Effect of Cluster-Zone Leaf Removal at Different Stages on Cabernet Sauvignon and Marselan (Vitis vinifera L.) Grape Phenolic and Volatile Profiles
Abstract
:1. Introduction
2. Results and Discussion
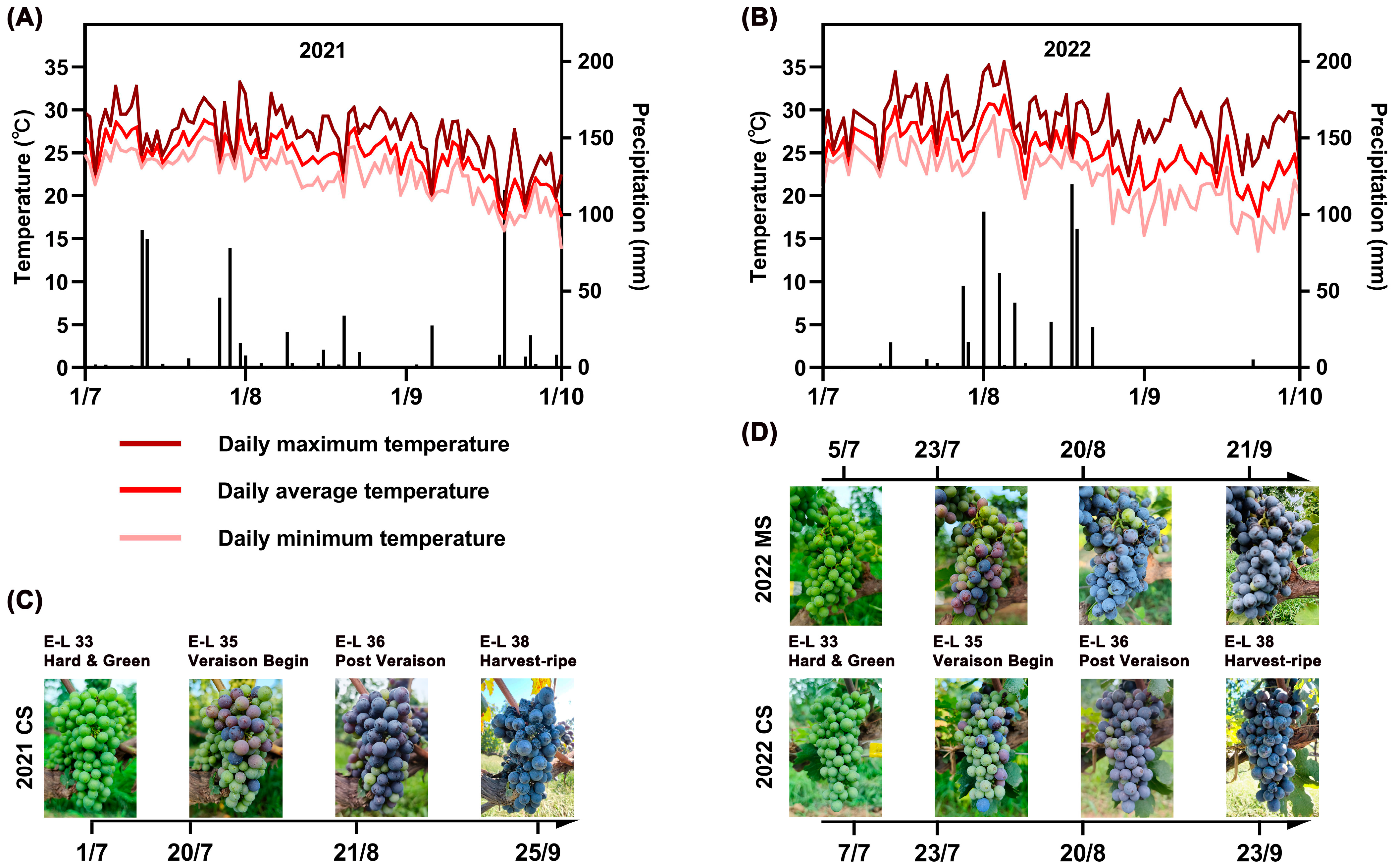
2.1. Meteorological Conditions and Grape Physicochemical Parameters
2.2. Impact of Cluster-Zone Leaf Removal on Grape Phenolic Profiles
2.2.1. Anthocyanins
2.2.2. Flavonols
2.2.3. Flavan-3-Ols
2.3. Grape Volatile Aromas of Grapes and Their Glycosylated Precursors
2.3.1. C6/C9 Compounds
2.3.2. Terpenes
| Compounds | Vintage and Variety | LR1 | LR2 | LR3 | CK |
|---|---|---|---|---|---|
| 1-Hexanol | 2021 Cabernet Sauvignon | 874.97 ± 251.56 ab | 1085.27 ± 73.2 a | 676.77 ± 134.92 b | 341.77 ± 129.16 c |
| 2022 Cabernet Sauvignon | 220.17 ± 26.08 | 225.96 ± 20.03 | 207.19 ± 44.75 | 253.82 ± 19.91 | |
| 2022 Marselan | 83.72 ± 6.07 | 117.07 ± 8.24 | 96.58 ± 42.14 | 84.09 ± 21.44 | |
| (E)-3-Hexen-1-ol | 2021 Cabernet Sauvignon | 1.76 ± 0.61 b | 2.71 ± 0.35 a | 1.12 ± 0.28 bc | 0.63 ± 0.27 c |
| 2022 Cabernet Sauvignon | 0.58 ± 0.1 | 0.59 ± 0.17 | 0.55 ± 0.13 | 0.51 ± 0.11 | |
| 2022 Marselan | 0.25 ± 0.06 | 0.28 ± 0.02 | 0.27 ± 0.01 | 0.26 ± 0.05 | |
| (Z)-3-Hexen-1-ol | 2021 Cabernet Sauvignon | 20.38 ± 5.37 ab | 25.46 ± 2.93 a | 16.06 ± 0.69 b | 22.07 ± 5.77 ab |
| 2022 Cabernet Sauvignon | 20.96 ± 1.88 b | 32.48 ± 3.28 a | 23.93 ± 1.95 b | 18.96 ± 5.76 b | |
| 2022 Marselan | 3.34 ± 1.19 b | 6.9 ± 0.95 a | 2.75 ± 0.46 b | 2.07 ± 0.53 b | |
| (E)-2-Hexen-1-ol | 2021 Cabernet Sauvignon | 34.03 ± 9.32 b | 48.4 ± 5.91 a | 28.54 ± 3.75 b | 12.54 ± 2.92 c |
| 2022 Cabernet Sauvignon | 19.18 ± 3.51 b | 23.42 ± 2.38 a | 19.87 ± 2.68 b | 18.92 ± 1.35 b | |
| 2022 Marselan | 3.96 ± 0.51 | 5.81 ± 0.91 | 4.28 ± 1.29 | 3.99 ± 0.92 | |
| 1-Nonanol | 2021 Cabernet Sauvignon | 0.3 ± 0.32 | 0.16 ± 0.07 | 0.13 ± 0.03 | 0.04 ± 0.01 |
| 2022 Cabernet Sauvignon | 0.01 ± 0.01 b | 0.01 ± 0.01 b | 0.01 ± 0.01 b | 0.02 ± 0.01 a | |
| 2022 Marselan | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | |
| Hexanal | 2021 Cabernet Sauvignon | 904.47 ± 137.91 c | 807.81 ± 77.27 c | 1168.99 ± 120.22 b | 1599.76 ± 106.62 a |
| 2022 Cabernet Sauvignon | 1741.02 ± 358.73 | 1715.35 ± 292.47 | 1689.5 ± 368.8 | 1601.67 ± 136.81 | |
| 2022 Marselan | 1369.63 ± 246.2 a | 968.46 ± 191.35 bc | 1266.12 ± 78.37 ab | 880.32 ± 136.6 c | |
| (E)-2-Hexenal | 2021 Cabernet Sauvignon | 841.82 ± 26.55 c | 1046.99 ± 82.52 b | 1218.98 ± 144.06 b | 1468.17 ± 103.45 a |
| 2022 Cabernet Sauvignon | 2370.67 ± 207.95 | 2732.25 ± 594.85 | 2281.23 ± 393.2 | 2654.72 ± 379.37 | |
| 2022 Marselan | 2047.78 ± 207.24 a | 1935.81 ± 136.68 a | 1984.76 ± 186.49 a | 1556.95 ± 116.68 b | |
| Nonanal | 2021 Cabernet Sauvignon | 1.17 ± 0.16 b | 0.96 ± 0.28 b | 2.16 ± 0.32 a | 1.9 ± 0.55 a |
| 2022 Cabernet Sauvignon | 1.21 ± 0.22 | 1.8 ± 0.33 | 1.14 ± 0.19 | 1.47 ± 0.5 | |
| 2022 Marselan | 1 ± 0.2 ab | 0.69 ± 0.09 ab | 1.01 ± 0.14 a | 0.67 ± 0.22 b | |
| (E,E)-2,4-Hexadienal | 2021 Cabernet Sauvignon | 3.24 ± 1.56 b | 4.16 ± 1.54 b | 5.48 ± 0.57 ab | 7.02 ± 0.62 a |
| 2022 Cabernet Sauvignon | 12.11 ± 2.14 | 12.21 ± 2.05 | 10.44 ± 1.78 | 11.58 ± 1.29 | |
| 2022 Marselan | 10.08 ± 1.34 a | 9.28 ± 0.9 ab | 10.2 ± 0.41 a | 7.79 ± 0.18 b | |
| Hexanoic acid | 2021 Cabernet Sauvignon | 3.04 ± 0.38 | 2.8 ± 0.42 | 2.94 ± 0.18 | 2.7 ± 0.45 |
| 2022 Cabernet Sauvignon | 1.62 ± 1.54 b | 4.6 ± 2.31 ab | 4.81 ± 0.69 ab | 5.33 ± 2.12 a | |
| 2022 Marselan | 2.97 ± 0.4 b | 5.03 ± 1.3 a | 3.12 ± 0.19 b | 3.9 ± 0.72 ab | |
| 1-Hexanol (Bound) | 2021 Cabernet Sauvignon | 15.19 ± 2.64 | 11.36 ± 1 | 13.81 ± 3.6 | 10.85 ± 3.68 |
| 2022 Cabernet Sauvignon | 21.79 ± 1.65 | 20.39 ± 4.45 | 23.55 ± 4.77 | 24.28 ± 3.79 | |
| 2022 Marselan | 47.37 ± 9.74 | 51.83 ± 5.79 | 43.23 ± 2.26 | 52.98 ± 8.87 | |
| (Z)-3-Hexen-1-ol (Bound) | 2021 Cabernet Sauvignon | 3.86 ± 0.52 a | 2.21 ± 1.24 b | 2.61 ± 0.63 ab | 2.52 ± 0.72 ab |
| 2022 Cabernet Sauvignon | 8.82 ± 1.05 | 7.26 ± 1.19 | 9.74 ± 1.73 | 7.92 ± 1.3 | |
| 2022 Marselan | 30.93 ± 2.91 | 33.23 ± 6.82 | 30.03 ± 9.57 | 34.51 ± 3.56 | |
| (E)-2-Hexen-1-ol (Bound) | 2021 Cabernet Sauvignon | 10.78 ± 3.01 | 8.07 ± 1.22 | 7.94 ± 0.72 | 9.46 ± 2.82 |
| 2022 Cabernet Sauvignon | 9.69 ± 6.1 ab | 5.22 ± 0.35 b | 7.06 ± 0.97 ab | 13.56 ± 3.74 a | |
| 2022 Marselan | 5.92 ± 0.12 | 6.36 ± 1.01 | 6.61 ± 2.62 | 6.62 ± 0.73 | |
| 1-Nonanol (Bound) | 2021 Cabernet Sauvignon | 0.05 ± 0.01 b | 0.05 ± 0.01 b | 0.07 ± 0.01 a | 0.05 ± 0.01 b |
| 2022 Cabernet Sauvignon | 0.07 ± 0.01 a | 0.03 ± 0.01 c | 0.05 ± 0.01 b | 0.06 ± 0.01 ab | |
| 2022 Marselan | 0.1 ± 0.02 b | 0.15 ± 0.03 a | 0.1 ± 0.01 b | 0.13 ± 0.04 ab | |
| Total C6/C9 Alcohol (Free) | 2021 Cabernet Sauvignon | 931.27 ± 217.85 a | 1162 ± 62.43 a | 722.61 ± 111.78 ab | 377.05 ± 108.29 b |
| 2022 Cabernet Sauvignon | 260.9 ± 25.03 | 282.46 ± 20.62 | 251.55 ± 38.1 | 292.23 ± 13.08 | |
| 2022 Marselan | 122.62 ± 45.8 | 130.07 ± 6.52 | 103.89 ± 35.79 | 105.43 ± 26.47 | |
| Total C6/C9 Aldehyde | 2021 Cabernet Sauvignon | 1750.7 ± 125.44 c | 1859.92 ± 81.62 c | 2395.61 ± 200.13 b | 3076.84 ± 169.43 a |
| 2022 Cabernet Sauvignon | 4125.02 ± 464.15 | 4461.61 ± 712.14 | 3982.32 ± 623.14 | 4269.42 ± 413.69 | |
| 2022 Marselan | 3428.49 ± 368.16 a | 2914.2 ± 260.77 ab | 3262.09 ± 207.93 a | 2445.73 ± 70.64 b | |
| Total C6/C9 Alcohol (Bound) | 2021 Cabernet Sauvignon | 29.88 ± 3.25 | 21.69 ± 1.66 | 24.43 ± 2.79 | 22.88 ± 5.72 |
| 2022 Cabernet Sauvignon | 40.36 ± 5.15 | 32.9 ± 4.32 | 40.4 ± 5.01 | 45.83 ± 2.55 | |
| 2022 Marselan | 86.97 ± 7.88 | 91.57 ± 5.85 | 79.97 ± 11.71 | 94.24 ± 10.49 | |
| Total C6/C9 Compounds | 2021 Cabernet Sauvignon | 2714.9 ± 180.58 b | 3046.4 ± 78.31 ab | 3145.6 ± 148.2 ab | 3479.48 ± 266.47 a |
| 2022 Cabernet Sauvignon | 4427.91 ± 440.03 | 4781.57 ± 710.35 | 4279.08 ± 588.76 | 4612.81 ± 408.39 | |
| 2022 Marselan | 3641.05 ± 346.82 a | 3140.8 ± 265.17 ab | 3449.08 ± 222.87 a | 2650.3 ± 35.54 b |
2.3.3. Norisoprenoids and Carotenoids
2.3.4. Aromatic Compounds
3. Materials and Methods
3.1. Reagents and Standards
3.2. Vineyard Experimental Design
3.3. Meteorological Data
3.4. Physicochemical Parameter Measurement
3.5. Quantitative Analysis of Phenolic Compounds
3.5.1. Extraction of Phenolic Compounds from Grape Skins
3.5.2. Analysis of Phenolic Compounds
3.5.3. Qualitative and Quantitative Analysis of Phenolic Compounds
3.6. Quantitative Analysis of Aroma Compounds
3.6.1. Free-Form Aroma Compound Analysis by HS-SPME-GC-MS
3.6.2. Bound-Form Volatile Compound Analysis by SPE-HS-SPME-GC-MS
3.6.3. Identification and Quantification of Aroma Compounds
3.7. Quantitative Analysis of Carotenoids
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, J.; Massonnet, M.; Cantu, D. The Genetic Basis of Grape and Wine Aroma. Hortic. Res. 2019, 6, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Takase, H.; Matsuyama, S.; Kobayashi, H.; Matsuo, H.; Ikoma, G.; Takata, R. Effect of Light Exposure on Linalool Biosynthesis and Accumulation in Grape Berries. Biosci. Biotechnol. Biochem. 2016, 80, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Asproudi, A.; Petrozziello, M.; Cavalletto, S.; Guidoni, S. Grape Aroma Precursors in Cv. Nebbiolo as Affected by Vine Microclimate. Food Chem. 2016, 211, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.-M.; Cushman, J.C.; Cramer, G.R. Water Deficit Alters Differentially Metabolic Pathways Affecting Important Flavor and Quality Traits in Grape Berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Mei, Y.; Chen, G.; Su-Zhou, C.; Li, Y.; Merkeryan, H.; Cui, P.; Liu, W.; Liu, X. Deficit Irrigation and Leaf Removal Modulate Anthocyanin and Proanthocyanidin Repartitioning of Cabernet Sauvignon (Vitis vinifera L.) Grape and Resulting Wine Profile. J. Sci. Food Agric. 2022, 102, 2937–2949. [Google Scholar] [CrossRef] [PubMed]
- Pardo-García, A.I.; de la Hoz, K.S.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of Vine Foliar Treatments on the Varietal Aroma of Monastrell Wines. Food Chem. 2014, 163, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.-B.; Liu, Y.; Lu, H.-C.; Hu, L.; Wang, Y.; Cheng, C.-F.; Chen, W.; Li, S.-D.; He, F.; Duan, C.-Q.; et al. Volatomics of ‘Cabernet Sauvignon’ Grapes and Wines under the Fan Training System Revealed the Nexus of Microclimate and Volatile Compounds. Food Chem. 2023, 403, 134421. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Ma, X.; Tang, Y.; Wang, Y.; Wu, B.; Jiao, X.; Zhang, Z.; Ju, Y. Effect of Cluster Zone Leaf Removal on Monoterpene Profiles of Sauvignon Blanc Grapes and Wines. Food Res. Int. 2020, 131, 109028. [Google Scholar] [CrossRef] [PubMed]
- Sivilotti, P.; Falchi, R.; Herrera, J.C.; Škvarč, B.; Butinar, L.; Sternad Lemut, M.; Bubola, M.; Sabbatini, P.; Lisjak, K.; Vanzo, A. Combined Effects of Early Season Leaf Removal and Climatic Conditions on Aroma Precursors in Sauvignon Blanc Grapes. J. Agric. Food Chem. 2017, 65, 8426–8434. [Google Scholar] [CrossRef]
- Young, P.R.; Eyeghe-Bickong, H.A.; du Plessis, K.; Alexandersson, E.; Jacobson, D.A.; Coetzee, Z.; Deloire, A.; Vivier, M.A. Grapevine Plasticity in Response to an Altered Microclimate: Sauvignon Blanc Modulates Specific Metabolites in Response to Increased Berry Exposure. Plant Physiol. 2016, 170, 1235–1254. [Google Scholar] [CrossRef]
- Feng, H.; Yuan, F.; Skinkis, P.A.; Qian, M.C. Influence of Cluster Zone Leaf Removal on Pinot Noir Grape Chemical and Volatile Composition. Food Chem. 2015, 173, 414–423. [Google Scholar] [CrossRef]
- Yue, X.; Zhao, Y.; Ma, X.; Jiao, X.; Fang, Y.; Zhang, Z.; Ju, Y. Effects of Leaf Removal on the Accumulation of Anthocyanins and the Expression of Anthocyanin Biosynthetic Genes in Cabernet Sauvignon (Vitis vinifera L.) Grapes. J. Sci. Food Agric. 2021, 101, 3214–3224. [Google Scholar] [CrossRef]
- Sivilotti, P.; Herrera, J.C.; Lisjak, K.; Baša Česnik, H.; Sabbatini, P.; Peterlunger, E.; Castellarin, S.D. Impact of Leaf Removal, Applied Before and After Flowering, on Anthocyanin, Tannin, and Methoxypyrazine Concentrations in ‘Merlot’ (Vitis vinifera L.) Grapes and Wines. J. Agric. Food Chem. 2016, 64, 4487–4496. [Google Scholar] [CrossRef]
- Georgiadou, E.C.; Mina, M.; Neoptolemou, V.; Koundouras, S.; D’Onofrio, C.; Bellincontro, A.; Mencarelli, F.; Fotopoulos, V.; Manganaris, G.A. The Beneficial Effect of Leaf Removal during Fruit Set on Physiological, Biochemical, and Qualitative Indices and Volatile Organic Compound Profile of the Cypriot Reference Cultivar ‘Xynisteri’. J. Sci. Food Agric. 2023, 103, 3776–3786. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, X.-Q.; Wang, Y.; Chen, W.-K.; Sun, R.-Z.; Cheng, G.; Liu, B.; Chen, W.; Duan, C.-Q.; Wang, J.; et al. Modulation of Volatile Compound Metabolome and Transcriptome in Grape Berries Exposed to Sunlight under Dry-Hot Climate. BMC Plant Biol. 2020, 20, 59. [Google Scholar] [CrossRef]
- Alessandrini, M.; Battista, F.; Panighel, A.; Flamini, R.; Tomasi, D. Effect of Pre-Bloom Leaf Removal on Grape Aroma Composition and Wine Sensory Profile of Semillon Cultivar. J. Sci. Food Agric. 2018, 98, 1674–1684. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry Temperature and Solar Radiation Alter Acylation, Proportion, and Concentration of Anthocyanin in Merlot Grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar] [CrossRef]
- Lu, H.-C.; Wang, Y.; Cheng, C.-F.; Chen, W.; Li, S.-D.; He, F.; Duan, C.-Q.; Wang, J. Distal Leaf Removal Made Balanced Source-Sink Vines, Delayed Ripening, and Increased Flavonol Composition in Cabernet Sauvignon Grapes and Wines in the Semi-Arid Xinjiang. Food Chem. 2022, 366, 130582. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol Profile Is a Reliable Indicator to Assess Canopy Architecture and the Exposure of Red Wine Grapes to Solar Radiation. Front. Plant Sci. 2019, 10, 430495. [Google Scholar] [CrossRef]
- Fanzone, M.; González-Manzano, S.; Pérez-Alonso, J.; Escribano-Bailón, M.T.; Jofré, V.; Assof, M.; Santos-Buelga, C. Evaluation of Dihydroquercetin-3-O-Glucoside from Malbec Grapes as Copigment of Malvidin-3-O-Glucoside. Food Chem. 2015, 175, 166–173. [Google Scholar] [CrossRef]
- Valentini, G.; Allegro, G.; Pastore, C.; Colucci, E.; Filippetti, I. Post-Veraison Trimming Slow down Sugar Accumulation without Modifying Phenolic Ripening in Sangiovese Vines. J. Sci. Food Agric. 2019, 99, 1358–1365. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.A.; Kang, H.-M.; Lee, Y.-T.; Islam, M.Z. Grape Terpenoids: Flavor Importance, Genetic Regulation, and Future Potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 1429–1447. [Google Scholar] [CrossRef] [PubMed]
- Timmins, J.J.B.; Kroukamp, H.; Paulsen, I.T.; Pretorius, I.S. The Sensory Significance of Apocarotenoids in Wine: Importance of Carotenoid Cleavage Dioxygenase 1 (CCD1) in the Production of β-Ionone. Molecules 2020, 25, 2779. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Pontin, M.; Berli, F.; Bottini, R.; Piccoli, P. Metabolism of Terpenes in the Response of Grape (Vitis vinifera L.) Leaf Tissues to UV-B Radiation. Phytochemistry 2012, 77, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dai, Z.; Ferrier, T.; Orduña, L.; Santiago, A.; Peris, A.; Wong, D.C.J.; Kappel, C.; Savoi, S.; Loyola, R.; et al. MYB24 Orchestrates Terpene and Flavonol Metabolism as Light Responses to Anthocyanin Depletion in Variegated Grape Berries. Plant Cell 2023, 35, koad228. [Google Scholar] [CrossRef] [PubMed]
- Skinkis, P.A.; Bordelon, B.P.; Butz, E.M. Effects of Sunlight Exposure on Berry and Wine Monoterpenes and Sensory Characteristics of Traminette. Am. J. Enol. Vitic. 2010, 61, 147–156. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Pieri, P.; Charon, J.; Pillet, J.; Hilbert, G.; Renaud, C.; Gomès, E.; Delrot, S.; Lecourieux, D. Dissecting the Biochemical and Transcriptomic Effects of a Locally Applied Heat Treatment on Developing Cabernet Sauvignon Grape Berries. Front. Plant Sci. 2017, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Liu, M.; Zhang, X.; Li, S.; Shi, Y.; Duan, C. Regional Variation of Chemical Characteristics in Young Marselan (Vitis vinifera L.) Red Wines from Five Regions of China. Foods 2022, 11, 787. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Ma, Y.; Xu, Y.; Nie, Y.; Tang, K. Characterization of the Key Aroma Compounds in Marselan Wine by Gas Chromatography-Olfactometry, Quantitative Measurements, Aroma Recombination, and Omission Tests. Molecules 2019, 24, 2978. [Google Scholar] [CrossRef]
- Sefton, M.A.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Occurrence, Sensory Impact, Formation, and Fate of Damascenone in Grapes, Wines, and Other Foods and Beverages. J. Agric. Food Chem. 2011, 59, 9717–9746. [Google Scholar] [CrossRef]
- Gök, R.; Bechtloff, P.; Ziegler, M.; Schmarr, H.-G.; Fischer, U.; Winterhalter, P. Synthesis of Deuterium-Labeled 1,1,6-Trimethyl-1,2-Dihydronaphthalene (TDN) and Quantitative Determination of TDN and Isomeric Vitispiranes in Riesling Wines by a Stable-Isotope-Dilution Assay. J. Agric. Food Chem. 2019, 67, 6414–6422. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. The Role of Xanthophyll Cycle Carotenoids in the Protection of Photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Downey, M.O.; Mazza, M.; Krstic, M.P. Development of a Stable Extract for Anthocyanins and Flavonols from Grape Skin. Am. J. Enol. Vitic. 2007, 58, 358–364. [Google Scholar] [CrossRef]
- Tian, M.-B.; Liu, Y.; Lu, H.-C.; Hu, L.; Wang, Y.; Cheng, C.-F.; Chen, W.; Li, S.-D.; He, F.; Duan, C.-Q.; et al. Cluster Spatial Positions Varied the Phenolics Profiles of ‘Cabernet Sauvignon’ Grapes and Wines under a Fan Training System with Multiple Trunks. Food Chem. 2022, 387, 132930. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.-N.; He, F.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Optimization of Sample Preparation and Phloroglucinol Analysis of Marselan Grape Skin Proanthocyanidins Using HPLC-DAD-ESI-MS/MS. S. Afr. J. Enol. Vitic. 2012, 33, 122–131. [Google Scholar]
- Yao, X.-C.; Zhang, H.-L.; Ma, X.-R.; Xia, N.-Y.; Duan, C.-Q.; Yang, W.-M.; Pan, Q.-H. Leaching and Evolution of Anthocyanins and Aroma Compounds during Cabernet Sauvignon Wine Fermentation with Whole-Process Skin-Seed Contact. Food Chem. 2024, 436, 137727. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.-T.; Li, H.-Q.; Lu, H.-C.; He, L.; Peng, W.-T.; Chen, W.; Li, S.-D.; Li, S.-P.; Duan, C.-Q.; et al. Microclimate Changes Caused by Black Inter-Row Mulch Decrease Flavonoids Concentrations in Grapes and Wines under Semi-Arid Climate. Food Chem. 2021, 361, 130064. [Google Scholar] [CrossRef]
- He, L.; Ren, Z.-Y.; Wang, Y.; Fu, Y.-Q.; Li, Y.; Meng, N.; Pan, Q.-H. Variation of Growth-to-Ripening Time Interval Induced by Abscisic Acid and Synthetic Auxin Affecting Transcriptome and Flavor Compounds in Cabernet Sauvignon Grape Berry. Plants 2020, 9, 630. [Google Scholar] [CrossRef]
- Kamffer, Z.; Bindon, K.A.; Oberholster, A. Optimization of a Method for the Extraction and Quantification of Carotenoids and Chlorophylls during Ripening in Grape Berries (Vitis vinifera Cv. Merlot). J. Agric. Food Chem. 2010, 58, 6578–6586. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, N.; Wang, Y.; Cheng, J.; Duan, C.; Pan, Q. Transcription Factor VvWRKY70 Inhibits Both Norisoprenoid and Flavonol Biosynthesis in Grape. Plant Physiol. 2023, 193, kiad423. [Google Scholar] [CrossRef]
| Vintage and Variety | Treatment | Weight of 100 Grapes (g) | Total Soluble Solids (°Brix) | pH Value | Titratable Acidity (g/L) |
|---|---|---|---|---|---|
| 2021 Cabernet Sauvignon | LR1 | 128.40 ± 4.04 | 20.43 ± 0.5 | 3.37 ± 0.02 ab | 7.26 ± 0.19 ab |
| LR2 | 134.60 ± 12.15 | 19.97 ± 0.12 | 3.4 ± 0.03 a | 6.82 ± 0.11 b | |
| LR3 | 139.93 ± 5.99 | 20.03 ± 0.45 | 3.39 ± 0.01 a | 7.13 ± 0.34 ab | |
| None | 133.60 ± 7.92 | 20.4 ± 0.44 | 3.32 ± 0.03 b | 7.51 ± 0.14 a | |
| p value | ns | ns | 0.0147 | 0.0252 | |
| 2022 Cabernet Sauvignon | LR1 | 118.29 ± 2.76 | 20.3 ± 1.31 | 3.23 ± 0.02 | 4.38 ± 0.05 b |
| LR2 | 126.09 ± 2.31 | 21.23 ± 0.42 | 3.26 ± 0.01 | 4.27 ± 0.08 b | |
| LR3 | 119.88 ± 3.39 | 20.97 ± 0.78 | 3.24 ± 0.01 | 4.29 ± 0.01 b | |
| None | 126.13 ± 6.37 | 21.43 ± 0.75 | 3.21 ± 0.03 | 4.53 ± 0.06 a | |
| p value | ns | ns | ns | 0.0018 | |
| 2022 Marselan | LR1 | 112.70 ± 3.62 | 21.4 ± 0.36 | 3.37 ± 0.03 | 6.75 ± 0.11 b |
| LR2 | 113.00 ± 1.22 | 21.63 ± 0.4 | 3.34 ± 0.03 | 6.94 ± 0.09 ab | |
| LR3 | 105.33 ± 4.4 | 21.07 ± 0.47 | 3.34 ± 0.03 | 6.82 ± 0.11 b | |
| None | 106.93 ± 8.77 | 21.53 ± 0.06 | 3.35 ± 0.02 | 7.17 ± 0.36 a | |
| p value | ns | ns | ns | 0.0124 |
| Compounds | Vintage and Variety | LR1 | LR2 | LR3 | CK |
|---|---|---|---|---|---|
| Monomeric anthocyanins | |||||
| Cyanidin-3-O-glucoside | 2021 Cabernet Sauvignon | 223.05 ± 19.4 a | 181.29 ± 10.79 b | 223.4 ± 15.58 a | 219.02 ± 4.21 a |
| 2022 Cabernet Sauvignon | 214.15 ± 3.83 b | 273.57 ± 8.91 a | 216.1 ± 16.04 b | 186.79 ± 4.7 c | |
| 2022 Marselan | 187.29 ± 8 a | 152.68 ± 2.52 b | 187.03 ± 7.31 a | 152.55 ± 6.58 b | |
| Peonidin-3-O-glucoside | 2021 Cabernet Sauvignon | 624.8 ± 21.57 a | 558.62 ± 14.1 b | 579.79 ± 26.91 b | 567.67 ± 4.17 b |
| 2022 Cabernet Sauvignon | 518.32 ± 5.72 b | 572.32 ± 7.23 a | 497.24 ± 17.02 c | 504.31 ± 4.66 bc | |
| 2022 Marselan | 408.65 ± 8.92 a | 377.38 ± 2.46 b | 402.11 ± 3.63 a | 331.67 ± 9.6 c | |
| Delphinidin-3-O-glucoside | 2021 Cabernet Sauvignon | 651.97 ± 59.17 | 608.53 ± 43.32 | 649.67 ± 58.29 | 657.27 ± 25.22 |
| 2022 Cabernet Sauvignon | 636.64 ± 31.18 b | 788.8 ± 32.27 a | 590.21 ± 38.14 b | 600.22 ± 21.39 b | |
| 2022 Marselan | 669.39 ± 64.53 a | 523.95 ± 21.05 bc | 572.28 ± 36.71 b | 456.52 ± 31.79 c | |
| Petunidin-3-O-glucoside | 2021 Cabernet Sauvignon | 343.8 ± 26.4 | 334.12 ± 16.98 | 353.27 ± 32.2 | 340.53 ± 7.94 |
| 2022 Cabernet Sauvignon | 357.85 ± 10.45 b | 424.37 ± 13.65 a | 322.91 ± 16.44 c | 331.18 ± 9.1 c | |
| 2022 Marselan | 560.93 ± 41.04 a | 470.3 ± 11.56 bc | 503.81 ± 32.49 b | 427.25 ± 13.24 c | |
| Malvidin-3-O-glucoside | 2021 Cabernet Sauvignon | 1822.93 ± 64.3 a | 1919.16 ± 53.43 a | 1803.66 ± 84.17 a | 1673.74 ± 11.37 b |
| 2022 Cabernet Sauvignon | 1937.1 ± 37.06 b | 2035.78 ± 39.18 a | 1723.4 ± 36.79 d | 1844.14 ± 25.95 c | |
| 2022 Marselan | 2767.63 ± 58.28 a | 2774.62 ± 25.77 a | 2767.31 ± 68.72 a | 2634.22 ± 55.26 b | |
| Acylated anthocyanins | |||||
| Cyanidin-3-O-(6-O-acetyl) glucoside | 2021 Cabernet Sauvignon | 39.16 ± 4.52 a | 27.77 ± 1.54 b | 39.87 ± 4.37 a | 38.67 ± 1.16 a |
| 2022 Cabernet Sauvignon | 33.45 ± 1.61 b | 43.88 ± 0.71 a | 31.29 ± 3.51 bc | 28.13 ± 1.65 c | |
| 2022 Marselan | 21.24 ± 1.81 a | 14.99 ± 0.49 b | 20.77 ± 2.17 a | 14.33 ± 1.26 b | |
| Peonidin-3-O-(6-O-acetyl) glucoside | 2021 Cabernet Sauvignon | 238.9 ± 8.33 a | 221.38 ± 4.73 b | 239.26 ± 12.19 a | 220.04 ± 1.6 b |
| 2022 Cabernet Sauvignon | 215.5 ± 2.98 b | 233.56 ± 2.54 a | 199.54 ± 7.32 c | 209.89 ± 3.58 b | |
| 2022 Marselan | 167.69 ± 5.03 a | 157.67 ± 0.52 b | 159.18 ± 6.45 b | 128.69 ± 1.9 c | |
| Delphinidin-3-O-(6-O-acetyl) glucoside | 2021 Cabernet Sauvignon | 142.43 ± 13.39 | 136.38 ± 7.4 | 146.51 ± 14.88 | 148.5 ± 2.63 |
| 2022 Cabernet Sauvignon | 141.68 ± 6.01 b | 174.29 ± 7.49 a | 128.87 ± 9.13 b | 130.58 ± 3.03 b | |
| 2022 Marselan | 115.2 ± 12.15 a | 92.67 ± 4.73 b | 99.13 ± 9.81 ab | 72.28 ± 6.72 c | |
| Petunidin-3-O-(6-O-acetyl) glucoside | 2021 Cabernet Sauvignon | 196.67 ± 18.36 | 190.03 ± 9.96 | 214.61 ± 22.59 | 197.62 ± 4.16 |
| 2022 Cabernet Sauvignon | 213.62 ± 8.67 b | 245.08 ± 7.28 a | 177.87 ± 14.37 c | 186.02 ± 8.41 c | |
| 2022 Marselan | 232.46 ± 15.14 a | 195.91 ± 8.16 bc | 215.55 ± 18.98 ab | 173.26 ± 7.34 c | |
| Malvidin-3-O-(6-O-acetyl) glucoside | 2021 Cabernet Sauvignon | 1397.61 ± 57 a | 1426.52 ± 38.75 a | 1407.32 ± 80.01 a | 1231.66 ± 5.99 b |
| 2022 Cabernet Sauvignon | 1602.35 ± 47.26 a | 1628.64 ± 32.29 a | 1390.87 ± 36.59 c | 1472.92 ± 30.21 b | |
| 2022 Marselan | 1789.73 ± 42.95 b | 1875.68 ± 37.02 a | 1774.26 ± 51.32 bc | 1702.95 ± 22.29 c | |
| Coumaroylated anthocyanins | |||||
| Cyanidin-3-O-(6-O-p-coumaryl) glucoside | 2021 Cabernet Sauvignon | 8.85 ± 1.15 | 8.87 ± 0.91 | 9.51 ± 1.14 | 9.09 ± 0.59 |
| 2022 Cabernet Sauvignon | 12.56 ± 0.29 b | 15.56 ± 0.63 a | 10.65 ± 1.48 c | 8.55 ± 0.59 d | |
| 2022 Marselan | 30.74 ± 2.59 a | 20.1 ± 0.9 c | 27.2 ± 1.6 b | 20.54 ± 0.35 c | |
| Peonidin-3-O-(6-O-p-coumaryl) glucoside | 2021 Cabernet Sauvignon | 139.2 ± 6.84 a | 149.23 ± 7.46 a | 143.31 ± 7.58 a | 121.44 ± 1.1 b |
| 2022 Cabernet Sauvignon | 146.4 ± 3.2 a | 152.98 ± 4.59 a | 126.22 ± 7.02 b | 123.6 ± 3.55 b | |
| 2022 - | 233.78 ± 5.14 a | 219.18 ± 1.68 b | 224.19 ± 7.14 b | 186.85 ± 0.67 c | |
| Delphinidin-3-O-(6-O-p-coumaryl) glucoside | 2021 Cabernet Sauvignon | 0.4 ± 0.24 c | 2.4 ± 0.19 a | 1.04 ± 0.19 b | 1.39 ± 0.21 b |
| 2022 Cabernet Sauvignon | 0.34 ± 0.95 c | 3.14 ± 0.78 a | 1.95 ± 0.96 ab | 1.17 ± 0.34 bc | |
| 2022 Marselan | 27.29 ± 3.84 a | 20.3 ± 1.12 b | 21.6 ± 1.32 b | 16.13 ± 1.12 c | |
| Petunidin-3-O-(6-O-p-coumaryl) glucoside | 2021 Cabernet Sauvignon | 25.39 ± 2.39 bc | 33.4 ± 2.8 a | 29.45 ± 3.04 ab | 24.04 ± 0.87 c |
| 2022 Cabernet Sauvignon | 34.52 ± 1.64 b | 38.8 ± 1.86 a | 29.53 ± 2.49 c | 27.07 ± 0.99 c | |
| 2022 Marselan | 160.39 ± 16.73 a | 142.61 ± 4.05 ab | 142.03 ± 7.41 ab | 125.78 ± 3.21 b | |
| Malvidin-3-O-(6-O-p-coumaryl) glucoside | 2021 Cabernet Sauvignon | 458.99 ± 22.13 b | 524.69 ± 31.73 a | 491.13 ± 26.8 ab | 390.34 ± 10.33 c |
| 2022 Cabernet Sauvignon | 537.49 ± 13.06 a | 525.23 ± 18.11 a | 444.06 ± 25.78 b | 446.76 ± 10.79 b | |
| 2022 Marselan | 1373.12 ± 64.74 b | 1463.14 ± 33.96 a | 1350.81 ± 31.7 b | 1329.69 ± 28.43 b | |
| Total anthocyanins | 2021 Cabernet Sauvignon | 6314.18 ± 321.38 | 6322.37 ± 231.46 | 6331.81 ± 386.6 | 5841.04 ± 64.99 |
| 2022 Cabernet Sauvignon | 6601.96 ± 159.33 b | 7156 ± 163.02 a | 5890.69 ± 224.22 c | 6101.32 ± 120.66 c | |
| 2022 Marselan | 8745.53 ± 330.49 a | 8501.18 ± 112.14 a | 8467.28 ± 280.59 a | 7772.72 ± 139.37 b | |
| Acetylation rate (%) | 2021 Cabernet Sauvignon | 31.91 ± 0.11 ab | 31.67 ± 0.21 b | 32.13 ± 0.18 a | 31.44 ± 0.12 b |
| 2022 Cabernet Sauvignon | 33.42 ± 0.27 a | 32.5 ± 0.07 c | 32.71 ± 0.21 bc | 33.23 ± 0.12 ab | |
| 2022 Marselan | 26.6 ± 0.15 b | 26.91 ± 0.16 b | 26.79 ± 0.12 b | 27.49 ± 0.15 a | |
| Coumaroylation rate (%) | 2021 Cabernet Sauvignon | 10.02 ± 0.01 c | 11.36 ± 0.24 a | 10.82 ± 0.17 b | 9.35 ± 0.09 d |
| 2022 Cabernet Sauvignon | 11.08 ± 0.03 a | 10.28 ± 0.14 b | 10.35 ± 0.24 b | 9.95 ± 0.14 b | |
| 2022 Marselan | 20.87 ± 0.25 b | 21.6 ± 0.33 ab | 21.27 ± 0.44 b | 21.94 ± 0.21 a | |
| Methylation rate (%) | 2021 Cabernet Sauvignon | 83.14 ± 0.57 b | 84.74 ± 0.35 a | 83.98 ± 0.88 b | 81.62 ± 0.27 c |
| 2022 Cabernet Sauvignon | 84.27 ± 0.27 ab | 81.85 ± 0.27 c | 82.79 ± 0.92 b | 84.34 ± 0.17 a | |
| 2022 Marselan | 88 ± 0.52 b | 90.58 ± 0.39 a | 89.82 ± 0.82 b | 90.3 ± 0.23 a | |
| Compounds | Vintage and Variety | LR1 | LR2 | LR3 | CK |
|---|---|---|---|---|---|
| kaempferol-3-O-glucoside | 2021 Cabernet Sauvignon | 3.05 ± 0.09 b | 3.97 ± 0.36 a | 3.77 ± 0.48 a | 2.21 ± 0.07 c |
| 2022 Cabernet Sauvignon | 8.38 ± 0.4 b | 8.38 ± 0.32 b | 9.18 ± 0.44 a | 5.86 ± 0.46 c | |
| 2022 Marselan | 4.74 ± 0.39 a | 3.96 ± 0.34 bc | 4.48 ± 0.45 ab | 3.74 ± 0.3 c | |
| kaempferol-3-O-galactoside | 2021 Cabernet Sauvignon | 0.78 ± 0.03 b | 0.92 ± 0.05 a | 0.92 ± 0.07 a | 0.51 ± 0.01 c |
| 2022 Cabernet Sauvignon | 2.28 ± 0.15 b | 2.29 ± 0.12 b | 2.57 ± 0.18 a | 1.46 ± 0.1 c | |
| 2022 Marselan | 1.25 ± 0.1 a | 0.96 ± 0.01 bc | 1.13 ± 0.16 ab | 0.91 ± 0.09 c | |
| kaempferol-3-O-glucuronide | 2021 Cabernet Sauvignon | 0.48 ± 0.01 b | 0.54 ± 0.05 a | 0.43 ± 0.03 b | 0.36 ± 0.02 c |
| 2022 Cabernet Sauvignon | 1.06 ± 0.05 a | 1.13 ± 0.05 a | 1.05 ± 0.08 a | 0.73 ± 0.06 b | |
| 2022 Marselan | 0.47 ± 0.03 a | 0.41 ± 0.01 b | 0.41 ± 0.05 ab | 0.38 ± 0.02 b | |
| quercetin-3-O-glucoside | 2021 Cabernet Sauvignon | 20.48 ± 0.36 b | 24.65 ± 1.48 a | 23.22 ± 1.21 a | 17.29 ± 0.38 c |
| 2022 Cabernet Sauvignon | 32.57 ± 0.96 a | 32.19 ± 1.02 a | 28.89 ± 1.02 b | 24.57 ± 1.34 c | |
| 2022 Marselan | 28.54 ± 0.86 a | 24.86 ± 0.72 b | 25.41 ± 1.45 b | 24.45 ± 0.91 b | |
| quercetin-3-O-galactoside | 2021 Cabernet Sauvignon | 6.99 ± 0.36 b | 9.23 ± 0.84 a | 8.28 ± 0.56 a | 5.39 ± 0.13 c |
| 2022 Cabernet Sauvignon | 16.05 ± 0.87 a | 15.36 ± 0.8 ab | 13.85 ± 0.76 b | 10.43 ± 0.96 c | |
| 2022 Marselan | 6.95 ± 0.62 a | 5.26 ± 0.51 b | 5.6 ± 0.98 b | 4.94 ± 0.47 b | |
| quercetin-3-O-glucuronide | 2021 Cabernet Sauvignon | 20.17 ± 0.53 | 22.06 ± 1.69 | 19.4 ± 1.68 | 19.98 ± 1.47 |
| 2022 Cabernet Sauvignon | 34.9 ± 0.74 a | 34.89 ± 1.2 a | 28.42 ± 1.75 b | 27.39 ± 1.73 b | |
| 2022 Marselan | 28.55 ± 0.64 a | 23.33 ± 0.24 c | 22.21 ± 2.32 c | 26.09 ± 0.51 b | |
| quercetin-3-O-rhamnoside | 2021 Cabernet Sauvignon | nd | nd | nd | nd |
| 2022 Cabernet Sauvignon | 4.7 ± 0.37 b | 5.63 ± 0.25 a | 3.21 ± 0.54 c | 1.67 ± 0.39 d | |
| 2022 Marselan | 6.33 ± 0.59 a | 6.74 ± 0.34 a | 4.04 ± 1.07 b | 4.19 ± 0.22 b | |
| quercetin-3-O-rutinoside | 2021 Cabernet Sauvignon | 0.9 ± 0.01 b | 0.89 ± 0.09 b | 0.89 ± 0.09 b | 1.17 ± 0.06 a |
| 2022 Cabernet Sauvignon | 2.71 ± 0.12 a | 2.37 ± 0.14 b | 2.16 ± 0.2 bc | 1.96 ± 0.24 c | |
| 2022 Marselan | 3.21 ± 0.15 a | 2.09 ± 0.04 b | 2.15 ± 0.23 b | 2.36 ± 0.04 b | |
| Isorhamnetin-3-O-glucoside | 2021 Cabernet Sauvignon | 4.79 ± 0.1 c | 6.41 ± 0.48 a | 5.69 ± 0.37 b | 4.16 ± 0.26 c |
| 2022 Cabernet Sauvignon | 7.43 ± 0.23 a | 7.32 ± 0.27 a | 7.08 ± 0.28 a | 5.37 ± 0.38 b | |
| 2022 Marselan | 6.76 ± 0.2 a | 5.89 ± 0.26 bc | 5.4 ± 0.48 c | 6.14 ± 0.25 b | |
| Isorhamnetin-3-O-glucuronide | 2021 Cabernet Sauvignon | 0.95 ± 0.05 b | 1.17 ± 0.11 a | 0.97 ± 0.08 b | nd |
| 2022 Cabernet Sauvignon | 1.51 ± 0.07 ab | 1.54 ± 0.09 a | 1.36 ± 0.07 b | 0.93 ± 0.1 c | |
| 2022 Marselan | 2.91 ± 0.09 a | 2.4 ± 0.3 ab | 1.9 ± 0.21 b | 2.71 ± 0.44 a | |
| myricetin-3-O-glucoside | 2021 Cabernet Sauvignon | 43.68 ± 0.6 b | 54.96 ± 5.22 a | 52.62 ± 4.67 a | 36.84 ± 2.55 b |
| 2022 Cabernet Sauvignon | 64.47 ± 3 a | 62.18 ± 3.83 ab | 57.47 ± 3.77 b | 46.95 ± 2.62 c | |
| 2022 Marselan | 74.74 ± 5.29 a | 56.31 ± 4.17 b | 61.59 ± 3.43 b | 70.87 ± 2.47 a | |
| myricetin-3-O-galactoside | 2021 Cabernet Sauvignon | 1.4 ± 0.1 a | 1.67 ± 0.2 a | 1.69 ± 0.19 a | 0.98 ± 0.08 b |
| 2022 Cabernet Sauvignon | 2.5 ± 0.12 a | 2.34 ± 0.11 a | 2 ± 0.03 b | 1.58 ± 0.24 c | |
| 2022 Marselan | 1.84 ± 0.1 a | 1.14 ± 0.14 c | 1.31 ± 0.12 c | 1.59 ± 0.06 b | |
| myricetin-3-O-glucuronide | 2021 Cabernet Sauvignon | 3.9 ± 0.04 b | 4.53 ± 0.39 a | 3.94 ± 0.33 b | 3.31 ± 0.25 c |
| 2022 Cabernet Sauvignon | 6.14 ± 0.25 a | 6.04 ± 0.29 a | 5.27 ± 0.42 b | 4.46 ± 0.26 c | |
| 2022 Marselan | 5.93 ± 0.46 a | 4.16 ± 0.21 b | 4.36 ± 0.4 b | 5.59 ± 0.17 a | |
| laricitrin-3-O-glucoside | 2021 Cabernet Sauvignon | 3.18 ± 0.05 bc | 4.18 ± 0.37 a | 3.61 ± 0.21 b | 2.81 ± 0.19 c |
| 2022 Cabernet Sauvignon | 5.23 ± 0.15 a | 4.57 ± 0.19 b | 4.53 ± 0.23 b | 3.83 ± 0.24 c | |
| 2022 Marselan | 7.48 ± 0.37 a | 6.62 ± 0.31 b | 6.27 ± 0.41 b | 7.45 ± 0.36 a | |
| syringetin-3-O-glucoside | 2021 Cabernet Sauvignon | 2.32 ± 0.01 b | 2.96 ± 0.21 a | 2.29 ± 0.16 b | 2.22 ± 0.17 b |
| 2022 Cabernet Sauvignon | 3.21 ± 0.1 a | 2.66 ± 0.08 b | 2.58 ± 0.11 bc | 2.41 ± 0.14 c | |
| 2022 Marselan | 6.41 ± 0.33 ab | 5.95 ± 0.28 bc | 5.44 ± 0.4 c | 7.07 ± 0.45 a | |
| Total flavonols | 2021 Cabernet Sauvignon | 113.06 ± 1.73 b | 138.14 ± 11.45 a | 127.73 ± 10 ab | 97.23 ± 5.41 c |
| 2022 Cabernet Sauvignon | 193.14 ± 7.38 a | 188.88 ± 8.17 a | 169.61 ± 9.79 b | 139.61 ± 9.04 c | |
| 2022 Marselan | 186.13 ± 4.59 a | 150.08 ± 6.81 c | 151.7 ± 11.47 c | 168.47 ± 6.18 b | |
| Proportion of kaempferol flavonols (%) | 2021 Cabernet Sauvignon | 3.81 ± 0.04 a | 3.93 ± 0.02 a | 4 ± 0.16 a | 3.19 ± 0.19 b |
| 2022 Cabernet Sauvignon | 6.07 ± 0.06 b | 6.24 ± 0.1 b | 7.55 ± 0.04 a | 5.77 ± 0.06 c | |
| 2022 Marselan | 3.47 ± 0.28 ab | 3.55 ± 0.09 a | 3.96 ± 0.17 a | 2.98 ± 0.11 b | |
| Proportion of quercetin flavonols (%) | 2021 Cabernet Sauvignon | 42.94 ± 0.33 b | 41.17 ± 0.39 c | 40.57 ± 0.4 c | 45.09 ± 0.52 a |
| 2022 Cabernet Sauvignon | 47.09 ± 0.26 a | 47.89 ± 0.53 a | 45.12 ± 0.09 b | 47.28 ± 0.3 a | |
| 2022 Marselan | 39.56 ± 1.64 a | 41.52 ± 0.82 a | 39.11 ± 0.91 a | 36.83 ± 0.15 b | |
| Proportion of Isorhamnetin flavonols (%) | 2021 Cabernet Sauvignon | 5.08 ± 0.06 b | 5.48 ± 0.04 a | 5.22 ± 0.05 b | 4.28 ± 0.07 c |
| 2022 Cabernet Sauvignon | 4.63 ± 0.03 b | 4.69 ± 0.06 b | 4.98 ± 0.09 a | 4.51 ± 0.04 b | |
| 2022 Marselan | 5.2 ± 0.16 a | 5.53 ± 0.27 a | 4.81 ± 0.09 b | 5.25 ± 0.17 a | |
| Proportion of myricetin flavonols (%) | 2021 Cabernet Sauvignon | 43.31 ± 0.41 bc | 44.25 ± 0.43 ab | 45.58 ± 0.41 a | 42.28 ± 0.58 c |
| 2022 Cabernet Sauvignon | 37.85 ± 0.26 | 37.34 ± 0.67 | 38.15 ± 0.21 | 37.97 ± 0.41 | |
| 2022 Marselan | 44.3 ± 1.89 ab | 41.02 ± 1.19 b | 44.39 ± 1.11 ab | 46.33 ± 0.31 a | |
| Proportion of laricitrin flavonols (%) | 2021 Cabernet Sauvignon | 2.82 ± 0.02 b | 3.02 ± 0.03 a | 2.83 ± 0.05 b | 2.89 ± 0.05 b |
| 2022 Cabernet Sauvignon | 2.71 ± 0.04 a | 2.42 ± 0.01 b | 2.67 ± 0.03 a | 2.75 ± 0.04 a | |
| 2022 Marselan | 4.02 ± 0.09 b | 4.41 ± 0.05 a | 4.13 ± 0.04 b | 4.42 ± 0.13 a | |
| Proportion of syringetin flavonols (%) | 2021 Cabernet Sauvignon | 2.05 ± 0.03 b | 2.15 ± 0.03 b | 1.8 ± 0.03 c | 2.28 ± 0.04 a |
| 2022 Cabernet Sauvignon | 1.66 ± 0.02 a | 1.41 ± 0.03 c | 1.52 ± 0.02 b | 1.73 ± 0.04 a | |
| 2022 Marselan | 3.44 ± 0.11 b | 3.97 ± 0.1 a | 3.59 ± 0.07 b | 4.19 ± 0.15 a |
| Compounds | Vintage and Variety | LR1 | LR2 | LR3 | CK |
|---|---|---|---|---|---|
| p-Cymene | 2021 Cabernet Sauvignon | 0.47 ± 0.17 | 0.35 ± 0.13 | 0.54 ± 0.21 | 0.38 ± 0.11 |
| 2022 Cabernet Sauvignon | 0.14 ± 0.02 b | 0.3 ± 0.09 a | 0.3 ± 0.11 a | 0.37 ± 0.06 a | |
| 2022 Marselan | 1.87 ± 0.86 | 1.78 ± 0.96 | 1.67 ± 0.91 | 0.9 ± 0.27 | |
| p-Cymenene | 2021 Cabernet Sauvignon | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.02 | 0.13 ± 0.01 |
| 2022 Cabernet Sauvignon | 0.1 ± 0.01 b | 0.13 ± 0.01 a | 0.13 ± 0.01 a | 0.14 ± 0.02 a | |
| 2022 Marselan | 0.2 ± 0.02 | 0.22 ± 0.05 | 0.22 ± 0.02 | 0.17 ± 0.02 | |
| Linalol | 2021 Cabernet Sauvignon | 0.11 ± 0.01 ab | 0.1 ± 0.01 b | 0.09 ± 0 b | 0.14 ± 0.03 a |
| 2022 Cabernet Sauvignon | nd | nd | nd | nd | |
| 2022 Marselan | 0.12 ± 0.02 | 0.16 ± 0.05 | 0.17 ± 0.04 | 0.17 ± 0.02 | |
| Levomenthol | 2021 Cabernet Sauvignon | 0.72 ± 0.08 | 0.48 ± 0.07 | 0.61 ± 0.11 | 0.63 ± 0.18 |
| 2022 Cabernet Sauvignon | 0.47 ± 0.06 b | 0.54 ± 0.11 ab | 0.47 ± 0.03 b | 0.71 ± 0.18 a | |
| 2022 Marselan | 0.64 ± 0.09 a | 0.44 ± 0.02 b | 0.42 ± 0.04 b | 0.41 ± 0.1 b | |
| α-Terpineol | 2021 Cabernet Sauvignon | 0.06 ± 0.01 b | 0.05 ± 0.01 b | 0.07 ± 0.01 b | 0.09 ± 0.01 a |
| 2022 Cabernet Sauvignon | nd | nd | nd | nd | |
| 2022 Marselan | 0.08 ± 0.01 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.1 ± 0.03 | |
| γ-Terpineol | 2021 Cabernet Sauvignon | 0.04 ± 0.01 b | 0.03 ± 0.01 b | 0.04 ± 0.01 b | 0.06 ± 0.01 a |
| 2022 Cabernet Sauvignon | nd | nd | nd | nd | |
| 2022 Marselan | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.01 | 0.07 ± 0.01 | |
| Levomenthol (Bound) | 2021 Cabernet Sauvignon | 2.37 ± 0.43 | 2.21 ± 0.23 | 2.03 ± 0.19 | 1.99 ± 0.37 |
| 2022 Cabernet Sauvignon | 2.92 ± 0.08 a | 1.68 ± 0.03 b | 2.64 ± 0.21 ab | 1.68 ± 0.15 b | |
| 2022 Marselan | 1.52 ± 0.05 | 2.01 ± 0.62 | 1.45 ± 0.09 | 2.02 ± 0.73 | |
| α-Terpineol (Bound) | 2021 Cabernet Sauvignon | 0.32 ± 0.04 | 0.26 ± 0.05 | 0.25 ± 0.03 | 0.28 ± 0.08 |
| 2022 Cabernet Sauvignon | 0.27 ± 0.08 a | 0.1 ± 0.04 b | 0.2 ± 0.06 ab | 0.13 ± 0.07 ab | |
| 2022 Marselan | 0.37 ± 0.09 | 0.39 ± 0.03 | 0.48 ± 0.17 | 0.31 ± 0.07 | |
| β-Citronellol (Bound) | 2021 Cabernet Sauvignon | 0.17 ± 0.03 | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.17 ± 0.04 |
| 2022 Cabernet Sauvignon | nd | nd | nd | nd | |
| 2022 Marselan | 0.22 ± 0.05 | 0.37 ± 0.11 | 0.29 ± 0.08 | 0.34 ± 0.07 | |
| Total Terpenes (Free) | 2021 Cabernet Sauvignon | 1.52 ± 0.22 | 1.13 ± 0.17 | 1.49 ± 0.27 | 1.45 ± 0.11 |
| 2022 Cabernet Sauvignon | 0.71 ± 0.06 b | 0.98 ± 0.05 ab | 0.9 ± 0.12 ab | 1.23 ± 0.18 a | |
| 2022 Marselan | 2.98 ± 0.71 | 2.8 ± 0.87 | 2.68 ± 0.75 | 1.81 ± 0.36 | |
| Total Terpenes (Bound) | 2021 Cabernet Sauvignon | 3.24 ± 0.27 a | 2.62 ± 0.16 ab | 2.43 ± 0.18 b | 2.36 ± 0.32 b |
| 2022 Cabernet Sauvignon | 3.2 ± 0.78 a | 1.78 ± 0.03 b | 2.85 ± 0.21 ab | 1.81 ± 0.18 b | |
| 2022 Marselan | 2.12 ± 0.09 | 2.76 ± 0.62 | 2.23 ± 0.15 | 2.67 ± 0.67 | |
| Total Terpenes | 2021 Cabernet Sauvignon | 4.76 ± 0.41 | 3.75 ± 0.33 | 3.92 ± 0.36 | 3.81 ± 0.4 |
| 2022 Cabernet Sauvignon | 3.91 ± 0.73 | 2.76 ± 0.05 | 3.75 ± 0.31 | 3.03 ± 0.34 | |
| 2022 Marselan | 5.1 ± 0.67 | 5.56 ± 0.35 | 4.91 ± 0.66 | 4.47 ± 0.4 |
| Compounds | Vintage and Variety | LR1 | LR2 | LR3 | CK |
|---|---|---|---|---|---|
| Norisoprenoids | |||||
| 6-methyl-5-Hepten-2-one | 2021 Cabernet Sauvignon | 0.31 ± 0.07 a | 0.21 ± 0.04 b | 0.3 ± 0.02 a | 0.27 ± 0.02 ab |
| 2022 Cabernet Sauvignon | 0.17 ± 0.02 b | 0.2 ± 0.01 b | 0.26 ± 0.09 ab | 0.33 ± 0.06 a | |
| 2022 Marselan | 0.32 ± 0.07 | 0.25 ± 0.05 | 0.29 ± 0.08 | 0.23 ± 0.02 | |
| Vitispirane | 2021 Cabernet Sauvignon | nd | nd | nd | 0.04 ± 0.02 |
| 2022 Cabernet Sauvignon | nd | 0.04 ± 0.02 b | 0.04 ± 0.02 b | 0.15 ± 0.03 a | |
| 2022 Marselan | 0.62 ± 0.29 | 0.93 ± 0.27 | 0.81 ± 0.04 | 0.93 ± 0.36 | |
| β-Ionone | 2021 Cabernet Sauvignon | 0.09 ± 0.02 | 0.1 ± 0.02 | 0.1 ± 0.01 | 0.11 ± 0 |
| 2022 Cabernet Sauvignon | 0.07 ± 0.01 b | 0.07 ± 0.01 b | 0.07 ± 0 b | 0.12 ± 0.03 a | |
| 2022 Marselan | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.06 ± 0.01 | |
| Theaspirane | 2021 Cabernet Sauvignon | nd | nd | nd | nd |
| 2022 Cabernet Sauvignon | nd | nd | nd | nd | |
| 2022 Marselan | 0.36 ± 0.08 | 0.27 ± 0.07 | 0.32 ± 0.05 | 0.29 ± 0.06 | |
| β-Damascenone | 2021 Cabernet Sauvignon | 1.78 ± 0.59 c | 3.15 ± 0.93 c | 6.94 ± 1.26 b | 11.3 ± 2.08 a |
| 2022 Cabernet Sauvignon | 1.67 ± 1.09 c | 4.85 ± 2.15 bc | 6.28 ± 2.11 b | 14.03 ± 1.39 a | |
| 2022 Marselan | 19.02 ± 3.97 | 23.57 ± 0.87 | 25.62 ± 4.6 | 21.89 ± 5.63 | |
| cis-Geranyl acetone | 2021 Cabernet Sauvignon | 0.34 ± 0.08 | 0.27 ± 0.06 | 0.33 ± 0.08 | 0.27 ± 0.03 |
| 2022 Cabernet Sauvignon | 0.13 ± 0.03 b | 0.18 ± 0.04 b | 0.23 ± 0.09 ab | 0.46 ± 0.25 a | |
| 2022 Marselan | 0.48 ± 0.1 | 0.39 ± 0.06 | 0.33 ± 0.17 | 0.32 ± 0.12 | |
| β-Damascenone (Bound) | 2021 Cabernet Sauvignon | nd | nd | nd | nd |
| 2022 Cabernet Sauvignon | 0.32 ± 0.15 a | 0.11 ± 0.02 b | 0.32 ± 0.04 a | 0.22 ± 0.05 ab | |
| 2022 Marselan | 0.21 ± 0.04 b | 0.45 ± 0.13 a | 0.42 ± 0.04 a | 0.53 ± 0.12 a | |
| Carotenoids | |||||
| β-catotene | 2021 Cabernet Sauvignon | 3.28 ± 0.09 b | 3.18 ± 0.33 b | 3.78 ± 0.23 a | 3.5 ± 0.21 ab |
| 2022 Cabernet Sauvignon | 4.88 ± 0.58 | 4.89 ± 0.62 | 3.75 ± 0.42 | 4.35 ± 0.81 | |
| 2022 Marselan | 6.46 ± 0.43 ab | 5.99 ± 0.5 b | 5.88 ± 0.26 b | 7.31 ± 0.21 a | |
| Xanthophyll | 2021 Cabernet Sauvignon | 24.55 ± 6.35 | 24.27 ± 1.47 | 29.7 ± 0.18 | 27.54 ± 3.26 |
| 2022 Cabernet Sauvignon | 33.58 ± 7.2 a | 36.04 ± 3.37 a | 25.65 ± 0.97 b | 25.75 ± 2.1 b | |
| 2022 Marselan | 31.83 ± 5.03 | 31.76 ± 4.16 | 32.58 ± 4.01 | 39.23 ± 2.67 | |
| Zeaxanthin | 2021 Cabernet Sauvignon | 2.84 ± 0.51 a | 1.79 ± 0.14 c | 2.64 ± 0.31 ab | 2.11 ± 0.03 bc |
| 2022 Cabernet Sauvignon | 5.17 ± 0.57 | 5.27 ± 0.73 | 4 ± 0.75 | 4.85 ± 0.84 | |
| 2022 Marselan | 3.79 ± 0.43 ab | 3.27 ± 0.42 b | 3.63 ± 0.38 ab | 4.31 ± 0.28 a | |
| Antheraxanthin | 2021 Cabernet Sauvignon | 0.5 ± 0.13 | 0.47 ± 0.16 | 0.62 ± 0.04 | 0.56 ± 0.17 |
| 2022 Cabernet Sauvignon | 1.15 ± 0.2 | 1.09 ± 0.33 | 0.82 ± 0.08 | 1.17 ± 0.2 | |
| 2022 Marselan | 1.13 ± 0.15 | 1.02 ± 0.09 | 1.09 ± 0.23 | 1.16 ± 0.08 | |
| Violaxanthin | 2021 Cabernet Sauvignon | 1.07 ± 0.12 b | 1.22 ± 0.35 ab | 1.55 ± 0.43 ab | 1.87 ± 0.38 a |
| 2022 Cabernet Sauvignon | 1.81 ± 0.32 | 1.88 ± 0.79 | 1.91 ± 0.6 | 2.29 ± 1.15 | |
| 2022 Marselan | 3.99 ± 1.65 | 3.23 ± 0.86 | 2.68 ± 0.08 | 3.17 ± 0.44 | |
| Neoxanthin | 2021 Cabernet Sauvignon | 1.07 ± 0.26 b | 1.1 ± 0.1 b | 1.44 ± 0.29 ab | 1.9 ± 0.21 a |
| 2022 Cabernet Sauvignon | 1.13 ± 0.17 | 1.38 ± 0.21 | 1.43 ± 0.32 | 1.57 ± 0.51 | |
| 2022 Marselan | 2.82 ± 1.13 | 2.39 ± 0.98 | 2.15 ± 0.07 | 3.03 ± 0.24 | |
| Total Norisoprenoids | 2021 Cabernet Sauvignon | 2.55 ± 0.4 c | 3.77 ± 0.75 c | 7.72 ± 0.97 b | 12.02 ± 1.66 a |
| 2022 Cabernet Sauvignon | 2.4 ± 0.96 b | 5.48 ± 1.77 b | 7.23 ± 1.78 b | 15.33 ± 1.38 a | |
| 2022 Marselan | 21.1 ± 3.58 | 25.93 ± 0.56 | 27.85 ± 3.71 | 24.25 ± 4.78 | |
| Total carotenoids | 2021 Cabernet Sauvignon | 33.31 ± 6.8 | 32.03 ± 1.62 | 39.72 ± 0.22 | 37.46 ± 3.88 |
| 2022 Cabernet Sauvignon | 47.72 ± 6.77 ab | 50.55 ± 4.55 a | 37.57 ± 2.54 b | 39.97 ± 1.75 ab | |
| 2022 Marselan | 50 ± 6.17 | 47.66 ± 6.91 | 48.01 ± 4.78 | 58.21 ± 2.87 | |
| V/(V + A + Z) (%) a | 2021 Cabernet Sauvignon | 24.63 ± 2.81 | 35.03 ± 7.57 | 32.13 ± 6.44 | 39.82 ± 8.16 |
| 2022 Cabernet Sauvignon | 22.24 ± 2.5 | 22.22 ± 3.38 | 28.01 ± 2.58 | 27.43 ± 10.97 | |
| 2022 Marselan | 43.75 ± 9.57 | 42.53 ± 3.36 | 36.36 ± 2.96 | 36.57 ± 4.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Wu, Y.; Lan, Y.; Cui, Y.; Shi, T.; Duan, C.; Pan, Q. Effect of Cluster-Zone Leaf Removal at Different Stages on Cabernet Sauvignon and Marselan (Vitis vinifera L.) Grape Phenolic and Volatile Profiles. Plants 2024, 13, 1543. https://doi.org/10.3390/plants13111543
Yao X, Wu Y, Lan Y, Cui Y, Shi T, Duan C, Pan Q. Effect of Cluster-Zone Leaf Removal at Different Stages on Cabernet Sauvignon and Marselan (Vitis vinifera L.) Grape Phenolic and Volatile Profiles. Plants. 2024; 13(11):1543. https://doi.org/10.3390/plants13111543
Chicago/Turabian StyleYao, Xuechen, Yangpeng Wu, Yibin Lan, Yanzhi Cui, Tonghua Shi, Changqing Duan, and Qiuhong Pan. 2024. "Effect of Cluster-Zone Leaf Removal at Different Stages on Cabernet Sauvignon and Marselan (Vitis vinifera L.) Grape Phenolic and Volatile Profiles" Plants 13, no. 11: 1543. https://doi.org/10.3390/plants13111543







