients as a cause of spring feeding by winter moth caterpillars. Ecology 1970, 51, 565–581. [Google Scholar] [CrossRef]
- Barry, T.N.; Manley, R.T. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep. 2. Quantitative digestion of carbohydrates and proteins. Br. J. Nutr. 1984, 51, 493–504. [Google Scholar] [CrossRef]
- Koleckar, V.; Kubikova, K.; Rehakova, Z.; Kuca, K.; Jun, D.; Jahodar, L.; Opletal, L. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Review. Med. Chem. 2008, 8, 436–447. [Google Scholar] [CrossRef]
- Kasolo, J.N.; Bimenya, G.S.; Ojok, L. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J. Med. Plant Res. 2010, 4, 753–757. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Kpoyizoun, P.K.; Metowogo, K.; Kantati, Y.T.; Missebukpo, A.; Dare, T.; Lawson-Evi, P.; Eklu-Gadegbeku, K.; Aklikokou, K.A. Antiinflammatory and antioxidant evaluation of Maytenus senegalensis hydroalcoholic roots extract fractions in allergic asthma. J. Phytopharmacol. 2020, 9, 252–257. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71S–88S. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Ghosh, P.; Biswas, M.; Biswas, S. Phytochemical screening, anti-oxidant and anti-microbial activity of leaves of Cleome rutidosperma DC. (Cleomaceae). J. Pharm. Sci. Res. 2019, 11, 1790–1795. [Google Scholar]
- Jain, D.; Meena, M.; Singh, D.; Janmeda, P. Structural characterisation of bioactive compounds of Gymnosporia senegalensis (Lam.) Loes. using advanced analytical technique like FT-IR, GC-MS and 1H-NMR spectroscopy. Nat. Prod. Res. 2023. [Google Scholar] [CrossRef]
- Ahmad, I.; Aqil, F.; Owais, M. Turning medicinal plants into drugs. Mod. Phytomed. 2006, 384, 67–72. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003–1008. [Google Scholar] [CrossRef]
- Patel, A.; Amit, P.; Patel, A.; Patel, N.M. Estimation of flavonoid, polyphenolic content and in-vitro antioxidant capacity of leaves of Tephrosia purpurea Linn. (Leguminosae). Int. J. Pharm. Sci. Res. 2010, 1, 66–77. [Google Scholar]
- Sahoo, A.; Marar, T. Phytochemical analysis, antioxidant assay and antimicrobial activity in leaves extracts of Cerbera odollam Gaertn. Pharmacogn. J. 2018, 10, 285–292. [Google Scholar] [CrossRef]
- Sun, B.S.; Ricardo-Da-Silva, J.M.; Spranger, M.I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Tepe, B.; Sokmen, A. Screening of the antioxidative properties and total phenolic contents of three endemic Tanacetum subspecies from Turkish flora. Bioresour. Technol. 2007, 98, 3076–3079. [Google Scholar] [CrossRef]
- Alves, C.Q.; David, J.M.; David, J.P.; Bahia, M.V.; Aguiar, R.M. Methods for determination of in vitro antioxidant activity for extracts and organic compounds. Quím. Nova 2010, 33, 2202–2210. [Google Scholar] [CrossRef]
- Mehta, T.; Meena, M.; Nagda, A. Bioactive compounds of Curvularia species as a source of various biological activities and biotechnological applications. Front. Microbiol. 2022, 13, 1069095. [Google Scholar] [CrossRef]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res. Int. 2014, 497606. [Google Scholar] [CrossRef]
- Dubey, M.K.; Meena, M.; Aamir, M.; Zehra, A.; Upadhyay, R.S. Regulation and role of metal ions in secondary metabolites production by microorganisms. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Gupta, V.K., Jogaiah, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 559–577. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Weli, A.M.; Al-Burtamani, R.S.; Al-Azzawi, M.S.; Akhtar, M.S.; Said, S.A. Antimicrobial, antioxidant and cytotoxic activity of Maytenus dhofarensis growing in Oman. J. Appl. Sci. 2019, 19, 229–234. [Google Scholar] [CrossRef]
- Jain, D.; Meena, M.; Singh, D.; Janmeda, P. Isolation, development and validation of HPTLC method for the estimation of β-carotene from Gymnosporia senegalensis (Lam.) Loes. Plant Physiol. Biochem. 2023, 201, 107843. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Çekiç, S.D.; Çetinkaya, A.; Avan, A.N.; Apak, R. Correlation of total antioxidant capacity with reactive oxygen species (ROS) consumption measured by oxidative conversion. J. Agric. Food Chem. 2013, 61, 5260–5270. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, C.; Yang, X.; Wang, J.; Long, G.; Zhou, J. Phytonanomaterials as therapeutic agents and drug delivery carriers. Adv. Drug Deliv. Rev. 2021, 176, 113868. [Google Scholar] [CrossRef]
- Jain, D.; Janmeda, P. Pharmacognostic standardization and qualitative analysis of Gymnosporia senegalensis. J. Appl. Biol. Chem. 2022, 3, 34–46. [Google Scholar] [CrossRef]
- Csepregi, K.; Marianna, K.; Eva, H. On the spectrophotometric determination of total phenolic and flavonoid contents. Acta Biol. Hung. 2013, 64, 500–509. [Google Scholar] [CrossRef]
- Ordoñez, A.A.L.; Gomez, J.D.; Vattuone, M.A.; Isla, M.I. Antioxidant activities of Sechium edule (Jacq.) Swart extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Sankhalkar, S.; Vernekar, V. Quantitative and qualitative analysis of phenolic and flavonoid content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacogn. Res. 2016, 8, 16–21. [Google Scholar] [CrossRef]
- Braca, A.; Tommasi, N.D.; Bari, L.D.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia terapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Jianxiong, Y.; Juan, G.; Jiangfeng, Y. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybednum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
| Parts | Extracts and Standards | Total Phenol (mg GAE/g) | Total Flavonoid (mg QE/g) | Total Flavonol (mg RE/g) | Total Tannin (mg TAE/g) | Total Saponin (mg SQE/g) |
|---|---|---|---|---|---|---|
| Leaf | PEL | 50.7 ± 0.06 | 37.7 ± 0.08 | 39.5 ± 0.03 | 39.2 ± 0.16 | 40.1 ± 0.05 |
| BEL | 44.6 ± 0.01 | 45.1 ± 0.03 | 35.7 ± 0.40 | 57.8 ± 0.04 | 39.1 ± 0.08 | |
| CEL | 44.9 ± 0.20 | 62.2 ± 0.01 | 46.2 ± 0.01 | 80.7 ± 0.13 | 36.6 ± 0.03 a | |
| EEL | 41.8 ± 0.03 | 53.3 ± 0.08 | 45.2 ± 0.07 | 97.5 ± 0.01 a | 56.3 ± 0.03 | |
| MEL | 79.8 ± 0.02 a | 39.0 ± 0.02 | 49.8 ± 0.04 | 42.8 ± 0.07 | 39.6 ± 0.04 | |
| AEL | 39.5 ± 0.09 | 97.1 ± 0.03 a | 96.7 ± 0.07 a | 55.8 ± 0.05 | 49.9 ± 0.08 | |
| Stem | PES | 47.5 ± 0.03 | 20.0 ± 0.07 | 44.7 ± 0.05 | 52.9 ± 0.44 | 15.1 ± 0.01 |
| BES | 59.1 ± 0.08 | 96.0 ± 0.01 a | 12.1 ± 0.05 | 51.4 ± 0.02 | 42.2 ± 0.01 | |
| CES | 97.7 ± 0.02 a | 52.1 ± 0.03 | 44.6 ± 0.07 | 63.9 ± 0.02 | 12.2 ± 0.09 | |
| EES | 40.1 ± 0.05 | 39.3 ± 0.08 | 41.4 ± 0.04 | 71.8 ± 0.01 | 48.2 ± 0.01 | |
| MES | 10.7 ± 0.01 a | 51.1 ± 0.05 | 47.8 ± 0.80 a | 50.3 ± 0.12 | 79.1 ± 0.06 a | |
| AES | 56.1 ± 0.07 | 62.9 ± 0.01 | 37.2 ± 0.03 | 65.4 ± 0.02 a | 57.8 ± 0.09 | |
| Bark | PEB | 44.8 ± 0.07 | 19.0 ± 0.03 | 59.7 ± 0.05 a | 45.7 ± 0.07 | 51.7 ± 0.07 |
| BEB | 74.7 ± 0.02 a | 70.7 ± 0.02 a | 58.0 ± 0.01 | 48.8 ± 0.06 | 24.7 ± 0.01 | |
| CEB | 42.7 ± 0.06 | 48.1 ± 0.02 | 46.2 ± 0.01 | 43.2 ± 0.01 | 13.5 ± 0.01 | |
| EEB | 52.7 ± 0.02 | 37.9 ± 0.06 | 41.9 ± 0.02 | 39.1 ± 0.04 | 63.6 ± 0.07 a | |
| MEB | 50.0 ± 0.03 | 39.8 ± 0.07 | 57.0 ± 0.01 | 53.4 ± 0.03 a | 38.3 ± 0.01 | |
| AEB | 38.9 ± 0.05 | 47.3 ± 0.01 | 56.2 ± 0.01 | 35.6 ± 0.04 | 11.2 ± 0.04 | |
| S | GA | 40.7 ± 0.04 | - | - | - | - |
| Q | - | 46.7 ± 0.01 | - | - | - | |
| R | - | - | 39.4 ± 0.05 | - | - | |
| TA | - | - | - | 38.6 ± 0.02 | - | |
| SQ | - | - | - | - | 40.9 ± 0.02 |
| Parts | Extracts and Standards | Half-Inhibitory Concentration (IC50) | Percentage (%) Inhibition | ||||
|---|---|---|---|---|---|---|---|
| DPPH (µg/mL) | Hydrogen Peroxide (µg/mL) | Superoxide (µg/mL) | Metal Chelation (µg/mL) | FRAP (mg/mL) | TAC (mg/mL) | ||
| Leaf | PEL | 3.31 ± 0.01 | 29.9 ± 0.11 | 1.23 ± 0.01 | 3.13 ± 0.04 | 0.73 ± 0.06 | 0.72 ± 0.03 |
| BEL | 3.45 ± 0.02 | 51.1 ± 0.19 | 3.99 ± 0.03 | 14.4 ± 0.06 | 7.55 ± 0.10 a | 1.21 ± 0.02 | |
| CEL | 3.63 ± 0.01 | 0.62 ± 0.02 | 3.13 ± 0.01 | 26.9 ± 0.11 a | 5.12 ± 0.01 | 1.75 ± 0.09 a | |
| EEL | 2.89 ± 0.01 | 0.11 ± 0.01 | 0.93 ± 0.01 | 4.60 ± 0.02 | 6.39 ± 0.05 | 0.15 ± 0.01 | |
| MEL | 40.9 ± 0.9 | 35.7 ± 0.13 | 10.8 ± 0.04 | 5.07 ± 0.02 | 5.99 ± 0.05 | 0.50 ± 0.01 | |
| AEL | 14.9 ± 0.07 | 63.3 ± 0.21 a | 17.7 ± 0.07 a | 12.5 ± 0.06 | 3.55 ± 0.01 | 0.27 ± 0.01 | |
| Stem | PES | 20.1 ± 0.05 | 43.9 ± 0.14 | 2.54 ± 0.02 | 1.39 ± 0.03 | 0.85 ± 0.01 | 0.19 ± 0.01 |
| BES | 3.41 ± 0.01 | 32.4 ± 0.11 | 0.76 ± 0.01 | 0.30 ± 0.01 | 3.12 ± 0.01 | 0.42 ± 0.01 | |
| CES | 3.22 ± 0.02 | 88.8 ± 1.12 | 2.56 ± 0.01 | 0.33 ± 0.01 | 2.65 ± 0.01 | 0.44 ± 0.08 | |
| EES | 1.91 ± 0.01 | 30.5 ± 0.11 | 19.5 ± 0.09 | 1.87 ± 0.02 a | 6.43 ± 0.04 | 1.51 ± 0.09 a | |
| MES | 2.04 ± 0.01 | 58.1 ± 0.15 | 22.8 ± 0.09 | 1.02 ± 0.02 | 6.69 ± 0.02 a | 1.43 ± 0.02 | |
| AES | 3.39 ± 0.02 | 26.8 ± 0.09 b | 24.2 ± 0.11 a | 2.04 ± 0.02 | 5.58 ± 0.02 | 0.55 ± 0.06 | |
| Bark | PEB | 11.1 ± 0.02 | 56.9 ± 0.09 a | 3.15 ± 0.02 | 3.25 ± 0.03 | 0.71 ± 0.01 | 0.35 ± 0.04 |
| BEB | 4.53 ± 0.01 | 42.4 ± 0.08 | 0.72 ± 0.01 | 1.17 ± 0.03 | 2.66 ± 0.01 | 0.38 ± 0.01 | |
| CEB | 4.04 ± 0.01 | 44.1 ± 0.08 | 0.16 ± 0.01 | 15.7 ± 0.06 a | 0.76 ± 0.01 | 1.43 ± 0.08 | |
| EEB | 8.34 ± 0.04 | 19.0 ± 0.07 | 0.82 ± 0.02 | 4.37 ± 0.03 | 1.42 ± 0.02 | 1.18 ± 0.05 | |
| MEB | 8.67 ± 0.04 | 21.3 ± 0.08 | 8.37 ± 0.02 | 1.51 ± 0.02 | 5.62 ± 0.01 a | 2.97 ± 0.01 a | |
| AEB | 4.61 ± 0.01 | 2.81 ± 0.03 | 43.9 ± 0.15 a | 0.91 ± 0.03 | 1.85 ± 0.01 | 0.31 ± 0.13 | |
| S | AC | 64.8 ± 0.12 | 22.4 ± 0.09 | - | 4.83 ± 0.01 | - | 1.27 ± 0.05 |
| BHA | - | - | 3.92 ± 0.01 | - | - | - | |
| BHT | - | - | - | - | 2.37 ± 0.01 | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jain, D.; Meena, M.; Janmeda, P.; Seth, C.S.; Arora, J. Analysis of Quantitative Phytochemical Content and Antioxidant Activity of Leaf, Stem, and Bark of Gymnosporia senegalensis (Lam.) Loes. Plants 2024, 13, 1425. https://doi.org/10.3390/plants13111425
Jain D, Meena M, Janmeda P, Seth CS, Arora J. Analysis of Quantitative Phytochemical Content and Antioxidant Activity of Leaf, Stem, and Bark of Gymnosporia senegalensis (Lam.) Loes. Plants. 2024; 13(11):1425. https://doi.org/10.3390/plants13111425
Chicago/Turabian StyleJain, Divya, Mukesh Meena, Pracheta Janmeda, Chandra Shekhar Seth, and Jaya Arora. 2024. "Analysis of Quantitative Phytochemical Content and Antioxidant Activity of Leaf, Stem, and Bark of Gymnosporia senegalensis (Lam.) Loes." Plants 13, no. 11: 1425. https://doi.org/10.3390/plants13111425
APA StyleJain, D., Meena, M., Janmeda, P., Seth, C. S., & Arora, J. (2024). Analysis of Quantitative Phytochemical Content and Antioxidant Activity of Leaf, Stem, and Bark of Gymnosporia senegalensis (Lam.) Loes. Plants, 13(11), 1425. https://doi.org/10.3390/plants13111425







