Responses of Soil Carbon and Microbial Residues to Degradation in Moso Bamboo Forest
Abstract
:1. Introduction
2. Results
2.1. Changes of Soil Environmental Factors and Soil C Pool under Different Degradation Time
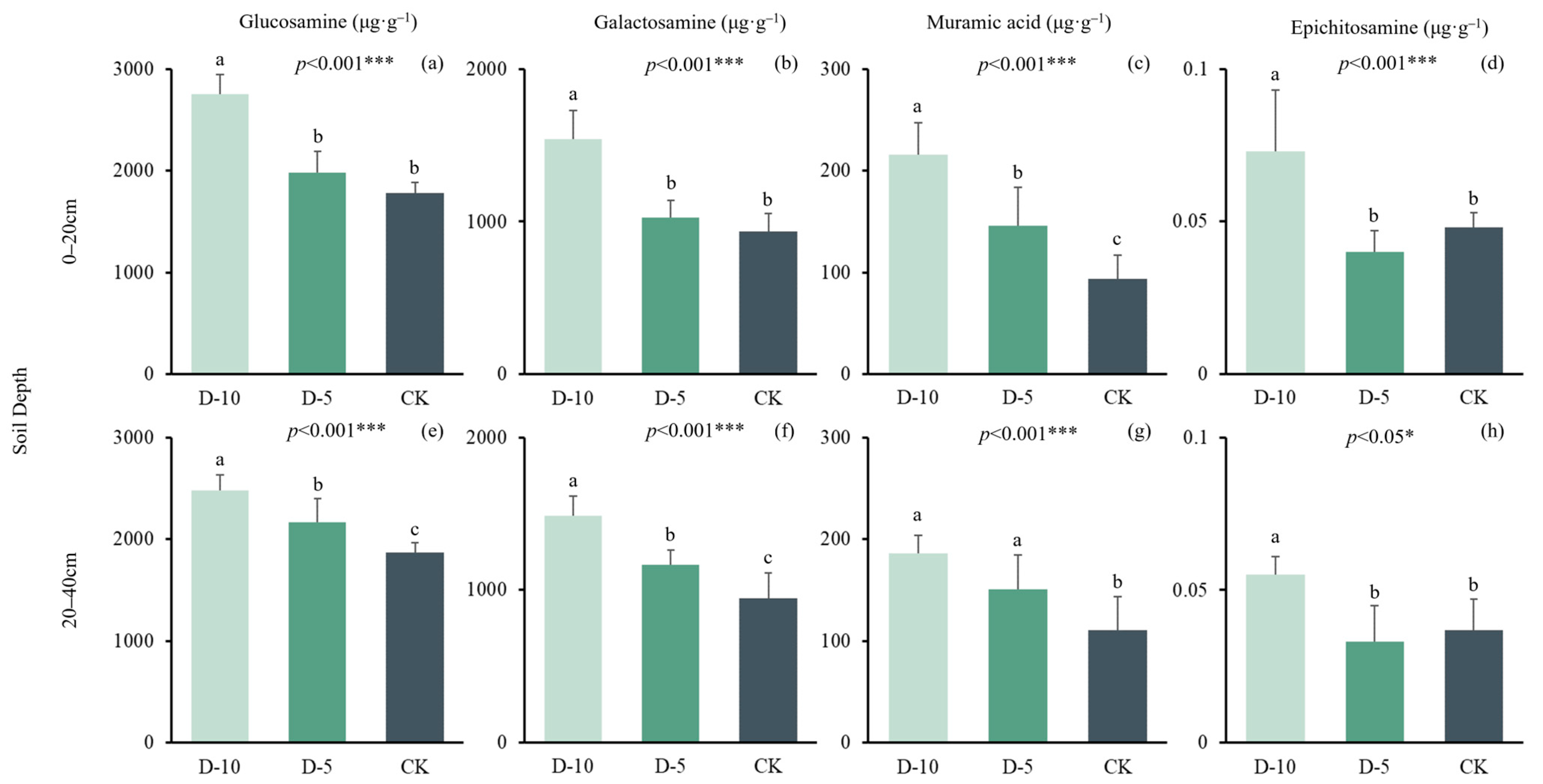
2.2. Changes of Soil Amino Sugars under Different Degradation Times
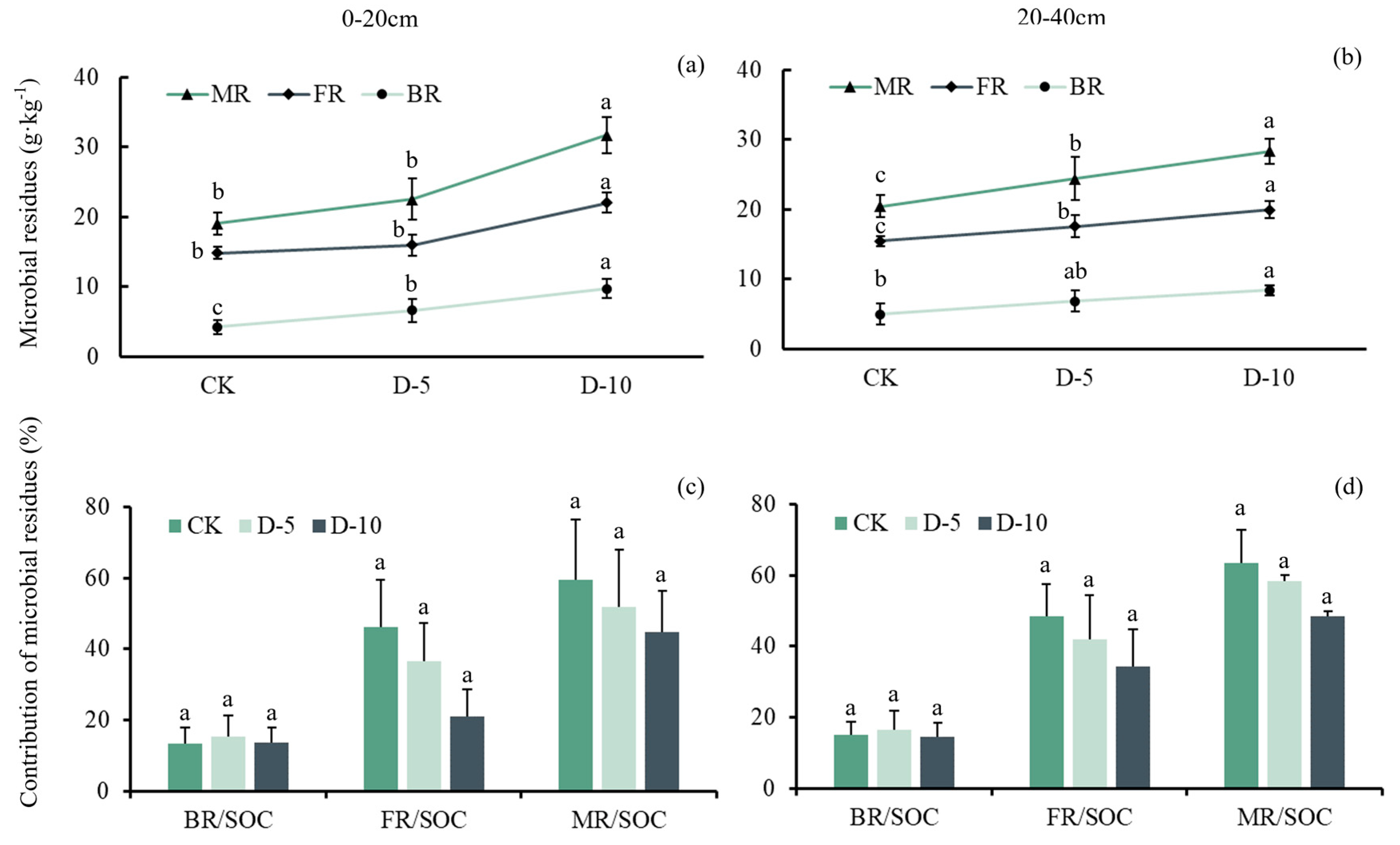
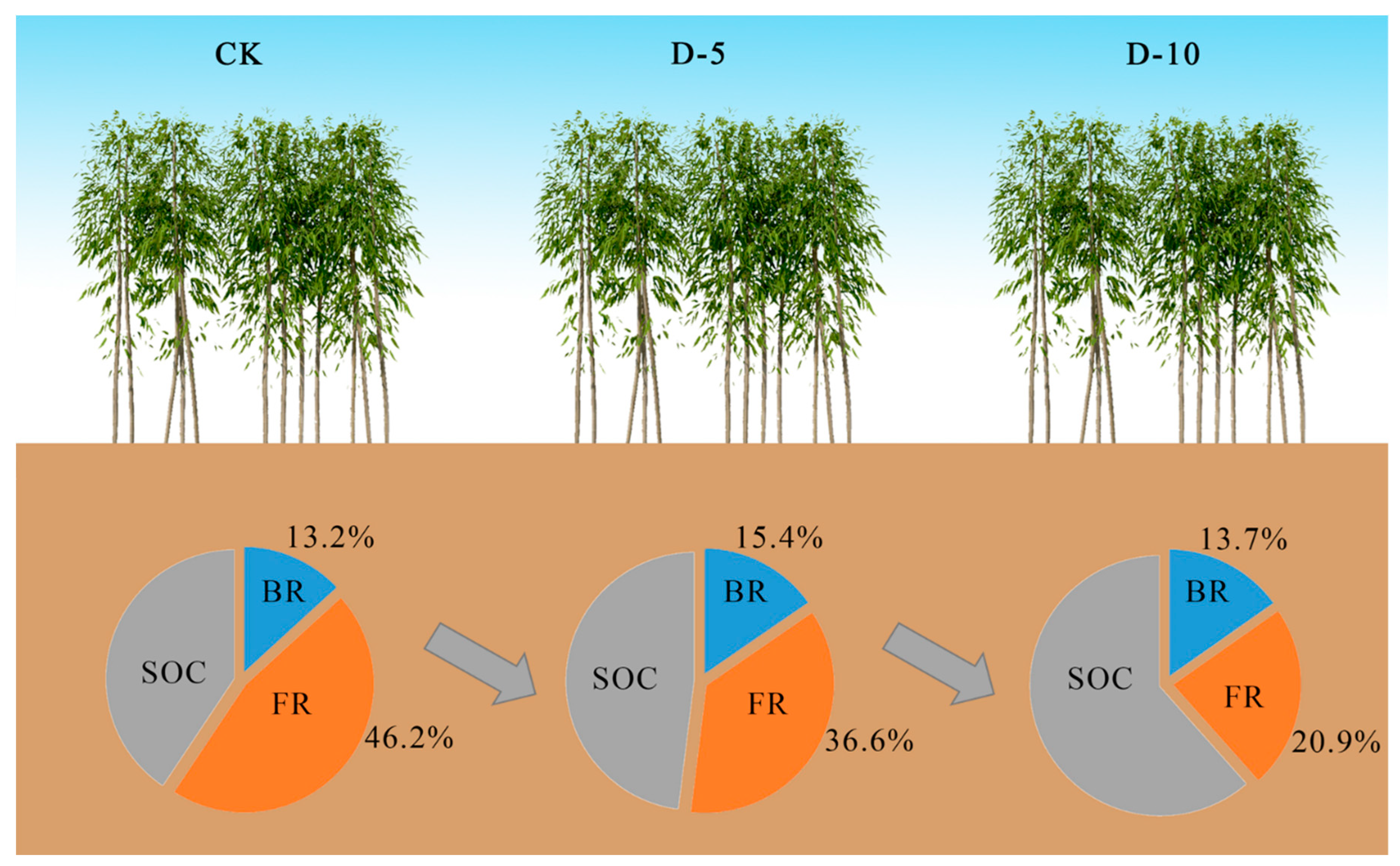
2.3. Changes of Soil Microbial Residues and Their Contribution to SOC
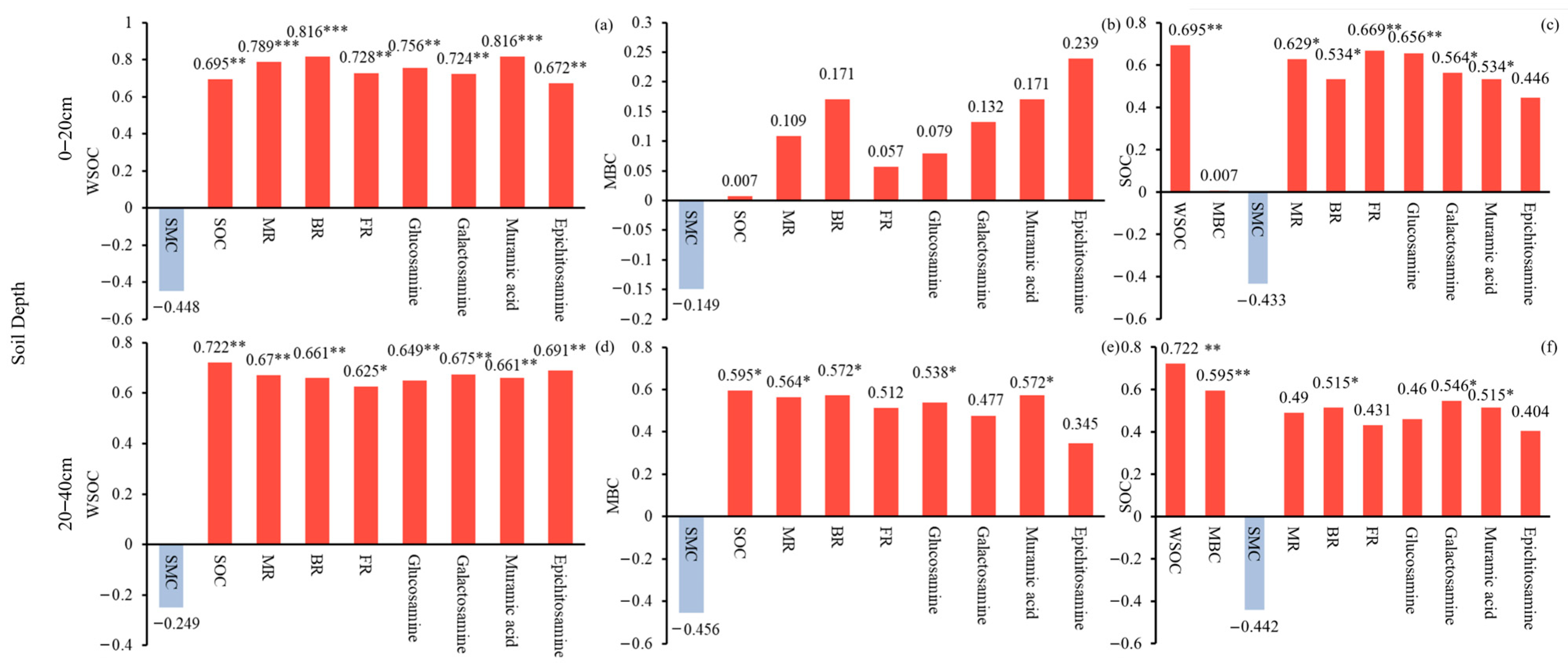
2.4. Influences of Soil C Pool on Moso Bamboo Soil Amino Sugars and Soil Microbial Residues
3. Discussion
3.1. Impacts of Degradation Time on Moso Bamboo Soil C Pool and C Components
3.2. Response of Soil Amino Sugars and MR to Degradation Time
3.3. Application of Microbial Residual C Research in Moso Bamboo Forest
3.4. Limitations
4. Materials and Methods
4.1. Description of Study Area
4.2. Experiment Design
4.3. Determination of Soil Microbial Residues
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De, P.L.O.; Machado, A. Carbono do solo e a mitigação da mudança climática global. Quim. Nova 2005, 28, 2. [Google Scholar]
- Law, B.E.; Harmon, M.E. Forest sector carbon management, measurement and verification, and discussion of policy related to climate change. Carbon Manag. 2011, 2, 73–84. [Google Scholar] [CrossRef]
- Achat, D.L.; Fortin, M.; Landmann, G.; Ringeval, B.; Augusto, L. Forest soil carbon is threatened by intensive biomass harvesting. Sci. Rep. 2015, 5, 15991. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liang, C.; Duan, X.; Chen, H.; Li, D. Variation of microbial residue contribution to soil organic carbon sequestration following land use change in a subtropical karst region. Geoderma 2019, 353, 340–346. [Google Scholar] [CrossRef]
- Hicks Pries, C.E.; Castanha, C.; Porras, R.C.; Torn, M.S. The whole-soil carbon flux in response to warming. Science 2017, 355, 6332. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Simpson, M.J.; Smith, E.; Kelleher, B.P. Microbially derived inputs to soil organic matter: Are current estimates too low? Environ. Sci. Technol. 2007, 41, 8070–8076. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Chang. Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Cheng, G.; Wixon, D.L.; Balser, T.C. An Absorbing Markov Chain approach to understanding the microbial role in soil carbon stabilization. Biogeochemistry 2011, 106, 303–309. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef]
- Hao, Z.; Zhao, Y.; Wang, X.; Wu, J.; Jiang, S.; Xiao, J.; Wang, K.; Zhou, X.; Liu, H.; Li, J.; et al. Thresholds in aridity and soil carbon-to-nitrogen ratio govern the accumulation of soil microbial residues. Commun. Earth Environ. 2021, 2, 236. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Bloem, J.; Baath, E.; Boeckx, P.; Rousk, J.; Bodé, S.; Forristal, D.; Jones, M.B. Abundance, production and stabilization of microbial biomass under conventional and reduced tillage. Soil Biol. Biochem. 2010, 42, 48–55. [Google Scholar] [CrossRef]
- Joergensen, R.G. Amino sugars as specific indices for fungal and bacterial residues in soil. Biol. Fertil. Soils 2018, 54, 559–568. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wichern, F. Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol. Biochem. 2008, 40, 2977–2991. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, S.; Zhou, Y.; Shi, Y.; Xu, L. The current status and potential research directions of soil microbial carbon in bamboo forest. Adv. Bamboo Sci. 2022, 1, 100005. [Google Scholar] [CrossRef]
- Ding, X.; Chen, S.; Zhang, B.; Liang, C.; He, H.; Horwath, W.R. Warming increases microbial residue contribution to soil organic carbon in an alpine meadow. Soil Biol. Biochem. 2019, 135, 13–19. [Google Scholar] [CrossRef]
- Yuan, N.; Wang, E.; Lv, S.; Tang, X.; Wang, T.; Wang, G.; Zhou, Y.; Zhou, G.; Shi, Y.; Xu, L. Degradation reduces greenhouse gas emissions while weakening ecosystem carbon sequestration of Moso bamboo forests. Sci. Total Environ. 2023, 877, 162915. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhu, Z.; Xu, X.; Liu, S.; Jones, D.L.; Kuzyakov, Y.; Shibistova, O.; Wu, J.; Ge, T. Carbon and nitrogen recycling from microbial necromass to cope with C:N stoichiometric imbalance by priming. Soil Biol. Biochem. 2020, 142, 107720. [Google Scholar] [CrossRef]
- Li, C.; Shi, Y.; Zhou, G.; Zhou, Y.; Xu, L.; Tong, L.; Liu, X. Effects of different management approaches on soil carbon dynamics in Moso bamboo forest ecosystems. CATENA 2018, 169, 59–68. [Google Scholar] [CrossRef]
- Deng, X.; Yin, J.; Xu, L.; Shi, Y.; Zhou, G.; Li, Y.; Chen, G.; Ye, Y.; Zhang, F.; Zhou, Y.; et al. Effects of abandonment management on soil C and N pools in Moso bamboo forests. Sci. Total Environ. 2020, 729, 138949. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, P.; Wang, H.; Zhou, G.; Wu, J.; Yang, F.; Qian, X. Seasonal soil CO2 efflux dynamics after land use change from a natural forest to Moso bamboo plantations in subtropical China. For. Ecol. Manag. 2011, 262, 1131–1137. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Jiang, H.; Yu, S.; Fu, J.; Li, W.; Wang, W.; Ma, Z.; Peng, C. Carbon sequestration by Chinese bamboo forests and their ecological benefits: Assessment of potential, problems, and future challenges. Environ. Rev. 2011, 19, 418–428. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, P.; Mo, L. Bamboo: A Possible Approach to the Control of Global Warming. Int. J. Nonlinear Sci. Numer. Simul. 2009, 10, 547–550. [Google Scholar]
- Zhou, G.; Meng, C.; Jiang, P.; Xu, Q. Review of Carbon Fixation in Bamboo Forests in China. Bot. Rev. 2011, 77, 262–270. [Google Scholar] [CrossRef]
- Yin, J.; Ge, Z.; Deng, X.; Shi, Y.; Xu, L.; Zhou, G.; Ye, Y.; Zhang, F.; Zhou, Y.; Li, C.; et al. Abandonment lead to structural degradation and changes in carbon allocation patterns in Moso bamboo forests. For. Ecol. Manag. 2019, 449, 117449. [Google Scholar] [CrossRef]
- Jandl, R.; Lindner, M.; Vesterdal, L.; Bauwens, B.; Baritz, R.; Hagedorn, F.; Johnson, D.W.; Minkkinen, K.; Byrne, K.A. How strongly can forest management influence soil carbon sequestration? Geoderma 2007, 137, 253–268. [Google Scholar] [CrossRef]
- Mancinelli, R.; Campiglia, E.; Di Tizio, A.; Marinari, S. Soil carbon dioxide emission and carbon content as affected by conventional and organic cropping systems in Mediterranean environment. Appl. Soil Ecol. 2010, 46, 64–72. [Google Scholar] [CrossRef]
- Sainju, U.M.; Jabro, J.D.; Stevens, W.B. Soil Carbon Dioxide Emission and Carbon Content as Affected by Irrigation, Tillage, ropping System, and Nitrogen Fertilization. J. Environ. Qual. 2008, 37, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fang, H.; Deng, X.; Ying, J.; Lv, W.; Shi, Y.; Zhou, G.; Zhou, Y. Biochar application increased ecosystem carbon sequestration capacity in a Moso bamboo forest. For. Ecol. Manag. 2020, 475, 118447. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Chang, S.X.; Jiang, P.; Zhou, G.; Fu, S.; Yan, E.; Wu, J.; Lin, L. Long-term intensive management effects on soil organic carbon pools and chemical composition in Moso bamboo (Phyllostachys pubescens) forests in subtropical China. For. Ecol. Manag. 2013, 303, 121–130. [Google Scholar] [CrossRef]
- Wander, M.M.; Traina, S.J.; Stinner, B.R.; Peters, S.E. Organic and Conventional Management Effects on Biologically Active Soil Organic Matter Pools. Soil Sci. Soc. Am. J. 1994, 58, 1130–1139. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Du, H.; Berninger, F.; Mao, F.; Li, X.; Chen, L.; Cui, L.; Li, Y.; Zhu, D. Soil respiration of a Moso bamboo forest significantly affected by gross ecosystem productivity and leaf area index in an extreme drought event. PeerJ 2018, 6, e5747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fang, Y.; Luo, Y.; Li, Y.; Ge, T.; Wang, Y.; Wang, H.; Yu, B.; Song, X.; Chen, J.; et al. Linking soil carbon availability, microbial community composition and enzyme activities to organic carbon mineralization of a bamboo forest soil amended with pyrogenic and fresh organic matter. Sci. Total Environ. 2021, 801, 149717. [Google Scholar] [CrossRef]
- Liu, S.; Xu, H.; Ding, J.; Chen, H.Y.H.; Wang, J.; Xu, Z.; Ruan, H.; Chen, Y. CO2 emission increases with damage severity in Moso bamboo forests following a winter storm in Southern China. Sci. Rep. 2016, 6, 30351. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liang, C.; Duncan, D.S.; Bao, X.; Xie, H.; He, H.; Wickings, K.; Zhang, X.; Chen, F. Impacts of vegetation type and climatic zone on neutral sugar distribution in natural forest soils. Geoderma 2016, 282, 139–146. [Google Scholar] [CrossRef]
- Spielvogel, S.; Prietzel, J.; Kögel-Knabner, I. Changes of lignin phenols and neutral sugars in different soil types of a high-elevation forest ecosystem 25 years after forest dieback. Soil Biol. Biochem. 2007, 39, 655–668. [Google Scholar] [CrossRef]
- Verchot, L.V.; Dutaur, L.; Shepherd, K.D.; Albrecht, A. Organic matter stabilization in soil aggregates: Understanding the biogeochemical mechanisms that determine the fate of carbon inputs in soils. Geoderma 2011, 161, 182–193. [Google Scholar] [CrossRef]
- Dou, S.; Shan, J.; Song, X.; Cao, R.; Wu, M.; Li, C.; Guan, S. Are humic substances soil microbial residues or unique synthesized compounds? A perspective on their distinctiveness. Pedosphere 2020, 30, 159–167. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Liang, C.; Duncan, D.S.; Balser, T.C.; Tiedje, J.M.; Jackson, R.D. Soil microbial residue storage linked to soil legacy under biofuel cropping systems in southern Wisconsin, USA. Soil Biol. Biochem. 2013, 57, 939–942. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Rubert, K.F.; Balser, T.C. Effect of plant materials on microbial transformation of amino sugars in three soil microcosms. Biol. Fertil. Soils 2007, 43, 631–639. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Chen, H.; Zheng, L.; Wang, K. Soil microbial community responses to forage grass cultivation in degraded karst soils, Southwest China. Land Degrad. Dev. 2018, 29, 4262–4270. [Google Scholar] [CrossRef]
- Craig, M.E.; Turner, B.L.; Liang, C.; Clay, K.; Johnson, D.J.; Phillips, R.P. Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Glob. Chang. Biol. 2018, 24, 3317–3330. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wen, L.; Yang, L.; Luo, P.; Xiao, K.; Chen, H.; Zhang, W.; He, X.; Chen, H.; Wang, K. Dynamics of soil organic carbon and nitrogen following agricultural abandonment in a karst region. J. Geophys. Res. Biogeosci. 2017, 122, 230–242. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.; Jastrow, J. The importance of anabolism in microbial control over soil carbon storage. Nat. Microb. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, G.; Du, H.; Lu, D.; Mo, L.; Xu, X.; Shi, Y.; Zhou, Y. Current and potential carbon stocks in Moso bamboo forests in China. J. Environ. Manag. 2015, 156, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brücken, B.; Kästner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W. Studies on Humus Formed by Microorganisms in Pure Culture and Soil. Ph.D. Thesis, Ji Lin Agricultural University, Changchun, China, 2006. (In Chinese). [Google Scholar]
- Dai, W.; Zhao, K.; Fu, W.; Jiang, P.; Li, Y.; Zhang, C.; Gielen, G.; Gong, X.; Li, Y.; Wang, H.; et al. Spatial variation of organic carbon density in topsoils of a typical subtropical forest, southeastern China. CATENA 2018, 167, 181–189. [Google Scholar] [CrossRef]
- Fang, H.; Ji, B.; Deng, X.; Ying, J.; Zhou, G.; Shi, Y.; Xu, L.; Tao, J.; Zhou, Y.; Li, C.; et al. Effects of topographic factors and aboveground vegetation carbon stocks on soil organic carbon in Moso bamboo forests. Plant Soil 2018, 433, 363–376. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, X.; Zhou, Y.; Zhang, J.; Zhang, X. Discrepancies in karst soil organic carbon in southwest china for different land use patterns: A case study of Guizhou Province. Int. J. Environ. Res. Public Health 2019, 16, 4199. [Google Scholar] [CrossRef]
- Nóbrega, G.N.; Ferreira, T.O.; Artur, A.G.; de Mendonça, E.S.; Raimundo, R.A.; Teixeira, A.S.; Otero, X.L. Evaluation of methods for quantifying organic carbon in mangrove soils from semi-arid region. J. Soils Sediments 2015, 15, 282–291. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of Biochars on Nitrous Oxide Emission and Nitrogen Leaching from Two Contrasting Soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]

| Soil Depth | Treatment | SBD | pH | SMC | df | N |
|---|---|---|---|---|---|---|
| (g·cm−3) | (% w/w) | |||||
| 0–20 cm | D-10 | 1.26 ± 0.06 a | 5.24 ± 0.34 a | 18.6 ± 5.72 b | 2 | 15 |
| D-5 | 1.30 ± 0.05 a | 5.32 ± 0.33 a | 17.2 ± 2.38 b | 2 | 15 | |
| CK | 1.26 ± 0.04 a | 5.44 ± 0.30 a | 25.9 ± 3.85 a | 2 | 15 |
| Soil Depth | Treatment | SBD | pH | SMC | df | N |
|---|---|---|---|---|---|---|
| (g·cm−3) | (% w/w) | |||||
| 20–40 cm | D-10 | 1.57 ± 0.02 a | 5.11 ± 0.17 a | 17.0 ± 4.80 a | 2 | 15 |
| D-5 | 1.58 ± 0.06 a | 5.11 ± 0.19 a | 17.7 ± 2.37 a | 2 | 15 | |
| CK | 1.54 ± 0.07 a | 5.12 ± 0.06 a | 24.2 ± 7.14 a | 2 | 15 |
| Soil Depth | Treatment | SOC | WSOC | MBC | df | N |
|---|---|---|---|---|---|---|
| (g·kg−1) | (mg·kg−1) | (mg·kg−1) | ||||
| 0–20 cm | D-10 | 74.3 ± 8.07 a | 370 ± 45.6 a | 536 ± 128 a | 2 | 15 |
| D-5 | 47.5 ± 8.24 b | 263 ± 77.4 b | 476 ± 130 a | 2 | 15 | |
| CK | 34.4 ± 5.29 b | 193 ± 49.0 b | 497 ± 174 a | 2 | 15 |
| Soil Depth | Treatment | SOC | WSOC | MBC | df | N |
|---|---|---|---|---|---|---|
| (g·kg−1) | (mg·kg−1) | (mg·kg−1) | ||||
| 20–40 cm | D-10 | 62.0 ± 7.30 a | 313 ± 55.9 a | 443 ± 67.3 a | 2 | 15 |
| D-5 | 45.5 ± 7.61 ab | 186 ± 24.9 b | 442 ± 57.4 a | 2 | 15 | |
| CK | 39.0 ± 5.59 b | 168 ± 35.7 b | 334 ± 55.0 b | 2 | 15 |
| Soil Depth | Treatment | Glucosamine | Galactosamine | Muramic Acid | Epichitosamine | df | N |
|---|---|---|---|---|---|---|---|
| (μg·g−1) | (μg·g−1) | (μg·g−1) | (μg·g−1) | ||||
| 0–20 cm | D-10 | 2760 ± 193 a | 1540 ± 188 a | 216 ± 13.9 a | 0.07 ± 0.02 a | 2 | 15 |
| D-5 | 1980 ± 211 b | 1030 ± 111 b | 146 ± 37.8 b | 0.04 ± 0.02 b | 2 | 15 | |
| CK | 1780 ± 108 b | 934 ± 118 b | 93.9 ± 22.9 c | 0.03 ± 0.01 b | 2 | 15 |
| Soil Depth | Treatment | Glucosamine | Galactosamine | Muramic Acid | Epichitosamine | df | N |
|---|---|---|---|---|---|---|---|
| (μg·g−1) | (μg·g−1) | (μg·g−1) | (μg·g−1) | ||||
| 20–40 cm | D-10 | 2480 ± 154 a | 1490 ± 131 a | 186 ± 7.66 a | 0.06 ± 0.01 a | 2 | 15 |
| D-5 | 2170 ± 229 b | 1170 ± 98.9 b | 151 ± 15.1 a | 0.03 ± 0.01 b | 2 | 15 | |
| CK | 1870 ± 90.5 c | 945 ± 166 c | 111 ± 14.6 b | 0.04 ± 0.01 b | 2 | 15 |
| Soil Depth | Treatment | MR | BR | FR | MR/SOC | FR/SOC | BR/SOC | df | N |
|---|---|---|---|---|---|---|---|---|---|
| (g·kg−1) | (g·kg−1) | (g·kg−1) | (%, w/w) | (%, w/w) | (%, w/w) | ||||
| 0–20 cm | D-10 | 31.7 ± 2.58 a | 9.71 ± 1.39 a | 22.0 ± 1.44 a | 44.6 ± 5.20 a | 46.2 ± 5.87 a | 13.7 ± 1.87 a | 2 | 15 |
| D-5 | 22.5 ± 2.94 b | 6.58 ± 1.69 b | 16.0 ± 1.55 b | 51.9 ± 7.20 a | 36.6 ± 4.82 a | 15.4 ± 2.59 a | 2 | 15 | |
| CK | 19.1 ± 1.53 c | 4.22 ± 1.03 c | 14.8 ± 0.82 b | 59.3 ± 7.61 a | 30.9 ± 3.39 a | 13.2 ± 2.00 a | 2 | 15 |
| Soil Depth | Treatment | MR | BR | FR | MR/SOC | FR/SOC | BR/SOC | df | N |
|---|---|---|---|---|---|---|---|---|---|
| (g·kg−1) | (g·kg−1) | (g·kg−1) | (%, w/w) | (%, w/w) | (%, w/w) | ||||
| 20–40 cm | D-10 | 28.3 ± 1.74 a | 8.38 ± 0.77 a | 20.0 ± 0.33 a | 48.4 ± 6.49 a | 34.2 ± 4.06 a | 14.3 ± 1.84 a | 2 | 15 |
| D-5 | 24.4 ± 3.11 b | 6.79 ± 1.52 a | 17.6 ± 0.74 b | 58.3 ± 7.87 a | 42.0 ± 5.56 a | 16.3 ± 2.49 a | 2 | 15 | |
| CK | 20.4 ± 1.59 c | 4.98 ± 1.46 b | 15.4 ± 0.57 c | 63.5 ± 4.17 a | 48.4 ± 4.70 a | 15.2 ± 1.57 a | 2 | 15 |
| Soil Depth | Degradation Years | Degradation Years × Soil Depth | |
|---|---|---|---|
| SOC | 0.30 | <0.001 | 0.61 |
| MR | 0.94 | <0.001 | <0.05 |
| FR | 0.91 | <0.001 | <0.05 |
| BR | 0.81 | <0.001 | 0.22 |
| MR/SOC | 0.38 | 0.09 | 0.97 |
| FR/SOC | 0.36 | <0.05 | 0.94 |
| BR/SOC | 0.61 | 0.63 | 0.93 |
| Soil Depth | Linear Regression Model | R2 | p | |
|---|---|---|---|---|
| FR | 0–20 cm | y = 0.043 + 0.116SOC − 0.114MBC + 0.360WSOC | 0.60 | <0.05 * |
| FR | 20–40 cm | y = 0.028 − 0.204SOC + 0.452MBC + 0.326WSOC | 0.63 | <0.01 ** |
| BR | 0–20 cm | y = 0.016 − 0.128SOC − 0.236MBC + 1.120WSOC | 0.69 | <0.01 ** |
| BR | 20–40 cm | y = −0.021 − 0.358SOC + 0.995MBC + 0.678WSOC | 0.68 | <0.01 ** |
| Plot | Elevation | Slope | Living Bamboo Density | D.B.H |
|---|---|---|---|---|
| (m) | (Trees ha−1) | (cm) | ||
| CK-1 | 103 | 9 | 2470 | 11.7 |
| CK-2 | 115 | 12 | 2496 | 12.5 |
| CK-3 | 151 | 13 | 2560 | 11.9 |
| CK-4 | 130 | 17 | 2673 | 13.2 |
| CK-5 | 97 | 11 | 2614 | 12.8 |
| D-5-1 | 76 | 21 | 1772 | 12.4 |
| D-5-2 | 143 | 18 | 1742 | 12.1 |
| D-5-3 | 135 | 20 | 1802 | 11.9 |
| D-5-4 | 126 | 21 | 1792 | 11.5 |
| D-5-5 | 112 | 17 | 1748 | 11.7 |
| D-10-1 | 140 | 10 | 1341 | 12.9 |
| D-10-2 | 173 | 5 | 1349 | 13.5 |
| D-10-3 | 92 | 12 | 1390 | 13.1 |
| D-10-4 | 86 | 14 | 1427 | 12.8 |
| D-10-5 | 166 | 28 | 1301 | 11.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Cheng, X.; Lv, Y.; Zhou, Y.; Zhou, G.; Shi, Y. Responses of Soil Carbon and Microbial Residues to Degradation in Moso Bamboo Forest. Plants 2024, 13, 1526. https://doi.org/10.3390/plants13111526
Liu S, Cheng X, Lv Y, Zhou Y, Zhou G, Shi Y. Responses of Soil Carbon and Microbial Residues to Degradation in Moso Bamboo Forest. Plants. 2024; 13(11):1526. https://doi.org/10.3390/plants13111526
Chicago/Turabian StyleLiu, Shuhan, Xuekun Cheng, Yulong Lv, Yufeng Zhou, Guomo Zhou, and Yongjun Shi. 2024. "Responses of Soil Carbon and Microbial Residues to Degradation in Moso Bamboo Forest" Plants 13, no. 11: 1526. https://doi.org/10.3390/plants13111526




