Effects of Organic Fertilizer Addition to Vegetation and Soil Bacterial Communities in Saline–Alkali-Degraded Grassland with Photovoltaic Panels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
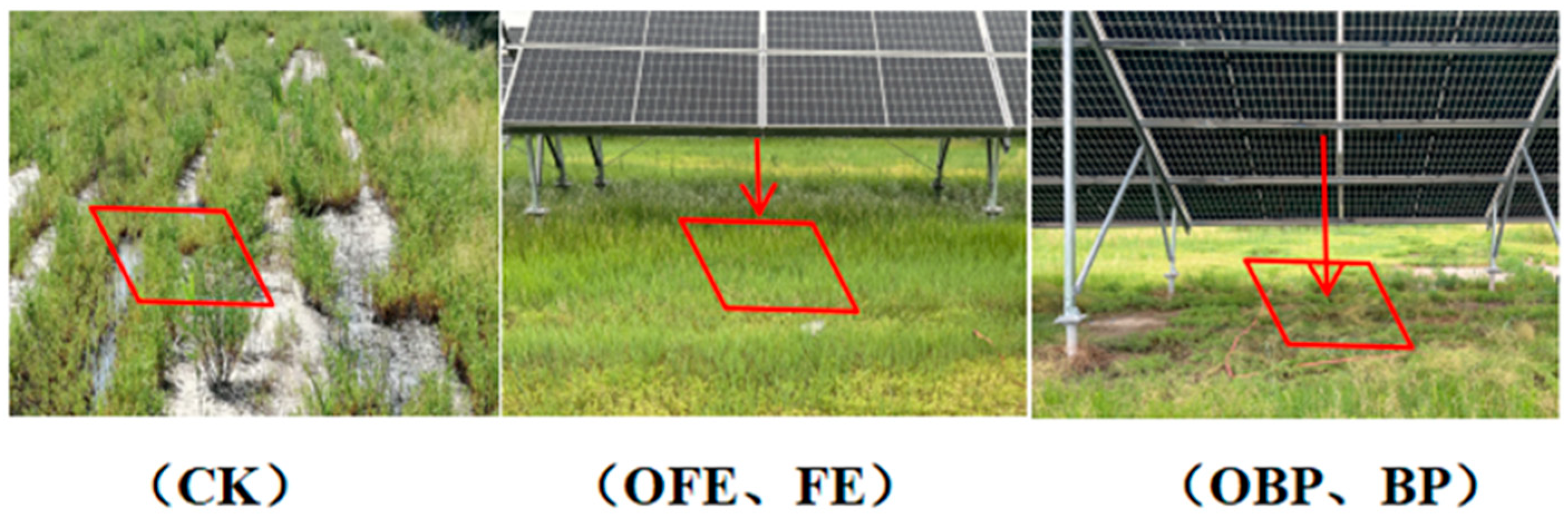
2.2. Experimental Design
2.3. Experimental Methods
2.3.1. Vegetation Determination Method
2.3.2. Soil Sampling and Analysis
2.3.3. Soil DNA Extraction, 16S rRNA Sequencing, and Data Analysis
2.4. Data Processing
3. Results
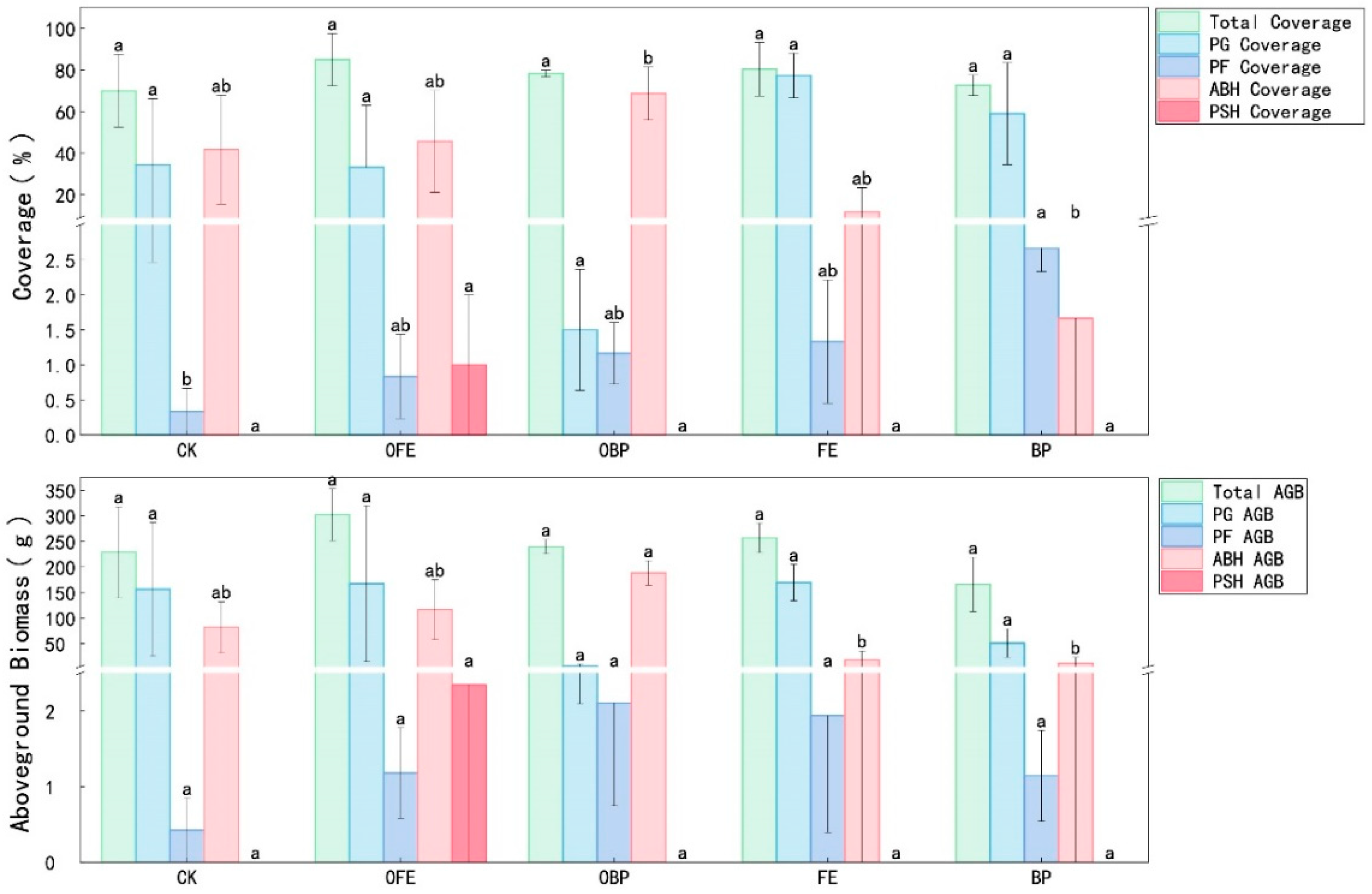
3.1. Effects of Organic Fertilizer Addition on Vegetation Community Composition and Diversity
3.2. Effects of Organic Fertilizer Addition on Soil Physicochemical Properties
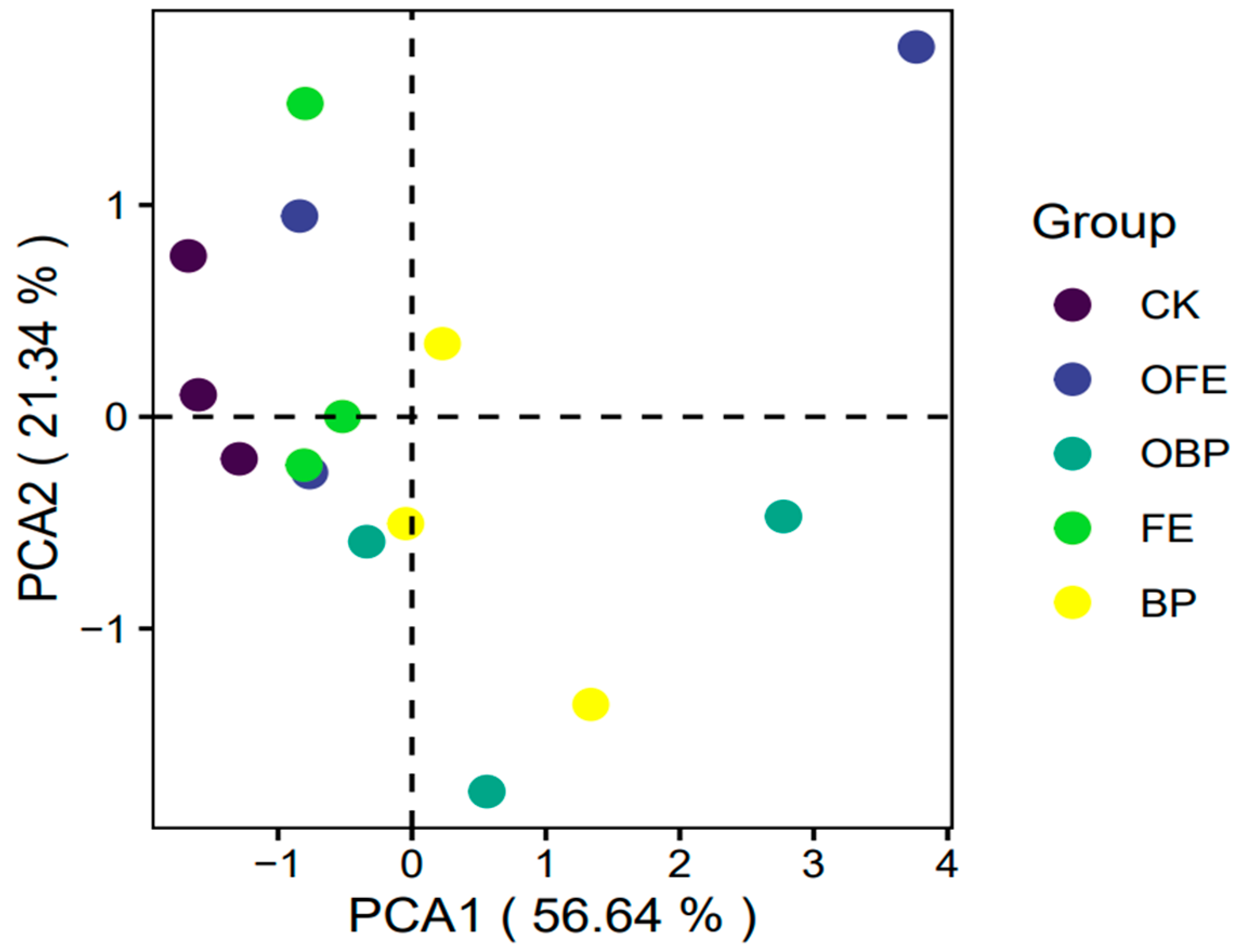
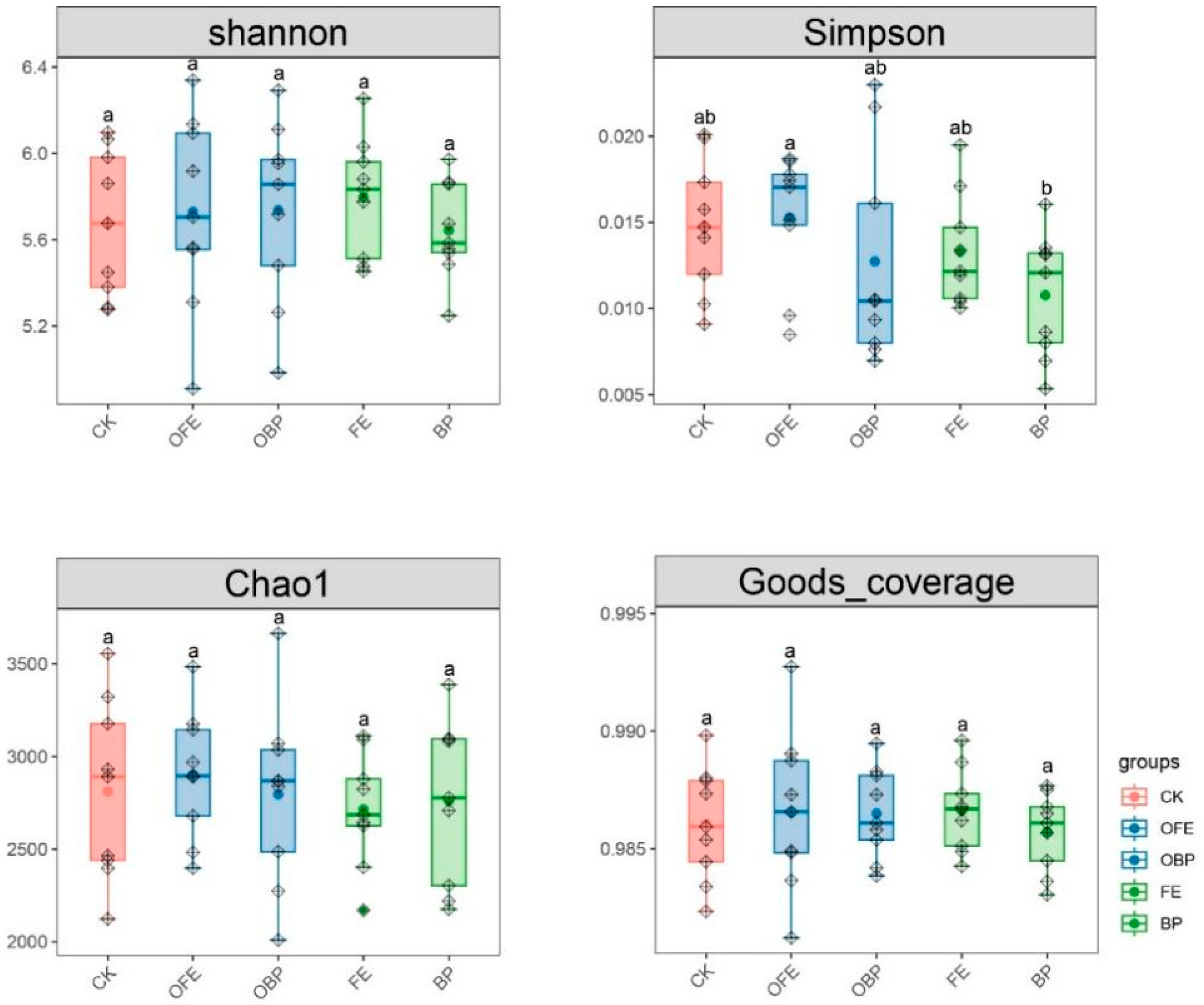
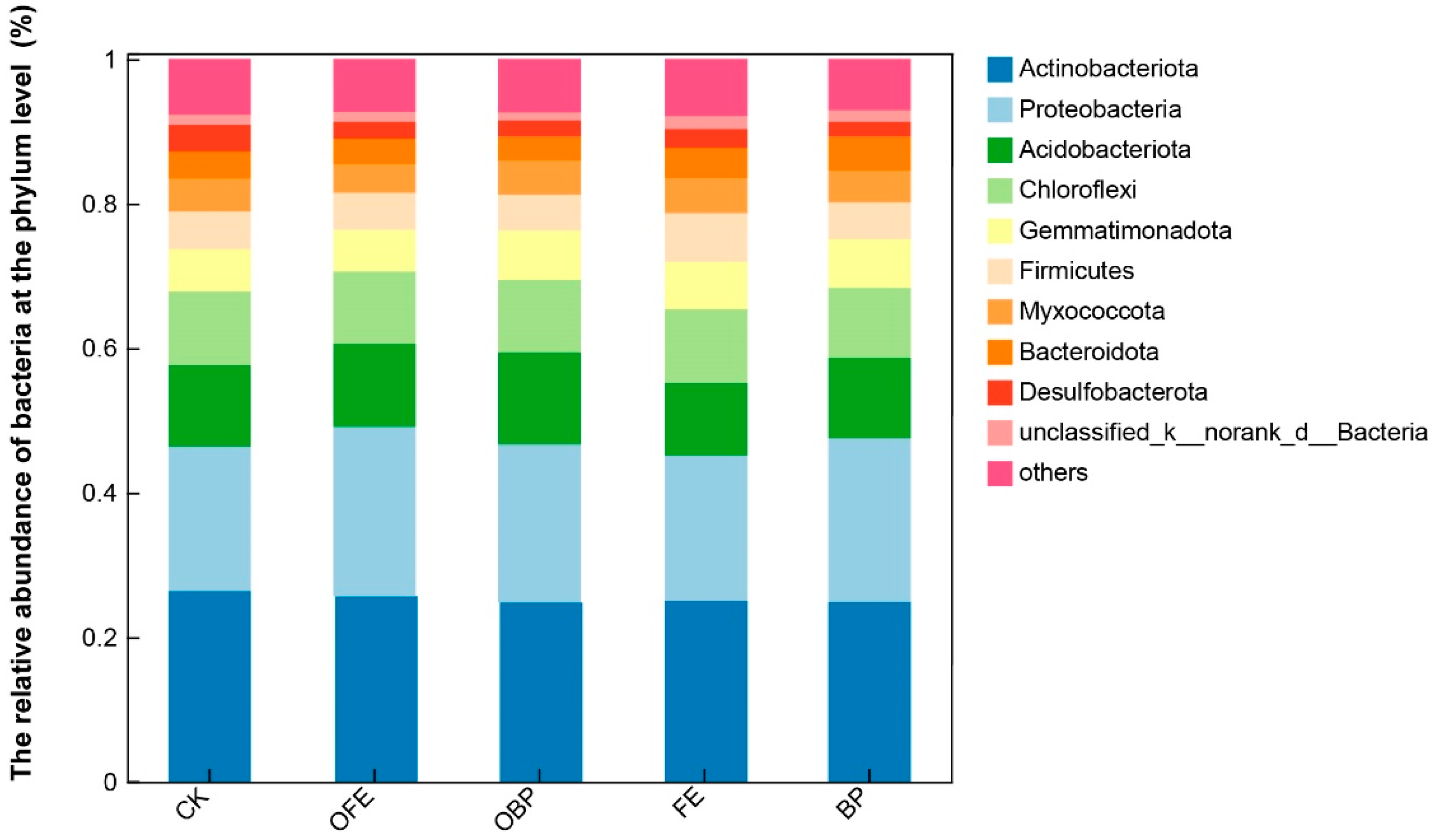
3.3. Effects of Organic Fertilizer Supplementation on Soil Bacterial Community Composition and Diversity
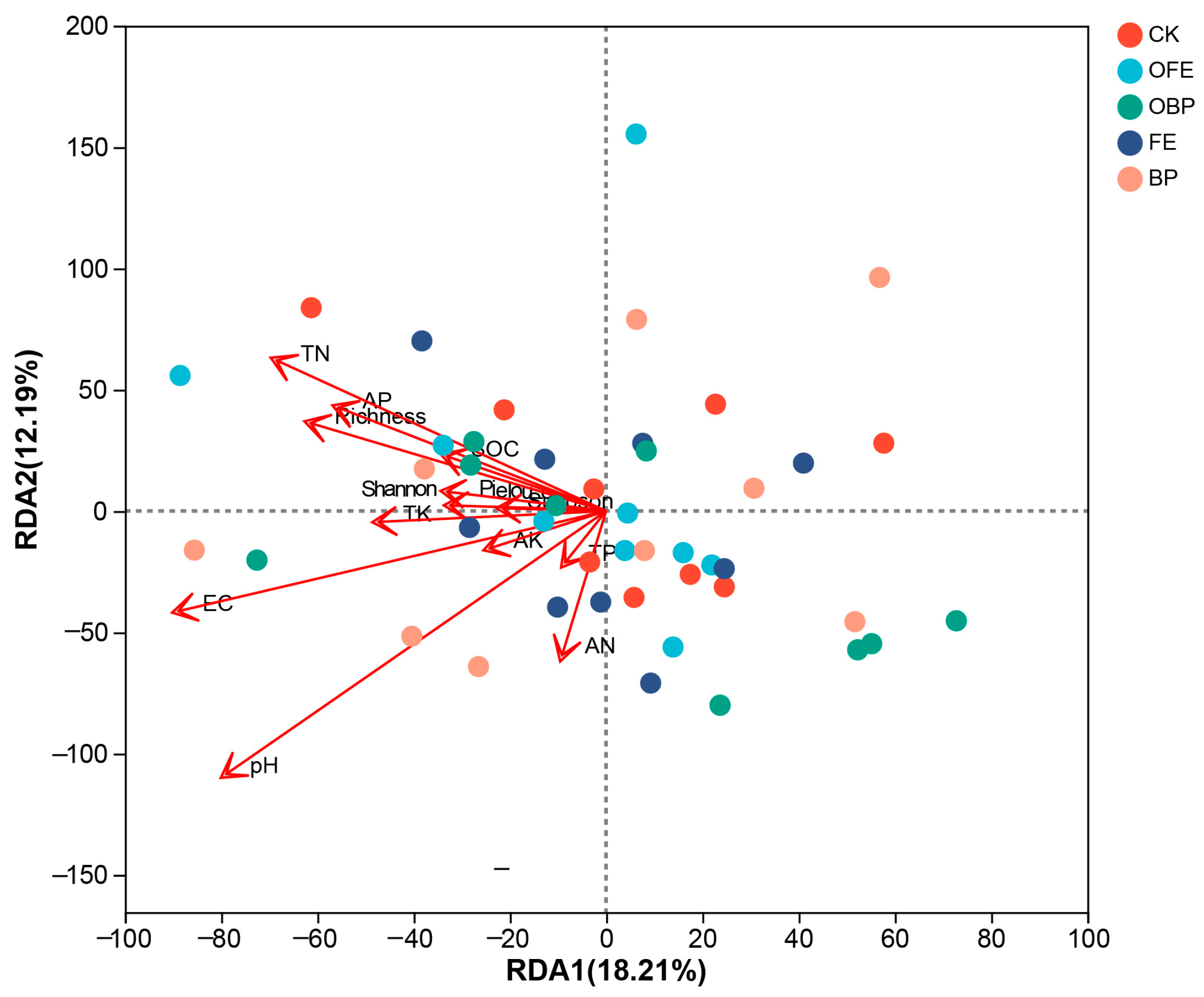
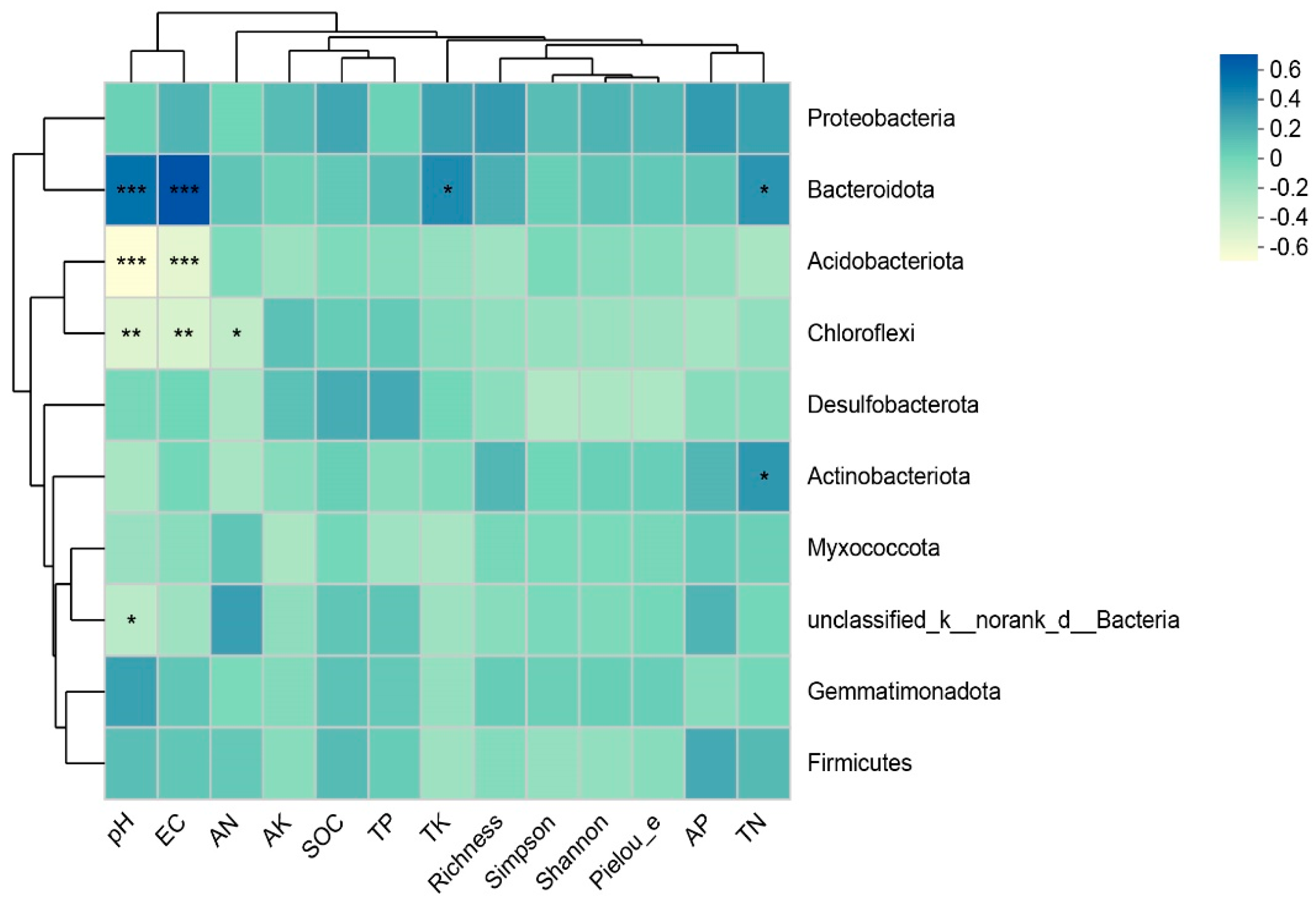
3.4. Relationship between Organic Fertilizer Addition and Vegetation and Soil and Microbial Community
4. Discussion
4.1. Effects of Organic Fertilizer Addition on Vegetation and Soil Physicochemical Properties
4.2. Effect of Organic Fertilizer Addition on Soil Bacterial Community
4.3. Correlation Analysis between Organic Fertilizer Addition and Vegetation, Soil, and Soil Bacteria
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- White, R.P.; Murray, S.; Rohweder, M.; Prince, S.D.; Thompson, K.M. Pilot analysis of global ecosystems: Grassland ecosystems. World Resour. Inst. 2000, 4, 275. [Google Scholar]
- Mannetje, L.T. The Role of Grasslands and Forests as Carbon Stores. Trop. Grassl. 2007, 41, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Z.; Wu, J. Grassland Ecosystem Services: A Systematic Review of Research Advances and Future Directions. Landsc. Ecol. 2020, 35, 793–814. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, T.; Shan, D.; Yan, R.; Zhang, L.; Wang, J.; Wuren, Q. Investigation into the Effects of Different Techniques on the Soil Nutrient Status in Degraded Stipa grandis Grassland. Agronomy 2024, 14, 57. [Google Scholar] [CrossRef]
- Wang, N.; Chen, H. Effect of saline–alkaline stresses on the interspecific competition between Aegilops tauschii and Triticum aestivum. Can. J. Soil Sci. 2023, 103, 462–470. [Google Scholar] [CrossRef]
- Yang, F.; An, F.; Ma, H.; Wang, Z.; Zhou, X.; Liu, Z. Variations on Soil Salinity and Sodicity and Its Driving Factors Analysis under Microtopography in Different Hydrological Conditions. Water 2016, 8, 227. [Google Scholar] [CrossRef]
- Zhang, T.; Zhan, X.Y.; Feng, H. Research advance and prospect of soil enzymes activities in saline-alkali soils. Chin. J. Soil Sci. 2017, 48, 495–500. [Google Scholar] [CrossRef]
- Li, Q. Discussion on the path of China’s energy transformation under the goal of carbon neutrality. China Coal 2021, 47, 1–7. [Google Scholar]
- Wilberforce, T.; Baroutaji, A.; El Hassan, Z.; Thompson, J.; Soudan, B.; Olabi, A.G. Prospects and challenges of concentrated solar photovoltaics and enhanced geothermal energy technologies. Sci. Total Environ. 2019, 659, 851–861. [Google Scholar] [CrossRef]
- Bogdanov, D.; Gulagi, A.; Fasihi, M. Full energy sector transition towards 100% renewable energy supply: Integrating power, heat, transport and industry sectors including desalination. Appl. Energy 2021, 283, 116273. [Google Scholar] [CrossRef]
- Helveston, J.P.; He, G.; Davidson, M.R. Quantifying the cost savings of global solar photovoltaic supply chains. Nature 2022, 612, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, L.; Ma, J.; Zhang, J.C. Economic analysis of user’s grid-connected PV system based on energy storage system. Acta. Energ. Sol. Sin 2012, 33, 1887–1892. [Google Scholar] [CrossRef]
- MacKay, D.J.C. Solar energy in the context of energy use, energy transportation and energy storage. Phil. Trans. R. Soc. A 2013, 371, 20110431. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.S.; Cagle, A.E.; Macknick, J.; Bloom, D.E.; Caplan, J.S.; Ravi, S. Effects of revegetation on soil physical and chemical properties in solar photovoltaic infrastructure. Front. Environ. Sci. 2020, 8, 140. [Google Scholar] [CrossRef]
- Yu, G.R.; Xu, X.L.; Wang, Q.F. Progress of the effects of global changes on the resource and environment carrying capacity of the ecological fragile areas. Chin. Basic Sci. 2020, 22, 16–20. [Google Scholar]
- Lambert, Q.; Bischoff, A.; Cueff, S. Effects of solar park construction and solar panels on soil quality, microclimate, CO2 fluxes, and vegetation under a Mediterranean climate. Land Degrad. Dev. 2021, 32, 5190–5202. [Google Scholar] [CrossRef]
- Lu, X. The Environment Effect Analysis of PV Power Plant Construction in Desert Gobi Lanzhou University. 2013. Available online: https://cdmd.cnki.com.cn/Article/CDMD-10730-1013237744.htm (accessed on 5 March 2024).
- Armstrong, A.; Ostle, N.J.; Whitaker, J. Solar park microclimate and vegetation management effects on grassland carbon cycling. Environ. Res. Lett. 2016, 11, 074016. [Google Scholar] [CrossRef]
- Zhao, P.Y. Effects of Photovoltaic Panels on Surface Soil Particles and Microclimate Inner Mongolia Agricultural University. 2016. Available online: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201701&filename=1016249431.nh (accessed on 5 March 2024).
- Yin, D.Y.; Ma, L.; Qu, J.J.; Zhao, S.P.; Yu, Y.; Tan, L.; Xiao, J.H. Effect of large photovoltaic power station on microclimate of desert region in Gonghe basin. Bull. Soil Water Conserv. 2017, 37, 15–21. [Google Scholar] [CrossRef]
- Zhai, B.; Dang, X.H.; Chen, X.; Liu, X.J.; Yang, S.R. Difference regularity of precipitation redistribution and soil water evapotranspiration in photovoltaic panels in typical steppe areas of Inner Mongolia. J. Chin. Agric. Univ. 2020, 25, 144–155. [Google Scholar]
- Lambert, Q.; Gros, R.; Bischoff, A. Ecological restoration of solar park plant communities and the effect of solar panels. Ecol. Eng. 2022, 182, 106722. [Google Scholar] [CrossRef]
- Fu, P.B.; Gan, Y.M.; Zhang, H.X.; Yang, P.G.; Guo, L.J.; Zeng, H. Effects of fertilizing on the forage production and quality of alpine grassland. Pratacul. Sci. 2015, 32, 1137–1142. [Google Scholar] [CrossRef]
- Zuzana, H.; Michal, H.; Vilémv, P.; Josef, H.; Michaela, K.; Jiří, M. Long-term dynamics of biomass production, soil chemical properties and plant species composition of alluvial grassland after the cessation of fertilizer application in the Czech Republic. Agric. Ecosyst. Environ. 2009, 130, 123–130. [Google Scholar] [CrossRef]
- Potter, P.; Ramankutty, N.; Bennett, E.M.; Donner, S.D. Characterizing the spatial patterns of global fertilizer application and manure production. Earth Interact. 2010, 14, 1–22. [Google Scholar] [CrossRef]
- Wang, C.T.; Wang, G.X.; Liu, W.; Wang, Q.L. Effects of fertilization gradients on plant community structure and soil characteristics in alpine meadow. Acta Ecol. Sin. 2013, 33, 3103–3113. [Google Scholar] [CrossRef]
- Heyburn, J.; Mckenzie, P.; Crawley, M.J.; Dario, A. Long-term belowground effects of grassland management: The key role of liming. Ecol. Appl. 2017, 27, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.E.; Hobbie, S.E.; Tilman, D.; Reich, P.B. Effects of plant diversity, N fertilization, and elevated carbon dioxide on grassland soil N cycling in a long-term experiment. Glob. Chang. Biol. 2013, 19, 1249–1261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, H.; Chen, Q.; Han, X.G. The counteractive effects of nitrogen addition and watering on soil bacterial communities in a steppe ecosystem. Soil Biol. Biochem. 2014, 72, 26–34. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, E.; Wu, J.; Ma, D.; Zhang, C.; Zhang, B.; Liu, Y.; Zhang, Z.; Tian, F.; Zhao, H.; et al. Soil fungal community is more sensitive than bacterial community to modified materials application in saline–alkali land of Hetao Plain. Front. Microbiol. 2024, 15, 1255536. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Periphyton: A Promising Bio-Organic Fertilizer Source in Agricultural Ecosystems; Elsevier: Boston, MA, USA, 2017; pp. 225–249. [Google Scholar] [CrossRef]
- Fernandez-Going, B.M.; Harrison, S.P.; Anacker, B.L.; Safford, H.D. Climate interacts with soil to produce beta diversity in Californian plant communities. Ecology 2013, 94, 2007–2018. [Google Scholar] [CrossRef]
- Liu, K.; Liang, T.; Qiang, W. Changes in seed germination strategy along the successional gradient from abandoned cropland to climax grassland in a subalpine meadow and some implications for rangeland restoration. Agric. Ecosyst. Environ. 2020, 289, 106746. [Google Scholar] [CrossRef]
- Zhao, L.P.; Cui, Z.; Liu, Y. Grazing exclusion erodes the forbs functional group without altering offspring recruitment composition in a typical steppe. Land Degrad. Dev. 2020, 31, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Zhou, D.W.; Li, Q.; Huang, Y.X. Effects of Fencing on Vegetation and Soil Nutrients of the Temperate Steppe Grasslands in Inner Mongolia. Agronomy 2021, 11, 1546. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; Chinese Agriculture Press: Beijing, China, 2008. [Google Scholar]
- Sparks, D.L.; Page, A.; Helmke, P.; Loeppert, R.; Soltanpour, P.; Tabatabai, M. Methods of Soil Analysis. Part 3-Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Lu, R. Soil Agricultural Chemical Analysis Method; Chinese Agriculture and Sciences Press: Beijing, China, 1999. [Google Scholar]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef] [PubMed]
- Mago, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Hautier, Y.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hillebrand, H. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature 2014, 508, 521–525. [Google Scholar] [CrossRef]
- Seitz, S.; Goebes, P.; Song, Z.S.; Bruelheide, H.; Hardtle, W.; Kuhn, P. Tree species and functional traits but not species richness affect interrill erosion processes in young subtropical forests. Soil 2016, 2, 49–61. [Google Scholar] [CrossRef]
- Tong, Z.; Quan, G.; Wan, L.; He, F.; Li, X. The Effect of Fertilizers on Biomass and Biodiversity on a Semi-Arid Grassland of Northern China. Sustainability 2019, 11, 2854. [Google Scholar] [CrossRef]
- Wang, T.; Wang, D.X.; Guo, T.D.; Zhang, G.G.; Zhao, S.X.; Niu, H.C. The impact of photovoltaic power construction on soil and vegetation. Res. Soil Water Conserv. 2016, 23, 90–94. [Google Scholar] [CrossRef]
- Du, H.; Sun, F. Preliminary study on the impact of photovoltaic power plant operation on vegetation. Environ. Dev. 2017, 29, 30–31. [Google Scholar]
- Zhai, B.; Gao, Y.; Dang, X.H.; Chen, X.; Cheng, B.; Liu, X.J. Effects of photovoltaic panels on the characteristics and diversity of Leymus chinensis community. Chin. J. Ecol. 2018, 37, 2237–2243. [Google Scholar]
- Mackie, K.A.; Zeiter, M.; Bloor, J.M.G.; Stampfli, A. Plant functional groups mediate drought resistance and recovery in a multisite grassland experiment. J. Ecol. 2019, 107, 937–949. [Google Scholar] [CrossRef]
- Sabir, M.S.; Shahzadi, F.; Ali, F.; Shakeela, Q.; Niaz, Z.; Ahmed, S. Comparative Effect of Fertilization Practices on Soil Microbial Diversity and Activity: An Overview. Curr. Microbiol. 2021, 78, 3644–3655. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, R.Q.; Huang, Z.; Cheng, Z.; Lopez-Vicente, M.; Ma, X.R. Solar photovoltaic panels significantly promote vegetation recovery by modifying the soil surface microhabitats in arid sandy ecosystem. Land Degrad. Dev. 2019, 30, 2177–2186. [Google Scholar] [CrossRef]
- Li, Y.; Kalnay, E.; Motesharrei, S.; Rivas, J.; Kucharski, F.; Kirk-Davidoff, D. Climate model shows large-scale wind and solar farms in the Sahara increase rain and vegetation. Science 2018, 361, 1019–1022. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Feng, Y.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong. China Soil Till. Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Xu, J.; Cao, K.; Wang, J.; Xu, C.; Cao, W. Green manure incorporation with reductions in chemical fertilizer inputs improves rice yield and soil organic matter accumulation. J. Soils Sediments 2020, 20, 2784–2793. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, R.; Xue, C.; Xun, W.; Sun, L.; Xu, Y. Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb. Ecol. 2014, 67, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gu, T.; Wang, W.; Hang, B.; Lin, X.; Huang, Q.; Shen, W. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2010, 326, 511–522. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.; Schadt, C.; Chang, S.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Chang. Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Sinkko, H.; Lukkari, K.; Sihvonen, L.M.; Sivonen, K.; Leivuori, M.; Rantanen, M. Bacteria contribute to sediment nutrient release and reflect progressed eutrophication-driven hypoxia in an organic-rich continental sea. PLoS ONE 2013, 8, e67061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, J.; Li, J.; Xin, Y.; Hao, Z.; Chen, C. Insights into bacterial diversity in compost: Core microbiome and prevalence of potential pathogenic bacteria. Sci. Total Environ. 2020, 718, 137304. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yu, X.; Chen, J.; Li, X.; Chen, J.; Li, J. Effects of compost as a soil amendment on bacterial community diversity in saline–alkali soil. Front. Microbiol. 2023, 14, 1253415. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G.; Deforest, J.; Marxsen, J.; Sinsabaugh, R.; Stromberger, M.E.; Wallenstein, M. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, J.; Manuel, D.-B.; de Beeck, M.O.; Shahbaz, M.; Chen, Y.; Deng, X.; Xu, Z.; Li, J.; Liu, Z. Rotation cropping and organic fertilizer jointly promote soil health and crop production. J. Environ. Manag. 2022, 315, 115190. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Aboveground Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change; Oxford University Press: Oxford, UK, 2010. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Knight, R. Global patterns in bacterial diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 11436–11440. [Google Scholar] [CrossRef]
- Cho, S.J.; Kim, M.H.; Lee, Y.O. Effect of pH on soil bacterial diversity. J. Ecol. Environ. 2016, 40, 10. [Google Scholar] [CrossRef]
- Rath, K.M.; Fierer, N.; Murphy, D.V.; Rousk, J. Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 2018, 13, 836–846. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Pavao-Zuckerman, M.A.; Minor, R.L.; Sutter, L.F.; Barnett-Moreno, I.; Blackett, D.T. Agrivoltaics provide mutual benefits across the food-energy-water nexus in drylands. Nat. Sustain. 2019, 2, 848–855. [Google Scholar] [CrossRef]
- Moscatelli, M.C.; Marabottini, R.; Massaccesi, L.; Marinari, S. Soil properties changes after seven years of ground mounted photovoltaic panels in Central Italy coastal area. Geoderma Reg. 2022, 29, e00500. [Google Scholar] [CrossRef]


| Name | CK | OFE | OBP | FE | BP | F | P |
|---|---|---|---|---|---|---|---|
| pH | 9.46 ± 0.14 a | 9.44 ± 0.13 a | 9.41 ± 0.05 a | 9.42 ± 0.08 a | 9.12 ± 0.07 a | 0.44 | 0.78 |
| EC (mS/cm) | 2.16 ± 0.33 ab | 2.89 ± 0.43 a | 1.89 ± 0.47 ab | 1.91 ± 0.42 ab | 1.25 ± 0.32 b | 2.21 | 0.09 |
| SOC (g/kg) | 5.91 ± 2.67 a | 6.80 ± 2.96 a | 6.61 ± 3.51 a | 6.30 ± 1.31 a | 5.80 ± 0.85 a | 0.23 | 0.92 |
| TK (g/kg) | 4.78 ± 0.97 a | 5.31 ± 0.84 a | 6.96 ± 3.11 a | 6.87 ± 1.80 a | 10.15 ± 3.52 a | 1.71 | 0.17 |
| AK (mg/kg) | 142.85 ± 5.32 ab | 140.79 ± 5.22 ab | 132.44 ± 4.15 b | 137.75 ± 6.54 ab | 144.37 ± 4.62 a | 0.81 | 0.52 |
| TP (g/kg) | 1.08 ± 0.04 a | 1.27 ± 0.05 a | 1.20 ± 0.10 a | 1.14 ± 0.06 a | 1.17 ± 0.08 a | 0.88 | 0.48 |
| AP (mg/kg) | 149.57 ± 11.26 a | 153.25 ± 9.82 a | 137.18 ± 11.11 a | 143.98 ± 9.37 a | 148.50 ± 10.22 a | 0.35 | 0.84 |
| TN (g/kg) | 0.99 ± 0.07 a | 1.22 ± 0.14 a | 1.12 ± 0.11 a | 1.22 ± 0.10 a | 1.02 ± 0.08 a | 1.43 | 0.24 |
| AN (mg/kg) | 178.32 ± 12.59 a | 176.45 ± 12.03 a | 146.24 ± 7.43 a | 151.68 ± 16.74 a | 152.94 ± 15.54 a | 1.30 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, A.; Bai, Z.; Gong, L.; Li, H.; Bai, Z.; Wang, M. Effects of Organic Fertilizer Addition to Vegetation and Soil Bacterial Communities in Saline–Alkali-Degraded Grassland with Photovoltaic Panels. Plants 2024, 13, 1491. https://doi.org/10.3390/plants13111491
Jia A, Bai Z, Gong L, Li H, Bai Z, Wang M. Effects of Organic Fertilizer Addition to Vegetation and Soil Bacterial Communities in Saline–Alkali-Degraded Grassland with Photovoltaic Panels. Plants. 2024; 13(11):1491. https://doi.org/10.3390/plants13111491
Chicago/Turabian StyleJia, Aomei, Zhenyin Bai, Liping Gong, Haixian Li, Zhenjian Bai, and Mingjun Wang. 2024. "Effects of Organic Fertilizer Addition to Vegetation and Soil Bacterial Communities in Saline–Alkali-Degraded Grassland with Photovoltaic Panels" Plants 13, no. 11: 1491. https://doi.org/10.3390/plants13111491







