Volatile Profile Characterization of Jujube Fruit via HS-SPME-GC/MS and Sensory Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Free and Bound Volatile Compositions
2.1.1. Esters
2.1.2. Aldehyde and Ketones
2.1.3. Acids
2.1.4. Alcohols
2.1.5. Benzenoid Aromatics
2.1.6. Terpenoids and Isoprenoids
2.1.7. Phenols and Others
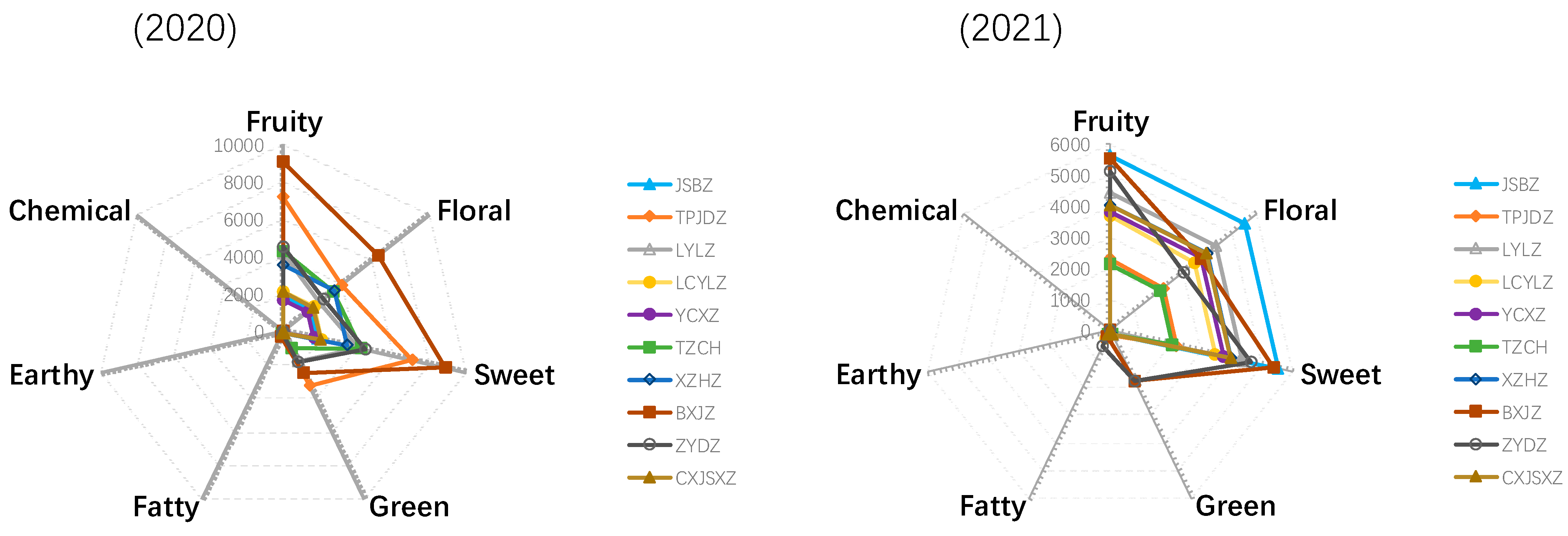
2.2. Aroma Series
| Compounds | Category | Threshold a | Descriptors b | Year | JSBZ | TPJDZ | LYLZ | LCYLZ | YCXZ | TZCH | XZHZ | BXJZ | ZYDZ | CXJSXZ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Acid | 2.5–250 | Pungent, sour, vinegar | 2020 | 5846.46 | 5676.31 | 4391.37 | 3028.99 | 3726.11 | 5043.30 | 8016.07 | 8086.92 | 3438.95 | 4670.85 |
| 2021 | 157.68 | 180.07 | 120.10 | 104.04 | 146.21 | 76.49 | 140.52 | 245.33 | 112.88 | 94.71 | ||||
| Butyric acid | Acid | 240 | Sour, cheese, butter, fruity | 2020 | 78.66 | 55.36 | 49.58 | 100.67 | 60.79 | 55.26 | 110.81 | 52.19 | 46.79 | 62.81 |
| 2021 | 78.73 | 51.42 | 66.68 | 64.39 | 72.14 | 50.29 | 57.90 | 75.52 | 49.00 | 66.70 | ||||
| Isovaleric acid | Acid | 120–700 | Sour, stinky, sweaty, cheese | 2020 | 98.29 | 36.01 | 33.54 | 300.68 | 95.19 | 70.48 | 290.17 | 1382.56 | 82.74 | 293.30 |
| 2021 | 27.57 | tr | tr | tr | tr | tr | 37.54 | 759.30 | 64.62 | tr | ||||
| Valeric acid | Acid | 3000 | Acidic | 2020 | 54.95 | 64.51 | 59.81 | 69.50 | 58.55 | 58.34 | 64.56 | 62.71 | 76.30 | 55.33 |
| 2021 | 98.14 | 75.64 | 90.99 | 73.74 | 87.23 | 73.20 | 103.76 | 139.72 | 109.99 | 58.92 | ||||
| Hexanoic acid | Acid | 3000 | Sour, fatty, cheese | 2020 | 237.10 | 685.67 | 584.00 | 1194.60 | 331.56 | 189.12 | 395.10 | 533.37 | 379.46 | 287.34 |
| 2021 | 997.57 | 1337.77 | 1452.96 | 755.07 | 974.28 | 260.64 | 992.05 | 1154.24 | 547.80 | 461.69 | ||||
| Octanoic acid | Acid | 3000 | Fatty, cheese | 2020 | 383.89 | 408.55 | 319.31 | 1009.92 | 468.97 | 303.77 | 405.71 | 368.97 | 341.68 | 491.86 |
| 2021 | 368.90 | 353.19 | 334.42 | 297.69 | 341.20 | 269.15 | 528.90 | 398.36 | 365.89 | 278.65 | ||||
| Nonanoic acid | Acid | 3000 | Cheese | 2020 | 249.92 | 256.81 | 254.44 | 259.60 | 244.89 | 243.70 | 252.58 | 254.19 | 240.54 | 251.00 |
| 2021 | 264.12 | 276.78 | 272.29 | 263.13 | 277.79 | 295.74 | 283.55 | 310.02 | 282.29 | 272.68 | ||||
| Decanoic acid | Acid | 10,000 | Rancid, sour, fatty, citrus | 2020 | 1555.22 | 1216.96 | 1589.38 | 6786.03 | 2937.21 | 1864.18 | 2613.48 | 2247.90 | 1291.36 | 3029.50 |
| 2021 | 927.35 | 813.82 | 876.27 | 1480.34 | 2180.98 | 607.21 | 2823.39 | 1801.22 | 962.75 | 1228.48 | ||||
| Dodecanoic acid | Acid | 10,000 | 2020 | 767.04 | 842.12 | 810.03 | 1174.03 | 891.33 | 938.74 | 1020.84 | 934.43 | 591.49 | 1320.53 | |
| 2021 | 523.08 | 393.99 | 798.13 | 561.26 | 555.64 | 370.64 | 887.98 | 526.03 | 527.07 | 461.79 | ||||
| Ethyl alcohol | Alcohol | 2020 | 7538.39 | 16,842.38 | 6261.72 | 1505.11 | 3267.59 | 5081.32 | 4032.40 | 9030.31 | 1260.89 | 6319.54 | ||
| 2021 | 334.84 | 376.47 | 408.11 | 246.66 | 238.23 | 216.34 | 220.57 | 335.92 | 321.65 | 231.46 | ||||
| Pent-1-en-3-ol | Alcohol | 400 | Green, vegetable, tropical fruity | 2020 | 1535.76 | 1604.58 | 211.63 | 158.69 | 86.04 | 79.59 | 63.52 | 137.86 | 240.03 | 66.85 |
| 2021 | 689.50 | 821.31 | 543.27 | 591.85 | 895.92 | 182.08 | 402.39 | 809.85 | 651.37 | 220.84 | ||||
| Methyl-2-butan-1-ol | Alcohol | 300 | fruity, fusel, alcoholic | 2020 | 40.43 | 99.83 | 50.59 | 35.02 | 33.30 | 34.22 | 42.59 | 65.78 | 34.83 | 55.49 |
| 2021 | 32.66 | 35.43 | 34.51 | 32.16 | 0.00 | 31.40 | 34.22 | 36.35 | 16.91 | 16.55 | ||||
| Oct-1-en-3-ol | Alcohol | 1 | Mushroom, earthy, green | 2020 | 1.81 | 5.20 | 4.46 | 2.46 | 3.10 | 1.95 | 3.15 | 4.36 | 2.66 | 2.73 |
| 2021 | 2.27 | 3.58 | 2.64 | 1.70 | 3.50 | 0.67 | 4.32 | 3.96 | 2.36 | 2.85 | ||||
| 2-Ethyl-1-hexanol | Alcohol | 270,000 | Citrus, floral, sweet | 2020 | 2.57 | 2.94 | 3.94 | 2.36 | 2.31 | 2.16 | 2.38 | 1.61 | 1.72 | 4.59 |
| 2021 | 1.15 | 0.94 | 0.85 | 1.24 | 0.83 | 1.95 | 1.03 | 1.01 | 1.15 | 1.05 | ||||
| Isopentanal | Aldehyde | 0.2–2 | Chocolate, peach, fatty | 2020 | 37.38 | 123.59 | 50.66 | 22.06 | 15.58 | 16.38 | 30.32 | 312.72 | 18.33 | 51.73 |
| 2021 | 10.91 | 12.42 | 12.61 | 10.40 | 12.42 | 9.25 | 13.91 | 150.05 | 10.27 | 13.28 | ||||
| (E)-But-2-en-1-al | Aldehyde | Flower | 2020 | 9.97 | 7.46 | 7.15 | 7.22 | 9.30 | 7.70 | 7.98 | 7.30 | 7.14 | 8.01 | |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Hexanal | Aldehyde | 4.5 | Grass-like, green | 2020 | 39.30 | 108.47 | 87.59 | 49.12 | 26.00 | 263.13 | 40.54 | 65.81 | 59.09 | 56.99 |
| 2021 | 89.43 | 150.63 | 86.46 | 55.63 | 135.92 | 37.43 | 70.67 | 109.72 | 85.54 | 102.04 | ||||
| Heptanal | Aldehyde | 3 | Fatty, green, herbal | 2020 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 2021 | tr | 11.70 | 10.66 | tr | tr | tr | tr | tr | 9.75 | tr | ||||
| (E)-Hex-2-en-1-al | Aldehyde | 17 | Green, banana, fatty, cheesy | 2020 | 8.73 | 11.28 | 11.24 | 11.59 | 9.71 | 8.60 | 7.86 | 9.09 | 9.86 | 8.76 |
| 2021 | 17.74 | 10.31 | 10.44 | 11.94 | 19.17 | 7.49 | 17.54 | 10.78 | 9.27 | 8.87 | ||||
| Octanal | Aldehyde | 0.7 | Citrus, orange, green, fatty | 2020 | tr | 5.55 | 3.95 | 4.12 | tr | 4.81 | 7.48 | 5.15 | 2.98 | tr |
| 2021 | 2.77 | 3.12 | 2.83 | 2.40 | tr | 2.70 | tr | 2.90 | 3.21 | 3.17 | ||||
| (Z)-Hept-2-en-1-al | Aldehyde | 13 | 2020 | 7.27 | 7.99 | 7.55 | 7.30 | 7.39 | 7.35 | 7.45 | 7.48 | 7.42 | 7.40 | |
| 2021 | 7.30 | 7.29 | 7.21 | 7.13 | 7.29 | 7.08 | 7.42 | 7.32 | 7.25 | 7.47 | ||||
| Nonanal | Aldehyde | 1 | Rose, orange | 2020 | tr | 4.33 | 3.54 | 2.60 | tr | 2.60 | 2.94 | 3.55 | 3.02 | tr |
| 2021 | 4.26 | 5.66 | 4.42 | 3.33 | 2.61 | 4.33 | 2.80 | 4.19 | 6.21 | 4.99 | ||||
| (E)-2-Oct-en-1-al | Aldehyde | 0.1 | Cucumber, green, herbal, fatty | 2020 | 7.62 | 10.16 | 8.98 | 7.56 | 7.70 | 8.16 | 7.82 | 8.45 | 8.22 | 7.94 |
| 2021 | 7.19 | 7.61 | 7.40 | 7.20 | 7.25 | 7.24 | 7.38 | 7.56 | 7.60 | 8.05 | ||||
| Decanal | Aldehyde | 0.1 | Oil, orange, peel | 2020 | 0.58 | 1.68 | 1.14 | 0.65 | 0.17 | 0.78 | 0.93 | 1.36 | 0.95 | 0.48 |
| 2021 | 4.29 | 4.02 | 2.18 | 18.19 | 3.68 | 3.31 | 2.28 | 2.49 | 42.54 | 1.51 | ||||
| Non-2-en-1-al | Aldehyde | 0.08–0.1 | Fatty, green, cucumber | 2020 | 2.27 | 2.81 | 2.52 | 2.23 | 2.27 | 2.33 | 2.42 | 2.50 | 2.35 | 2.28 |
| 2021 | 2.21 | 2.27 | 2.24 | 3.26 | 2.21 | 2.18 | 2.27 | 2.33 | 4.53 | 2.31 | ||||
| (E)-Dec-2-en-1-al | Aldehyde | 0.3–0.4 | Fatty, earthy, coriander, green, mushroom | 2020 | 1.35 | 5.77 | 4.63 | 1.79 | tr | tr | 1.37 | 2.31 | 3.50 | 1.33 |
| 2021 | tr | tr | tr | tr | 1.08 | 1.09 | 1.06 | 1.20 | 1.26 | 1.37 | ||||
| Benzaldehyde | Aldehyde | 350–3500 | Sweet, cherry | 2020 | 10.23 | 193.23 | 317.56 | 36.02 | 43.38 | 26.45 | 69.95 | 312.52 | 17.33 | 586.44 |
| 2021 | 6.67 | 194.98 | 228.02 | 9.83 | 12.90 | 6.91 | 152.62 | 440.11 | 12.37 | 82.26 | ||||
| Cumaldehyde | Aldehyde | 400 | 2020 | 8.36 | 8.58 | 7.90 | 7.09 | 7.46 | 6.37 | 6.19 | 6.43 | 6.28 | 2.86 | |
| 2021 | tr | tr | 2.68 | tr | 2.77 | tr | tr | 5.40 | tr | tr | ||||
| Pentan-3-one | Ketones | 3.17–49.35 | Ethereal, acetone | 2020 | 0.00 | tr | tr | 107.29 | tr | tr | tr | tr | tr | 80.53 |
| 2021 | 193.61 | tr | tr | 86.49 | 168.58 | 554.97 | 126.64 | tr | 132.93 | 120.07 | ||||
| Hex-4-en-3-one | Ketones | Green | 2020 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Pent-3-en-2-one | Ketones | 15 | Fruity | 2020 | 7.23 | 7.46 | 7.16 | 7.07 | tr | 7.17 | 7.16 | 7.18 | 7.08 | 7.34 |
| 2021 | 7.07 | 7.07 | 7.07 | 7.05 | 7.05 | tr | 3.54 | 7.07 | 7.08 | 7.06 | ||||
| Isobutyl ketone | Ketones | 0.66–1.86 | Green, fruity, pineapple, banana | 2020 | 4.41 | 4.62 | 4.46 | 4.38 | 4.17 | 6.44 | 4.26 | 4.22 | 4.13 | 4.31 |
| 2021 | 36.68 | 36.70 | 31.92 | 33.35 | 34.88 | 34.86 | 30.93 | 35.16 | 33.67 | 26.65 | ||||
| Heptan-2-one | Ketones | 140 | Fruity, sweet, herbal, coconut | 2020 | 16.30 | 16.18 | 13.85 | 22.28 | 6.06 | 11.58 | 21.42 | 12.77 | 18.60 | 17.87 |
| 2021 | 58.24 | 29.84 | 38.77 | 17.51 | 59.33 | 20.72 | 33.37 | 64.68 | 39.52 | 31.86 | ||||
| Acetoin | Ketones | 800 | Sweet, buttery, creamy, dairy, milky, fatty | 2020 | 119.57 | 38.88 | 8.99 | 40.46 | 60.12 | 50.94 | 89.99 | 36.77 | tr | 74.56 |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| 1-Hepten-3-one | Ketones | Metallic | 2020 | 7.38 | 8.56 | 7.78 | 7.37 | 7.55 | 7.51 | 7.69 | 7.61 | 7.46 | 7.56 | |
| 2021 | tr | 7.35 | 7.21 | 3.57 | tr | 7.10 | 7.33 | 7.31 | 7.30 | 7.56 | ||||
| 2-Methyl-3-octanone | Ketones | 2020 | 2.74 | 4.66 | 3.26 | 2.72 | 2.92 | 3.40 | 3.17 | 4.47 | 2.90 | 2.76 | ||
| 2021 | 2.79 | 3.87 | 3.87 | 2.72 | 2.68 | 2.43 | 3.09 | 4.47 | 3.18 | 2.70 | ||||
| Nonan-2-one | Ketones | 5–200 | Sweet, green, earthy, herbal | 2020 | 2.29 | 2.44 | 3.33 | 4.84 | 2.45 | 2.30 | 3.79 | 2.42 | 2.26 | 2.94 |
| 2021 | 15.25 | 5.34 | 3.60 | 8.41 | 33.22 | 4.04 | 6.83 | 11.52 | 4.31 | 9.24 | ||||
| Octan-2-one | Ketones | 5 | Earthy, herbal | 2020 | 1.08 | 2.39 | 1.07 | 1.07 | tr | tr | 2.30 | tr | 2.37 | 1.04 |
| 2021 | 3.71 | 2.87 | 3.59 | 2.24 | 3.39 | 2.55 | 2.96 | 3.58 | 3.05 | 2.97 | ||||
| Styrene | Benzenoid | 730 | Sweet, balsam, floral | 2020 | 7.00 | 9.22 | 7.16 | 6.70 | 6.65 | 6.58 | 6.92 | 7.55 | 6.85 | 7.70 |
| 2021 | 6.24 | 6.01 | 5.91 | 6.01 | 6.41 | 6.47 | 6.00 | 5.98 | 6.05 | 6.85 | ||||
| Ethyl benzenecarboxylate | Benzenoid | 60 | 2020 | 6.15 | 3.56 | 2.29 | 2.73 | 2.58 | 7.62 | 13.89 | 5.79 | 3.80 | 3.75 | |
| 2021 | 2.44 | tr | tr | 2.14 | tr | 2.16 | tr | 1.14 | 3.18 | 1.01 | ||||
| Naphthalene | Benzenoid | Pungent | 2020 | 11.90 | 11.44 | 9.78 | 8.59 | 7.90 | 7.71 | 8.00 | 8.42 | 7.46 | 7.85 | |
| 2021 | 8.73 | 7.89 | 7.43 | 7.38 | 8.30 | 7.97 | 9.28 | 7.88 | 7.46 | 7.54 | ||||
| Analgit | Benzenoid | 2020 | 7.15 | 7.23 | 7.00 | 6.74 | 6.92 | 6.54 | 6.52 | 3.29 | 6.51 | 3.19 | ||
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Ethyl salicylate | Benzenoid | 84 | Sweet, mint, floral, balsam | 2020 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Isobutyl benzoate | Benzenoid | Sweet, fruity, musty, balsam | 2020 | 0.92 | 0.93 | tr | tr | tr | tr | 1.93 | nd | 0.94 | nd | |
| 2021 | 1.99 | tr | tr | tr | tr | tr | tr | nd | 2.08 | nd | ||||
| Benzyl alcohol | Benzenoid | 20,000 | Floral, rose, balsamic | 2020 | 36.91 | 145.54 | 203.53 | nd | nd | nd | 55.37 | 279.77 | 19.87 | 243.59 |
| 2021 | 18.91 | 131.05 | 85.06 | nd | nd | nd | 57.21 | 243.02 | 19.53 | 55.12 | ||||
| Ethyl benzenepropanoate | Benzenoid | 2020 | 1.90 | 2.42 | tr | tr | tr | tr | tr | tr | tr | tr | ||
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| β-Methylnaphthalene | Benzenoid | 2020 | 5.91 | 6.11 | 5.89 | 5.81 | 5.95 | 5.86 | 5.98 | 6.12 | 5.88 | 6.00 | ||
| 2021 | 5.66 | 7.72 | 5.65 | 5.68 | 5.70 | 5.73 | 5.74 | 5.79 | 5.64 | 5.65 | ||||
| α-Calacorene | Benzenoid | 2020 | 6.69 | 9.09 | 7.17 | 8.04 | 5.65 | 8.23 | 8.37 | 8.06 | tr | 9.69 | ||
| 2021 | 7.03 | 7.48 | 25.41 | 5.74 | 6.14 | 20.05 | 9.19 | 7.89 | 6.37 | 10.49 | ||||
| Phenol | Phenols | Plastic, rubber | 2020 | 0.86 | 1.25 | 1.52 | 0.51 | 0.18 | 0.15 | 0.23 | 0.87 | tr | 0.98 | |
| 2021 | 0.20 | 6.09 | 0.47 | 0.38 | 1.72 | 0.59 | 1.09 | 1.54 | 0.33 | 0.04 | ||||
| Eugenol | Phenols | 6 | Sweet | 2020 | nd | 3.11 | 3.77 | nd | nd | 2.99 | tr | tr | 2.86 | tr |
| 2021 | nd | 3.02 | 2.92 | nd | nd | tr | tr | tr | tr | tr | ||||
| 2,4-Bis(1,1-dimethylethyl)phenol | Phenols | 2020 | 50.80 | 30.86 | 43.57 | 42.75 | 32.38 | 61.42 | 60.61 | 68.25 | 52.21 | 68.67 | ||
| 2021 | 22.29 | 18.34 | 11.51 | 21.98 | 42.67 | 44.51 | 24.15 | 32.35 | 29.69 | 29.25 | ||||
| Methyl acetate | Esters | Sweet, fruity | 2020 | 141.00 | 148.25 | 159.91 | 293.07 | 105.51 | 235.13 | 195.28 | 214.77 | 135.67 | 534.11 | |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Ethyl Acetate | Esters | 5000 | Fruity, sweet | 2020 | 8791.03 | 9775.09 | 3351.95 | 2200.36 | 1079.32 | 4631.17 | 5680.06 | 8517.00 | 1302.71 | 7985.80 |
| 2021 | 54.69 | 65.42 | 98.09 | 97.27 | 49.52 | 44.79 | 62.47 | 173.29 | 66.87 | 46.81 | ||||
| Ethyl propanoate | Esters | 10 | Sweet, fruity, grape, pineapple | 2020 | tr | 6.49 | tr | tr | tr | 3.09 | 2.60 | 3.27 | tr | 2.25 |
| 2021 | 0.45 | tr | tr | tr | tr | 0.79 | tr | tr | 0.39 | tr | ||||
| Ethyl 2-methylbutanoate | Esters | 0.006 | Sweet, green, apple, fruity | 2020 | nd | 18.41 | 9.91 | nd | nd | 5.22 | nd | 14.15 | 10.25 | nd |
| 2021 | nd | tr | tr | nd | nd | tr | nd | 9.83 | 9.77 | nd | ||||
| Isoamyl acetate | Esters | 2 | Sweet, fruity, banana | 2020 | nd | 11.62 | nd | tr | nd | tr | 5.09 | 17.15 | 10.41 | 10.73 |
| 2021 | nd | 5.08 | nd | 10.59 | nd | 5.26 | 5.06 | 5.25 | 5.12 | 5.25 | ||||
| Ethyl hexanoate | Esters | 1 | Apple, peel, fruity | 2020 | 10.94 | 18.21 | 11.42 | 9.90 | 10.69 | 9.88 | 10.64 | 12.50 | 9.29 | 11.22 |
| 2021 | 9.54 | 9.89 | 9.84 | 9.52 | 9.56 | 9.60 | 9.31 | 9.59 | 9.50 | 9.43 | ||||
| Hexyl acetate | Esters | 2 | Fruity, apple, banana, green, floral | 2020 | 1.65 | 1.68 | 1.52 | 1.62 | tr | tr | tr | tr | tr | tr |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Ethyl heptanoate | Esters | 2.2 | Fruity, pineapple | 2020 | tr | 0.20 | 0.02 | tr | tr | tr | tr | 0.03 | tr | tr |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Ethyl lactate | Esters | 14,000 | Fruity, buttery | 2020 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Ethyl 2-hexenoate | Esters | Fruity, green, sweet | 2020 | tr | 0.93 | 0.10 | tr | tr | tr | tr | 0.31 | 0.17 | tr | |
| 2021 | 0.78 | 0.13 | 0.09 | tr | 0.01 | tr | 0.04 | 0.22 | 0.17 | 0.19 | ||||
| Ethyl caprate | Esters | Sweet, fruity, apple, grape | 2020 | 63.73 | 34.19 | 11.66 | 23.62 | 37.02 | 26.10 | 57.30 | 22.39 | 3.46 | 82.08 | |
| 2021 | 0.92 | 0.60 | 0.78 | 0.75 | 0.80 | 0.38 | 1.24 | 0.00 | 0.63 | 0.19 | ||||
| Ethyl dodecanoate | Esters | 5900 | Sweet, floral | 2020 | 9.87 | 10.58 | 6.32 | 5.53 | 6.82 | 7.05 | 9.47 | 6.46 | tr | 12.82 |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Eucalyptol | Isoprenoids | Eucalyptus, herbal, camphor | 2020 | 1.00 | 1.06 | 1.02 | 1.01 | 1.03 | 1.04 | 1.04 | 0.97 | 1.03 | 0.97 | |
| 2021 | 0.91 | 0.49 | 0.93 | 1.02 | 1.01 | 0.94 | 1.10 | 0.47 | 0.95 | 0.93 | ||||
| Sulcatone | Isoprenoids | Citrus, green, apple | 2020 | 2.66 | 5.61 | 3.95 | 2.17 | 2.64 | 3.05 | 2.70 | 4.60 | 5.37 | 3.19 | |
| 2021 | 2.41 | 3.47 | 3.03 | 2.15 | 2.34 | 2.14 | 2.44 | 3.41 | 10.26 | 2.82 | ||||
| Camphor | Isoprenoids | 460 | Camphoreous | 2020 | tr | 0.02 | 0.01 | tr | tr | tr | tr | 0.01 | tr | tr |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Linalool | Isoprenoids | 15 | Citrus, floral, sweet, rose, blueberry | 2020 | 0.01 | 0.01 | 0.01 | tr | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | tr |
| 2021 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | tr | 0.01 | tr | ||||
| α-Ionene | Isoprenoids | 2020 | tr | 1.39 | 0.23 | 0.57 | tr | 1.25 | 2.35 | 1.78 | 0.81 | tr | ||
| 2021 | 3.57 | tr | 0.91 | 0.82 | 1.51 | tr | 2.86 | 0.54 | 1.16 | 0.83 | ||||
| Hotrienol | Isoprenoids | Sweet | 2020 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Levomenthol | Isoprenoids | Peppermint, minty | 2020 | tr | 0.95 | 0.93 | 0.93 | 0.92 | 0.93 | 0.93 | 0.94 | 0.92 | 0.95 | |
| 2021 | 0.93 | tr | tr | tr | 0.90 | 0.93 | tr | tr | 0.92 | 0.91 | ||||
| α-Terpineol | Isoprenoids | 330 | Pine, lilac, citrus, woody, floral | 2020 | 0.90 | 0.94 | 0.91 | 0.90 | 0.89 | 0.91 | 0.91 | 0.91 | 0.91 | 0.90 |
| 2021 | 0.91 | 0.90 | 0.92 | 0.90 | 0.90 | 0.89 | 0.90 | 0.89 | 0.90 | 0.44 | ||||
| β-Damascenone | Isoprenoids | 0.0009 | Sweet, fruity, flora, honey, baked apple | 2020 | 1.68 | 3.56 | 2.21 | 1.86 | 1.48 | 3.04 | 3.13 | 5.83 | 2.49 | 1.84 |
| 2021 | 4.98 | 1.96 | 3.91 | 3.11 | 3.36 | 1.84 | 3.57 | 3.35 | 2.70 | 3.56 | ||||
| cis-Geranylacetone | Isoprenoids | 60 | Rose, floral, green, magnolia, fruity | 2020 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 2021 | tr | 62.05 | 61.88 | 124.87 | 124.43 | tr | 62.67 | 62.69 | 66.33 | tr | ||||
| 3,3,5-Trimethylcyclohexene | Others | 2020 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| 3,4,4-Trimethyl-2-cyclopenten-1-one | Others | 2020 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| γ-Caprolactone | Others | Herbal, sweet, tobacco | 2020 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| Compounds | Category | Threshold a | Describe b | Year | JSBZ | TPJDZ | LYLZ | LCYLZ | YCXZ | TZCH | XZHZ | BXJZ | ZYDZ | CXJSXZ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Acid | 2.5–250 | Pungent, sour, vinegar | 2020 | 355.25 | 336.35 | 480.60 | 334.95 | 320.35 | 329.55 | 376.10 | 363.50 | 332.50 | 307.95 |
| 2021 | 317.30 | 330.75 | 317.80 | 502.55 | 502.70 | 333.00 | 367.05 | 322.10 | 363.60 | 411.30 | ||||
| Formic acid | Acid | 450,000 | Vinegar | 2020 | 780.95 | 719.25 | 213.85 | 851.75 | 729.05 | 932.15 | 571.10 | 694.30 | 1377.25 | 464.85 |
| 2021 | 719.20 | 1157.65 | 768.80 | 1195.45 | 1569.40 | 795.55 | 1056.70 | 975.70 | 1006.60 | 854.80 | ||||
| Hexanoic acid | Acid | 3000 | Sour, fatty, cheese | 2020 | 196.15 | 170.40 | 164.25 | 166.20 | 163.60 | 164.65 | 166.30 | 170.00 | 161.90 | 161.20 |
| 2021 | 160.20 | 163.20 | 158.85 | 162.00 | 168.00 | 160.05 | 160.50 | 161.00 | 162.35 | 163.30 | ||||
| Octanoic acid | Acid | 3000 | Fatty, cheese | 2020 | 1140.65 | 1144.15 | 1138.85 | 1138.15 | 1136.10 | 1140.30 | 1162.05 | 1165.35 | 1139.55 | 1131.25 |
| 2021 | 1139.70 | 1134.95 | 1133.75 | 1140.40 | 1148.65 | 1136.55 | 1134.15 | 1134.65 | 1127.60 | 1163.55 | ||||
| Nonanoic acid | Acid | 3000 | Cheese | 2020 | 1266.05 | 1316.95 | 1298.70 | 1306.30 | 1292.00 | 1299.45 | 1344.35 | 1377.15 | 1312.25 | 1264.80 |
| 2021 | 1298.80 | 1228.05 | 1270.25 | 1239.90 | 1290.20 | 1305.90 | 1251.45 | 1276.40 | 1200.05 | 1338.15 | ||||
| Decanoic acid | Acid | 10,000 | Rancid, sour, fatty, citrus | 2020 | 1241.65 | 1207.95 | 1199.95 | 1203.80 | 1194.60 | 1188.95 | 1212.65 | 1217.25 | 1525.00 | 1185.25 |
| 2021 | 1200.15 | 1170.15 | 1206.15 | 1179.90 | 1199.70 | 1194.35 | 1232.50 | 1191.10 | 1133.30 | 1263.50 | ||||
| 3-Methyl-butan-2-ol | Alcohol | 250–300 | Fruity | 2020 | 260.10 | 277.20 | 284.60 | 279.50 | 283.15 | 284.50 | 295.20 | 273.15 | 281.80 | 286.25 |
| 2021 | 270.50 | 250.15 | 269.50 | 237.45 | 240.05 | 256.70 | 269.05 | 263.45 | 244.00 | 260.35 | ||||
| Hexan-2-ol | Alcohol | Chemical, fruity, fatty | 2020 | 30.40 | 31.30 | 30.90 | 30.85 | 31.55 | 31.40 | 33.05 | 32.80 | 31.50 | 31.35 | |
| 2021 | 30.55 | 30.30 | 30.35 | 30.55 | 30.15 | 30.10 | 30.70 | 30.25 | 31.10 | 30.55 | ||||
| 2,7-Dimethyl-4-octanol | Alcohol | 2020 | 1.90 | 1.85 | 1.85 | 1.85 | 1.85 | 1.90 | 1.90 | 1.95 | 1.85 | 1.85 | ||
| 2021 | 1.85 | 1.90 | 1.85 | 2.00 | 2.05 | 1.85 | 1.85 | 1.85 | 2.10 | 1.95 | ||||
| 4-Methyl-2-heptanol | Alcohol | 2020 | 8.40 | 8.60 | 8.75 | 8.40 | 8.40 | 8.70 | 9.35 | 9.05 | 8.60 | 8.15 | ||
| 2021 | 8.40 | 8.65 | 7.90 | 9.95 | 9.80 | 8.20 | 8.35 | 8.55 | 10.50 | 8.20 | ||||
| 5-Methyl-2-heptanol | Alcohol | 2020 | 2.30 | 2.35 | 2.40 | 2.30 | 2.35 | 2.40 | 2.45 | 2.45 | 2.35 | 2.35 | ||
| 2021 | 2.30 | 2.30 | 2.25 | 2.50 | 2.50 | 2.30 | 2.30 | 2.35 | 2.70 | 2.30 | ||||
| Dodecan-5-ol | Alcohol | 2020 | 1.80 | 1.80 | 1.80 | 1.80 | 1.80 | 1.85 | 1.90 | 1.90 | 1.85 | 1.80 | ||
| 2021 | 1.80 | 1.80 | 1.75 | 1.95 | 1.95 | 1.80 | 1.80 | 1.80 | 2.10 | 1.90 | ||||
| Oct-1-en-3-ol | Alcohol | 1 | Mushroom, earthy, green | 2020 | 2.20 | 1.60 | 1.25 | 1.60 | 1.30 | 1.35 | 1.50 | 3.20 | 0.95 | 2.00 |
| 2021 | 1.60 | 1.55 | 1.20 | 1.85 | 2.50 | 0.95 | 1.60 | 1.75 | 3.00 | 2.85 | ||||
| 2-Ethyl-1-hexanol | Alcohol | 270,000 | Citrus, floral, sweet | 2020 | 74.90 | 51.30 | 71.70 | 102.00 | 41.55 | 86.45 | 159.50 | 63.30 | 78.70 | 54.25 |
| 2021 | 59.90 | 80.35 | 62.35 | 40.80 | 39.35 | 86.45 | 33.60 | 145.50 | 56.90 | 34.15 | ||||
| Dodecan-1-ol | Alcohol | 0.0152–0.0533 | Earthy, soapy, waxy, fatty | 2020 | 0.90 | 0.85 | 0.95 | 0.85 | 0.85 | 0.90 | 0.95 | 1.00 | 0.90 | 0.85 |
| 2021 | 0.85 | 0.80 | 0.75 | 0.90 | 0.75 | 0.75 | 0.75 | 0.70 | 0.95 | 0.90 | ||||
| Undecan-1-ol | Alcohol | Waxy, rose, soapy, floral, citrus | 2020 | 1.01 | 1.14 | 1.12 | 1.25 | 1.05 | 1.21 | 1.15 | 1.45 | 1.13 | 1.11 | |
| 2021 | 1.22 | 0.95 | 1.05 | 1.20 | 1.15 | 1.05 | 1.20 | 1.23 | 1.52 | 1.25 | ||||
| 2,4-Bis(1,1-dimethylethyl)phenol | Phenols | 2020 | 1748.65 | 1543.65 | 1798.60 | 1732.45 | 1722.20 | 2057.20 | 2096.55 | 1982.05 | 2256.25 | 1805.95 | ||
| 2021 | 1962.50 | 1825.05 | 1714.65 | 2007.65 | 1727.50 | 1599.85 | 1847.10 | 1551.10 | 3648.40 | 1395.30 | ||||
| Hotrienol | Terpenoids | Sweet | 2020 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | |
| 2021 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr | ||||
| Levomenthol | Terpenoids | Peppermint, minty | 2020 | 4.65 | 0.00 | 4.70 | 4.60 | 4.65 | 4.75 | 6.20 | 6.10 | 4.70 | 4.65 | |
| 2021 | 4.75 | 4.70 | 4.65 | 4.95 | 4.85 | 4.65 | 4.85 | 4.65 | 4.95 | 5.20 |
| Compounds (μg/L) | Category | Threshold | Describe | Aroma Feature | Year | JSBZ | TPJDZ | LYLZ | LCYLZ | YCXZ | TZCH | XZHZ | BXJZ | ZYDZ | CXJSXZ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Acid | 2.5–250 | Pungent, sour, vinegar | Fatty | 2020 | 1.42 | 1.35 | 1.92 | 1.34 | 1.28 | 1.32 | 1.50 | 1.45 | 1.33 | 1.23 |
| 2021 | 1.27 | 1.32 | 1.27 | 2.01 | 2.01 | 1.33 | 1.47 | 1.29 | 1.45 | 1.65 | |||||
| Oct-1-en-3-ol | Alcohol | 1 | Mushroom, earthy, green | Green, Earthy | 2020 | 2.20 | 1.60 | 1.25 | 1.60 | 1.30 | 1.35 | 1.50 | 3.20 | 2.00 | |
| 2021 | 1.60 | 1.55 | 1.20 | 1.85 | 2.50 | 1.60 | 1.75 | 3.00 | 2.85 | ||||||
| Dodecan-1-ol | Alcohol | 0.0152–0.0533 | Earthy, soapy, waxy, fatty | Fatty, Earthy | 2020 | 16.89 | 15.95 | 17.82 | 15.95 | 15.95 | 16.89 | 17.82 | 18.76 | 16.89 | 15.95 |
| 2021 | 15.95 | 15.01 | 14.07 | 16.89 | 14.07 | 14.07 | 14.07 | 13.13 | 17.82 | 16.89 |
| Compounds (μg/L) | Category | Threshold | Describe | Aroma Feature | Year | JSBZ | TPJDZ | LYLZ | LCYLZ | YCXZ | TZCH | XZHZ | BXJZ | ZYDZ | CXJSXZ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetic acid | Acid | 2.5–250 | Pungent, sour, vinegar | Fatty | 2020 | 23.39 | 22.71 | 17.57 | 12.12 | 14.90 | 20.17 | 32.06 | 32.35 | 13.76 | 18.68 |
| 2021 | 15.77 | 18.01 | 12.01 | 10.40 | 14.62 | 7.65 | 14.05 | 24.53 | 11.29 | 9.47 | |||||
| Isovaleric acid | Acid | 120–700 | Sour, stinky, sweaty, cheese | Fatty | 2020 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.98 | 0.00 | 0.00 |
| 2021 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.08 | 0.00 | 0.00 | |||||
| Pent-1-en-3-ol | Alcohol | 400.00 | Green, vegetable, tropical fruity | Fruity, green | 2020 | 3.84 | 4.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2021 | 1.72 | 2.05 | 1.36 | 1.48 | 2.24 | 0.00 | 1.01 | 2.02 | 1.63 | 0.00 | |||||
| Oct-1-en-3-ol | Alcohol | 1.00 | Mushroom, earthy, green | Green, earthy | 2020 | 1.81 | 5.20 | 4.46 | 2.46 | 3.10 | 1.95 | 3.15 | 4.36 | 2.66 | 2.73 |
| 2021 | 2.27 | 3.58 | 2.64 | 1.70 | 3.50 | 0.00 | 4.32 | 3.96 | 2.36 | 2.85 | |||||
| Isopentanal | Aldehyde | 0.2–2 | Chocolate, peach, fatty | Fruity, fatty | 2020 | 18.69 | 61.80 | 25.33 | 11.03 | 7.79 | 8.19 | 15.16 | 156.36 | 9.17 | 25.87 |
| 2021 | 5.46 | 6.21 | 6.31 | 5.20 | 6.21 | 4.63 | 6.96 | 75.03 | 5.14 | 6.64 | |||||
| Pentanal | Aldehyde | 12.00 | Fruity, nutty, berry | Fruity | 2020 | 5.06 | 15.61 | 11.65 | 7.27 | 3.26 | 8.08 | 5.70 | 13.14 | 6.08 | 5.31 |
| 2021 | 0.00 | 25.28 | 18.80 | 4.94 | 0.00 | 0.00 | 0.00 | 22.96 | 10.77 | 10.54 | |||||
| Hexanal | Aldehyde | 4.50 | Grass-like, green | Green | 2020 | 8.73 | 24.10 | 19.46 | 10.92 | 5.78 | 58.47 | 9.01 | 14.62 | 13.13 | 12.66 |
| 2021 | 19.87 | 33.47 | 19.21 | 12.36 | 30.20 | 8.32 | 15.70 | 24.38 | 19.01 | 22.68 | |||||
| Heptanal | Aldehyde | 3.00 | Fatty, green, herbal | Green, fatty | 2020 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2021 | 0.00 | 3.90 | 3.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.25 | 0.00 | |||||
| (E)-Hex-2-en-1-al | Aldehyde | 17.00 | Green, banana, fatty, cheesy | Fruity, green, fatty | 2020 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2021 | 1.04 | 0.00 | 0.00 | 0.00 | 1.13 | 0.00 | 1.03 | 0.00 | 0.00 | 0.00 | |||||
| Octanal | Aldehyde | 0.70 | Citrus, orange, green, fatty | Fruity, green, fatty | 2020 | 0.00 | 7.93 | 5.64 | 5.89 | 0.00 | 6.87 | 10.69 | 7.36 | 4.26 | 0.00 |
| 2021 | 3.96 | 4.46 | 4.04 | 3.43 | 0.00 | 3.86 | 0.00 | 4.14 | 4.59 | 4.53 | |||||
| Nonanal | Aldehyde | 1.00 | Rose, orange | Fruity, floral | 2020 | 0.00 | 4.33 | 3.54 | 2.60 | 0.00 | 2.60 | 2.94 | 3.55 | 3.02 | 0.00 |
| 2021 | 4.26 | 5.66 | 4.42 | 3.33 | 2.61 | 4.33 | 2.80 | 4.19 | 6.21 | 4.99 | |||||
| (E)-2-Oct-en-1-al | Aldehyde | 0.10 | Cucumber, green, herbal, fatty | Green, fatty | 2020 | 76.20 | 101.60 | 89.80 | 75.60 | 77.00 | 81.60 | 78.20 | 84.50 | 82.20 | 79.40 |
| 2021 | 71.90 | 76.10 | 74.00 | 72.00 | 72.50 | 72.40 | 73.80 | 75.60 | 76.00 | 80.50 | |||||
| Decanal | Aldehyde | 0.10 | Oil, orange, peel | Fruity, fatty | 2020 | 5.80 | 16.80 | 11.40 | 6.50 | 1.70 | 7.80 | 9.30 | 13.60 | 9.50 | 4.80 |
| 2021 | 42.90 | 40.20 | 21.80 | 181.90 | 36.80 | 33.10 | 22.80 | 24.90 | 425.40 | 15.10 | |||||
| Non-2-en-1-al | Aldehyde | 0.08–0.1 | Fatty, green, cucumber | Green, fatty | 2020 | 22.70 | 28.10 | 25.20 | 22.30 | 22.70 | 23.30 | 24.20 | 25.00 | 23.50 | 22.80 |
| 2021 | 22.10 | 22.70 | 22.40 | 32.60 | 22.10 | 21.80 | 22.70 | 23.30 | 45.30 | 23.10 | |||||
| (E)-Dec-2-en-1-al | Aldehyde | 0.3–0.4 | Fatty, earthy, coriander, green, mushroom | Green, fatty, earthy | 2020 | 3.38 | 14.43 | 11.58 | 4.48 | 0.00 | 0.00 | 3.43 | 5.78 | 8.75 | 3.33 |
| 2021 | 0.00 | 0.00 | 0.00 | 0.00 | 2.70 | 2.73 | 2.65 | 3.00 | 3.15 | 3.43 | |||||
| Pentan-3-one | Ketones | 3.17–49.35 | Ethereal, acetone | Chemical | 2020 | 0.00 | 0.00 | 0.00 | 2.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.63 |
| 2021 | 3.92 | 0.00 | 0.00 | 1.75 | 3.42 | 11.25 | 2.57 | 0.00 | 2.69 | 2.43 | |||||
| Isobutyl ketone | Ketones | 0.66–1.86 | Green, fruity, pineapple, banana | Fruity, green | 2020 | 2.37 | 2.48 | 2.40 | 2.35 | 2.24 | 3.46 | 2.29 | 2.27 | 2.22 | 2.32 |
| 2021 | 19.72 | 19.73 | 17.16 | 17.93 | 18.75 | 18.74 | 16.63 | 18.90 | 18.10 | 14.33 | |||||
| Methyl benzoate | Benzene | 0.52 | Floral | Floral | 2020 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.21 | 0.00 | 0.00 | 0.00 |
| 2021 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| Ethyl Acetate | Esters | 5000.00 | Fruity, sweet | Fruity, sweet | 2020 | 1.76 | 1.96 | 0.00 | 0.00 | 0.00 | 0.00 | 1.14 | 1.70 | 0.00 | 1.60 |
| 2021 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||
| Ethyl 2-methylbutanoate | Esters | 0.01 | Sweet, green, apple, fruity | Fruity, sweet, green | 2020 | 0.00 | 3068.33 | 1651.67 | 0.00 | 0.00 | 870.00 | 0.00 | 2358.33 | 1708.33 | 0.00 |
| 2021 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1638.33 | 1628.33 | 0.00 | |||||
| Isoamyl acetate | Esters | 2.00 | Sweet, fruity, banana | Fruity, sweet | 2020 | 0.00 | 5.81 | 0.00 | 0.00 | 0.00 | 0.00 | 2.55 | 8.58 | 5.21 | 5.37 |
| 2021 | 0.00 | 2.54 | 0.00 | 5.30 | 0.00 | 2.63 | 2.53 | 2.63 | 2.56 | 2.63 | |||||
| Ethyl hexanoate | Esters | 1.00 | Apple, peel, fruity | Fruity | 2020 | 10.94 | 18.21 | 11.42 | 9.90 | 10.69 | 9.88 | 10.64 | 12.50 | 9.29 | 11.22 |
| 2021 | 9.54 | 9.89 | 9.84 | 9.52 | 9.56 | 9.60 | 9.31 | 9.59 | 9.50 | 9.43 | |||||
| β-Damascenone | Terpenoids | 0.00 | Sweet, fruity, flora, honey, baked apple | Fruity, floral, sweet | 2020 | 1866.67 | 3955.56 | 2455.56 | 2066.67 | 1644.44 | 3377.78 | 3477.78 | 6477.78 | 2766.67 | 2044.44 |
| 2021 | 5533.33 | 2177.78 | 4344.44 | 3455.56 | 3733.33 | 2044.44 | 3966.67 | 3722.22 | 3000.00 | 3955.56 | |||||
| cis-Geranylacetone | Terpenoids | 60.00 | Rose, floral, green, magnolia, fruity | Fruity, floral, green | 2020 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2021 | 0.00 | 1.03 | 1.03 | 2.08 | 2.07 | 0.00 | 1.04 | 1.04 | 1.11 | 0.00 |
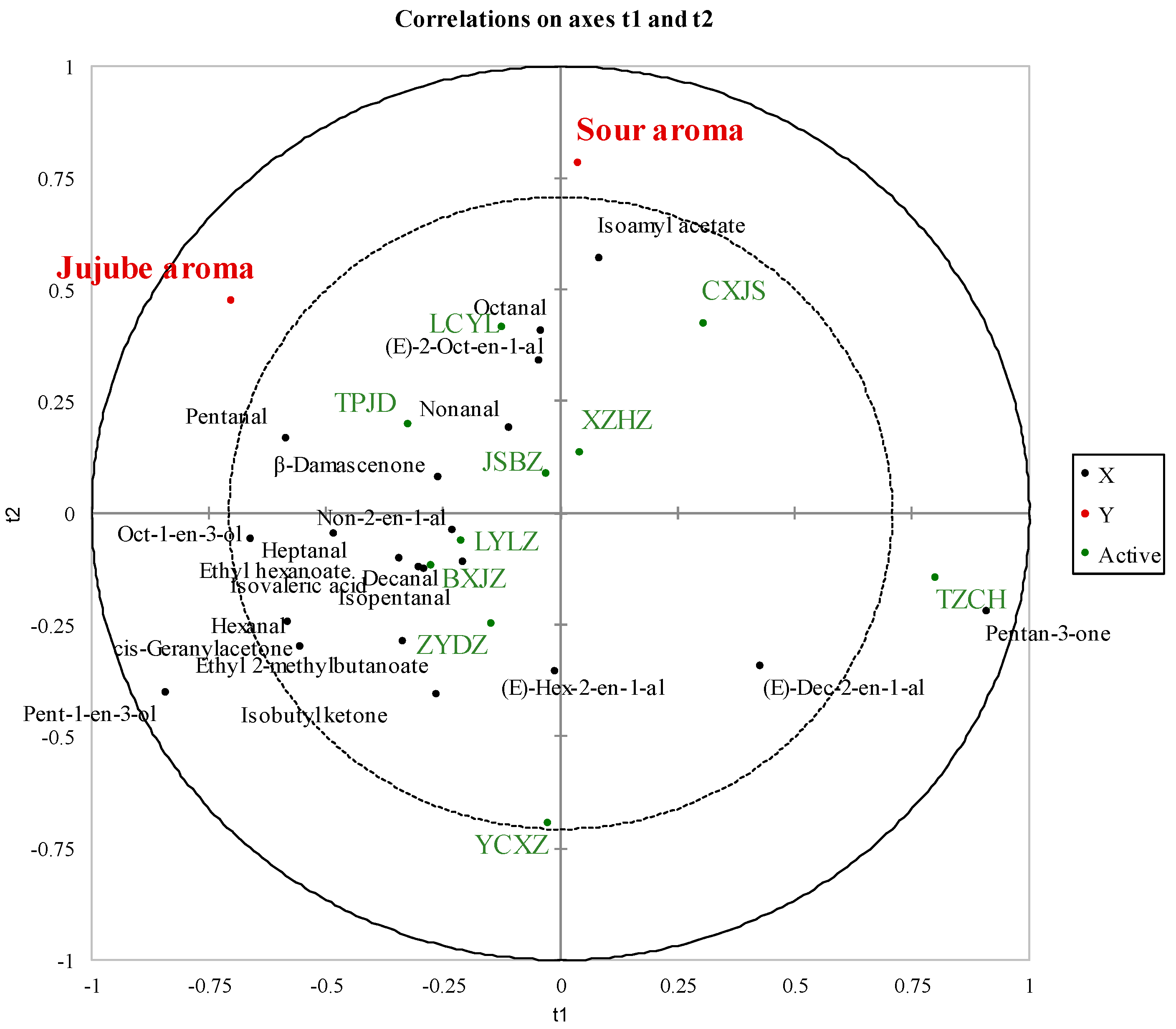
2.3. Correlation between Volatiles and Sensory Attributes in Jujube Using PLSR
3. Materials and Methods
3.1. Materials
3.2. Reagents
3.3. Sample Pre-Treatment
3.4. Free Volatile Extraction
3.5. Bound Volatile Extraction
3.6. GC-MS Analysis
3.7. Quantitative and OAVs Calculation
3.8. Sensory Evaluation
3.9. Statistical Analysis Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, S. Past, Present, and Future of Jujubes—Chinese Dates in the United States. HortScience 2013, 48, 672–680. [Google Scholar] [CrossRef]
- Sheng, J.P.; Shen, L. Chinese Jujube (Ziziphus jujuba Mill.) and Indian Jujube (Ziziphus mauritiana Lam.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2011; pp. 299–326. [Google Scholar] [CrossRef]
- Al-Saeedi, A.H.; Al-Ghafri, M.T.H.; Hossain, M.A. Comparative Evaluation of Total Phenols, Flavonoids Content and Antioxidant Potential of Leaf and Fruit Extracts of Omani Ziziphus jujuba L. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 78–83. [Google Scholar] [CrossRef]
- Jin, X. Jujuba—Ziziphus jujuba. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 263–269. [Google Scholar] [CrossRef]
- Abdoul-Azize, S. Potential Benefits of Jujube (Zizyphus lotus L.) Bioactive Compounds for Nutrition and Health. J. Nutr. Metab. 2016, 2016, 2867470. [Google Scholar] [CrossRef] [PubMed]
- Rashwan, A.K.; Karim, N.; Shishir, M.R.I.; Bao, T.; Lu, Y.; Chen, W. Jujube Fruit: A Potential Nutritious Fruit for the Development of Functional Food Products. J. Funct. Foods 2020, 75, 104205. [Google Scholar] [CrossRef]
- Kuscu, A.; Bulantekin, Ö. Determination of Phenolics, Organic Acids, Minerals and Volatile Compounds of Jujube (Ziziphus jujuba Miller) Jam Produced by under Vacuum Evaporation Compared with Open Pan Method. J. Food Meas. Charact. 2021, 15, 1–12. [Google Scholar] [CrossRef]
- Chen, K.; Fan, D.; Fu, B.; Zhou, J.; Li, H. Comparison of Physical and Chemical Composition of Three Chinese Jujube (Ziziphus jujuba Mill.) Cultivars Cultivated in Four Districts of Xinjiang Region in China. Food Sci. Technol. 2018, 39, 912–921. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Wang, W.; Zheng, F.; Chen, F. Comparison of Volatile Compositions of 15 Different Varieties of Chinese Jujube (Ziziphus jujuba Mill.). J. Food Sci. Technol. 2019, 56, 1631–1640. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Li, P.; Xi, H.; Wu, L.; Zhang, J.; Peng, G.; Su, Y. Direct Analysis of Volatile Components from Intact Jujube by Carbon Fiber Ionization Mass Spectrometry. BMC Chem. 2019, 13, 125. [Google Scholar] [CrossRef]
- Hjelmeland, A.K.; Ebeler, S.E. Glycosidically Bound Volatile Aroma Compounds in Grapes and Wine: A Review. Am. J. Enol. Vitic. 2015, 66, 1–11. [Google Scholar] [CrossRef]
- Song, J.; Bi, J.; Chen, Q.; Wu, X.; Lyu, Y.; Meng, X. Assessment of Sugar Content, Fatty Acids, Free Amino Acids, and Volatile Profiles in Jujube Fruits at Different Ripening Stages. Food Chem. 2019, 270, 344–352. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of Volatile Profile from Ten Different Varieties of Chinese Jujubes by HS-SPME/GC-MS Coupled with E-Nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Han, J.; Fu, L.; Shang, H.; Yang, L. Assessment of Characteristics Aroma of Heat Pump Drying (HPD) Jujube Based on HS-SPME/GC-MS and e-Nose. J. Food Compos. Anal. 2022, 110, 104402. [Google Scholar] [CrossRef]
- Liu, Y.; Sang, Y.; Guo, J.; Zhang, W.; Zhang, T.; Wang, H.; Cheng, S.; Chen, G. Analysis of Volatility Characteristics of Five Jujube Varieties in Xinjiang Province, China, by HS-SPME-GC/MS and E-Nose. Food Sci. Nutr. 2021, 9, 6617–6626. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Bi, J.; Chen, Q.; Wu, X.; Gou, M.; Hou, H.; Jin, X.; Purcaro, G. Volatile Profile Characterization of Winter Jujube from Different Regions via HS-SPME-GC/MS and GC-IMS. J. Food Qual. 2021, 2021, 9958414. [Google Scholar] [CrossRef]
- Yan, X.; Pan, S.; Liu, X.; Tan, M.; Zheng, X.; Du, W.; Wu, M.; Song, Y. Profiling the Major Aroma-Active Compounds of Microwave-Dried Jujube Slices through Molecular Sensory Science Approaches. Foods 2023, 12, 3012. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Ren, J.; Yuan, G.; Li, Y.; Zhang, B.; Zhu, B. Characterization of Free and Bound Volatile Compounds in Six Ribes nigrum L. Blackcurrant Cultivars. Food Res. Int. 2018, 103, 301–315. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Wang, X.; Wang, R.; Ren, F.; Zhang, Q.; Shan, Y.; Ding, S. Characterization of Volatile Component Changes in Jujube Fruits during Cold Storage by Using Headspace-Gas Chromatography-Ion Mobility Spectrometry. Molecules 2019, 24, 3904. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Methyl Jasmonate in Conjunction with Ethanol Treatment Increases Antioxidant Capacity, Volatile Compounds and Postharvest Life of Strawberry Fruit. Eur. Food Res. Technol. 2005, 221, 731–738. [Google Scholar] [CrossRef]
- Neri, F.; Cappellin, L.; Spadoni, A.; Cameldi, I.; Algarra Alarcon, A.; Aprea, E.; Romano, A.; Gasperi, F.; Biasioli, F. Role of Strawberry Volatile Organic Compounds in the Development of Botrytis Cinerea Infection. Plant Pathol. 2015, 64, 709–717. [Google Scholar] [CrossRef]
- Qin, G.; Tao, S.; Zhang, H.; Huang, W.; Wu, J.; Xu, Y.; Zhang, S. Evolution of the Aroma Volatiles of Pear Fruits Supplemented with Fatty Acid Metabolic Precursors. Molecules 2014, 19, 20183–20196. [Google Scholar] [CrossRef]
- Hernández, F.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A.; Legua, P. Physico-Chemical, Nutritional, and Volatile Composition and Sensory Profile of Spanish Jujube (Ziziphus jujuba Mill.) Fruits. J. Sci. Food Agric. 2016, 96, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Galindo, A.; Noguera-Artiaga, L.; Cruz, Z.N.; Burló, F.; Hernández, F.; Torrecillas, A.; Carbonell-Barrachina, Á.A. Sensory and Physico-Chemical Quality Attributes of Jujube Fruits as Affected by Crop Load. LWT Food Sci. Technol. 2015, 63, 899–905. [Google Scholar] [CrossRef]
- Song, J.; Chen, Q.; Bi, J.; Meng, X.; Wu, X.; Qiao, Y.; Lyu, Y. GC/MS Coupled with MOS e-Nose and Flash GC e-Nose for Volatile Characterization of Chinese Jujubes as Affected by Different Drying Methods. Food Chem. 2020, 331, 127201. [Google Scholar] [CrossRef] [PubMed]
- Reche, J.; Hernández, F.; Almansa, M.S.; Carbonell-Barrachina, Á.A.; Legua, P.; Amorós, A. Physicochemical and Nutritional Composition, Volatile Profile and Antioxidant Activity Differences in Spanish Jujube Fruits. LWT Food Sci. Technol. 2018, 98, 1–8. [Google Scholar] [CrossRef]
- Sun, X.; Gu, D.; Fu, Q.; Gao, L.; Shi, C.; Zhang, R.; Qiao, X. Content Variations in Compositions and Volatile Component in Jujube Fruits during the Blacking Process. Food Sci. Nutr. 2019, 7, 1387–1395. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, J.; Wang, Y.; Wang, X.; Chen, F.; Wang, X. Characterization of Aroma-Impact Compounds in Dry Jujubes (Ziziphus jujube Mill.) by Aroma Extract Dilution Analysis (AEDA) and Gas Chromatography-Mass Spectrometer (GC-MS). Int. J. Food Prop. 2018, 21, 1844–1853. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.; Xiao, Z.; Niu, Y. Characterization of the Key Aroma Compounds in Mulberry Fruits by Application of Gas Chromatography-Olfactometry (GC-O), Odor Activity Value (OAV), Gas Chromatography-Mass Spectrometry (GC-MS) and Flame Photometric Detection (FPD). Food Chem. 2018, 245, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, B.; Zhu, B.; Lan, Y.; Gao, Y.; Wang, D.; Reeves, M.J.; Duan, C. Differences in Volatile Profiles of Cabernet Sauvignon Grapes Grown in Two Distinct Regions of China and Their Responses to Weather Conditions. Plant Physiol. Biochem. 2015, 89, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Baldwin, E.A.; Plotto, A.; Luo, W.; Raithore, S.; Yu, Z.; Bai, J. Effect of Methyl Salicylate and Methyl Jasmonate Pre-Treatment on the Volatile Profile in Tomato Fruit Subjected to Chilling Temperature. Postharvest Biol. Technol. 2015, 108, 28–38. [Google Scholar] [CrossRef]
- Wiśniewska, P.; Śliwińska, M.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The Analysis of Raw Spirits—A Review of Methodology. J. Inst. Brew. 2016, 122, 5–10. [Google Scholar] [CrossRef]
- Smit, B.A.; Engels, W.J.M.; Smit, G. Branched Chain Aldehydes: Production and Breakdown Pathways and Relevance for Flavour in Foods. Appl. Microbiol. Biotechnol. 2009, 81, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; He, F.; Zhu, B.; Lan, Y.; Pan, Q.; Li, C.; Reeves, M.J.; Wang, J. Free and Glycosidically Bound Aroma Compounds in Cherry (Prunus avium L.). Food Chem. 2014, 152, 29–36. [Google Scholar] [CrossRef]
- Wang, D.; Cai, J.; Zhu, B.; Wu, G.; Duan, C.; Chen, G.; Shi, Y. Study of Free and Glycosidically Bound Volatile Compounds in Air-Dried Raisins from Three Seedless Grape Varieties Using HS–SPME with GC–MS. Food Chem. 2015, 177, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.V.; Quek, S.Y.; Stevenson, R.J.; Winz, R.A. Characterisation of Bound Volatile Compounds of a Low Flavour Kiwifruit Species: Actinidia eriantha. Food Chem. 2012, 134, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhu, B.; Wang, Y.; Lu, L.; Lan, Y.; Reeves, M.; Duan, C. Influence of Pre-Fermentation Cold Maceration Treatment on Aroma Compounds of Cabernet Sauvignon Wines Fermented in Different Industrial Scale Fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhu, Y.; Kortesniemi, M.; Zhu, B.; Li, H. Aromatic Characteristics of Passion Fruit Wines Measured by E-Nose, GC-Quadrupole MS, GC-Orbitrap-MS and Sensory Evaluation. Foods 2022, 11, 3789. [Google Scholar] [CrossRef]
| Compounds (μg/L) | Category | Standard | Qualitative a | Curve | RI b | Quantify c |
|---|---|---|---|---|---|---|
| Acetic acid | Acid | Butyric Acid | B | y = 21,865x + 42.766 | 1433 | 43 |
| Butyric acid | Acid | Butyric Acid | A | y = 21,865x + 42.766 | 1620 | 60 |
| Isovaleric acid | Acid | Butyric Acid | B | y = 21,865x + 42.766 | 1660 | 60 |
| Formic acid | Acid | Butyric Acid | B | y = 21,865x + 42.766 | 1494 | 46 |
| Valeric acid | Acid | Butyric Acid | B | y = 21,865x + 42.766 | 1711 | 60 |
| Hexanoic acid | Acid | Hexanoic acid | A | y = 8519.9x + 30.542 | 1829 | 60 |
| Octanoic acid | Acid | Octanoic Acid | A | y = 35,255x + 219.26 | 2035 | 60 |
| Nonanoic acid | Acid | Octanoic Acid | B | y = 35,255x + 219.26 | 2138 | 60 |
| Decanoic acid | Acid | Octanoic Acid | B | y = 35,255x + 219.26 | 2265 | 60 |
| Dodecanoic acid | Acid | Octanoic Acid | B | y = 35,255x + 219.26 | 2503 | 73 |
| 3-Methyl-butan-2-ol | Alcohol | Isopentanol | B | y = 19,779x + 31.211 | 1100 | 45 |
| Hexan-2-ol | Alcohol | Hexan-1-ol | B | y = 1546.3x − 5.0952 | 1219 | 45 |
| 2,7-Dimethyl-4-octanol | Alcohol | Octan-3-ol | B | y = 238.79x + 0.2758 | 1336 | 69 |
| 4-Methyl-2-heptanol | Alcohol | Octan-3-ol | B | y = 238.79x + 0.2758 | 1349 | 45 |
| 5-Methyl-2-heptanol | Alcohol | Octan-3-ol | B | y = 238.79x + 0.2758 | 1353 | 45 |
| Dodecan-5-ol | Alcohol | Octan-3-ol | B | y = 238.79x + 0.2758 | 1390 | 69 |
| Ethyl alcohol | Alcohol | Isopentanol | B | y = 19,779x + 31.211 | 930 | 31 |
| Pent-1-en-3-ol | Alcohol | Isopentanol | B | y = 19,779x + 31.211 | 1164 | 57 |
| Methyl-2-butan-1-ol | Alcohol | Isopentanol | B | y = 19,779x + 31.211 | 1208 | 70 |
| Oct-1-en-3-ol | Alcohol | (E)-Hex-3-en-1-ol | B | y = 728.12x + 0.0508 | 1428 | 57 |
| 2-Ethyl-1-hexanol | Alcohol | Octan-3-ol | B | y = 238.79x + 0.2758 | 1466 | 57 |
| Dodecan-1-ol | Alcohol | Octan-1-ol | B | y = 70.259x + 0.1283 | 1953 | 43 |
| Undecan-1-ol | Alcohol | Octan-1-ol | B | y = 70.259x + 0.1283 | 1889 | 69 |
| Isopentanal | Aldehyde | Hexanal | B | y = 7081x + 8.15 | 906 | 58 |
| (E)-But-2-en-1-al | Aldehyde | (E)-Hex-2-en-1-al | B | y = 117.26x +7.04 | 1062 | 70 |
| Hexanal | Aldehyde | Hexanal | A | y = 7081x + 8.15 | 1098 | 56 |
| Heptanal | Aldehyde | Hexanal | B | y = 7081x + 8.15 | 1199 | 57 |
| (E)-Hex-2-en-1-al | Aldehyde | (E)-Hex-2-en-1-al | A | y = 117.26x +7.04 | 1228 | 41 |
| Octanal | Aldehyde | Nonanal | B | y = 648.61x + 1.9932 | 1287 | 43 |
| (Z)-Hept-2-en-1-al | Aldehyde | (E)-Hex-2-en-1-al | B | y = 117.26x + 7.04 | 1321 | 41 |
| Nonanal | Aldehyde | Nonanal | A | y = 648.61x + 1.9932 | 1382 | 57 |
| (E)-2-Oct-en-1-al | Aldehyde | (E)-Hex-2-en-1-al | B | y = 117.26x + 7.04 | 1416 | 41 |
| Decanal | Aldehyde | Decanal | A | y = 2272.1x − 0.2855 | 1491 | 43 |
| Non-2-en-1-al | Aldehyde | Nonanal | B | y = 648.61x + 1.9932 | 1516 | 43 |
| (E)-Dec-2-en-1-al | Aldehyde | Nonanal | B | y = 648.61x + 1.9932 | 1646 | 43 |
| Benzaldehyde | Aldehyde | Benzaldehyde | A | y = 1782x + 1.4342 | 1512 | 106 |
| Cumaldehyde | Aldehyde | Styrene | B | y = 1228x + 5.2634 | 1781 | 133 |
| Pentan-3-one | Ketones | Hexanal | B | y = 7081x + 8.15 | 996 | 57 |
| Hex-4-en-3-one | Ketones | (E)-Hex-2-en-1-al | B | y = 117.26x +7.04 | 1197 | 69 |
| Pent-3-en-2-one | Ketones | (E)-Hex-2-en-1-al | B | y = 117.26x +7.04 | 1139 | 69 |
| Isobutyl ketone | Ketones | Nonanal | B | y = 648.61x + 1.9932 | 1168 | 57 |
| Heptan-2-one | Ketones | Hexanal | B | y = 7081x + 8.15 | 1195 | 43 |
| Acetoin | Ketones | Hexanal | B | y = 7081x + 8.15 | 1285 | 45 |
| 1-Hepten-3-one | Ketones | (E)-Hex-2-en-1-al | B | y = 117.26x + 7.04 | 1298 | 55 |
| 2-Methyl-3-octanone | Ketones | Nonanal | B | y = 648.61x + 1.9932 | 1318 | 43 |
| Nonan-2-one | Ketones | Nonanal | B | y = 648.61x + 1.9932 | 1379 | 43 |
| Octan-2-one | Ketones | Nonanal | B | y = 648.61x + 1.9932 | 1283 | 43 |
| Styrene | Benzenoid | Styrene | A | y = 1228x + 5.2634 | 1262 | 104 |
| Ethyl benzenecarboxylate | Benzenoid | Ethyl salicylate | B | y = 537.33x + 1.7861 | 1666 | 105 |
| Naphthalene | Benzenoid | Styrene | B | y = 1228x + 5.2634 | 1741 | 128 |
| Analgit | Benzenoid | Analgit | A | y = 378.52x + 6.2031 | 1755 | 120 |
| Ethyl salicylate | Benzenoid | Ethyl salicylate | A | y = 537.33x + 1.7861 | 1793 | 120 |
| Isobutyl benzoate | Benzenoid | Ethyl salicylate | B | y = 537.33x + 1.7861 | 1835 | 105 |
| Benzyl alcohol | Benzenoid | Benzyl alcohol | A | y = 36338x + 36.142 | 1844 | 108 |
| Ethyl benzenepropanoate | Benzenoid | Ethyl salicylate | B | y = 537.33x + 1.7861 | 1862 | 104 |
| β-Methylnaphthalene | Benzenoid | Styrene | B | y = 1228x + 5.2634 | 1872 | 142 |
| α-Calacorene | Benzenoid | Styrene | B | y = 1228x + 5.2634 | 1887 | 157 |
| Phenol | phenols | Phenol | A | y = 5832.8x − 1.562 | 2011 | 94 |
| Eugenol | phenols | p-Ethylguaiacol | B | y = 729.32x + 2.8065 | 2156 | 164 |
| 2,4-Bis(1,1-dimethylethyl)phenol | phenols | Phenol | B | y = 5832.8x − 1.562 | 2315 | 191 |
| Methyl acetate | Esters | Ethyl Acetate | B | y = 8635.7x + 18.973 | 804 | 43 |
| Ethyl Acetate | Esters | Ethyl Acetate | A | y = 8635.7x + 18.973 | 878 | 43 |
| Ethyl propanoate | Esters | Propyl acetate | B | y = 1187.9x − 0.515 | 964 | 57 |
| Ethyl 2-methylbutanoate | Esters | Isoamyl acetate | B | y = 948.91x + 9.6091 | 1069 | 102 |
| Isoamyl acetate | Esters | Isoamyl acetate | A | y = 948.91x + 9.6091 | 1132 | 43 |
| Ethyl hexanoate | Esters | Ethyl hexanoate | A | y = 628.86x + 9.0529 | 1236 | 88 |
| Hexyl acetate | Esters | Hexyl acetate | A | y = 631.81x + 1.396 | 1269 | 43 |
| Ethyl heptanoate | Esters | Ethyl heptanoate | A | y = 511.01x − 0.0218 | 1325 | 88 |
| Ethyl lactate | Esters | Ethyl butanoate | B | y = 1624.7x + 6.7119 | 1334 | 45 |
| Ethyl 2-hexenoate | Esters | Ethyl 2-hexenoate | A | y = 521.8x − 0.1164 | 1340 | 55 |
| Ethyl caprate | Esters | Ethyl caprate | A | y = 3321.8x − 0.1688 | 1635 | 88 |
| Ethyl dodecanoate | Esters | Ethyl caprylate | B | y = 1049.9x + 4.7153 | 1819 | 88 |
| Eucalyptol | Isoprenoids | α-Terpineol | B | y = 154.66x + 0.8754 | 1216 | 43 |
| Sulcatone | Isoprenoids | Sulcatone | A | y = 645.87x + 0.9362 | 1330 | 43 |
| Camphor | Isoprenoids | Limonene | B | y = 3.7759x + 0.0009 | 1501 | 95 |
| Linalool | Isoprenoids | Linalool | A | y = 3.4965x + 0.0045 | 1520 | 71 |
| α-Ionene | Isoprenoids | α-ionone | A | y = 753.46x + 0.0117 | 1559 | 159 |
| Hotrienol | Isoprenoids | β-Myrcene | A | y = 1.2298x + 9E-05 | 1588 | 71 |
| Levomenthol | Isoprenoids | α-Terpineol | B | y = 154.66x + 0.8754 | 1642 | 71 |
| α-Terpineol | Isoprenoids | α-Terpineol | A | y = 154.66x + 0.8754 | 1683 | 59 |
| β-Damascenone | Isoprenoids | β-Damascenone | A | y = 65.15x + 1.447 | 1803 | 69 |
| cis-Geranylacetone | Isoprenoids | Neral | B | y = 2583.1x + 121.51 | 1832 | 69 |
| 3,3,5-Trimethylcyclohexene | Others | Limonene | B | y = 3.7759x + 0.0009 | 1572 | 109 |
| 3,4,4-Trimethyl-2-cyclopenten-1-one | Others | Limonene | B | y = 3.7759x + 0.0009 | 1573 | 109 |
| γ-Caprolactone | Others | Limonene | B | y = 3.7759x + 0.0009 | 1693 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Ma, L.; Meng, X.; Zhang, S.; Cao, M.; Kong, D.; Chen, X.; Li, Z.; Pang, X.; Bo, W. Volatile Profile Characterization of Jujube Fruit via HS-SPME-GC/MS and Sensory Evaluation. Plants 2024, 13, 1517. https://doi.org/10.3390/plants13111517
Liu R, Ma L, Meng X, Zhang S, Cao M, Kong D, Chen X, Li Z, Pang X, Bo W. Volatile Profile Characterization of Jujube Fruit via HS-SPME-GC/MS and Sensory Evaluation. Plants. 2024; 13(11):1517. https://doi.org/10.3390/plants13111517
Chicago/Turabian StyleLiu, Ruojin, Ling Ma, Xiangyu Meng, Shuwei Zhang, Ming Cao, Decang Kong, Xuexun Chen, Zhiqin Li, Xiaoming Pang, and Wenhao Bo. 2024. "Volatile Profile Characterization of Jujube Fruit via HS-SPME-GC/MS and Sensory Evaluation" Plants 13, no. 11: 1517. https://doi.org/10.3390/plants13111517





