Desiccation Tolerance of Epiphytic Macrolichens in an Evergreen Temperate Rain Forest (Alerce Costero National Park, Chile)
Abstract
1. Introduction
2. Results
2.1. Diversity and Distribution of Macrolichens
2.2. Microclimate Measurements
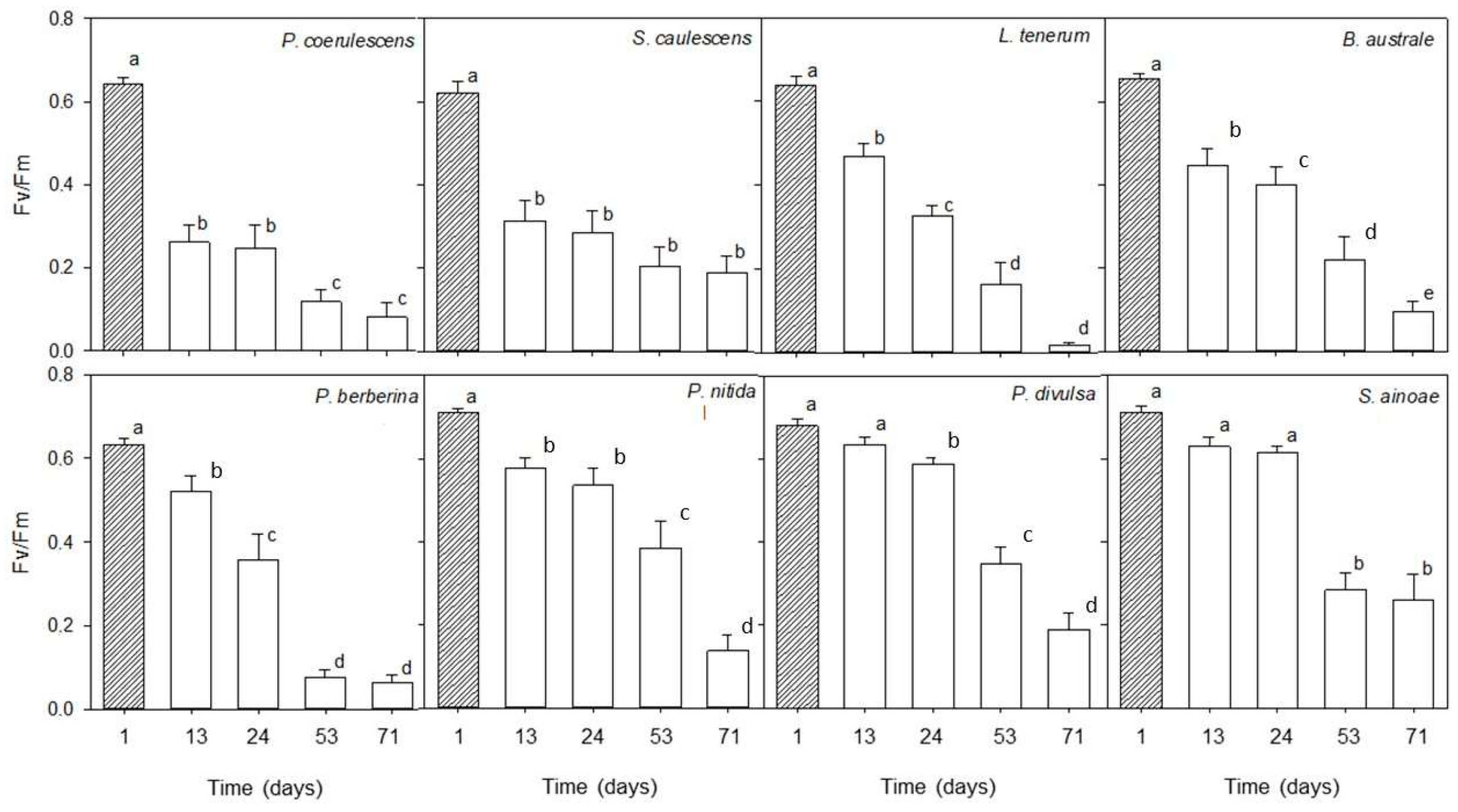
2.3. Desiccation Tolerance
2.4. Response to Light
3. Discussion
4. Materials and Methods
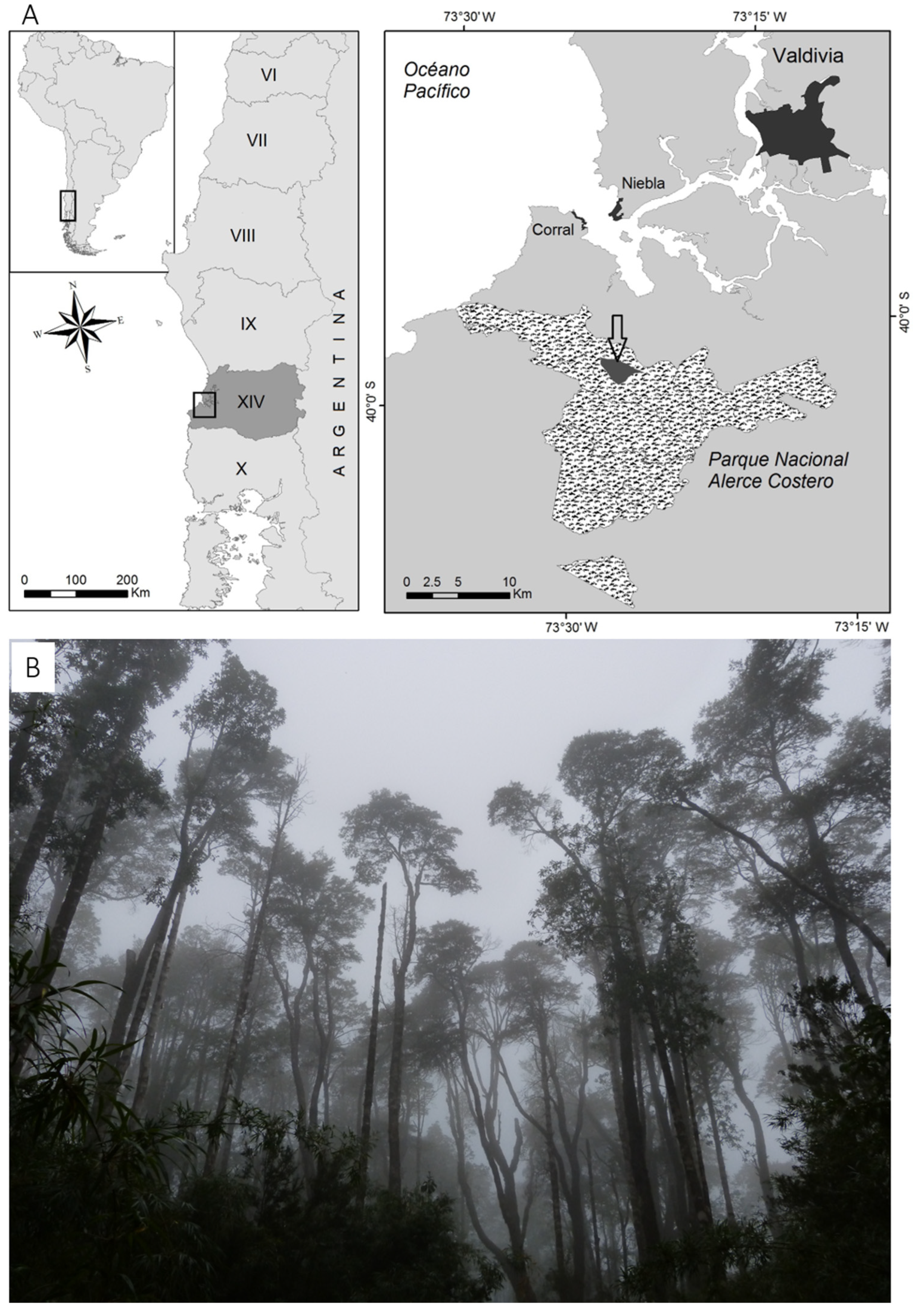
4.1. Study Area
4.2. Censuses of the Lichen Flora
4.3. Microclimate
4.4. Sample Collection
4.5. Desiccation Tolerance
4.6. Response to Light
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neira, E. Chile’s Frontier Forests; Global Forest Watch, World Resources Institute: Washington, DC, USA, 2002; ISBN 978-1-56973-495-7. [Google Scholar]
- Villagran, C.; Hinojosa, F. Historia de los bosques del sur de Sudamérica, II: Análisis fitogeográfico. Rev. Chil. Hist. Nat. 1997, 70, 241–267. [Google Scholar]
- Armesto, J.J.; Rozzi, R.; Smith-Ramírez, C.; Arroyo, M.T.K. Conservation Targets in South American Temperate Forests. Science 1998, 282, 1271–1272. [Google Scholar] [CrossRef]
- Galloway, D.J. Los Líquenes Del Bosque Templado de Chile. In Ecología de los Bosques Nativos de Chile; Editorial Universitaria: Santiago, Chile, 1996; pp. 101–112. [Google Scholar]
- Woda, C.; Huber, A.; Dohrenbusch, A. Vegetación Epifita y Captación de Neblina En Bosques Siempreverdes En La Cordillera Pelada, Sur de Chile. Bosque 2006, 27, 231–240. [Google Scholar] [CrossRef]
- Rodríguez, R.; Alarcón, D.; Espejo, J. Helechos Nativos del Centro y sur de Chile; Corporación Chilena de la Madera: Concepción, Chile, 2009. [Google Scholar]
- Marticorena, A.; Alarcón, D.; Abello, L.; Atala, C. Plantas Trepadoras, Epífitas y Parásitas Nativas de Chile; Corporación Chilena de la Madera: Concepción, Chile, 2010. [Google Scholar]
- Ellis, C.J. Lichen Epiphyte Diversity: A Species, Community and Trait-Based Review. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 131–152. [Google Scholar] [CrossRef]
- Asplund, J.; Wardle, D.A. How Lichens Impact on Terrestrial Community and Ecosystem Properties. Biol. Rev. 2017, 92, 1720–1738. [Google Scholar] [CrossRef]
- Sillett, S.C.; Rambo, T.R. Vertical Distribution of Dominant Epiphytes in Douglas-Fir Forests of Central Oregon Cascades. Northwest Sci. 2000, 74, 44–49. [Google Scholar]
- Li, S.; Liu, W.-Y.; Li, D.-W.; Song, L.; Shi, X.-M.; Lu, H.-Z. Species Richness and Vertical Stratification of Epiphytic Lichens in Subtropical Primary and Secondary Forests in Southwest China. Fungal Ecol. 2015, 17, 30–40. [Google Scholar] [CrossRef]
- Green, T.G.A.; Lange, O.L.; Cowan, I.R. Ecophysiology of Lichen Photosynthesis: The Role of Water Status and Thallus Diffusion Resistances. Cryptogam. Bot. 1994, 4, 166–178. [Google Scholar]
- Sancho, L.G.; Kappen, L. Photosynthesis and Water Relations and the Role of Anatomy in Umbilicariaceae (Lichenes) from Central Spain. Oecologia 1989, 81, 473–480. [Google Scholar] [CrossRef]
- Lakatos, M.; Rascher, U.; Büdel, B. Functional Characteristics of Corticolous Lichens in the Understory of a Tropical Lowland Rain Forest. New Phytol. 2006, 172, 679–695. [Google Scholar] [CrossRef]
- Beckett, R.P. Some Aspects of the Water Relations of Lichens from Habitats of Contrasting Water Status Studied Using Thermocouple Psychrometry. Ann. Bot. 1995, 76, 211–217. [Google Scholar] [CrossRef]
- Giordani, P.; Incerti, G.; Rizzi, G.; Rellini, I.; Nimis, P.L.; Modenesi, P. Functional Traits of Cryptogams in Mediterranean Ecosystems Are Driven by Water, Light and Substrate Interactions. J. Veg. Sci. 2014, 25, 778–792. [Google Scholar] [CrossRef]
- Rosabal, D.; Burgaz, A.R.; Altamirano, A.; Aragón, G. Differences in Diversity of Corticolous Lichens between Interior and Edge of the Monte Barranca Semi-Deciduous Forest, Santiago de Cuba. Bryologist 2012, 115, 333–340. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Odor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Dullinger, S.; Essl, F.; Rabitsch, W.; Erb, K.-H.; Gingrich, S.; Haberl, H.; Hülber, K.; Jarošík, V.; Krausmann, F.; Kühn, I.; et al. Europe’s Other Debt Crisis Caused by the Long Legacy of Future Extinctions. Proc. Natl. Acad. Sci. USA 2013, 110, 7342–7347. [Google Scholar] [CrossRef]
- Sancho, L.G.; de la Torre, R.; Horneck, G.; Ascaso, C.; de Los Rios, A.; Pintado, A.; Wierzchos, J.; Schuster, M. Lichens Survive in Space: Results from the 2005 LICHENS Experiment. Astrobiology 2007, 7, 443–454. [Google Scholar] [CrossRef]
- Raggio, J.; Pintado, A.; Ascaso, C.; De La Torre, R.; De Los Ríos, A.; Wierzchos, J.; Horneck, G.; Sancho, L.G. Whole Lichen Thalli Survive Exposure to Space Conditions: Results of Lithopanspermia Experiment with Aspicilia Fruticulosa. Astrobiology 2011, 11, 281–292. [Google Scholar] [CrossRef]
- Lange, O.L. Experimentell-Ökologische Untersuchungen an Flechten Der Negev-Wüste. Flora Oder Allg. Bot. Zeitung. Abt. B Morphol. Geobot. 1969, 158, 324–359. [Google Scholar] [CrossRef]
- Péli, E.R.; Lei, N.; Pócs, T.; Laufer, Z.; Porembski, S.; Tuba, Z. Ecophysiological Responses of Desiccation-Tolerant Cryptobiotic Crusts. Cent. Eur. J. Biol. 2011, 6, 838–849. [Google Scholar] [CrossRef]
- Green, T.G.A.; Sancho, L.G.; Pintado, A. Ecophysiology of Desiccation/Rehydration Cycles in Mosses and Lichens. In Plant Desiccation Tolerance; Lüttge, U., Beck, E., Bartels, D., Eds.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2011; pp. 89–120. ISBN 978-3-642-19106-0. [Google Scholar]
- Kranner, I.; Zorn, M.; Turk, B.; Wornik, S.; Beckett, R.P.; Batič, F. Biochemical Traits of Lichens Differing in Relative Desiccation Tolerance. New Phytol. 2003, 160, 167–176. [Google Scholar] [CrossRef]
- Challabathula, D.; Zhang, Q.; Bartels, D. Protection of Photosynthesis in Desiccation-Tolerant Resurrection Plants. J. Plant Physiol. 2018, 227, 84–92. [Google Scholar] [CrossRef]
- Kershaw, K.A.; MacFarlane, J.D. Physiological-Environmental Interactions in Lichens. X. Light as an Ecological Factor. New Phytol. 1980, 84, 687–702. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. Differences in the Susceptibility to Light Stress Between Epiphytic Lichens of Ancient and Young Boreal Forest Stands. Funct. Ecol. 1996, 10, 344–354. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Solhaug, K.A. High-Light-Intensity Damage to the Foliose Lichen Lobaria Pulmonaria within Natural Forest: The Applicability of Chlorophyll Fluorescence Methods. Lichenologist 2000, 32, 271–289. [Google Scholar] [CrossRef]
- Beckett, R.P.; Minibayeva, F.; Solhaug, K.A.; Roach, T. Photoprotection in Lichens: Adaptations of Photobionts to High Light. Lichenologist 2021, 53, 21–33. [Google Scholar] [CrossRef]
- Seaward, M.R.D. Environmental Role of Lichens. In Lichen Biology; Nash, I., Thomas, H., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 274–298. ISBN 978-0-521-87162-4. [Google Scholar]
- Scheidegger, C.; Werth, S. Conservation Strategies for Lichens: Insights from Population Biology. Fungal Biol. Rev. 2009, 23, 55–66. [Google Scholar] [CrossRef]
- Green, T.G.A.; Lange, O.L.; Cowan, I.R. Ecophysiological Adaptations of the Lichen Genera Pseudocyphellaria and Sticta to South Temperate Rainforests. Lichenologist 1991, 23, 267–282. [Google Scholar] [CrossRef]
- Lange, O.L.; Kilian, E.; Ziegler, H. Water Vapor Uptake and Photosynthesis of Lichens: Performance Differences in Species with Green and Blue-Green Algae as Phycobionts. Oecologia 1986, 71, 104–110. [Google Scholar] [CrossRef]
- Araya-Osses, D.; Casanueva, A.; Román-Figueroa, C.; Uribe, J.M.; Paneque, M. Climate Change Projections of Temperature and Precipitation in Chile Based on Statistical Downscaling. Clim. Dyn. 2020, 54, 4309–4330. [Google Scholar] [CrossRef]
- Atala, C.; Schneider, C.; Bravo, G.; Quilodrán, M.; Vargas, R. Anatomical, Physiological and Chemical Differences between Populations of Pseudocyphellaria flavicans (Hook. f. & Taylor) Vain. from Chile. Gayana Bot. 2015, 72, 21–26. [Google Scholar] [CrossRef]
- González-Reyes, Á.; Muñoz, A.A. Cambios En La Precipitación de La Ciudad de Valdivia (Chile) Durante Los Últimos 150 Años. Bosque 2013, 34, 200–213. [Google Scholar] [CrossRef]
- Sillett, S.C.; McCune, B. Survival and Growth of Cyanolichen Transplants in Douglas-Fir Forest Canopies. Bryologist 1998, 101, 20–31. [Google Scholar] [CrossRef]
- Villagra, J.; Montenegro, D.; San Martín, C.; Ramírez, C.; Álvarez, I. Estudio de La Flora Liquénica de Las Turberas de La Comuna de Tortel (Región de Aisén), Patagonia Chilena. An. Inst. Patagon. 2009, 37, 53–62. [Google Scholar] [CrossRef]
- Bustamante, R.; Serey, I.; Guzmán, G. Distribución y Abundancia de Epífitos En Bosques de Lenga (Nothofagus Pumilio), Isla Navarino, Región de Magallanes y de La Antártica Chilena. Ser. Cient. Inst. Antárt. Chil. 1989, 39, 59–67. [Google Scholar]
- Rubio, C.; Saavedra, M.; Cuéllar, M.; Díaz, R.; Quilhot, W. Epiphytic Lichens of Conguill{io National Park, Soutern Chile. Gayana Bot. 2013, 70, 66–81. [Google Scholar] [CrossRef]
- McCune, B. Lichen Communities as Indicators of Forest Health. Bryologist 2000, 103, 353–356. [Google Scholar] [CrossRef]
- Hauck, M.; Meißner, T. Epiphytic Lichen Abundance on Branches and Trunks of Abies Balsamea on Whiteface Mountain, New York. Lichenologist 2002, 34, 443–446. [Google Scholar] [CrossRef]
- Marmor, L.; Tõrra, T.; Saag, L.; Randlane, T. Species Richness of Epiphytic Lichens in Coniferous Forests: The Effect of Canopy Openness. Ann. Bot. Fenn. 2012, 49, 352–358. [Google Scholar] [CrossRef]
- Díaz, I.A.; Sieving, K.E.; Peña-Foxon, M.E.; Larraín, J.; Armesto, J.J. Epiphyte Diversity and Biomass Loads of Canopy Emergent Trees in Chilean Temperate Rain Forests: A Neglected Functional Component. For. Ecol. Manag. 2010, 259, 1490–1501. [Google Scholar] [CrossRef]
- Normann, F.; Weigelt, P.; Gehrig-Downie, C.; Gradstein, S.R.; Sipman, H.J.M.; Obregon, A.; Bendix, J. Diversity and Vertical Distribution of Epiphytic Macrolichens in Lowland Rain Forest and Lowland Cloud Forest of French Guiana. Ecol. Indic. 2010, 10, 1111–1118. [Google Scholar] [CrossRef]
- Barkman, J.J. Phytosociology and Ecology of Cryptogamic Epiphytes (Including a Taxonomic Survey and Description of Their Vegetation Units in Europe); Van Gorcum & Comp. N.V.: Assen, The Netherlands, 1969. [Google Scholar]
- Rambo, T.R. Structure and Composition of Corticolous Epiphyte Communities in a Sierra Nevada Old-Growth Mixed-Conifer Forest. Bryologist 2010, 113, 55–71. [Google Scholar] [CrossRef]
- Villagra, J.; Sancho, L.G.; Alors, D. Macrolichen Communities Depend on Phorophyte in Conguillío National Park, Chile. Plants 2023, 12, 2452. [Google Scholar] [CrossRef]
- Parra, M.J.; Acuña, K.; Corcuera, L.J.; Saldaña, A. Vertical Distribution of Hymenophyllaceae Species among Host Tree Microhabitats in a Temperate Rain Forest in Southern Chile. J. Veg. Sci. 2009, 20, 588–595. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Ohlson, M.; Solhaug, K.A.; Bilger, W.; Nybakken, L. Aspect-Dependent High-Irradiance Damage in Two Transplanted Foliose Forest Lichens, Lobaria Pulmonaria and Parmelia Sulcata. Can. J. For. Res. 2001, 31, 1639–1649. [Google Scholar] [CrossRef]
- Green, T.G.A.; Nash, T.H.; Lange, O.L. Physiological Ecology of Carbon Dioxide Exchange. In Lichen Biology; Nash, I., Thomas, H., Eds.; Cambridge University Press: Cambridge, UK, 2008; pp. 152–181. ISBN 978-0-521-87162-4. [Google Scholar]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H. Desiccation-Tolerance in Lichens: A Review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Ndhlovu, N.T.; Minibayeva, F.; Smith, F.R.; Beckett, R.P. Lichen Substances Are More Important for Photoprotection in Sun than Shade Collections of Lichens from the Same Species. Bryologist 2023, 126, 180–190. [Google Scholar] [CrossRef]
- Galloway, D.J. Studies in Pseudocyphellaria (Lichens) III. The South American Species. Bibl. Lichenol. 1992, 46, 275. [Google Scholar] [CrossRef]
- Kermit, T.; Gauslaa, Y. The Vertical Gradient of Bark pH of Twigs and Macrolichens in a Picea Abies Canopy Not Affected by Acid Rain. Lichenologist 2001, 33, 353–359. [Google Scholar] [CrossRef]
- Schneider, P.H.; Schmitt, J.L. Composition, Community Structure and Vertical Distribution of Epiphytic Ferns on Alsophila Setosa Kaulf., in a Semideciduous Seasonal Forest, Morro Reuter, RS, Brazil. Acta Bot. Bras. 2011, 25, 557–565. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Máguas, C.; Adams, W.W.; Meyer, A.; Kilian, E.; Lange, O.L. Effect of High Light on the Efficiency of Photochemical Energy Conversion in a Variety of Lichen Species with Green and Blue-Green Phycobionts. Planta 1990, 180, 400–409. [Google Scholar] [CrossRef]
- Lüttge, U. Desiccation Tolerance of the Epilithic Lichen Lecanora Muralis (Schreb.) Rabenh. in the Temperate Climate. Flora—Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 233–237. [Google Scholar] [CrossRef]
- Garreaud, R.D. Cambio Climático: Bases Físicas e Impactos en Chile; Universidad de Chile: Santiago, Chile, 2011. [Google Scholar]
- Martínez-Retureta, R.; Aguayo, M.; Abreu, N.J.; Stehr, A.; Duran-Llacer, I.; Rodríguez-López, L.; Sauvage, S.; Sánchez-Pérez, J.-M. Estimation of the Climate Change Impact on the Hydrological Balance in Basins of South-Central Chile. Water 2021, 13, 794. [Google Scholar] [CrossRef]
- Luebert, F.; Pliscoff, P. Sinopsis Bioclimática y Vegetacional de Chile—Facultad de Ciencias Forestales y de la Conservación de la Naturaleza; Universidad de Chile: Santiago, Chile, 2006; ISBN 978-956-11-2575-9. [Google Scholar]
- Di Castri, F.; Hajek, E.R. Representaciones gráficas de pares climáticas. In Bioclimatología de Chile; Universidad Católica de Chile: Santiago, Chile, 1976; pp. 13–52. [Google Scholar]
- Gatica, A.; Pereira, Í.; Vallejos, O. Líquenes epífitos: Una herramienta para estudiar la continuidad ecológica en Isla Mocha, Chile. Gayana Bot. 2011, 68, 226–235. [Google Scholar] [CrossRef]
- Green, T.G.A.; Büdel, B.; Meyer, A.; Zellner, H.; Lange, O.L. Temperate Rainforest Lichens in New Zealand: Light Response of Photosynthesis. N. Z. J. Bot. 1997, 35, 493–504. [Google Scholar] [CrossRef]
- Pardow, A.; Hartard, B.; Lakatos, M. Morphological, Photosynthetic and Water Relations Traits Underpin the Contrasting Success of Two Tropical Lichen Groups at the Interior and Edge of Forest Fragments. AoB Plants 2010, 2010, plq004. [Google Scholar] [CrossRef]
- Pintado, A.; Sancho, L.G.; Green, T.G.A.; Blanquer, J.M.; Lázaro, R. Functional Ecology of the Biological Soil Crust in Semiarid SE Spain: Sun and Shade Populations of Diploschistes Diacapsis (Ach.) Lumbsch. Lichenologist 2005, 37, 425–432. [Google Scholar] [CrossRef]
- Reiter, R.; Höftberger, M.; Allan Green, T.G.; Türk, R. Photosynthesis of Lichens from Lichen-Dominated Communities in the Alpine/Nival Belt of the Alps—II: Laboratory and Field Measurements of CO2 Exchange and Water Relations. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 34–46. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Palmqvist, K.; Solhaug, K.A.; Holien, H.; Hilmo, O.; Nybakken, L.; Myhre, L.C.; Ohlson, M. Growth of Epiphytic Old Forest Lichens across Climatic and Successional Gradients. Can. J. For. Res. 2007, 37, 1832–1845. [Google Scholar] [CrossRef]
- Gauslaa, Y.; Goward, T. Relative Growth Rates of Two Epiphytic Lichens, Lobaria Pulmonaria and Hypogymnia Occidentalis, Transplanted within and Outside of Populus Dripzones. Botany 2012, 90, 954–965. [Google Scholar] [CrossRef]
- Heber, U.; Bilger, W.; Bligny, R.; Lange, O.L. Phototolerance of Lichens, Mosses and Higher Plants in an Alpine Environment: Analysis of Photoreactions. Planta 2000, 211, 770–780. [Google Scholar] [CrossRef]
- Veblen, T.T.; Donoso, C.; Kitzberger, T.; Robertus, A.J. Ecology of Southern Chilean and Argentinean Nothofagus Forests. In The Ecology and Biogeography of Nothofagus Forests; Yale University Press: New Haven, CT, USA, 1996; pp. 293–353. [Google Scholar]
- Oyarzun, C.E.; Godoy, R.; Sepulveda, A. Water and Nutrient Fluxes in a Cool Temperate Rainforest at the Cordillera de La Costa in Southern Chile. Hydrol. Process. 1998, 12, 1067–1077. [Google Scholar] [CrossRef]
- Galloway, D.J. Studies on the Lichen Genus Sticta (Schreber) Ach.: I. Southern South American Species. Lichenologist 1994, 26, 223–282. [Google Scholar] [CrossRef]
- Wedin, M. The Lichen Family Sphaerophoracae (Caliciales, Ascomycotina) in Temperate Areas of the Southern Hemisphere; Almqvist & Wiksell: Uppsala, Sweden, 1995. [Google Scholar]
- Parmasto, E. Nova Hedwigia; Schweizerbart Science Publishers: Stuttgart, Germany, 1978; pp. 99–144. [Google Scholar]
- Rascher, U.; Liebig, M.; Lüttge, U. Evaluation of Instant Light-Response Curves of Chlorophyll Fluorescence Parameters Obtained with a Portable Chlorophyll Fluorometer on Site in the Field. Plant Cell Environ. 2000, 23, 1397–1405. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A Reappraisal of the Use of DMSO for the Extraction and Determination of Chlorophylls a and b in Lichens and Higher Plants. Environ. Exp. Bot. 1992, 32, 85–100. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R. Rapid Light Curves: A Powerful Tool to Assess Photosynthetic Activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence—A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Jensen, M. Measurement of Chlorophyll Fluorescence in Lichens. In Protocols in Lichenology: Culturing, Biochemistry, Ecophysiology and Use in Biomonitoring; Kranner, I.C., Beckett, R.P., Varma, A.K., Eds.; Springer Lab Manuals; Springer: Berlin/Heidelberg, Germany, 2002; pp. 135–151. ISBN 978-3-642-56359-1. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research; Statistical Tables; W. H. Freeman: San Francisco, CA, USA, 1970; Volume 19. [Google Scholar]
| Family | Species Name | Distribution | Nn | Sc | Dw |
|---|---|---|---|---|---|
| Coccotremataceae | Lepolichen coccophorus | Endemic | 2.8 | ||
| Collemataceae | Collema laeve | Austral | 1.3 | ||
| Lobariaceae | Pseudocyphellaria berberina | Endemic | 7.2 | 22.0 | 48.0 |
| Pseudocyphellaria coerulescens | Endemic | 5.3 | 8.7 | ||
| Pseudocyphellaria divulsa | Endemic | 20.4 | 27.6 | 6.9 | |
| Pseudocyphellaria nitida | Endemic | 17.8 | 31.5 | 25.9 | |
| Sticta ainoae | Endemic | 34.4 | |||
| Sticta caulescens | Endemic | 3.9 | 0.84 | ||
| Sticta hypochra | Endemic | 1.5 | |||
| Parmeliaceae | Platismatia glauca | Cosmopolitan | 5.6 | ||
| Sphaerophoraceae | Bunodophoron australe | Austral | 4.6 | 5.3 | |
| Bunodophoron ramuliferum | Austral | 1.5 | |||
| Leifidium tenerum | Austral | 6.1 | 7.5 | 2.2 | |
| Atheliaceae | Cora glabrata | Cosmopolitan | 1.3 |
| Macrolichen Species | Tree Species | ||
|---|---|---|---|
| Nn | Sc | Dw | |
| Stratum A %RC | |||
| S. ainoae | 63.52 ± 4.87 | Not found | Not found |
| S. hypocra | 2.87 ± 2.39 | Not found | Not found |
| C. laeve | 2.87 ± 4.41 | Not found | Not found |
| S. caulescens | 7.38 ± 0.76 | 2.04 ± 2.50 | Not found |
| P. divulsa | 14.75 ± 6.00 | 44.22 ± 2.90 | 20.37 ± 2.50 |
| P. nitida | 8.61 ± 3.15 | 29.93 ± 5.20 | 30.56 ± 11.68 |
| P. berberina | Not found | 23.81 ± 5.67 | 49.07 ± 4.29 |
| Macrolichen species | Stratum B %RC | ||
| B. ramuliferum | 2.74 ± 5.00 | Not found | Not found |
| S. ainoae | 1.37 ± 2.50 | Not found | Not found |
| C. laeve | 2.74 ± 2.89 | Not found | Not found |
| D. glabratum | 1.37 ± 0.00 | Not found | Not found |
| B. australe | 9.59 ± 2.81 | 8.96 ± 4.00 | Not found |
| P. divulsa | 26.48 ± 6.05 | 16.04 ± 5.95 | Not found |
| P. nitida | 27.85 ± 4.91 | 32.55 ± 3.26 | 23.47 ± 3.98 |
| P. berberina | 15.07 ± 4.59 | 20.75 ± 3.90 | 47.42 ± 3.50 |
| L. tenerum | 12.79 ± 4.63 | 12.74 ± 6.63 | 3.29 ± 2.50 |
| P. coerulescens | Not found | 8.96 ± 4.41 | 13.15 ± 7.68 |
| L. coccophorus | Not found | Not found | 4.23 ± 3.75 |
| P. glauca | Not found | Not found | 8.45 ± 7.64 |
| Tree Species | Humidity (%) | Temperature (°C) | Vapor Pressure Deficit (VPD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | p | A | B | p | A | B | p | |
| Nn | 91.6 ± 0.2 | 87.8 ± 0.4 | >0.001 | 9.8 ± 0.1 | 10.3 ± 0.1 | 0.0009 | 19.3 ± 0.007 | 29.9 ± 0.01 | >0.001 |
| Sc | 91.6 ± 0.3 | 85.6 ± 0.4 | >0.001 | 9.7 ± 0.1 | 9.9 ± 0.1 | 0.4 | 19.9 ± 0.009 | 34 ± 0.01 | >0.001 |
| Dw | 89.1 ± 0.3 | 87.5 ± 0.3 | 0.002 | 9.7 ± 0.1 | 10.0 ± 0.1 | 0.002 | 25.1 ± 0.01 | 29.4 ± 0.01 | 0.16 |
| Species | Y (II) | PPFD 24 | PPFD 55 | PPFD 367 |
|---|---|---|---|---|
| p ≤ 0.01 | p = 0.04 | p = 0.026 | p = 0.070 | |
| P. coerulescens | 0.64 ± 0.015 | 84.42 ± 14.7 a | 41.06 ± 4.9 ab | 4.66 ± 1.3 a |
| S. caulescens | 0.62 ± 0.027 | 78.42 ± 16.8 a | 50.98 ± 11.7 b | 4.80 ± 1.3 a |
| L. tenerun | 0.64 ± 0.02 | 89.61 ± 19.5 a | 39.09 ± 4.9 ab | 3.17 ± 0.7 a |
| B. australe | 0.66 ± 0.011 | 76.26 ± 13.9 a | 37.26 ± 4.7 ab | 4.34 ± 1.4 a |
| P. berberina | 0.63 ± 0.014 | 70.96 ± 9.6 a | 29.16 ± 3.6 ab | 4.85 ± 0.5 a |
| P. nitida | 0.70 ± 0.011 | 83.50 ± 10.5 a | 46.19 ± 3.6 b | 7.93 ± 1.5 a |
| P. divulsa | 0.68 ± 0.017 | 71.43 ± 16.2 a | 21.86 ± 3.7 a | 2.44 ± 0.5 a |
| S. ainoae | 0.68 ± 0.017 | 68.30 ± 12.2 a | 22.74 ± 1.6 ab | 3.65 ± 0.8 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villagra, J.; Raggio, J.; Alors, D.; Sancho, L.G. Desiccation Tolerance of Epiphytic Macrolichens in an Evergreen Temperate Rain Forest (Alerce Costero National Park, Chile). Plants 2024, 13, 1519. https://doi.org/10.3390/plants13111519
Villagra J, Raggio J, Alors D, Sancho LG. Desiccation Tolerance of Epiphytic Macrolichens in an Evergreen Temperate Rain Forest (Alerce Costero National Park, Chile). Plants. 2024; 13(11):1519. https://doi.org/10.3390/plants13111519
Chicago/Turabian StyleVillagra, Johana, José Raggio, David Alors, and Leopoldo G. Sancho. 2024. "Desiccation Tolerance of Epiphytic Macrolichens in an Evergreen Temperate Rain Forest (Alerce Costero National Park, Chile)" Plants 13, no. 11: 1519. https://doi.org/10.3390/plants13111519
APA StyleVillagra, J., Raggio, J., Alors, D., & Sancho, L. G. (2024). Desiccation Tolerance of Epiphytic Macrolichens in an Evergreen Temperate Rain Forest (Alerce Costero National Park, Chile). Plants, 13(11), 1519. https://doi.org/10.3390/plants13111519








