Comparative Metabolome and Transcriptome Analyses Reveal Differential Enrichment of Metabolites with Age in Panax notoginseng Roots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Tissue Collection
2.2. Metabolome Analysis
2.3. Transcriptome Analysis
2.4. Statistical Analysis
3. Results
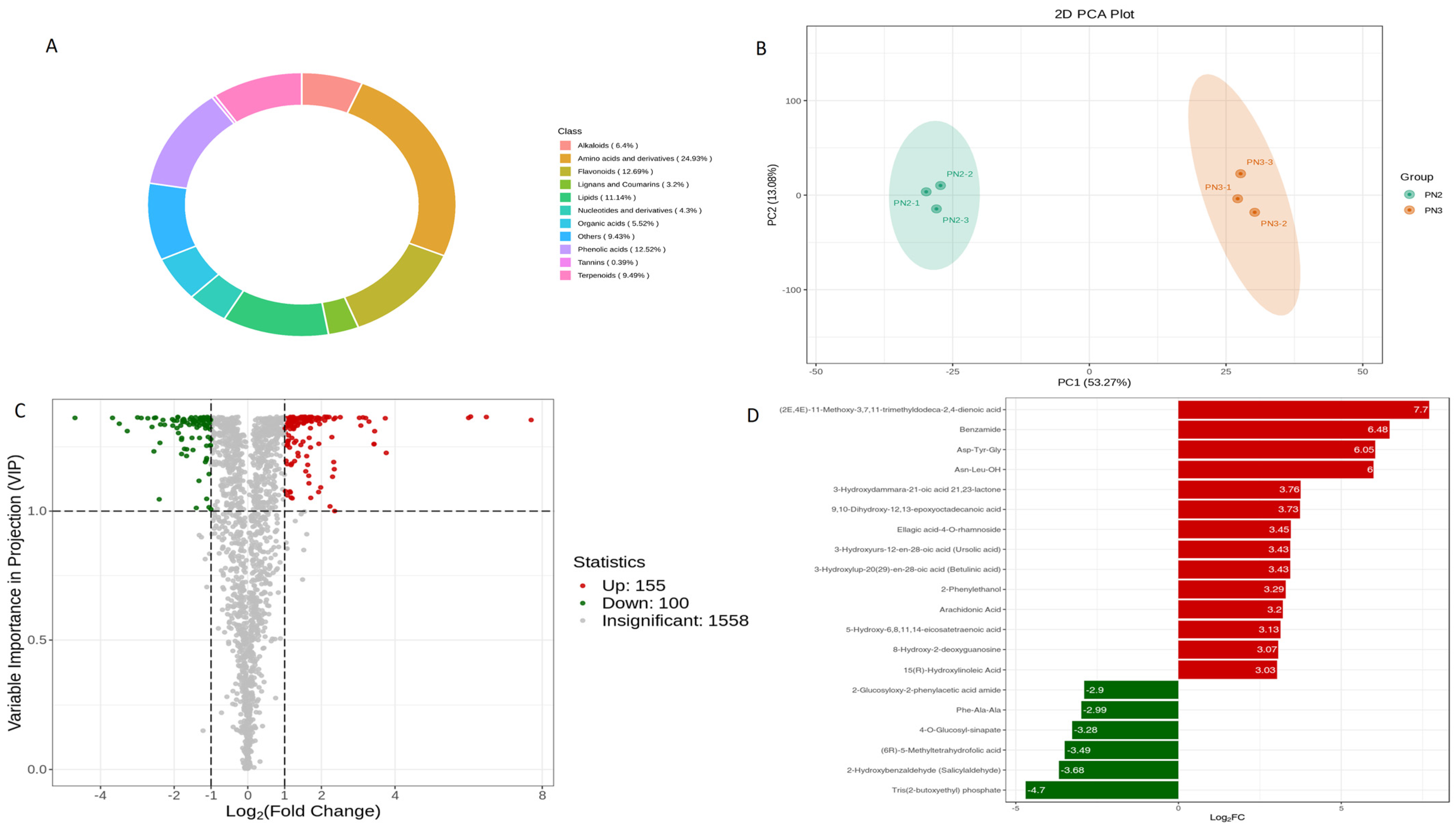
3.1. Metabolome of P. notoginseng (PN2 vs. PN3)
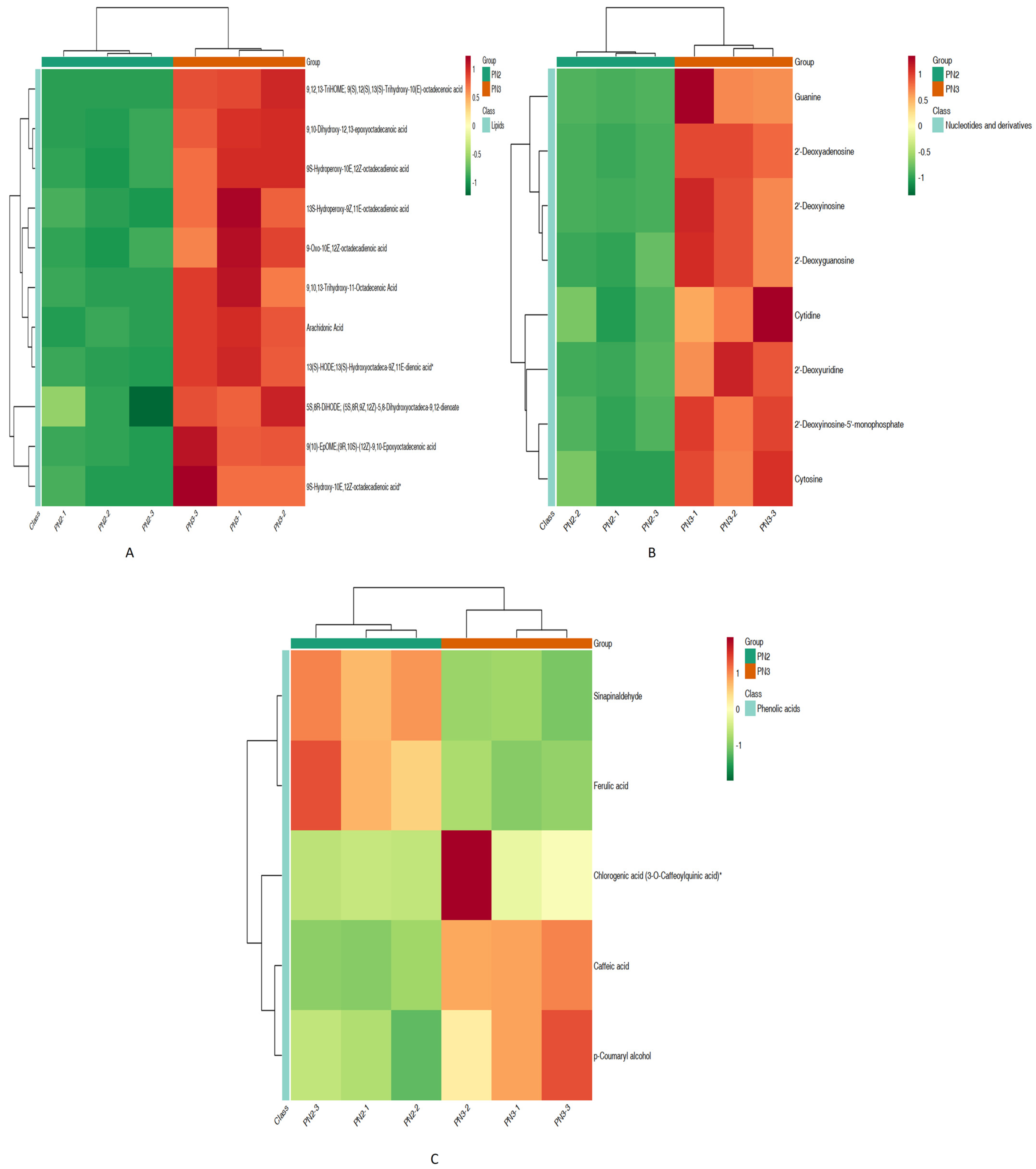
3.1.1. Differential Metabolites
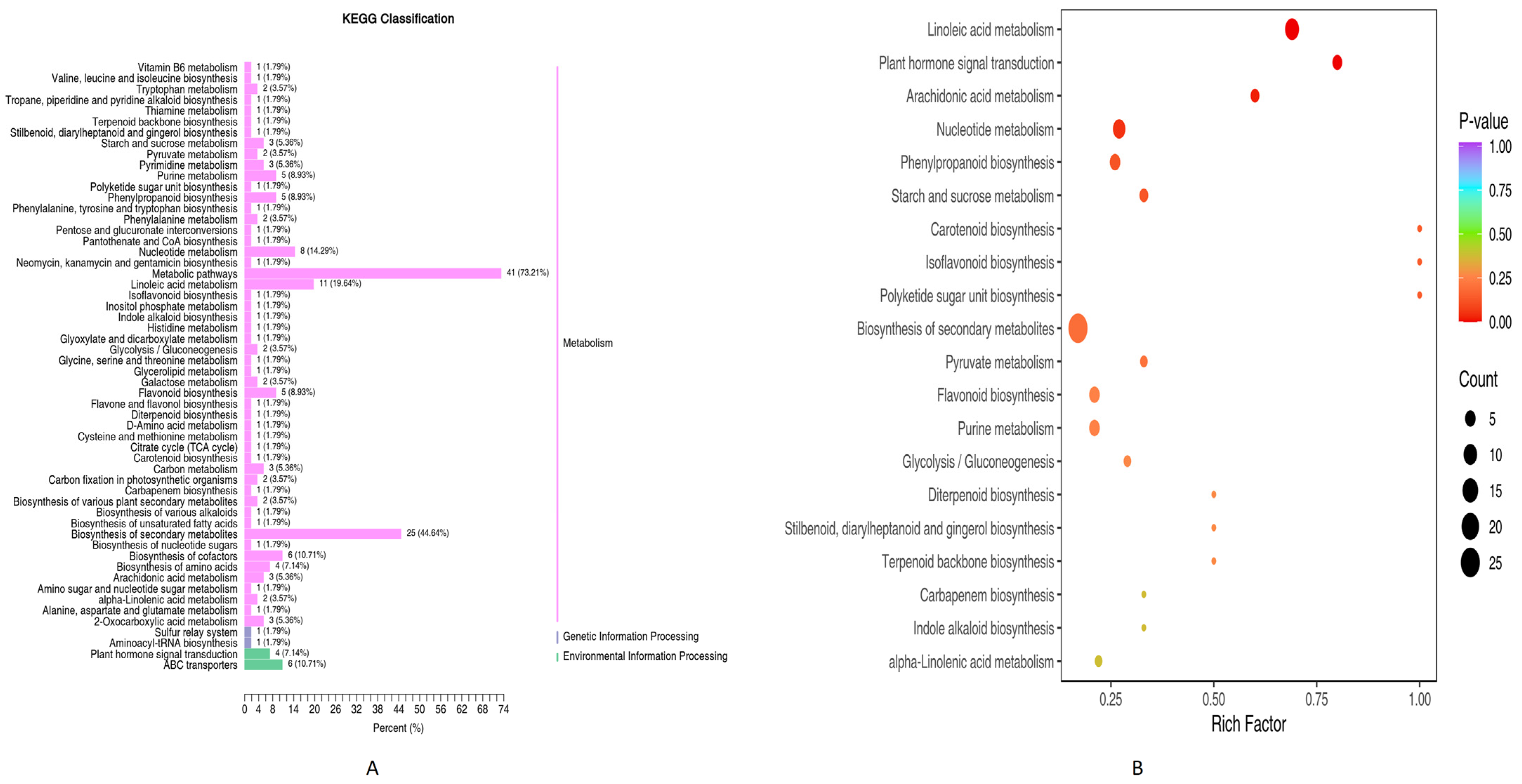
3.1.2. KEGG Differential Metabolites Enrichment Analysis (PN2 vs. PN3)
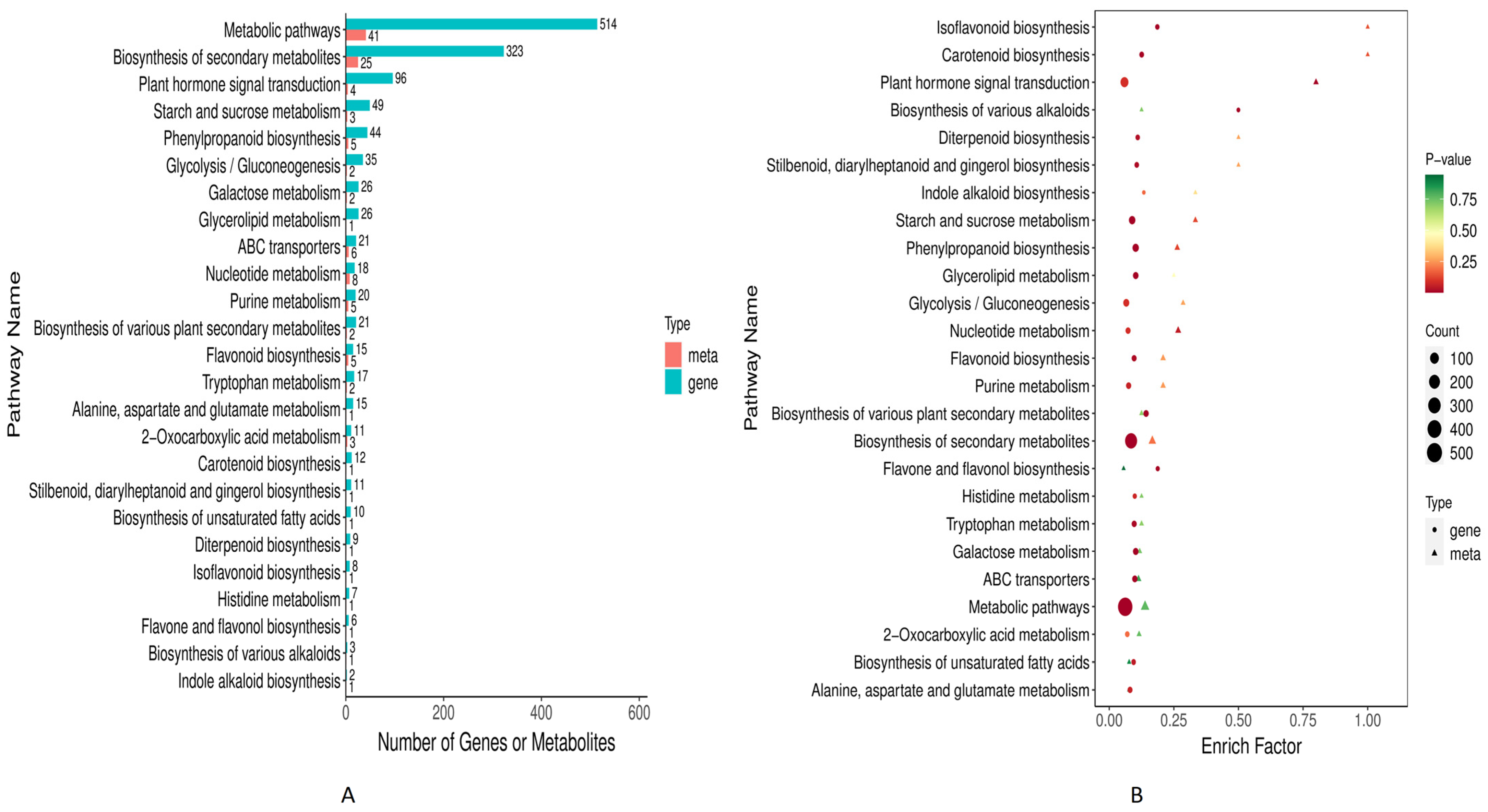
3.1.3. KEGG Pathways Enrichment Analysis
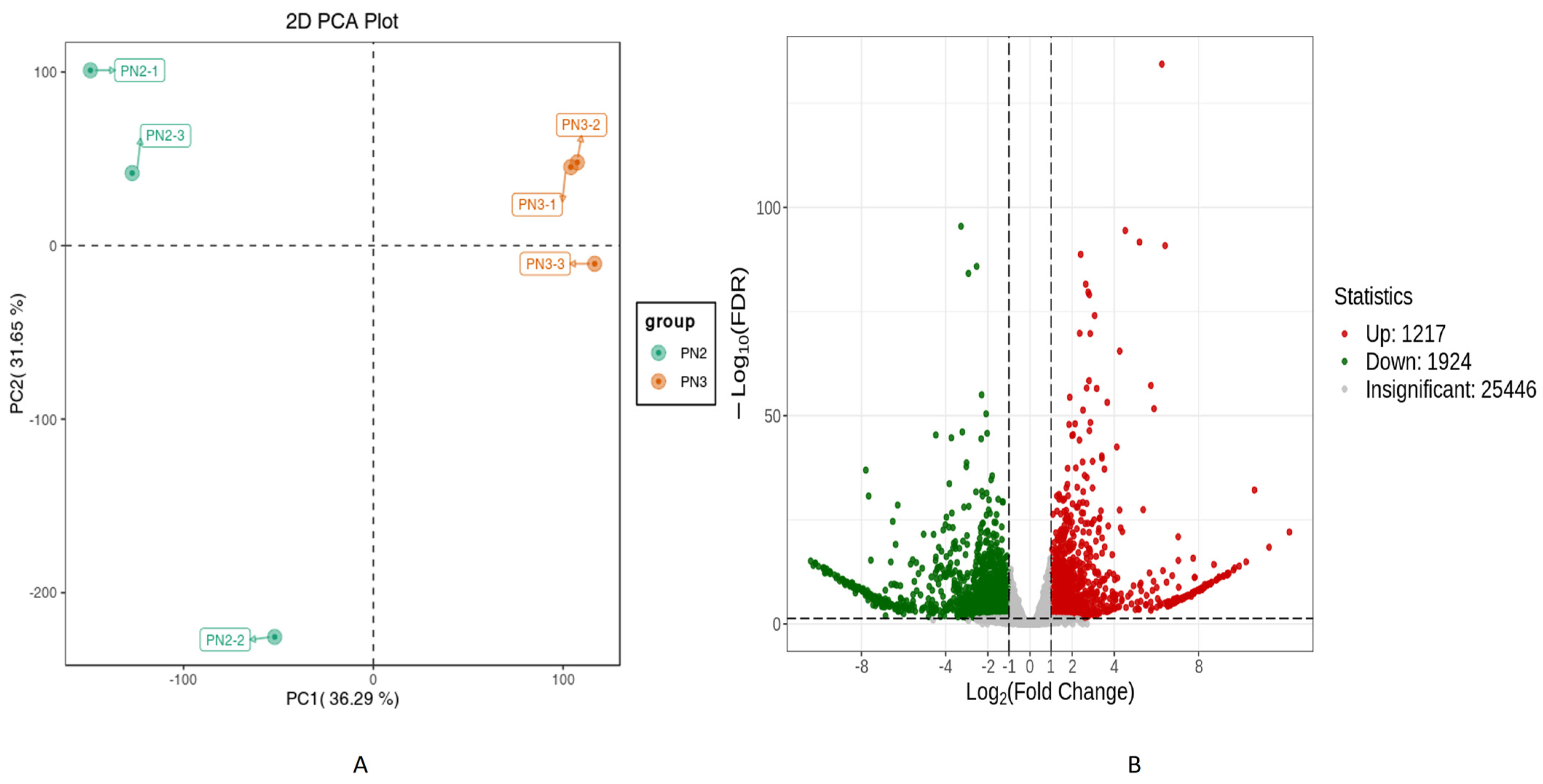
3.2. Transcriptome of Panax notoginseng (PN2 vs. PN3)
Differentially Expressed Gene Analysis
3.3. Gene Ontology Pathways Enrichment (GO) Analysis
3.4. Combined Metabolome and Transcriptome KEGG Enrichment Analysis
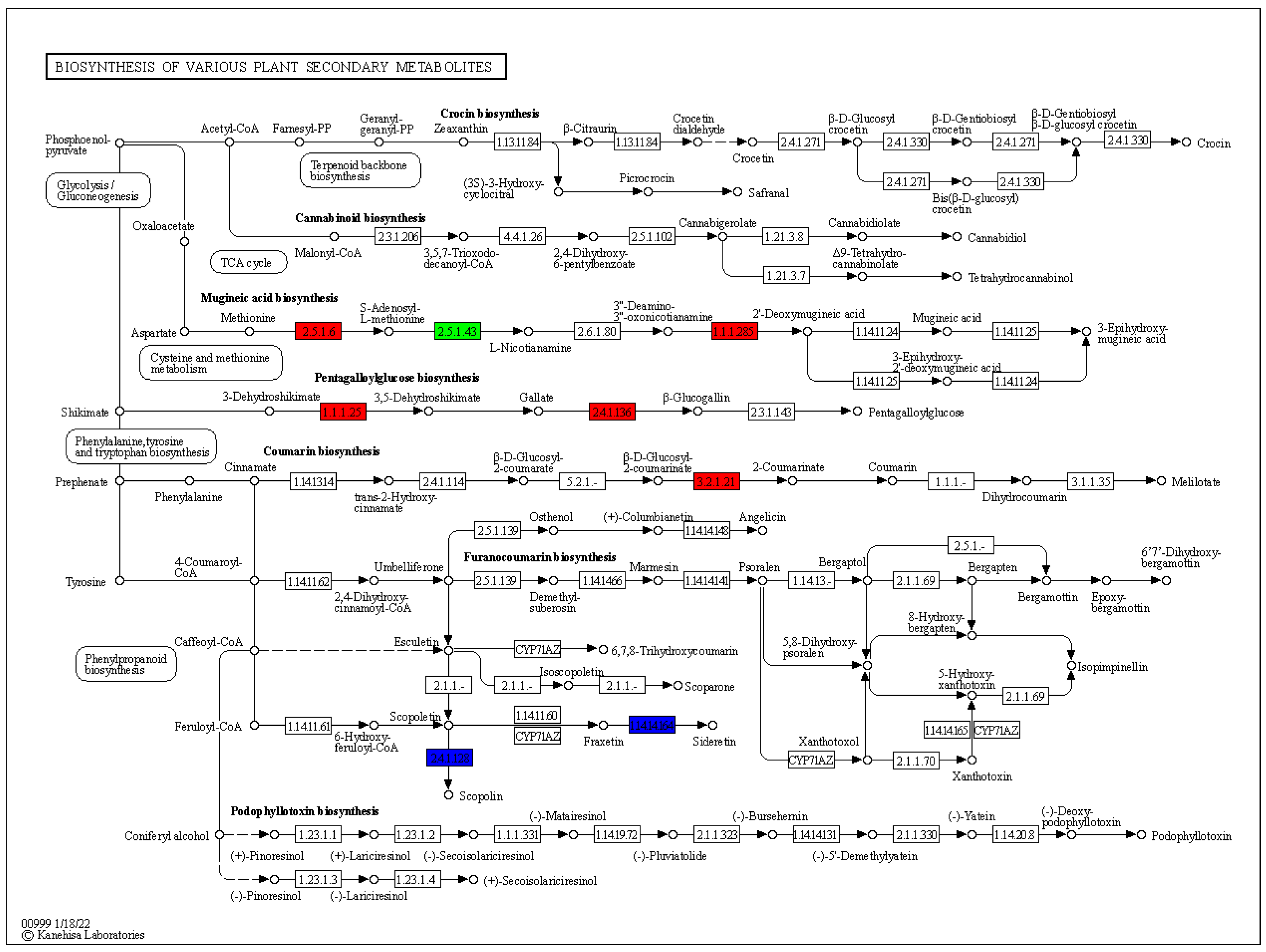
3.5. Expression Changes in Various Plants Secondary Metabolic Pathways
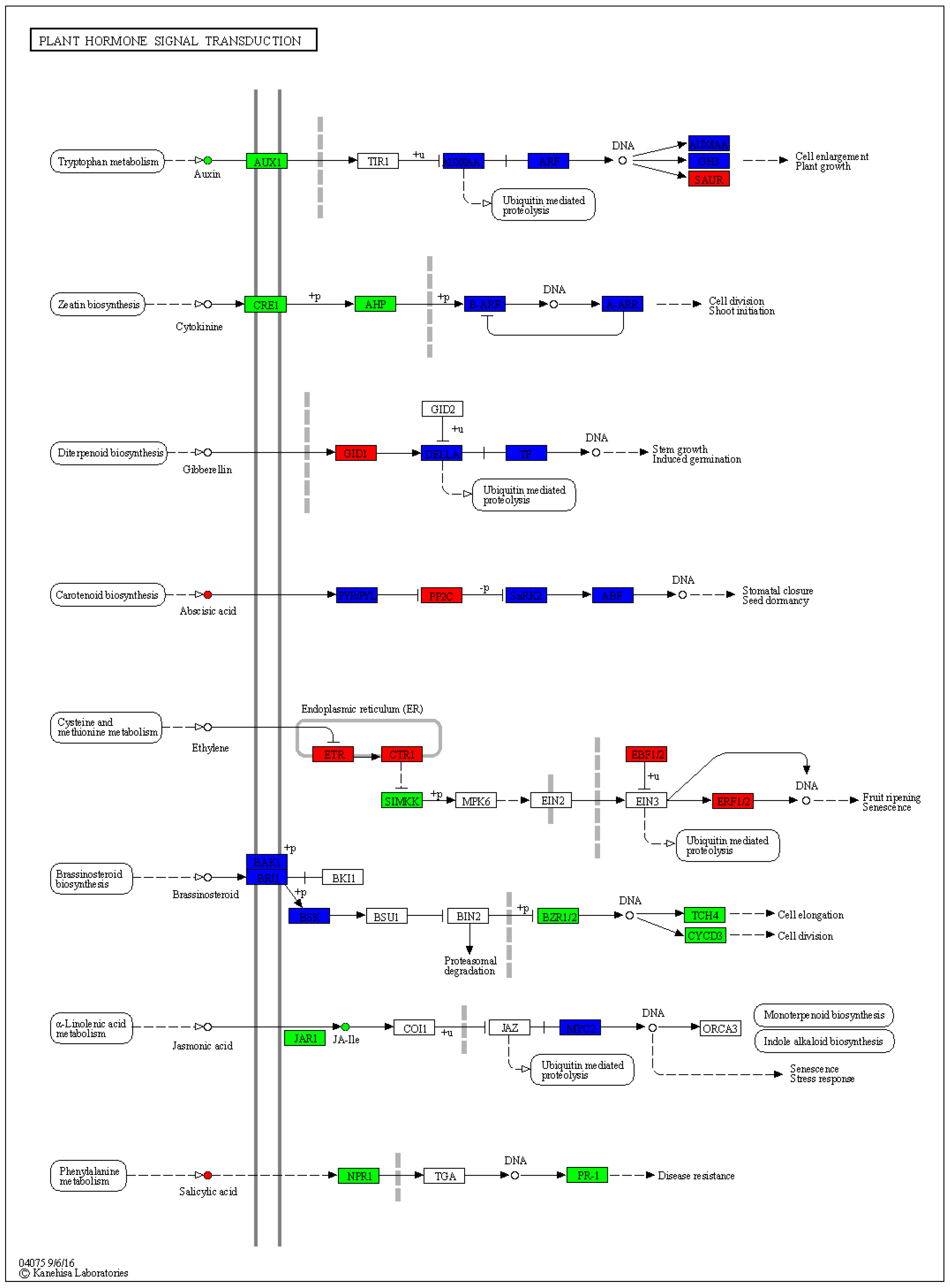
3.6. Expression Changes in Phytohormone Signaling Pathways
4. Discussion
4.1. Metabolomic Analysis
4.2. Transcriptome Analysis and Expression Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wei, G.; Dong, L.; Yang, J.; Zhang, L.; Xu, J.; Yang, F.; Cheng, R.; Xu, R.; Chen, S. Integrated Metabolomic and Transcriptomic Analyses Revealed the Distribution of Saponins in Panax notoginseng. Acta Pharm. Sin. B 2018, 8, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Fan, J.; Han, J. Ameliorating Effects of Traditional Chinese Medicine Preparation, Chinese Materia Medica and Active Compounds on Ischemia/Reperfusion-Induced Cerebral Microcirculatory Disturbances and Neuron Damage. Acta Pharm. Sin. B 2015, 5, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Yang, M.; Zhu, S.S. Strategies to Solve the Problem of Soil Sickness of Panax notoginseng (Family: Araliaceae). Allelopath. J. 2019, 47, 37–56. [Google Scholar] [CrossRef]
- Wei, W.; Ye, C.; Huang, H.-C.; Yang, M.; Mei, X.-Y.; Du, F.; He, X.-H.; Zhu, S.-S.; Liu, Y.-X. Appropriate Nitrogen Application Enhances Saponin Synthesis and Growth Mediated by Optimizing Root Nutrient Uptake Ability. J. Ginseng Res. 2020, 44, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, R.; Zhou, G.; Zhou, X.; Kou, Z.; Sui, F.; Li, C.; Tang, L.; Wang, Z. Traditional Uses, Botany, Phytochemistry, Pharmacology and Toxicology of Panax notoginseng (Burk.) FH Chen: A Review. J. Ethnopharmacol. 2016, 188, 234–258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fang, K.; Ding, Z.; Wu, J.; Lin, J.; Xu, D.; Zhong, J.; Xia, F.; Feng, J.; Shen, G. Untargeted Metabolomics Analysis Revealed the Difference of Component and Geographical Indication Markers of Panax notoginseng in Different Production Areas. Foods 2023, 12, 2377. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, X.; Cui, X.; Hu, D.; Chen, Y.; Zhao, J.; Li, S. High-performance Anion-exchange Chromatography Coupled with Diode Array Detection for the Determination of Dencichine in Panax Notoginseng and Related Species. J. Sep. Sci. 2013, 36, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.-X.; Li, R.; Liu, K.; Yang, J.; Li, H.-J.; Li, S.-L.; Liu, J.-Q.; Liu, L.-F.; Xin, G.-Z. Structural Characterization and Discrimination of Chinese Medicinal Materials with Multiple Botanical Origins Based on Metabolite Profiling and Chemometrics Analysis: Clematidis Radix et Rhizoma as a Case Study. J. Chromatogr. A 2015, 1425, 129–140. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, C.; Zuo, T.; Li, W.; Jia, L.; Wang, X.; Qian, Y.; Guo, D.; Yang, W. In-Depth Profiling, Characterization, and Comparison of the Ginsenosides among Three Different Parts (the Root, Stem Leaf, and Flower Bud) of Panax quinquefolius L. by Ultra-High Performance Liquid Chromatography/Quadrupole-Orbitrap Mass Spectrometry. Anal. Bioanal. Chem. 2019, 411, 7817–7829. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Yang, S.; Zhang, W.; Zhang, J.; Zhao, W.; Chen, L.; Wen, Y.; Zhang, Y.; Lu, K. Strategy for Comparative Untargeted Metabolomics Reveals Honey Markers of Different Floral and Geographic Origins Using Ultrahigh-Performance Liquid Chromatography-Hybrid Quadrupole-Orbitrap Mass Spectrometry. J. Chromatogr. A 2017, 1499, 78–89. [Google Scholar] [CrossRef]
- Xu, X.-F.; Gao, Y.; Xu, S.-Y.; Liu, H.; Xue, X.; Zhang, Y.; Zhang, H.; Liu, M.-N.; Xiong, H.; Lin, R.-C. Remarkable Impact of Steam Temperature on Ginsenosides Transformation from Fresh Ginseng to Red Ginseng. J. Ginseng Res. 2018, 42, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-S.; Park, H.-W.; In, G.; Seo, H.K.; Won, T.H.; Jang, K.H.; Cho, B.-G.; Han, C.K.; Shin, J. Metabolomic Approach for Discrimination of Four-and Six-Year-Old Red Ginseng (Panax Ginseng) Using UPLC-QToF-MS. Chem. Pharm. Bull. 2016, 64, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, J.; Chen, N.; Li, M. The Complete Chloroplast Genome of Traditional Chinese Medical Plants Paris Polyphylla Var. Yunnanensis. Mitochondrial DNA Part A 2017, 28, 159–160. [Google Scholar] [CrossRef] [PubMed]
- De Rijke, E.; Fellner, C.; Westerveld, J.; Lopatka, M.; Cerli, C.; Kalbitz, K.; De Koster, C.G. Determination of N-Alkanes in C. Annuum (Bell Pepper) Fruit and Seed Using GC-MS: Comparison of Extraction Methods and Application to Samples of Different Geographical Origin. Anal. Bioanal. Chem. 2015, 407, 5729–5738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-C.; Li, G.; Jiang, C.; Yang, B.; Yang, H.-J.; Xu, H.-Y.; Huang, L.-Q. Tissue-Specific Distribution of Ginsenosides in Different Aged Ginseng and Antioxidant Activity of Ginseng Leaf. Molecules 2014, 19, 17381–17399. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.-Z.; Zhu, X.-Y.; Wan, J.-Y.; Zhang, J.; Li, W.; Ruan, C.-C.; Yuan, C.-S. Dynamic Changes in Neutral and Acidic Ginsenosides with Different Cultivation Ages and Harvest Seasons: Identification of Chemical Characteristics for Panax Ginseng Quality Control. Molecules 2017, 22, 734. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.; Fang, X.; Tian, Y.; Yu, M.; Xiao, H. Integrative Metabolome and Transcriptome Analyses Reveal the Regulatory Network of Ginsenoside Synthesis in Ginseng. 2022; preprint. [Google Scholar]
- Hui, F. Application of Transcriptome Sequencing in Study of Medicinal Plants. Chin. Tradit. Herb. Drugs 2019, 6149–6155. [Google Scholar]
- Fang, X.; Wang, M.; Zhou, X.; Wang, H.; Wang, H.; Xiao, H. Effects of Growth Years on Ginsenoside Biosynthesis of Wild Ginseng and Cultivated Ginseng. BMC Genom. 2022, 23, 325. [Google Scholar] [CrossRef]
- Jayakodi, M.; Lee, S.-C.; Lee, Y.S.; Park, H.-S.; Kim, N.-H.; Jang, W.; Lee, H.O.; Joh, H.J.; Yang, T.-J. Comprehensive Analysis of Panax Ginseng Root Transcriptomes. BMC Plant Biol. 2015, 15, 138. [Google Scholar] [CrossRef]
- Jo, I.-H.; Lee, S.-H.; Kim, Y.-C.; Kim, D.-H.; Kim, H.-S.; Kim, K.-H.; Chung, J.-W.; Bang, K.-H. De Novo Transcriptome Assembly and the Identification of Gene-Associated Single-Nucleotide Polymorphism Markers in Asian and American Ginseng Roots. Mol. Genet. Genom. 2015, 290, 1055–1065. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kui, L.; Zhang, G.; Zhu, S.; Zhang, J.; Wang, X.; Yang, M.; Huang, H.; Liu, Y.; Wang, Y. Whole-Genome Sequencing and Analysis of the Chinese Herbal Plant Panax notoginseng. Mol. Plant 2017, 10, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar]
- Chen, L.; Zhang, Y.-H.; Wang, S.; Zhang, Y.; Huang, T.; Cai, Y.-D. Prediction and Analysis of Essential Genes Using the Enrichments of Gene Ontology and KEGG Pathways. PLoS ONE 2017, 12, e0184129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-Y.; Xu, X.-Z.; Kuang, S.-B.; Cun, Z.; Wu, H.-M.; Shuang, S.-P.; Chen, J.-W. Constitutive Activation of Genes Involved in Triterpene Saponins Enhances the Accumulation of Saponins in Three-Year-Old Panax notoginseng Growing under Moderate Light Intensity. Ind. Crops Prod. 2021, 171, 113938. [Google Scholar] [CrossRef]
- Hwang, H.; Winkler-Moser, J.K.; Liu, S.X. Study on Antioxidant Activity of Amino Acids at Frying Temperatures and Their Interaction with Rosemary Extract, Green Tea Extract, and Ascorbic Acid. J. Food Sci. 2019, 84, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, L.; Liu, Y.; Zou, S.; Lu, M.; An, H. Metabolomics Combined with Transcriptomics Analysis Revealed the Amino Acids, Phenolic Acids, and Flavonol Derivatives Biosynthesis Network in Developing Rosa Roxburghii Fruit. Foods 2022, 11, 1639. [Google Scholar] [CrossRef]
- Harmon, M.A.; Boehm, M.F.; Heyman, R.A.; Mangelsdorf, D.J. Activation of Mammalian Retinoid X Receptors by the Insect Growth Regulator Methoprene. Proc. Natl. Acad. Sci. USA 1995, 92, 6157–6160. [Google Scholar] [CrossRef] [PubMed]
- Schoff, P.K.; Ankley, G.T. Effects of Methoprene, Its Metabolites, and Breakdown Products on Retinoid-activated Pathways in Transfected Cell Lines. Environ. Toxicol. Chem. An Int. J. 2004, 23, 1305–1310. [Google Scholar] [CrossRef]
- Kuda, O.; Brezinova, M.; Rombaldova, M.; Slavikova, B.; Posta, M.; Beier, P.; Janovska, P.; Veleba, J.; Kopecky Jr, J.; Kudova, E. Docosahexaenoic Acid–Derived Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) with Anti-Inflammatory Properties. Diabetes 2016, 65, 2580–2590. [Google Scholar] [CrossRef]
- Lee, J.; Moraes-Vieira, P.M.; Castoldi, A.; Aryal, P.; Yee, E.U.; Vickers, C.; Parnas, O.; Donaldson, C.J.; Saghatelian, A.; Kahn, B.B. Branched Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) Protect against Colitis by Regulating Gut Innate and Adaptive Immune Responses. J. Biol. Chem. 2016, 291, 22207–22217. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Bansode, R.R.; Trappey II, A.; Bawadi, H.A.; Truax, R. In Vitro Anti-Proliferative Activities of Ellagic Acid. J. Nutr. Biochem. 2004, 15, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-M.; Zhao, L.; Li, H.; Xu, H.; Chen, W.-W.; Tao, L. Research Progress on the Anticarcinogenic Actions and Mechanisms of Ellagic Acid. Cancer Biol. Med. 2014, 11, 92. [Google Scholar]
- Fontaine, B.M.; Nelson, K.; Lyles, J.T.; Jariwala, P.B.; García-Rodriguez, J.M.; Quave, C.L.; Weinert, E.E. Identification of Ellagic Acid Rhamnoside as a Bioactive Component of a Complex Botanical Extract with Anti-Biofilm Activity. Front. Microbiol. 2017, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Mlala, S.; Oyedeji, A.O.; Gondwe, M.; Oyedeji, O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules 2019, 24, 2751. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xu, P. Recent Advances in Biotechnological Production of 2-Phenylethanol. Biotechnol. Adv. 2011, 29, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Khan, H.; Xiao, J.; Cheang, W.S. Effects of Arachidonic Acid Metabolites on Cardiovascular Health and Disease. Int. J. Mol. Sci. 2021, 22, 12029. [Google Scholar] [CrossRef] [PubMed]
- Powell, W.S.; Rokach, J. Targeting the OXE Receptor as a Potential Novel Therapy for Asthma. Biochem. Pharmacol. 2020, 179, 113930. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, S.K.; Boguszewska, K.; Szewczuk, M.; Kaźmierczak-Barańska, J.; Karwowski, B.T. 8-Oxo-7, 8-Dihydro-2′-Deoxyguanosine (8-OxodG) and 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) as a Potential Biomarker for Gestational Diabetes Mellitus (GDM) Development. Molecules 2020, 25, 202. [Google Scholar] [CrossRef]
- Paluchova, V.; Vik, A.; Cajka, T.; Brezinova, M.; Brejchova, K.; Bugajev, V.; Draberova, L.; Draber, P.; Buresova, J.; Kroupova, P. Triacylglycerol-rich Oils of Marine Origin Are Optimal Nutrients for Induction of Polyunsaturated Docosahexaenoic Acid Ester of Hydroxy Linoleic Acid (13-DHAHLA) with Anti-inflammatory Properties in Mice. Mol. Nutr. Food Res. 2020, 64, 1901238. [Google Scholar] [CrossRef]
- Chernikov, M.V.; Sosnovskaya, A.V.; Pozdnyakov, D.I. Study of Some Aspets Neuroprotective Activity of Phenylacetic Acid Amides under Conditions of Focal Brain Ischemia. J. Volgogr. State Med. Univ. 2021, 18, 163–167. [Google Scholar] [CrossRef]
- Li, W.; Wen, L.; Chen, Z.; Zhang, Z.; Pang, X.; Deng, Z.; Liu, T.; Guo, Y. Study on Metabolic Variation in Whole Grains of Four Proso Millet Varieties Reveals Metabolites Important for Antioxidant Properties and Quality Traits. Food Chem. 2021, 357, 129791. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.N.; Jansen, H.; Kennedy, R.L.; Malabu, U.H. Hydroxyoctadecadienoic Acids: Oxidised Derivatives of Linoleic Acid and Their Role in Inflammation Associated with Metabolic Syndrome and Cancer. Eur. J. Pharmacol. 2016, 785, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.; Martín-Venegas, R.; Moreno, J.J. Differential Cell Growth/Apoptosis Behavior of 13-Hydroxyoctadecadienoic Acid Enantiomers in a Colorectal Cancer Cell Line. Am. J. Physiol. Liver Physiol. 2014, 307, G664–G671. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic Acid and Mental Diseases: From Chemistry to Medicine. Curr. Neuropharmacol. 2017, 15, 471. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef] [PubMed]
- Tasca, C.I.; Lanznaster, D.; Oliveira, K.A.; Fernández-Dueñas, V.; Ciruela, F. Neuromodulatory Effects of Guanine-Based Purines in Health and Disease. Front. Cell. Neurosci. 2018, 12, 376. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.M.; Maowa, J.; Alam, A.; Dey, S.; Hosen, A.; Hasan, I.; Fujii, Y.; Ozeki, Y.; Kawsar, S.M.A. In Silico DFT Study, Molecular Docking, and ADMET Predictions of Cytidine Analogs with Antimicrobial and Anticancer Properties. Silico Pharmacol. 2021, 9, 42. [Google Scholar] [CrossRef]
- Ali, E.S.; Ben-Sahra, I. Regulation of Nucleotide Metabolism in Cancers and Immune Disorders. Trends Cell Biol. 2023, 33, 950–966. [Google Scholar] [CrossRef]
- Li, B.; Gao, G.; Zhang, W.; Li, B.; Yang, C.; Jiang, X.; Tian, Y.; Liang, H. Metabolomics Analysis Reveals an Effect of Homocysteine on Arachidonic Acid and Linoleic Acid Metabolism Pathway. Mol. Med. Rep. 2018, 17, 6261–6268. [Google Scholar] [CrossRef]
- Zhang, J.; Diao, F.; Hao, B.; Xu, L.; Jia, B.; Hou, Y.; Ding, S.; Guo, W. Multiomics Reveals Claroideoglomus Etunicatum Regulates Plant Hormone Signal Transduction, Photosynthesis and La Compartmentalization in Maize to Promote Growth under La Stress. Ecotoxicol. Environ. Saf. 2023, 262, 115128. [Google Scholar] [CrossRef]
- Sun, J.; Du, L.; Liang, W.; Qu, Z.; Zhao, H.; Guan, W. Effect of Postharvest Processing on Quality Traits of Radix Gentianae Macrophyllae: A Integrative Analysis of Metabolomics and Proteomics. Plant Physiol. Biochem. 2023, 204, 108099. [Google Scholar] [CrossRef]
- Liu, R.; Hu, J. HemeBIND: A Novel Method for Heme Binding Residue Prediction by Combining Structural and Sequence Information. BMC Bioinform. 2011, 12, 207. [Google Scholar] [CrossRef]
- Ochiiwa, H.; Ailiken, G.; Yokoyama, M.; Yamagata, K.; Nagano, H.; Yoshimura, C.; Muraoka, H.; Ishida, K.; Haruma, T.; Nakayama, A. TAS4464, a NEDD8-Activating Enzyme Inhibitor, Activates Both Intrinsic and Extrinsic Apoptotic Pathways via c-Myc-Mediated Regulation in Acute Myeloid Leukemia. Oncogene 2021, 40, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary Metabolites of Plants and Their Role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 293–304. [Google Scholar]
- Li, J.; Ma, L.; Zhang, S.; Zuo, C.; Song, N.; Zhu, S.; Wu, J. Transcriptome Analysis of 1-and 3-Year-Old Panax notoginseng Roots and Functional Characterization of Saponin Biosynthetic Genes DS and CYP716A47-Like. Planta 2019, 249, 1229–1237. [Google Scholar] [CrossRef]
- Boiteau, R.M.; Markillie, L.M.; Hoyt, D.W.; Hu, D.; Chu, R.K.; Mitchell, H.D.; Pasa-Tolic, L.; Jansson, J.K.; Jansson, C. Metabolic Interactions between Brachypodium and Pseudomonas Fluorescens under Controlled Iron-Limited Conditions. Msystems 2021, 6, e00580-20. [Google Scholar] [CrossRef]
- Li, Y.; Wang, N.; Zhao, F.; Song, X.; Yin, Z.; Huang, R.; Zhang, C. Changes in the Transcriptomic Profiles of Maize Roots in Response to Iron-Deficiency Stress. Plant Mol. Biol. 2014, 85, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002; ISBN 0471496413. [Google Scholar]
- Kim, T.-W.; Paveen, S.; Lee, Y.H.; Lee, Y.S. Comparison of Cytotoxic Effects of Pentagalloylglucose, Gallic Acid, and Its Derivatives against Human Cancer MCF-7 and MDA MB-231 Cells. Bull. Korean Chem. Soc. 2014, 35, 987–988. [Google Scholar] [CrossRef]
- Pei, Y.; Chen, Z.-P.; Ju, H.-Q.; Komatsu, M.; Ji, Y.; Liu, G.; Guo, C.; Zhang, Y.-J.; Yang, C.-R.; Wang, Y.-F. Autophagy Is Involved in Anti-Viral Activity of Pentagalloylglucose (PGG) against Herpes Simplex Virus Type 1 Infection in Vitro. Biochem. Biophys. Res. Commun. 2011, 405, 186–191. [Google Scholar] [CrossRef]
- Zhao, J.; Li, P.; Xia, T.; Wan, X. Exploring Plant Metabolic Genomics: Chemical Diversity, Metabolic Complexity in the Biosynthesis and Transport of Specialized Metabolites with the Tea Plant as a Model. Crit. Rev. Biotechnol. 2020, 40, 667–688. [Google Scholar] [CrossRef]
- Roepke, J.; Bozzo, G.G. Arabidopsis Thaliana β-Glucosidase BGLU15 Attacks Flavonol 3-O-β-Glucoside-7-O-α-Rhamnosides. Phytochemistry 2015, 109, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; S Parmar, V.; K Prasad, A.; Saso, L. Coumarins as Antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, E.; Breen, S. Interplay between Phytohormone Signalling Pathways in Plant Defence–Other than Salicylic Acid and Jasmonic Acid. Essays Biochem. 2022, 66, 657–671. [Google Scholar]
- Gomes, G.L.B.; Scortecci, K.C. Auxin and Its Role in Plant Development: Structure, Signalling, Regulation and Response Mechanisms. Plant Biol. 2021, 23, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Swarup, R.; Bhosale, R. Developmental Roles of AUX1/LAX Auxin Influx Carriers in Plants. Front. Plant Sci. 2019, 10, 1306. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Z.; Yao, X.; Chen, J.; Chen, X.; Zhou, H.; Lou, Y.; Ming, F.; Jin, Y. Genome-Wide Identification and Characterization of Small Auxin-up RNA (SAUR) Gene Family in Plants: Evolution and Expression Profiles during Normal Growth and Stress Response. BMC Plant Biol. 2021, 21, 4. [Google Scholar] [CrossRef]
- Wan, L.; Huang, Q.; Ji, X.; Song, L.; Zhang, Z.; Pan, L.; Fu, J.; Elbaiomy, R.G.; Eldomiaty, A.S.; Rather, S.A. RNA Sequencing in Artemisia Annua L Explored the Genetic and Metabolic Responses to Hardly Soluble Aluminum Phosphate Treatment. Funct. Integr. Genom. 2023, 23, 141. [Google Scholar] [CrossRef]
- Laffont, C.; Rey, T.; André, O.; Novero, M.; Kazmierczak, T.; Debellé, F.; Bonfante, P.; Jacquet, C.; Frugier, F. The CRE1 Cytokinin Pathway Is Differentially Recruited Depending on Medicago Truncatula Root Environments and Negatively Regulates Resistance to a Pathogen. PLoS ONE 2015, 10, e0116819. [Google Scholar] [CrossRef]
- Deng, Y.; Dong, H.; Mu, J.; Ren, B.; Zheng, B.; Ji, Z.; Yang, W.-C.; Liang, Y.; Zuo, J. Arabidopsis Histidine Kinase CKI1 Acts Upstream of Histidine Phosphotransfer Proteins to Regulate Female Gametophyte Development and Vegetative Growth. Plant Cell 2010, 22, 1232–1248. [Google Scholar] [CrossRef]
- Wu, K.; Xu, H.; Gao, X.; Fu, X. New Insights into Gibberellin Signaling in Regulating Plant Growth–Metabolic Coordination. Curr. Opin. Plant Biol. 2021, 63, 102074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Z.; Luo, S.; Li, X.; Lyu, J.; Liu, Z.; Wan, Z.; Yu, J. Genome-Wide Identification and Expression Analysis of the Cucumber PP2C Gene Family. BMC Genomics 2022, 23, 563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-L.; Hoang, N.-H.; Tao, F.; Fu, T.-T.; Guo, S.-J.; Guo, C.-M.; Zhou, C.-B.; Thanh, T.-L.; Buensanteai, K. Transcriptomics and Phytohormone Metabolomics Provide Comprehensive Insights into the Response Mechanism of Tea against Blister Blight Disease. Sci. Hortic. 2024, 324, 112611. [Google Scholar] [CrossRef]
- Geraats, B.P.J.; Bakker, P.A.H.M.; Lawrence, C.B.; Achuo, E.A.; Höfte, M.; Van Loon, L.C. Ethylene-Insensitive Tobacco Shows Differentially Altered Susceptibility to Different Pathogens. Phytopathology 2003, 93, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, L.; Zhao, Z.; Li, P.; Tan, S. Chitosan Oligosaccharide as a Plant Immune Inducer on the Passiflora Spp.(Passion Fruit) CMV Disease. Front. Plant Sci. 2023, 14, 1131766. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Ni, Y.; Liu, X.; Zhao, H.; Chen, Y.; Jia, M.; Liu, M.; Liu, H.; Tian, B. The Mechanism of Sesame Resistance against Macrophomina phaseolina Was Revealed via a Comparison of Transcriptomes of Resistant and Susceptible Sesame Genotypes. BMC Plant Biol. 2021, 21, 159. [Google Scholar] [CrossRef]
- Ren, R.; Wei, Y.; Ahmad, S.; Jin, J.; Gao, J.; Lu, C.; Zhu, G.; Yang, F. Identification and Characterization of NPR1 and PR1 Homologs in Cymbidium Orchids in Response to Multiple Hormones, Salinity and Viral Stresses. Int. J. Mol. Sci. 2020, 21, 1977. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Zhang, A.; Guan, Y.; Jiao, J.; Ghanim, M.; Zhang, Y.; He, X.; Shi, R. Comparative Metabolome and Transcriptome Analyses Reveal Differential Enrichment of Metabolites with Age in Panax notoginseng Roots. Plants 2024, 13, 1441. https://doi.org/10.3390/plants13111441
Yan X, Zhang A, Guan Y, Jiao J, Ghanim M, Zhang Y, He X, Shi R. Comparative Metabolome and Transcriptome Analyses Reveal Differential Enrichment of Metabolites with Age in Panax notoginseng Roots. Plants. 2024; 13(11):1441. https://doi.org/10.3390/plants13111441
Chicago/Turabian StyleYan, Xinru, Ao Zhang, Yiming Guan, Jinlong Jiao, Murad Ghanim, Yayu Zhang, Xiahong He, and Rui Shi. 2024. "Comparative Metabolome and Transcriptome Analyses Reveal Differential Enrichment of Metabolites with Age in Panax notoginseng Roots" Plants 13, no. 11: 1441. https://doi.org/10.3390/plants13111441





