Effects of Biochar and Straw Amendment on Soil Fertility and Microbial Communities in Paddy Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental Design
2.2. Sample Collection and Determination
2.3. Data Analysis
3. Results
3.1. Effect of Various Treatments on Soil Chemical Properties in Paddy Soils
3.2. Effect of the Various Treatments on Three-Dimensional Fluorescence Spectral Characteristics of Soil DOM
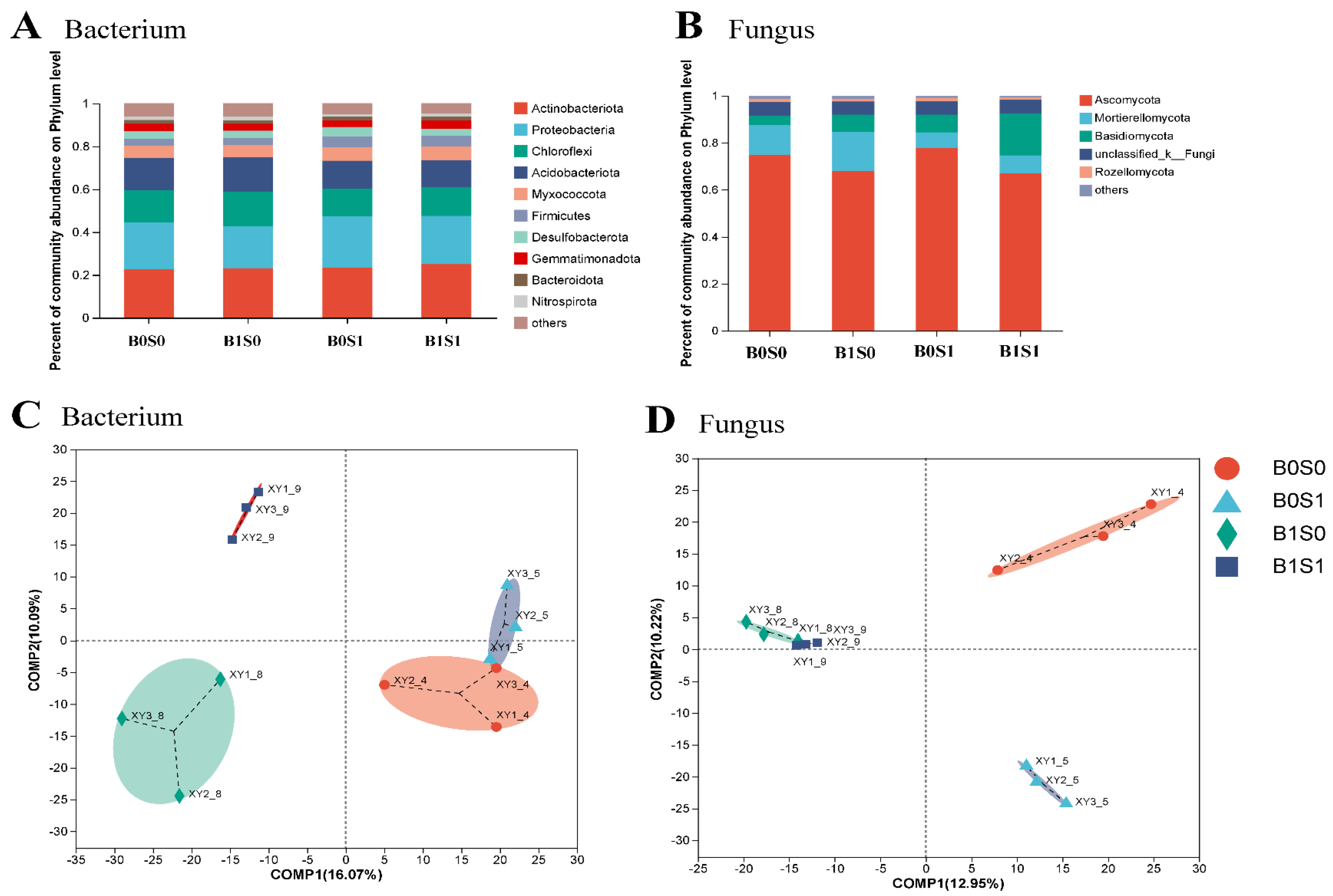
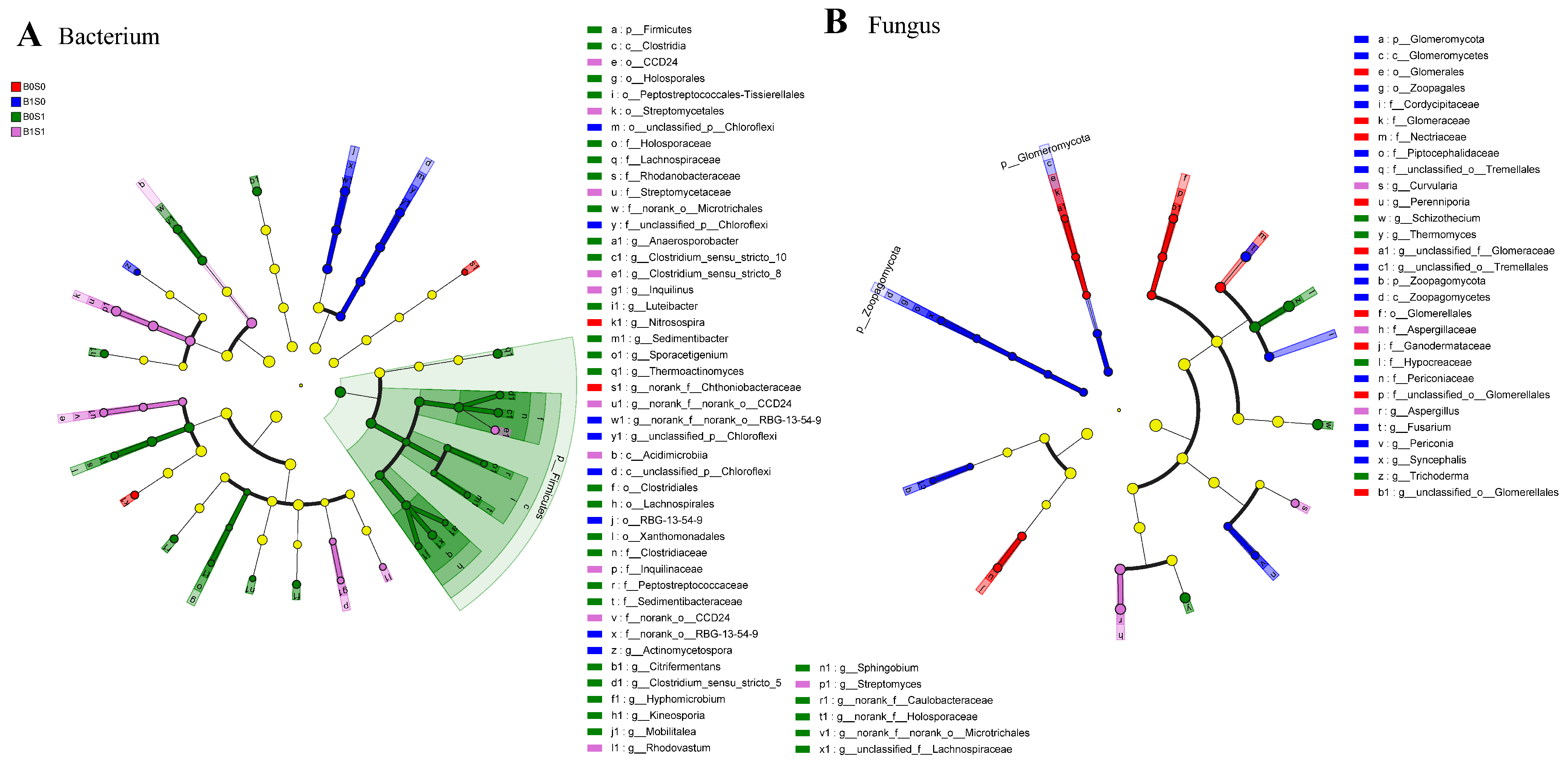
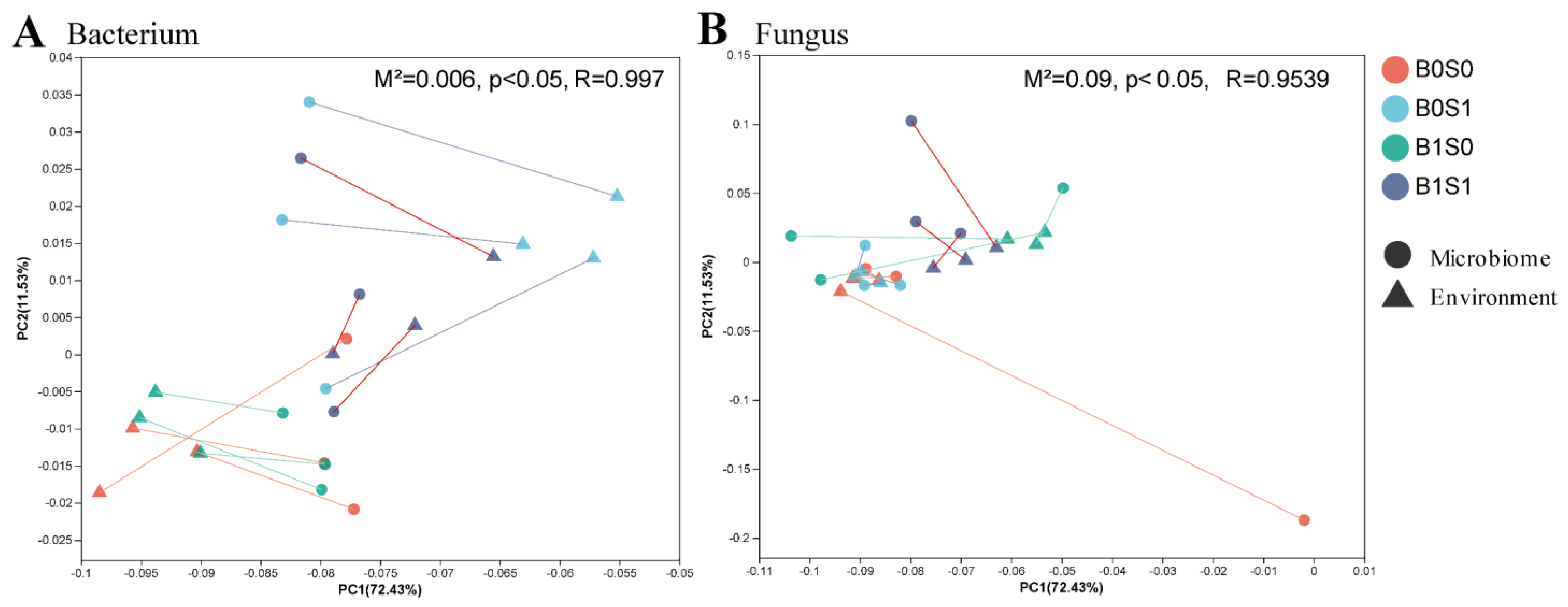
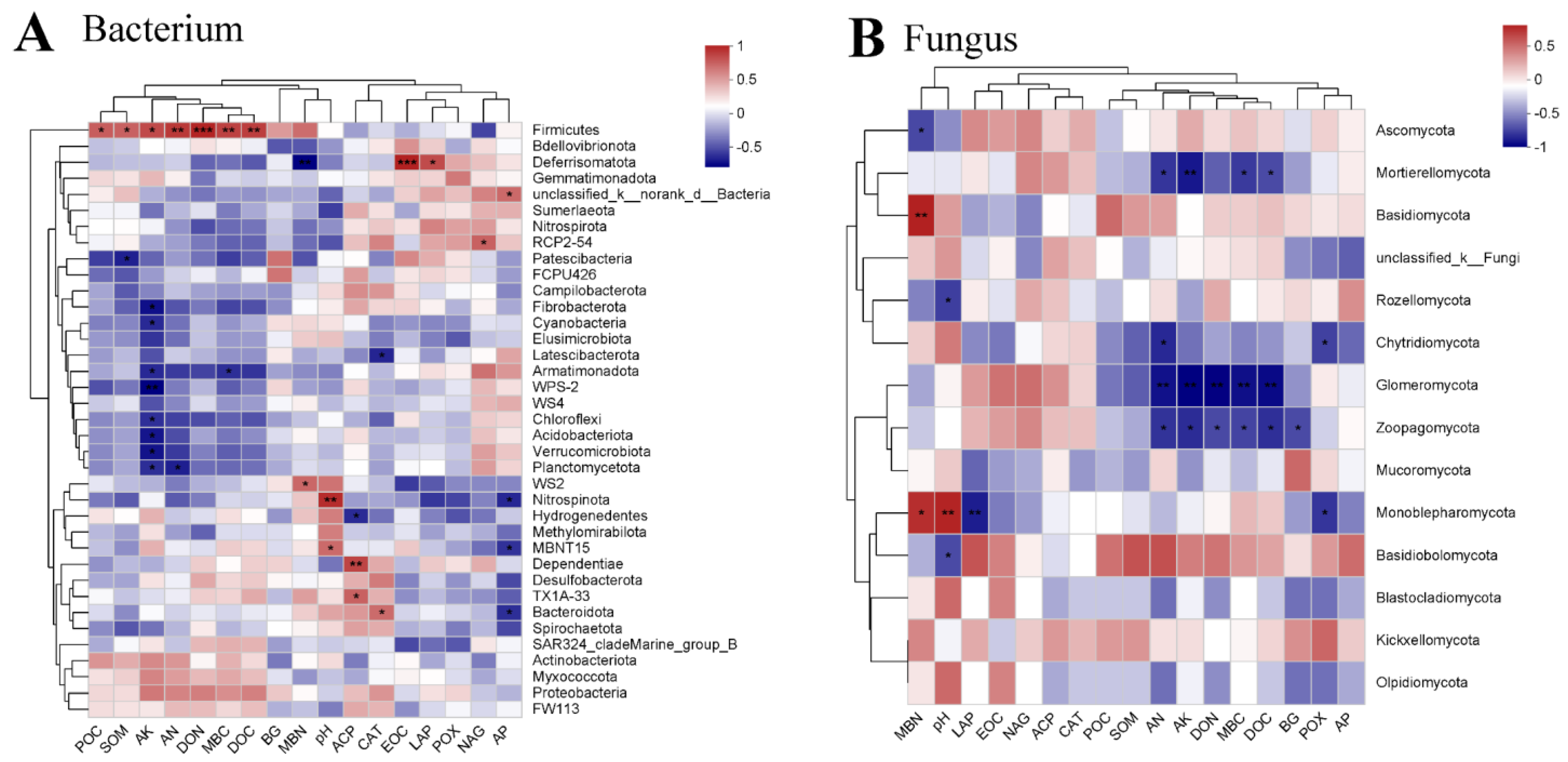
3.3. Effect of the Various Treatments on Soil Bacteria and Fungi in Paddy Soils
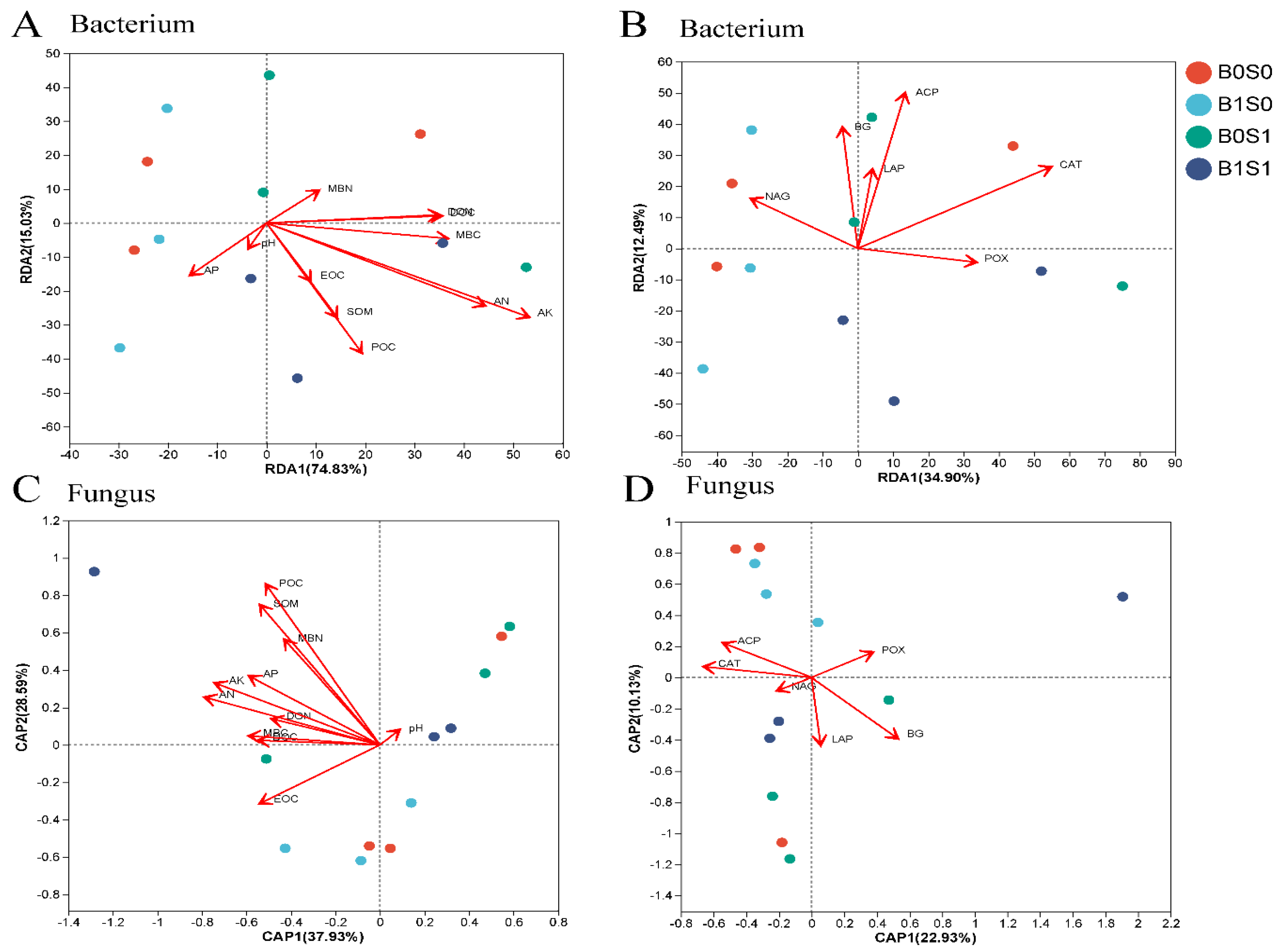
3.4. The Relationship between Soil Environment and Microorganisms under Different Treatments
4. Discussion
4.1. Effects of Biochar and Straw on the Soil Chemical Properties in Paddy Soils
4.2. Effects of Biochar and Straw on Soil 3D Fluorescence Spectral Characteristics of Soil DOM in Paddy Soils
4.3. Effect of Various Treatments on Soil Microorganisms in Paddy Soils
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berendse, F.; van Ruijven, J.; Jongejans, E.; Keesstra, S. Loss of Plant Species Diversity Reduces Soil Erosion Resistance. Ecosystems 2015, 18, 881–888. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, L.; Guo, J.; Ray, J.L.; He, J. Impact of long-term fertilization practices on the abundance and composition of soil bacterial communities in Northeast China. Appl. Soil Ecol. 2010, 46, 119–124. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Li, Y.X.; Riaz, M.; Xia, H.; Wang, J.Y.; Wang, X.L.; Jiang, C.C. Effect of biochar and nitrogen fertilizers on maize seedling growth and enzyme activity relating to nitrification. Soil Use Manag. 2023, 39, 1467–1476. [Google Scholar] [CrossRef]
- Zhou, K.; Cheng, T.; Zhu, Y.; Cao, W.; Ustin, S.L.; Zheng, H.; Yao, X.; Tian, Y. Assessing the Impact of Spatial Resolution on the Estimation of Leaf Nitrogen Concentration Over the Full Season of Paddy Rice Using Near-Surface Imaging Spectroscopy Data. Front. Plant Sci. 2018, 9, 964. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Gao, J.; Peng, F.; Gao, P. Long-term combined application of manure and chemical fertilizer sustained higher nutrient status and rhizospheric bacterial diversity in reddish paddy soil of Central South China. Sci. Rep. 2018, 8, 16554. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Hu, X.; Ma, J.; Ye, J.; Sun, W.; Wang, Q.; Lin, H. Effects of long-term organic material applications on soil carbon and nitrogen fractions in paddy fields. Soil Tillage Res. 2020, 196, 104483. [Google Scholar] [CrossRef]
- Liu, B.; Xia, H.; Jiang, C.; Riaz, M.; Yang, L.; Chen, Y. 14 year applications of chemical fertilizers and crop straw effects on soil labile organic carbon fractions, enzyme activities and microbial community in rice-wheat rotation of middle China. Sci. Total Environ. 2022, 841, 156608. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; He, J.; Li, H.; Wang, Q.; Lu, C.; Zheng, K.; Liu, W.; Zhao, H.; Lou, S. Effect of straw retention on crop yield, soil properties, water use efficiency and greenhouse gas emission in China: A meta-analysis. Int. J. Plant Prod. 2019, 13, 347–367. [Google Scholar] [CrossRef]
- Zhang, W.M.; Chen, W.F.; Meng, J.; Jin, L.; Guo, W.; Zhao, H.L. Study of Straw-Biochar on Utilization Potential, Industry Model and Developing Strategy in Northeast China. Sci. Agric. Sin. 2019, 52, 2406–2424. (In Chinese) [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Tang, X.; Yan, L.; El-Desouki, Z.; Li, Y.X.; Wang, X.L.; Jiang, C.C. Insight into mechanisms of biochar-fertilizer induced of microbial community and microbiology of nitrogen cycle in acidic soil. J. Environ. Manag. 2023, 336, 117602. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; de Pasquale, C. Effect of biochar on the physical and structural properties of a sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar addition to agricultural soil increased CH4 uptake and water holding capacity-Results from a short-term pilot field study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Fu, Q.; Zhao, H.; Li, H.; Li, T.; Hou, R.; Liu, D.; Ji, Y.; Gao, Y.; Yu, P. Effects of biochar application during different periods on soil structures and water retention in seasonally frozen soil areas. Sci. Total Environ. 2019, 694, 133732. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.Y.; Tang, G.M.; Ge, C.H.; Ma, H.G.; Zhang, Z.D.; Xu, W.L. Effects of straw incorporation modes on microbial activity and functional diversity in sandy soil. Chin. J. Eco-Agric. 2016, 24, 489–498. (In Chinese) [Google Scholar]
- Shi, Z.; Deng, C.; Luo, S.S.; Wang, S.J.; Gao, Q. Effects of Straw and Biochar on the Activities of Key Enzymes in Carbon, Nitrogen and Phosphorus Cycle of Aggregates in Northeast Black Soil. Chin. J. Soil Sci. 2023, 54, 1128–1136. (In Chinese) [Google Scholar]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Liu, E.; Teclemariam, S.G.; Yan, C.; Yu, J.; Gu, R.; Liu, S.; He, W.; Liu, Q. Long-term effects of no-tillage management practice on soil organic carbon and its fractions in the northern China. Geoderma 2014, 213, 379–384. [Google Scholar] [CrossRef]
- Xu, J.H.; Sun, Y.; Gao, L.; Cui, X.Y. A review of the factors influencing soil organic carbon stability. Chin. J. Eco-Agric. 2018, 26, 222–230. (In Chinese) [Google Scholar]
- Jin, Z.; Shah, T.; Zhang, L.; Liu, H.; Peng, S.; Nie, L. Effect of straw returning on soil organic carbon in rice-wheat rotation system: A review. Food Energy Secur. 2020, 9, e200. [Google Scholar] [CrossRef]
- Malhi, S.S.; Lemke, R.; Wang, Z.H.; Chhabra, B.S. Tillage, nitrogen and crop residue effects on crop yield, nutrient uptake, soil quality, and greenhouse gas emissions. Soil Tillage Res. 2006, 90, 171–183. [Google Scholar] [CrossRef]
- Hou, J.X.; Zhang, S.M.; Yuan, J.C. Effects of maize straw-derived organic materials on improving soil nutrient availability and enzyme activities in a mollisol. J. Plant Nutr. Fertil. 2021, 27, 610–618. (In Chinese) [Google Scholar]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Chang. Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Busch, D.; Kammann, C.; Grünhage, L. Simple biotoxicity tests for evaluation of carbonaceous soil additives: Establishment and reproducibility of four test procedures. J. Environ. Qual. 2012, 41, 1023–1032. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G. Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: Chemical and physical characterization. Ind. Crops Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Zi, H.B.; Hu, L.; Wang, C.T. Responses of soil bacterial community and enzyme activity to experimental warming of an alpine meadow: Effects of warming on soil microorganisms. Eur. J. Soil Sci. 2018, 69, 429–438. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Saba, B.; Yan, L.; Li, X.Y.; Wang, J.Y.; Jiang, C.C. Assessing the impact of biochar on microbes in acidic soils: Alleviating the toxicity of aluminum and acidity. J. Environ. Manag. 2023, 345, 118796. [Google Scholar] [CrossRef]
- Bao, S.D. Soil And Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Deforest, J.L. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and l-DOPA. Soil Biol. Biochem. 2009, 41, 1180–1186. [Google Scholar] [CrossRef]
- Liu, C.; Bai, X.D.; Zhao, H.C.; Huang, Z.H.; Liu, S.T.; Lu, H.B.; Liu, Z.G.; Liu, X.L. The effect mechanism of spring maize straw returning method on soil DOM spectral characteristics in cold and arid regions of China. Ecol. Environ. Sci. 2023, 32, 1419–1432. (In Chinese) [Google Scholar]
- Chen, H.L.; Zhou, J.M.; Xiao, B.H. Characterization of dissolved organic matter derived from rice straw at different stages of decay. J. Soils Sediments 2010, 10, 915–922. [Google Scholar] [CrossRef]
- Li, Q.; Gu, F.; Zhou, Y.; Xu, T.; Wang, L.; Zuo, Q.; Xiao, L.; Liu, J.; Tian, Y. Changes in the Impacts of Topographic Factors, Soil Texture, and Cropping Systems on Topsoil Chemical Properties in the Mountainous Areas of the Subtropical Monsoon Region from 2007 to 2017: A Case Study in Hefeng, China. Int. J. Environ. Res. Public Health 2021, 18, 832. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Tian, D.; Liu, J.; Lv, S.; He, X.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to stover and stover-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Zhao, H.L.; Abdul, G.S.; Li, S.; Chen, Y.; Shi, J.; Zhang, X. Effect of straw return mode on soil aggregation and aggregate carbon content in an annual maize-wheat double cropping system. Soil Tillage Res. 2018, 175, 178–186. [Google Scholar] [CrossRef]
- Li, C.; Li, X.P. Effect of biochar preparation and its application amount on soil carbon pool and plant growth. J. South. Agric. 2015, 46, 1786–1791. (In Chinese) [Google Scholar]
- Robertson, S.J.; Rutherford, P.M.; López-Gutiérrez Juan, C.; Massicotte, H.B. Biochar enhances seedling growth and alters root symbioses and properties of sub-boreal forest soils. Can. J. Soil Sci. 2012, 92, 329–340. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Zhang, M.Y.; Liu, B.; Li, Y.; El-Desouki, Z. Biochar-N fertilizer interaction increases N utilization efficiency by modifying soil C/N component under N fertilizer deep placement modes. Chemosphere 2022, 286, 131594. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, W.; Mao, Y.; Yang, T.; Chen, Y. Biochar Addition Alters C: N: P Stoichiometry in Moss Crust-Soil Continuum in Gurbantünggüt Desert. Plants 2022, 11, 814. [Google Scholar] [CrossRef]
- Niu, Z.Y.; Yan, S.; Guo, Q.Q. Effects of biochar on yield an quality of flue-cured tobacco and nutrients and carbon pool in two typical soils planted with tobacco. Chin. J. Soil Sci. 2017, 48, 155–161. (In Chinese) [Google Scholar]
- Tomczyk, A.; Szewczuk-Karpisz, K. Effect of Biochar Modification by Vitamin C, Hydrogen Peroxide or Silver Nanoparticles on Its Physicochemistry and Tetracycline Removal. Materials 2022, 15, 5379. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.H.; Zhao, Y.; Wei, D. Insight into transformation of dissolved organic matter in the Heilongjiang River. Environ. Sci. Pollut. Res. 2019, 26, 3340–3349. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.F.; Zhou, H.J.; Yu, X.N.; Zhang, X.F.; Li, Z.P.; Fu, Z.Y.; Meng, Q. Physiochemical properties and yields of corn-stalk-biochar under different pyrolyzed temperatures. J. Plant Nutr. Fertil. 2017, 23, 1268–1275. (In Chinese) [Google Scholar]
- Zhang, H.; Voroney, R.; Price, G. Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol. Biochem. 2015, 83, 19–28. [Google Scholar] [CrossRef]
- Wang, J.X.; Riaz, M.; Babar, S.; Xia, H.; Xia, X.Y.; Wang, J.Y.; Jiang, C.C. Iron-modified biochar reduces nitrogen loss and improves nitrogen retention in Luvisols by adsorption and microbial regulation. Sci. Total Environ. 2023, 879, 163196. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lv, J.; Xie, J.; Yu, J.; Li, J.; Zhang, J.; Tang, C.; Niu, T.; Patience, B.E. Effect of slow-release fertilizer on soil fertility and growth and quality of wintering Chinese chives (Allium tuberm Rottler ex Spreng.) in greenhouses. Sci. Rep. 2021, 11, 8070. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Z.; Yan, X.; Wang, B.; Liu, R.T.; An, H. Effects of desertification on soil nutrients and extracellular enzyme activities in desert grassland. Ecol. Environ. Sci. 2018, 27, 1082–1088. (In Chinese) [Google Scholar]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Farooq, M.; Nawaz, A.; Al-Sadi, A.M.; Solaiman, Z.M.; Alghamdi, S.S.; Ammara, U.; Ok, Y.S.; Siddique, K.H.M. Biochar for crop production: Potential benefits and risks. J. Soils Sediments 2017, 17, 685–716. [Google Scholar] [CrossRef]
- He, Y.H.; Zhou, X.H.; Jiang, L.L.; Li, M.; Du, Z.G.; Zhou, G.Y.; Shao, J.J.; Wang, X.H.; Xu, Z.H.; Bai, S.H.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. GCB Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Zhang, A.F.; Cheng, G.; Hussain, Q.; Zhang, M.; Feng, H.; Dyck, M.; Sun, B.H.; Zhao, Y.; Chen, H.X.; Chen, J.; et al. Contrasting effects of straw and straw–derived biochar application on net global warming potential in the Loess Plateau of China. Field Crops Res. 2017, 205, 45–54. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xiong, Z.Q.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Bernd, M.; Karsten, K. Controls of bioavailability and biodegradability of dissolved organic matter in soils. Geoderma 2003, 113, 211–235. [Google Scholar]
- Welikala, D.; Robinson, B.H.; Moltchanova, E. Soil cadmium mobilisation by dissolved organic matter from soil amendments. Chemosphere 2021, 271, 129536. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Pei, D.; Zhang, X.; Lai, Q.; He, F.; Fu, C.; Liu, J.; Li, W. The Distribution of DOM in the Wanggang River Flowing into the East China Sea. Int. J. Environ. Res. Public Health 2022, 19, 9219. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, K.; Han, G.; Wang, H.; Tan, N.; Yan, Z. Integrated diagnosis and time-series sensitivity evaluation of nutrient deficiencies in medicinal plant (Ligusticum chuanxiong Hort.) based on UAV multispectral sensors. Front. Plant Sci. 2023, 13, 1092610. [Google Scholar] [CrossRef] [PubMed]
- Biederbeck, V.O.; Janzen, H.H.; Campbell, C.A. Labile soil organic matter as influenced by cropping practices in an arid environment. Soil Biol. Biochem. 1994, 26, 1647–1656. [Google Scholar] [CrossRef]
- Li, H.Y.; Lin, T.; Wang, Z.J.; Zhao, H.C.; Zheng, J.P.; Lv, Y.D.; Qian, Y.D.; Fan, M.Y. Effects of biochar continuous returning on soil nutrients, enzyme activities and humus components in soda saline-alkali paddy field soil. Agric. Res. Arid. Areas 2022, 40, 135–142. (In Chinese) [Google Scholar]
- Zhang, H.Y.; Yang, Q.X.; Yang, Q. Compositional characteristics and photochemical activity of dissolved organic matter derived from straw in aquatic environment. China Environ. Sci. 2020, 40, 2521–2528. (In Chinese) [Google Scholar]
- Wu, Y.P.; Liu, T.; Peng, Q.A. Greenhouse gas emissions in red soil as influenced by different C/N residues under nitrogen applications. J. Agro-Environ. Sci. 2014, 33, 2053–2062. (In Chinese) [Google Scholar]
- Mitchell, P.J.; André, J.S.; Soong, R.; Simpson, M.J. Shifts in microbial community and water-extractable organic matter composition with biochar amendment in a temperate forest soil. Soil Biol. Biochem. 2015, 81, 244–254. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Cai, Z.C.; Huang, X.Q.; Zhao, J. Principles and Practice of Building Healthy Microbial Community to Control Soil-borne Crop Disease in Intensive Agriculture. Acta Pedol. Sin. 2023, 60, 1213–1220. (In Chinese) [Google Scholar]
- Zhou, X.; Liu, L.L.; Zhao, J. High carbon resource diversity enhances the certainty of successful plant pathogen and disease control. New Phytol. 2023, 237, 1333–1346. [Google Scholar] [CrossRef]
- Putten, W.H.V.D.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T. Plant–soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Tan, C.L.; Liu, Y.; Huang, X.G.; Zhang, J.Y.; Luo, W.H. Effect of biochar on soil microbial metabolic activities. Chin. J. Eco-Agric. 2022, 30, 333–342. (In Chinese) [Google Scholar]
- Zhao, Z.; Ma, Y.; Feng, T. Assembly processes of abundant and rare microbial communities in orchard soil under a cover crop at different periods. Geoderma 2022, 406, 115543. [Google Scholar] [CrossRef]
- Lyu, B.C.; Sun, H.; Qian, J.Q.; Liang, H.; Zhu, J.P.; Zhang, Q.S.; Shan, C.; Zhang, Y.Y. Interaction between root exudates of medicinal plants and rhizosphere microorganisms and its application in ecological planting of Chinese medicinal materials. China J. Chin. Mater. Med. 2024, 49, 2128–2137. (In Chinese) [Google Scholar]
- Xu, M.; Xian, Y.; Wu, J.; Gu, Y.; Yang, G.; Zhang, X. Effect of biogas slurry addition on soil properties, yields, and bacterial composition in the rice-rape rotation ecosystem over 3 years. J. Soils Sediments 2019, 19, 2534–2542. [Google Scholar] [CrossRef]
- Górska, E.B.; Stępien, W.; Cunha, A. Microbial diversity as an indicator of a diversified cropping system for luvisoils in a moderate climate. Case study–Long term experiments from Poland. Ecol. Indic. 2022, 141, 109–133. [Google Scholar] [CrossRef]
- Che, J.; Zhao, X.Q.; Zhou, X.; Jia, Z.J.; Shen, R.F. High pH-enhanced soil nitrification was associated with ammonia-oxidizing bacteria rather than archaea in acidic soils. Appl. Soil Ecol. 2015, 85, 2129. [Google Scholar] [CrossRef]
- Tang, H.; Yu, M.; Wang, Y.; Han, X.; Wei, D. Effects of long-term fertilization on nifH gene diversity in agricultural black soil. Afr. J. Microbiol. Res. 2012, 6, 2659–2666. [Google Scholar]
- Gao, G.; Yan, L.; Tong, K.Q.; Yu, H.L.; Lu, M.; Wang, L.; Niu, Y.S. The potential and prospects of modified biochar for comprehensive management of salt-affected soils and plants: A critical review. Sci. Total Environ. 2024, 912, 169618. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Volpe, V.; Chitarra, W.; Cascone, P. The association with two different Arbuscular mycorrhizal fungi differently affects water stress tolerance in tomato. Front. Plant Sci. 2018, 9, 1480. [Google Scholar] [CrossRef]
| Treatment | pH | SOM (g/kg) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) |
|---|---|---|---|---|---|
| B0S0 | 5.61 ± 0.12 a | 24.66 ± 0.67 c | 97.86 ± 7.08 b | 29.05 ± 3.02 a | 91.04 ± 4.72 b |
| B1S0 | 5.76 ± 0.17 a | 29.47 ± 2.13 b | 92.81 ± 7.12 b | 30.11 ± 3.85 a | 99.36 ± 6.48 b |
| B0S1 | 5.65 ± 0.23 a | 30.92 ± 2.22 ab | 113.49 ± 5.83 a | 30.68 ± 12.07 a | 145.54 ± 20.62 a |
| B1S1 | 5.61 ± 0.15 a | 33.22 ± 2.03 a | 120.23 ± 2.2 a | 34.24 ± 3.87 a | 147.2 ± 10.47 a |
| B | 0.28 | 10.78 * | 0.06 | 0.35 | 0.50 |
| S | 0.32 | 21.43 ** | 39.80 ** | 0.54 | 52.44 ** |
| B*S | 0.86 | 1.35 | 2.98 | 0.10 | 0.22 |
| Treatment | MBN (mg·kg−1) | MBC (mg·kg−1) | DON (mg·kg−1) | DOC (mg·kg−1) | EOC (mg·kg−1) | POC (mg·kg−1) | MBC/MBN | DOC/DON |
|---|---|---|---|---|---|---|---|---|
| B0S0 | 4.59 ± 0.67 a | 174.67 ± 24.57 c | 5.10 ± 0.60 b | 79.65 ± 3.85 c | 21.91 ± 2.13 a | 4.91 ± 0.27 c | 38.11 ± 2.90 ab | 15.71 ± 1.26 a |
| B1S0 | 5.81 ± 0.32 a | 190.75 ± 5.43 c | 5.13 ± 0.48 b | 81.12 ± 1.16 c | 21.08 ± 2.56 a | 8.20 ± 1.43 b | 32.95 ± 2.83 b | 15.92 ± 1.64 a |
| B0S1 | 6.75 ± 1.80 a | 354.43 ± 13.00 a | 7.17 ± 0.50 a | 108.75 ± 2.79 a | 19.79 ± 1.94 a | 7.71 ± 1.25 b | 55.39 ± 16.07 a | 15.23 ± 1.36 a |
| B1S1 | 6.28 ± 1.53 a | 270.97 ± 36.37 b | 6.46 ± 0.08 a | 92.07 ± 6.86 b | 22.98 ± 2.19 a | 11.07 ± 1.33 a | 43.98 ± 6.02 ab | 14.27 ± 1.15 a |
| B | 3.37 | 95.41 ** | 41.18 ** | 67.88 ** | 0.36 | 0.01 * | 7.73 * | 1.85 |
| S | 0.27 | 6.41 * | 1.67 | 9.78 * | 0.01 | 0.86 * | 2.65 | 0.23 |
| B*S | 1.39 | 13.99 ** | 1.96 | 13.93 ** | 1.03 | 2.47 * | 0.38 | 0.56 |
| Treatment | Fluorescence Index (FI) | Biological Index (BIX) | UV254 | The Distribution of Fluorescence Components | ||
|---|---|---|---|---|---|---|
| C1% | C2% | C3% | ||||
| B0S0 | 2.10 ± 0.03 a | 0.62 ± 0.04 a | 0.14 ± 0.02 c | 49.28 ± 1.48 a | 43.38 ± 2.47 a | 7.34 ± 0.99 a |
| B1 S0 | 1.98 ± 0.03 b | 0.55 ± 0.02 a | 0.26 ± 0.01 b | 49.72 ± 2.46 a | 44.43 ± 6.22 a | 9.18 ± 1.85 a |
| B0S1 | 2.06 ± 0.05 ab | 0.57 ± 0.08 a | 0.23 ± 0.02 b | 46.32 ± 8.66 a | 44.41 ± 7.47 a | 9.28 ± 1.20 a |
| B1S1 | 2.02 ± 0.09 ab | 0.55 ± 0.04 a | 0.33 ± 0.01 a | 46.41 ± 4.37 a | 44.34 ± 2.58 a | 9.25 ± 2.61 a |
| B | 5.83 * | 1.81 | 124.12 | 0.01 | 0.03 | 0.78 |
| S | 0.01 | 0.68 | 71.79 ** | 1.15 | 0.02 | 0.95 |
| B*S | 1.24 | 0.89 | 1.19 | 0 | 0.03 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Shen, J.; Riaz, M.; Jiang, C.; Zu, C.; Jiang, C.; Liu, B. Effects of Biochar and Straw Amendment on Soil Fertility and Microbial Communities in Paddy Soils. Plants 2024, 13, 1478. https://doi.org/10.3390/plants13111478
Xia H, Shen J, Riaz M, Jiang C, Zu C, Jiang C, Liu B. Effects of Biochar and Straw Amendment on Soil Fertility and Microbial Communities in Paddy Soils. Plants. 2024; 13(11):1478. https://doi.org/10.3390/plants13111478
Chicago/Turabian StyleXia, Hao, Jia Shen, Muhammad Riaz, Cuncang Jiang, Chaolong Zu, Chaoqiang Jiang, and Bo Liu. 2024. "Effects of Biochar and Straw Amendment on Soil Fertility and Microbial Communities in Paddy Soils" Plants 13, no. 11: 1478. https://doi.org/10.3390/plants13111478
APA StyleXia, H., Shen, J., Riaz, M., Jiang, C., Zu, C., Jiang, C., & Liu, B. (2024). Effects of Biochar and Straw Amendment on Soil Fertility and Microbial Communities in Paddy Soils. Plants, 13(11), 1478. https://doi.org/10.3390/plants13111478







