Can Simulated Microgravity and Darkness Conditions Influence the Phytochemical Content and Bioactivity of the Sprouts?—A Preliminary Study on Selected Fabaceae Species
Abstract
1. Introduction
2. Results and Discussion
2.1. Growth Parameters, Biomass, and Germination Rate
2.2. Amount of Bioactive Compounds
2.3. Abscisic Acid Amount and Phenylalanine Ammonia-Lyase Activity
2.4. Antioxidant Activity
2.5. Cytotoxic Activity
2.5.1. Prostate Cancer
2.5.2. Breast Cancer
2.6. Safety Studies
3. Materials and Methods
3.1. Plant Material
3.2. Microgravity Simulation Setup
3.3. Extracts Preparation
3.3.1. Qualitative and Quantitative Analysis and Antioxidant Activity
3.3.2. Cytotoxic Activity
3.3.3. Abscisic Acid Amount and Phenylalanine Ammonia-Lyase Activity
3.4. Biomass and Germination Rate
3.5. Germination Rate
3.6. Qualitative and Quantitative HPLC Analysis
3.7. Antioxidant Activity
3.8. Cell Cultures and Viability Assays
3.9. Abscisic Acid (ABA) Amount and Phenylalanine Ammonia-Lyase (PAL) Activity
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grudzińska, M.; Galanty, A.; Paśko, P. Can Edible Sprouts Be the Element of Effective Chemopreventive Strategy?—A Systematic Review of in Vitro and in Vivo Study. Trends Food Sci. Technol. 2023, 139, 104130. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; González-Arceo, M.; Fernández-Quintela, A.; Eseberri, I.; Trepiana, J.; Puy Portillo, M. Scientific Evidence Supporting the Beneficial Effects of Isoflavones on Human Health. Nutrients 2020, 12, 3853. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Zagrodzki, P.; Miret, M.; Paśko, P. Chickpea and Lupin Sprouts, Stimulated by Different LED Lights, As Novel Examples of Isoflavones-Rich Functional Food, and Their Impact on Breast and Prostate Cells. Molecules 2022, 27, 9030. [Google Scholar] [CrossRef] [PubMed]
- Galanty, A.; Prochownik, E.; Grudzińska, M.; Paśko, P. Chickpea Sprouts as a Potential Dietary Support in Different Prostate Disorders—A Preliminary In Vitro Study. Molecules 2024, 29, 1044. [Google Scholar] [CrossRef] [PubMed]
- Lim, I.; Kang, M.; Kim, B.C.; Ha, J. Metabolomic and Transcriptomic Changes in Mungbean (Vigna radiata (L.) R. Wilczek) Sprouts under Salinity Stress. Front. Plant Sci. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Halstead, T.W.; Dutcher, F.R. Plants in space. Annu. Rev. Plant. Physiol. 1987, 38, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, L.K.; Levine, L.H.; Musgrave, M.E. Plant Secondary Metabolism in Altered Gravity. In Methods in Molecular Biology, Protocols for In Vitro Culturesand Secondary Metabolite Analysis of Aromatic and Medicinal Plants; Mohan Jain, S., Saxena, P.K., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2009; Volume 547, pp. 373–386. [Google Scholar]
- Nakajima, S.; Nagata, M.; Ikehata, A. Mechanism for Enhancing the Growth of Mung Bean Seedlings under Simulated Microgravity. npj Microgravity 2021, 7, 1030677. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Ogawa, Y.; Suzuki, T.; Kondo, N. Enhanced Antioxidant Activity in Mung Bean Seedlings Grown under Slow Clinorotation. Microgravity Sci. Technol. 2019, 31, 395–401. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Soga, K.; Kamisaka, S.; Hoson, T. Changes in Levels of Cell Wall Constituents in Wheat Seedlings Grown under Continuous Hypergravity Conditions. Adv. Space Res. 2005, 36, 1292–1297. [Google Scholar] [CrossRef]
- Faraoni, P.; Sereni, E.; Gnerucci, A.; Cialdai, F.; Monici, M.; Ranaldi, F. Glyoxylate Cycle Activity in Pinus Pinea Seeds during Germination in Altered Gravity Conditions. Plant Physiol. Biochem. 2019, 139, 389–394. [Google Scholar] [CrossRef]
- Xie, Q.; Essemine, J.; Pang, X.; Chen, H.; Jin, J.; Cai, W. Abscisic Acid Regulates the Root Growth Trajectory by Reducing Auxin Transporter PIN2 Protein Levels in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 632676. [Google Scholar] [CrossRef]
- Li, J.; Ou-Lee, T.-M.; Raba, R.; Amundson, R.G.; Lastatbi1, R.L. Arabidopsis Flavonoid Mutants Are Hypersensitive to UV-6 Lrradiation. Plant Cell 1993, 5, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.N.; Bercovich, Y.; Mashinskiy, A.L.; Meleshko, G.I. The First “Space” Vegetables Have Been Grown in the “SVET” Greenhouse by Means of Controlled Environmental Conditions. Acta Astronaut. 1993, 8, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.P.S.; Meyers, A.D.; Basu, P.; Hutchinson, S.; Luesse, D.R.; Wyatt, S.E. Spaceflight Induces Novel Regulatory Responses in Arabidopsis Seedling as Revealed by Combined Proteomic and Transcriptomic Analyses. BMC Plant Biol. 2020, 20, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, L.; Dipierro, N.; Paciolla, C. Effects of Darkness and Light Spectra on Nutrients and Pigments in Radish, Soybean, Mung Bean and Pumpkin Sprouts. Antioxidants 2020, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Battistelli, A.; Proietti, S.; Moscatello, S.; Rouphael, Y.; Cardarelli, M.; Casucci, M. Rocket seedling production on the International Space Station: Growth and Nutritional properties. Microgravity Sci. Technol. 2007, XIX-2, 118–121. [Google Scholar] [CrossRef]
- Galanty, A.; Niepsuj, M.; Grudzińska, M.; Zagrodzki, P.; Podolak, I.; Paśko, P. In the Search for Novel, Isoflavone-Rich Functional Foods—Comparative Studies of Four Clover Species Sprouts and Their Chemopreventive Potential for Breast and Prostate Cancer. Pharmaceuticals 2022, 15, 806. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.H.; Levine, H.G.; Stryjewski, E.C.; Prima, V.; Piastuch, W.C. Effect of Spaceflight on Isoflavonoid Accumulation in Etiolated Soybean Seedlings. J. Gravit. Physiol. 2001, 8, 21–27. [Google Scholar]
- Kicel, A.; Wolbiś, M. Phenolic Acids in Flowers and Leaves of Trifolium repens L. and Trifolium pratense L. Herba Pol. 2006, 52, 51–57. [Google Scholar]
- Kicel, A.; Wolbiś, M. Study on the Phenolic Constituents of the Flowers and Leaves of Trifolium repens L. Nat. Prod. Res. 2012, 26, 2050–2054. [Google Scholar] [CrossRef]
- Tundis, R.; Marrelli, M.; Conforti, F.; Tenuta, M.C.; Bonesi, M.; Menichini, F.; Loizzo, M.R. Trifolium Pratense and T. repens (Leguminosae): Edible Flower Extracts as Functional Ingredients. Foods 2015, 4, 338–348. [Google Scholar] [CrossRef]
- Popović, Z.; Smiljanić, M.; Kostić, M.; Nikić, P.; Janković, S. Wild Flora and Its Usage in Traditional Phytotherapy (Deliblato Sands, Serbia, South East Europe). Indian J. Trad. Knowl. 2014, 13, 9–35. [Google Scholar]
- Whetten, R.; Sederoff, R. Lignin Biosynthesis. Plant Cell 1995, 7, 1001–1013. [Google Scholar] [CrossRef]
- Cowles, J.R.; Scheld, H.W.; LeMay, R.; Peterson, C. Experiments on Plants Grown in Space: Growth and Lignification in Seedlings Exposed to Eight Days of Microgravity. Ann. Bot. 1984, 54, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R. Abscisic Acid Synthesis and Response. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2013; Volume 11, pp. 1–36. [Google Scholar]
- Schulze, A.; Jensen, P.J.; Desrosiers, M.; Buta, J.G.; Bandurski, R.S. Studies on the Growth and Lndole-3-Acetic Acid and Abscisic Acid Content of Zea Mays Seedlings Grown in Microgravity. Plant Physiol. 1992, 100, 692–698. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Mekky, R.H.; Thabet, M.M.; Rodríguez-Pérez, C.; Elnaggar, D.M.Y.; Mahrous, E.A.; Segura-Carretero, A.; Abdel-Sattar, E. Comparative Metabolite Profiling and Antioxidant Potentials of Seeds and Sprouts of Three Egyptian Cultivars of Vicia faba L. Food Res. Int. 2020, 136, 109537. [Google Scholar] [CrossRef]
- Okumura, K.; Hosoya, T.; Kawarazaki, K.; Izawa, N.; Kumazawa, S. Antioxidant Activity of Phenolic Compounds from Fava Bean Sprouts. J. Food Sci. 2016, 81, 1394–1398. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and Breast Cancer: In Vitro Anticancer Activities of Isoflavones, Lignans, Coumestans, Stilbenes and Their Analogs and Derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef]
- Hajirahimkhan, A.; Dietz, B.M.; Bolton, J.L. Botanical Modulation of Menopausal Symptoms: Mechanisms of Action? Planta Med. 2013, 79, 538–553. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, A. Altered Gravity Experiments During Analog Missions. Ann. Astron. Novae 2023, 4, 299–310. [Google Scholar]
- Borst, A.G.; Van Loon, J.J.W.A. Technology and Developments for the Random Positioning Machine, RPM. Microgravity Sci. Technol. 2009, 21, 287–292. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Żmudzki, P.; Bieniek, U.; Prochownik, E.; Domínguez-Álvarez, E.; Bierła, K.; Łobiński, R.; Szpunar, J.; et al. Varied Effect of Fortification of Kale Sprouts with Novel Organic Selenium Compounds on the Synthesis of Sulphur and Phenolic Compounds in Relation to Cytotoxic, Antioxidant and Anti-Inflammatory Activity. Microchem. J. 2022, 179, 107509. [Google Scholar] [CrossRef]
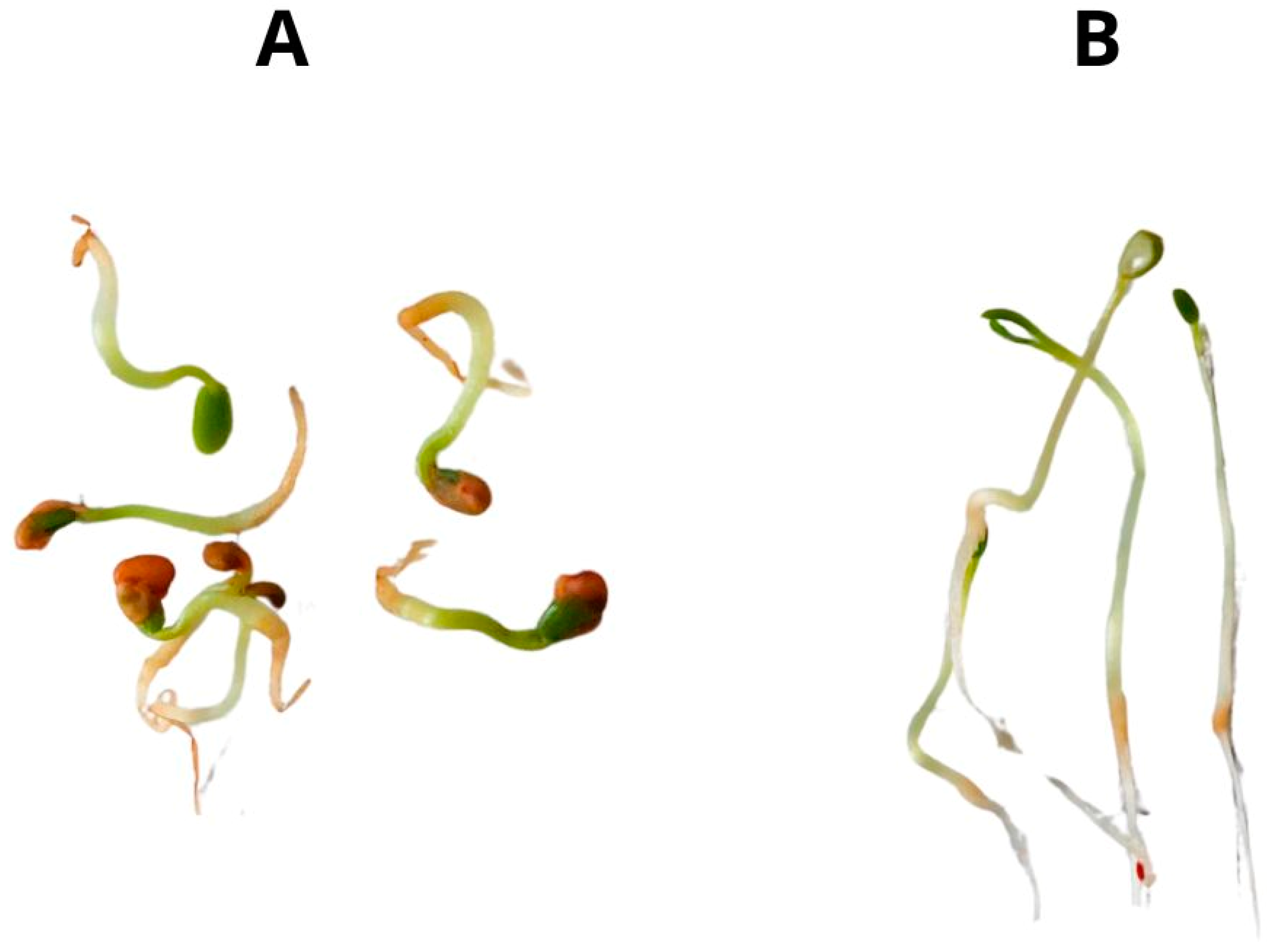
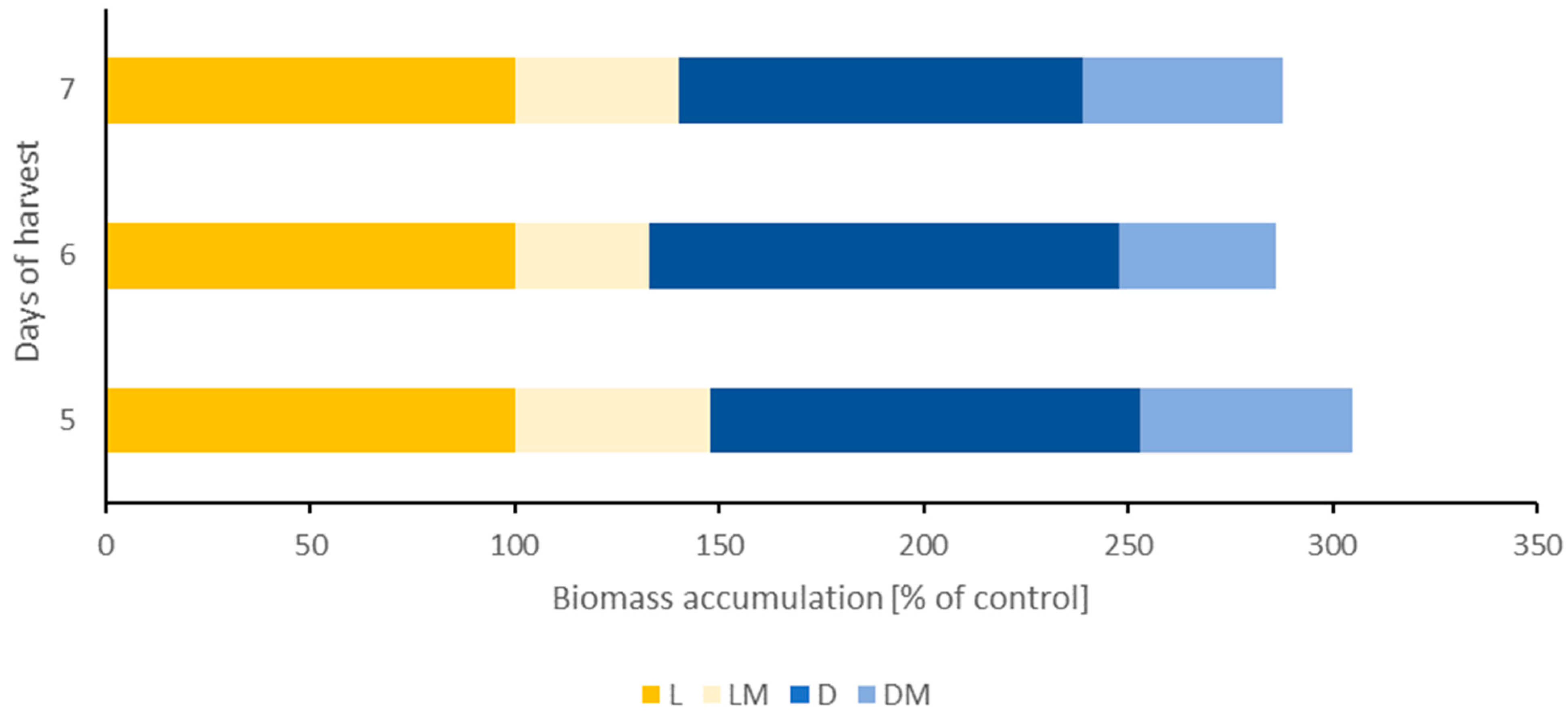
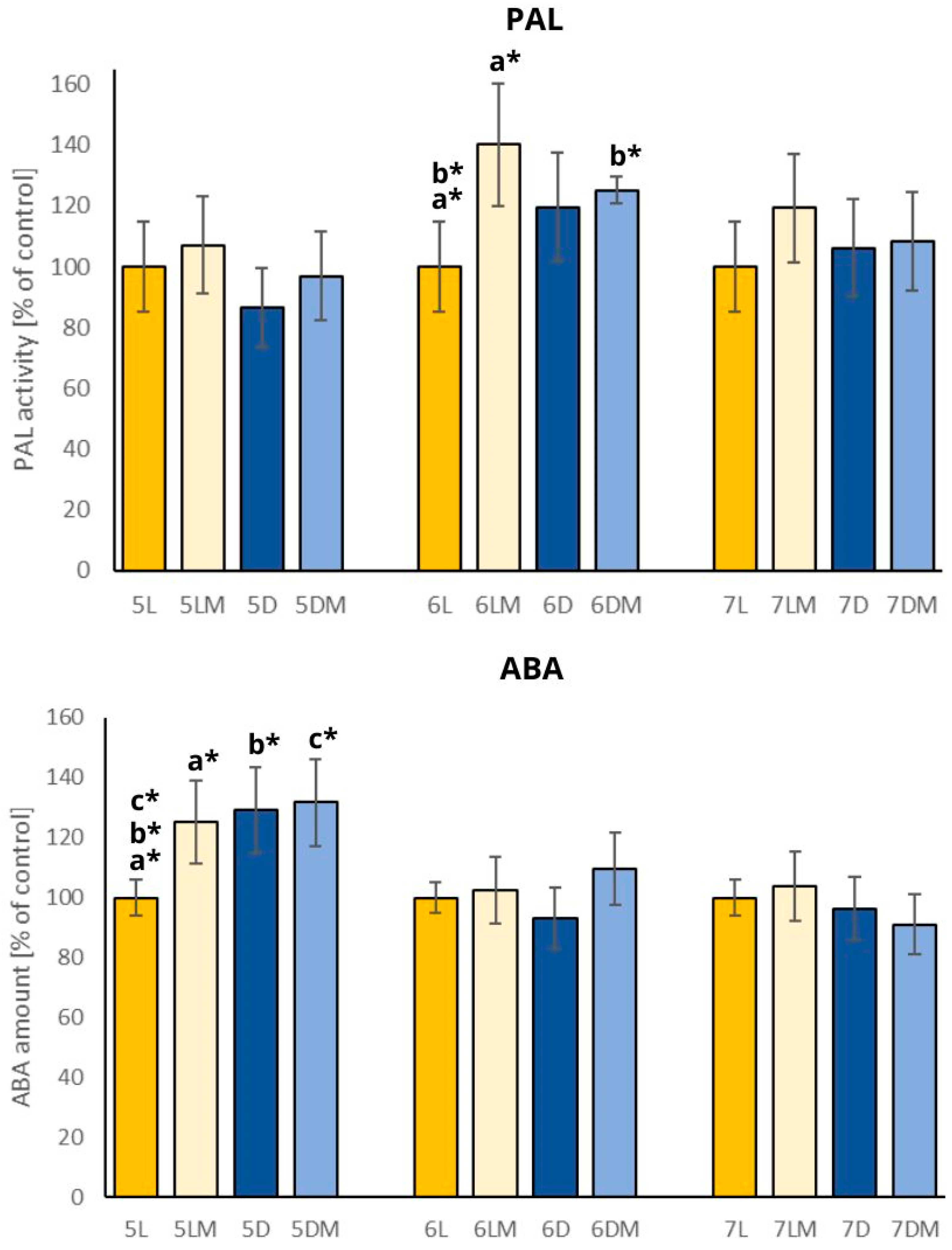


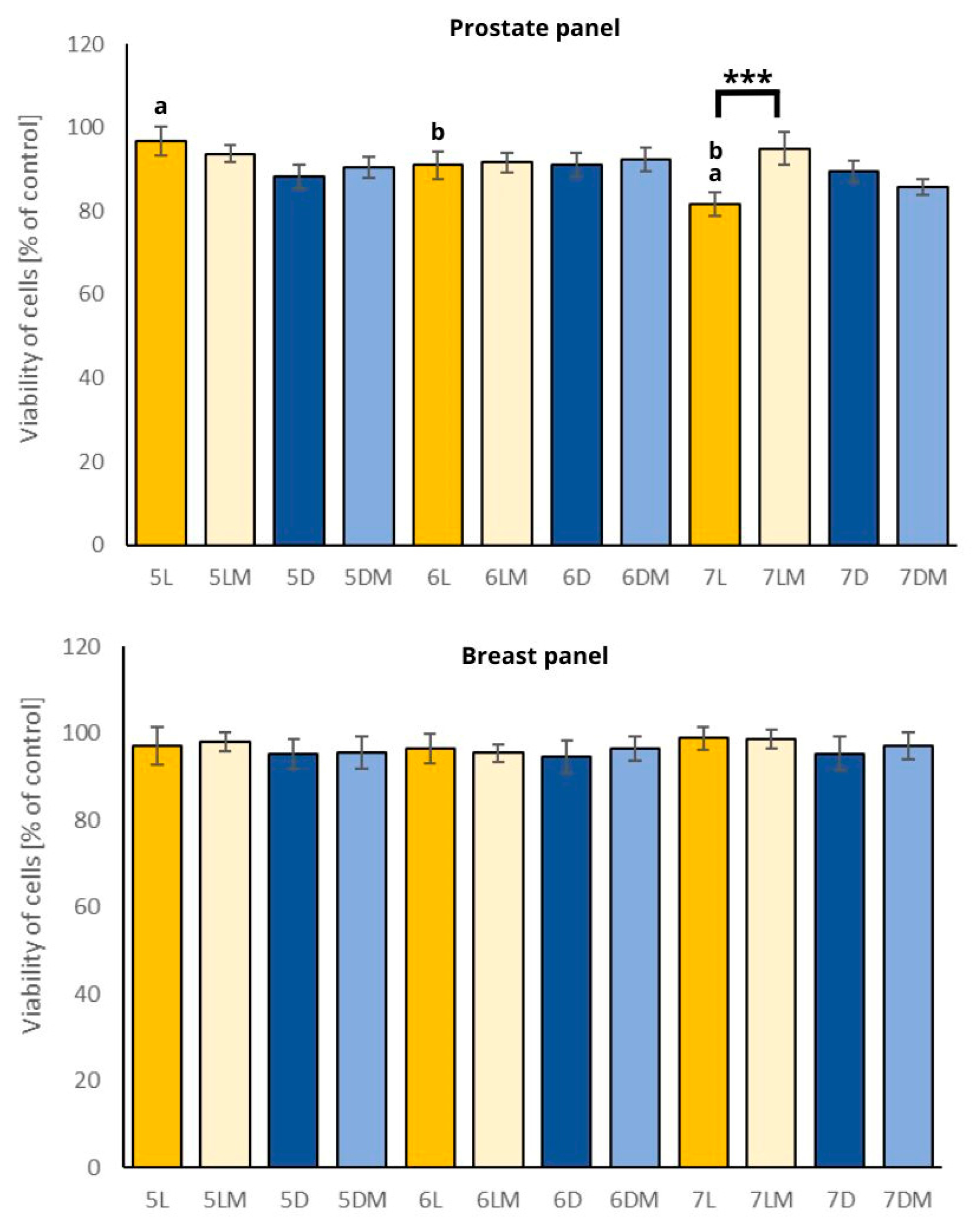
| Sample | Seedling Length [mm] | Stem Length [mm] | Roots Length [mm] |
|---|---|---|---|
| 5L | 12.67 ± 2.08 cd | 8.67 ± 1.15 c | 3.00 ± 2.65 |
| 5LM | 9.33 ± 0.58 | 5.33 ± 0.58 | 2.67 ± 0.58 |
| 6L | 25.33 ± 0.58 ace | 14.33 ± 3.05 a | 8.67 ± 2.52 |
| 6LM | 8.00 ± 1.73 a | 4.00 ± 0.60 a | 2.00 ± 1.00 |
| 7L | 28.33 ± 0.58 bdfg | 19.67 ± 2.52 bcde | 6.33 ± 3.21 |
| 7LM | 18.00 ± 5.57 b | 9.00 ± 1.00 b | 7.33 ± 4.73 |
| 5D | 18.00 ± 6.24 jk | 13.00 ± 6.08 h | 4.67 ± 1.53 |
| 5DM | 9.67 ± 2.08 | 5.67 ± 1.15 | 2.67 ± 1.15 |
| 6D | 30.33 ± 1.53 hjl | 22.00 ± 4.36 fi | 6.67 ± 3.21 |
| 6DM | 9.00 ± 1.73 eh | 6.00 ± 1.73 f | 2.00 ± 1.73 |
| 7D | 46.33 ± 6.80 gikl | 32.33 ± 6.43 eghi | 11.67 ± 6.66 |
| 7DM | 14.33 ± 2.08 fi | 9.67 ± 0.58 dg | 3.33 ± 2.08 |
| Sample | Daidzin | Ononin | Formononetin | Isoflavone Sum | Protocatechuic Acid | Caffeic Acid | Chlorogenic Acid | Phenolic Acid Sum |
|---|---|---|---|---|---|---|---|---|
| 5L | 6.76 ± 0.10 cij | 0.97 ± 0.02 bhij | 3.87 ± 0.40 ghi | 11.6 | 7.73 ± 0.94 bfg | 85.52 ± 5.0 cj | 38.01 ± 3.90 cij | 131.26 |
| 5LM | 15.14 ± 0.81 abcd | 9.49 ± 1.26 abc | 3.63 ± 0.20 ab | 28.26 | 9.69 ± 0.75 ab | 21.50 ± 1.05 abcd | 2.02 ± 0.30 abcd | 33.21 |
| 6L | 9.16 ± 0.80 eikl | 2.75 ± 0.20 ehkl | 5.50 ± 0.30 dgj | 17.41 | 10.08 ± 0.80 df | 100.32 ± 9.50 fkl | 66.88 ± 9.20 eik | 177.28 |
| 6LM | 5.58 ± 0.10 aef | 4.78 ± 0.30 adef | 3.59 ± 0.70 cde | 13.95 | 5.84 ± 0.23 acde | 3.59 ± 0.50 efi | 39.65 ± 1.40 aef | 49.08 |
| 7L | 7.66 ± 0.20 gk | <LOD gik | 7.66 ± 0.60 fhjk | 15.32 | 8.51 ± 1.50 | 117.46 ± 2.90 gjkm | 81.71 ± 11.10 gj | 207.68 |
| 7LM | 5.30 ± 0.40 bgh | 8.29 ± 0.70 dg | 5.07 ± 0.40 acf | 18.66 | 8.29 ± 0.97c | 87.09 ± 6.20 begh | 60.83 ± 5.90 bgh | 156.21 |
| 5D | 4.95 ± 0.64 jnrs | <LOD jo | 2.70 ± 0.10 in | 7.65 | 5.40 ± 0.45 ghk | 84.53 ± 6.30 ot | 56.95 ± 6.60 op | 147.78 |
| 5DM | 2.59 ± 0.35 dmn | 11.55 ± 0.35 cmno | 1.19 ± 0.1 blmn | 15.33 | 10.75 ± 0.60 h | 150.32 ± 2.80 dno | 55.95 ± 6.0 dl | 218.21 |
| 6D | 2.65 ± 0.10 lrt | <LOD lr | 5.30 ± 0.90 p | 7.95 | 10.60 ± 0.99 ikl | 149.68 ± 9.40 lrtu | 108.62 ± 4.60 kno | 268.9 |
| 6DM | 2.36 ± 0.30 fo | 3.37 ± 0.30 fmpr | 2.52 ± 0.33 elop | 8.25 | 8.08 ± 0.75 ei | 105.32 ± 10.40 inpr | 63.97 ± 8.60 fmn | 178.38 |
| 7D | 7.10 ± 0.20 pst | <LOD s | 4.74 ± 0.48 kr | 11.86 | 7.12 ± 0.64 jl | 79.08 ± 7.20 msu | 86.20 ± 6.80 p | 172.4 |
| 7DM | 8.77 ± 0.30 hmop | 2.34 ± 0.20 nps | 5.26 ± 0.40 mor | 16.37 | 9.64 ± 0.41 j | 164.83 ± 9.10 hps | 87.97 ± 9.60 hlm | 263.32 |
| Sample | FRAP [mM Fe2+/100 g dw] | DPPH [mM TROLOX/100 g dw] |
|---|---|---|
| 5L | 4.34 ± 0.10 ad | 1.70 ± 0.15 aef |
| 5LM | 6.44 ± 0.20 abcp | 2.79 ± 0.10 abcd |
| 6L | 5.38 ± 0.10 eg | 2.19 ± 0.04 gi |
| 6LM | 3.13 ± 0.20 befr | 1.58 ± 0.06 bgh |
| 7L | 4.75 ± 0.20 i | 2.32 ± 0.10 el |
| 7LM | 4.35 ± 1.20 ch | 2.01 ± 0.21 cjk |
| 5D | 3.33 ± 0.17 jlp | 1.45 ± 0.12 dmo |
| 5DM | 6.21 ± 0.20 djk | 2.67 ± 0.11 fmn |
| 6D | 6.58 ± 0.10 glmnr | 3.14 ± 0.42 hiops |
| 6DM | 4.66 ± 0.10 fkm | 2.05 ± 0.14 npr |
| 7D | 3.43 ± 0.20 ino | 1.31 ± 0.12 klst |
| 7DM | 5.20 ± 0.10 ho | 2.76 ± 0.15 jrt |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grudzińska, M.; Galanty, A.; Prochownik, E.; Kołodziejczyk, A.; Paśko, P. Can Simulated Microgravity and Darkness Conditions Influence the Phytochemical Content and Bioactivity of the Sprouts?—A Preliminary Study on Selected Fabaceae Species. Plants 2024, 13, 1515. https://doi.org/10.3390/plants13111515
Grudzińska M, Galanty A, Prochownik E, Kołodziejczyk A, Paśko P. Can Simulated Microgravity and Darkness Conditions Influence the Phytochemical Content and Bioactivity of the Sprouts?—A Preliminary Study on Selected Fabaceae Species. Plants. 2024; 13(11):1515. https://doi.org/10.3390/plants13111515
Chicago/Turabian StyleGrudzińska, Marta, Agnieszka Galanty, Ewelina Prochownik, Agata Kołodziejczyk, and Paweł Paśko. 2024. "Can Simulated Microgravity and Darkness Conditions Influence the Phytochemical Content and Bioactivity of the Sprouts?—A Preliminary Study on Selected Fabaceae Species" Plants 13, no. 11: 1515. https://doi.org/10.3390/plants13111515
APA StyleGrudzińska, M., Galanty, A., Prochownik, E., Kołodziejczyk, A., & Paśko, P. (2024). Can Simulated Microgravity and Darkness Conditions Influence the Phytochemical Content and Bioactivity of the Sprouts?—A Preliminary Study on Selected Fabaceae Species. Plants, 13(11), 1515. https://doi.org/10.3390/plants13111515






