Effects of Jasmonic Acid on Stress Response and Quality Formation in Vegetable Crops and Their Underlying Molecular Mechanisms
Abstract
1. Introduction
2. Jasmonic Acid (JA)
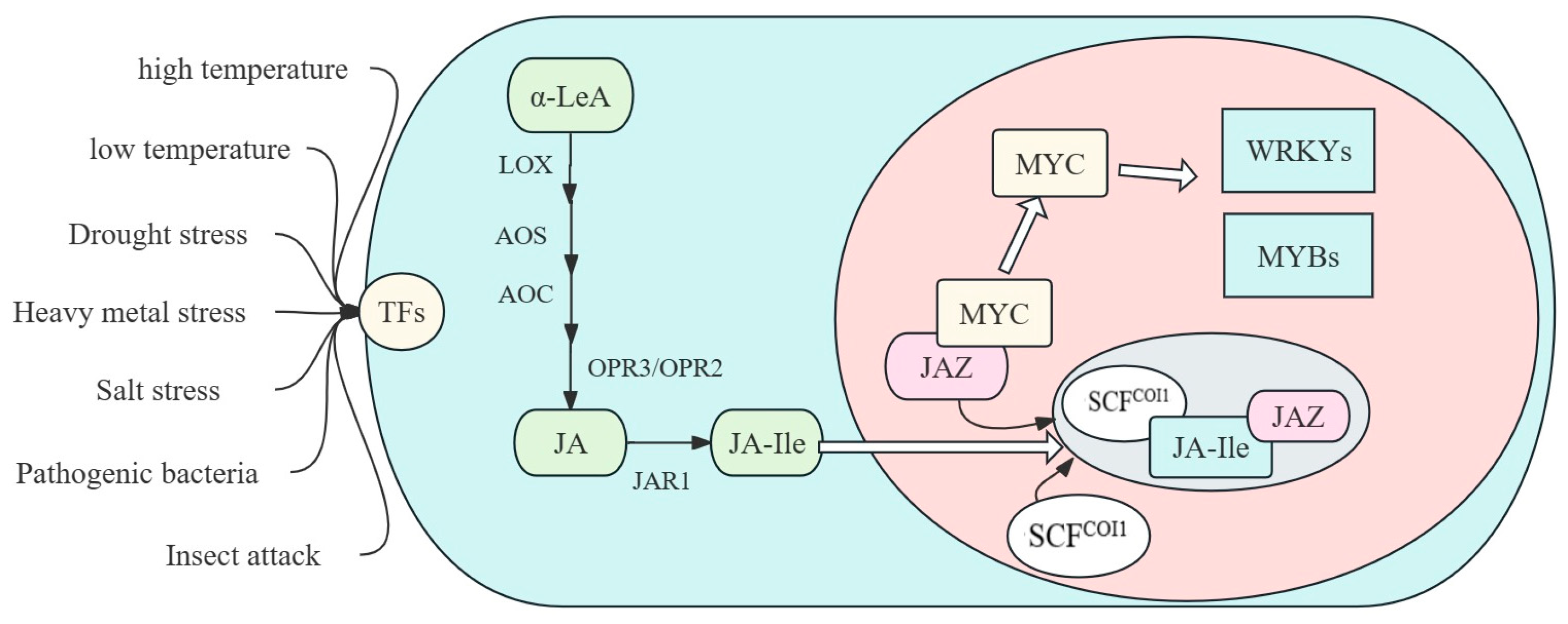
3. JA Biosynthesis and Signaling
3.1. JA Biosynthesis
3.2. JA Signaling
4. JA Regulation
4.1. Growth Accumulation
4.2. The Influence of External Factors
4.3. Molecular Regulation
4.3.1. Synthase Regulation in Signal Transduction
4.3.2. Transcription Factors Regulation
5. The Role of JA in Vegetable Crop Resistance
5.1. Disease and Pest Resistance
5.2. Abiotic Stress
5.2.1. Salt Stress
5.2.2. Drought Stress
5.2.3. High- and Low-Temperature Stress
5.2.4. Heavy Metal Stress
6. Quality
6.1. Vitamin C
6.2. Flavonoid Substances
6.3. Phenolic Compounds
6.4. Terpenoids
6.5. Lignin
7. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, L.; Hao, N.; Wu, T.; Cao, J. Advances in Understanding and Harnessing the Molecular Regulatory Mechanisms of Vegetable Quality. Front. Plant Sci. 2022, 13, 836515. [Google Scholar] [CrossRef] [PubMed]
- Pasković, I.; Soldo, B.; Goreta Ban, S.; Radić, T.; Lukić, M.; Urlić, B.; Mimica, M.; Brkić Bubola, K.; Colla, G.; Rouphael, Y.; et al. Fruit quality and volatile compound composition of processing tomato as affected by fertilisation practices and arbuscular mycorrhizal fungi application. Food Chem. 2021, 359, 129961. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Chauhan, S.; Dhillon, T.S. Genome editing for vegetable crop improvement: Challenges and future prospects. Front. Genet. 2022, 13, 1037091. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Hsu, W.H.; Mao, W.T.; Yang, C.H. The Oncidium Ethylene Synthesis Gene Oncidium 1-Aminocyclopropane-1 Carboxylic Acid Synthase 12 and Ethylene Receptor Gene Oncidium ETR1 Affect GA-DELLA and Jasmonic Acid Signaling in Regulating Flowering Time, Anther Dehiscence, and Flower Senescence in Arabidopsis. Front. Plant Sci. 2022, 13, 785441. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Suzuki, T.; Takebayashi, Y.; Ishiguro, R.; Higashiyama, T.; Sakakibara, H.; Ishiguro, S. Jasmonic acid facilitates flower opening and floral organ development through the upregulated expression of SlMYB21 transcription factor in tomato. Biosci. Biotechnol. Biochem. 2018, 82, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate Signaling Network Activates Root Stem Cells and Promotes Regeneration. Cell 2019, 177, 942–956.e914. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Liang, X.; Zhang, Y.; Fernie, A.R. Mass spectrometric exploration of phytohormone profiles and signaling networks. Trends Plant Sci. 2023, 28, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Shikha, D.; Jakhar, P.; Satbhai, S.B. Role of jasmonate signaling in the regulation of plant responses to nutrient deficiency. J. Exp. Bot. 2023, 74, 1221–1243. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Gomi, K. Jasmonic Acid Pathway in Plants 2.0. Int. J. Mol. Sci. 2021, 22, 3506. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Lan, K.; Liu, Y.; Chen, R.; Hu, T.; Zhao, S.; Yin, X.; Xie, T. Transcriptome analysis reveals regulation mechanism of methyl jasmonate-induced terpenes biosynthesis in Curcuma wenyujin. PLoS ONE 2022, 17, e0270309. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.X.; Fang, Y.; Li, D.; Mao, Z.; Zhu, Z.; Chen, X.S.; Feng, S.Q. MdERF1B-MdMYC2 module integrates ethylene and jasmonic acid to regulate the biosynthesis of anthocyanin in apple. Hortic. Res. 2022, 9, uhac142. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, W.; Tong, T.; Chen, G.; Zeng, F.; Jang, S.; Gao, W.; Li, Z.; Mak, M.; Deng, F.; et al. Molecular Interaction and Evolution of Jasmonate Signaling with Transport and Detoxification of Heavy Metals and Metalloids in Plants. Front. Plant Sci. 2021, 12, 665842. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Oliver, D.J. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 1998, 10, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Qiu, J.; Zhang, Y.; Li, M.; Liu, P. Jasmonates Coordinate Secondary with Primary Metabolism. Metabolites 2023, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional Roles in Stress Tolerance. Front. Plant Sci. 2016, 7, 813. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Nandi, A.K. AtOZF1 positively regulates JA signaling and SA-JA cross-talk in Arabidopsis thaliana. J. Biosci. 2022, 47, 8. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, C.; Wang, L. Novel Crosstalks between Circadian Clock and Jasmonic Acid Pathway Finely Coordinate the Tradeoff among Plant Growth, Senescence and Defense. Int. J. Mol. Sci. 2019, 20, 5254. [Google Scholar] [CrossRef]
- Yi, R.; Shan, X. Post-translational modifications: Emerging regulators manipulating jasmonate biosynthesis and signaling. Plant Cell Rep. 2023, 42, 215–222. [Google Scholar] [CrossRef]
- Fonseca, S.; Chico, J.M.; Solano, R. The jasmonate pathway: The ligand, the receptor and the core signalling module. Curr. Opin. Plant Biol. 2009, 12, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C. Perception, signaling and cross-talk of jasmonates and the seminal contributions of the Daoxin Xie’s lab and the Chuanyou Li’s lab. Plant Cell Rep. 2014, 33, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Forner, S.; Strnad, M.; Hause, B. Jasmonates in flower and seed development. Biochimie 2013, 95, 79–85. [Google Scholar] [CrossRef] [PubMed]
- León, J. Role of plant peroxisomes in the production of jasmonic acid-based signals. Subcell Biochem. 2013, 69, 299–313. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, X.; Li, Z.; Song, Q.; Xu, C.; Luo, K. Jasmonic acid regulates lignin deposition in poplar through JAZ5-MYB/NAC interaction. Front. Plant Sci. 2023, 14, 1232880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yao, J.; Ke, J.; Zhang, L.; Lam, V.Q.; Xin, X.F.; Zhou, X.E.; Chen, J.; Brunzelle, J.; Griffin, P.R.; et al. Structural basis of JAZ repression of MYC transcription factors in jasmonate signalling. Nature 2015, 525, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhao, J.; Tzeng, D.T.W.; Liu, Y.; Deng, L.; Yang, T.; Zhai, Q.; Wu, F.; Huang, Z.; Zhou, M.; et al. MYC2 Orchestrates a Hierarchical Transcriptional Cascade That Regulates Jasmonate-Mediated Plant Immunity in Tomato. Plant Cell 2017, 29, 1883–1906. [Google Scholar] [CrossRef]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef]
- Zhou, X.E.; Zhang, Y.; Yao, J.; Zheng, J.; Zhou, Y.; He, Q.; Moreno, J.; Lam, V.Q.; Cao, X.; Sugimoto, K.; et al. Assembly of JAZ-JAZ and JAZ-NINJA complexes in jasmonate signaling. Plant Commun. 2023, 4, 100639. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Zhang, Y.; Peng, W.; Wang, Z.; Xie, D. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 2009, 60, 3849–3860. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Chico, J.M.; Sánchez-Serrano, J.J.; Solano, R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Gimenez-Ibanez, S.; Goossens, A.; Solano, R. Redundancy and specificity in jasmonate signalling. Curr. Opin. Plant Biol. 2016, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant 2019, 165, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Serna-Escolano, V.; Valverde, J.M.; García-Pastor, M.E.; Valero, D.; Castillo, S.; Guillén, F.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M. Pre-harvest methyl jasmonate treatments increase antioxidant systems in lemon fruit without affecting yield or other fruit quality parameters. J. Sci. Food Agric. 2019, 99, 5035–5043. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Huang, W.; Li, M.; Xu, Z.; Wang, F.; Xiong, A. Expression profiles of genes involved in jasmonic acid biosynthesis and signaling during growth and development of carrot. Acta Biochim. Biophys. Sin. 2016, 48, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Y.; Wang, S.; Qi, T.; Song, S. Jasmonate action and crosstalk in flower development and fertility. J. Exp. Bot. 2023, 74, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Krajncic, B.; Kristl, J.; Janzekovic, I. Possible role of jasmonic acid in the regulation of floral induction, evocation and floral differentiation in Lemna minor L. Plant Physiol. Biochem. 2006, 44, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Diallo, A.O.; Agharbaoui, Z.; Badawi, M.A.; Ali-Benali, M.A.; Moheb, A.; Houde, M.; Sarhan, F. Transcriptome analysis of an mvp mutant reveals important changes in global gene expression and a role for methyl jasmonate in vernalization and flowering in wheat. J. Exp. Bot. 2014, 65, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Gong, W.; Yu, X.; Ji, K.; Jiang, Y.; Chang, Y.; Yuan, D. Transcriptome and Anatomical Comparisons Reveal the Effects of Methyl Jasmonate on the Seed Development of Camellia oleifera. J. Agric. Food Chem. 2023, 71, 6747–6762. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Li, C.; Peng, W.; Gao, B. New perspective of jasmonate function in leaf senescence. Plant Signal. Behav. 2011, 6, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Luo, F.; Li, P.; Zhou, Q.; Zhou, X.; Wei, B.; Cheng, S.; Zhou, H.; Ji, S. Potential of jasmonic acid (JA) in accelerating postharvest yellowing of broccoli by promoting its chlorophyll degradation. Food Chem. 2020, 309, 125737. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Yi, G.; Seo, J.; Kang, B.C.; Choi, J.H.; Lee, E.J. Jasmonic acid and ERF family genes are involved in chilling sensitivity and seed browning of pepper fruit after harvest. Sci. Rep. 2020, 10, 17949. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Yuan, H.; Kanwar, M.K.; Bhardwaj, R.; Thukral, A.K.; Zheng, B. Jasmonic Acid Seed Treatment Stimulates Insecticide Detoxification in Brassica juncea L. Front. Plant Sci. 2018, 9, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of jasmonic acid in plants: The molecular point of view. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.; Atzorn, R.; Brash, A.; Kuhn, H.; Wasternack, C.; Willmitzer, L.; Pena-Cortes, H. Expression of a Flax Allene Oxide Synthase cDNA Leads to Increased Endogenous Jasmonic Acid (JA) Levels in Transgenic Potato Plants but Not to a Corresponding Activation of JA-Responding Genes. Plant Cell 1995, 7, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Lulai, E.; Huckle, L.; Neubauer, J.; Suttle, J. Coordinate expression of AOS genes and JA accumulation: JA is not required for initiation of closing layer in wound healing tubers. J. Plant Physiol. 2011, 168, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Ogawa, D.; Katou, S.; Kamada, H.; Nakajima, N.; Saji, H.; Soyano, T.; Sasabe, M.; Machida, Y.; Mitsuhara, I.; et al. A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant Cell Physiol. 2005, 46, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Rahman, M.A.; Skalicky, M.; Alabdallah, N.M.; Waseem, M.; Jahan, M.S.; Ahammed, G.J.; El-Mogy, M.M.; El-Yazied, A.A.; Ibrahim, M.F.M.; et al. Ozone Induced Stomatal Regulations, MAPK and Phytohormone Signaling in Plants. Int. J. Mol. Sci. 2021, 22, 6304. [Google Scholar] [CrossRef] [PubMed]
- Grantz, D.A.; Vu, H.B. Root and shoot gas exchange respond additively to moderate ozone and methyl jasmonate without induction of ethylene: Ethylene is induced at higher O3 concentrations. J. Exp. Bot. 2012, 63, 4303–4313. [Google Scholar] [CrossRef] [PubMed]
- Nawkar, G.M.; Maibam, P.; Park, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. UV-Induced cell death in plants. Int. J. Mol. Sci. 2013, 14, 1608–1628. [Google Scholar] [CrossRef] [PubMed]
- Vanhaelewyn, L.; Prinsen, E.; Van Der Straeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef]
- Ðinh, S.T.; Gális, I.; Baldwin, I.T. UVB radiation and 17-hydroxygeranyllinalool diterpene glycosides provide durable resistance against mirid (Tupiocoris notatus) attack in field-grown Nicotiana attenuata plants. Plant Cell Environ. 2013, 36, 590–606. [Google Scholar] [CrossRef] [PubMed]
- Izaguirre, M.M.; Scopel, A.L.; Baldwin, I.T.; Ballaré, C.L. Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol. 2003, 132, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhang, S.; Wang, C.; Yang, X.; Wang, Y.; Su, X.; Du, J.; Yang, C. Arabidopsis CPR5 independently regulates seed germination and postgermination arrest of development through LOX pathway and ABA signaling. PLoS ONE 2011, 6, e19406. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Miszczuk, E.; Bajguz, A.; Hayat, S. Specific Roles of Lipoxygenases in Development and Responses to Stress in Plants. Plants 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vargas, R.; Saltveit, M.E. Involvement of putative chemical wound signals in the induction of phenolic metabolism in wounded lettuce. Physiol. Plant 2002, 114, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Brown, R.; Köllner, T.G.; Fu, J.; Chen, X.; Wong, G.K.; Gershenzon, J.; Peters, R.J.; Chen, F. Origin and early evolution of the plant terpene synthase family. Proc. Natl. Acad. Sci. USA 2022, 119, e2100361119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lu, S.; Yu, W.; Ehsan, S.; Zhang, Y.; Jia, H.; Fang, J. Jasmonate increases terpene synthase expression, leading to strawberry resistance to Botrytis cinerea infection. Plant Cell Rep. 2022, 41, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; Mosandl, A.; Wüst, M. Induction of de novo volatile terpene biosynthesis via cytosolic and plastidial pathways by methyl jasmonate in foliage of Vitis vinifera L. J. Agric. Food Chem. 2005, 53, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Liao, X.; Luo, X.; Zhu, Y.; Feng, S.; Yu, C.; Lu, J.; Shen, C.; Wang, H. Comparative Metabolomic and Proteomic Analyses Reveal the Regulation Mechanism Underlying MeJA-Induced Bioactive Compound Accumulation in Cutleaf Groundcherry (Physalis angulata L.) Hairy Roots. J. Agric. Food Chem. 2018, 66, 6336–6347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, X.; Chen, W.; Xu, Z.; Chen, M.; Wang, H.; Yu, D. The emerging role of jasmonate in the control of flowering time. J. Exp. Bot. 2022, 73, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef]
- Han, X.; Kui, M.; He, K.; Yang, M.; Du, J.; Jiang, Y.; Hu, Y. Jasmonate-regulated root growth inhibition and root hair elongation. J. Exp. Bot. 2023, 74, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Li, Z.; Min, D.; Zhang, X.; Ai, W.; Li, J.; Zhou, J.; Li, Z.; Li, F.; Li, X. CRISPR/Cas9-Mediated SlMYC2 Mutagenesis Adverse to Tomato Plant Growth and MeJA-Induced Fruit Resistance to Botrytis cinerea. J. Agric. Food Chem. 2020, 68, 5529–5538. [Google Scholar] [CrossRef]
- Gupta, N.; Prasad, V.B.; Chattopadhyay, S. LeMYC2 acts as a negative regulator of blue light mediated photomorphogenic growth, and promotes the growth of adult tomato plants. BMC Plant Biol. 2014, 14, 38. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Shan, J.; Li, C.; Suo, H.; Liu, J.; An, K.; Li, X.; Xiong, X. A Genome-Wide View of the Transcriptome Dynamics of Fresh-Cut Potato Tubers. Genes 2023, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Strategies for Enhanced Crop Resistance to Insect Pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, S.; Ye, N.; Jiang, M.; Cao, J.; Zhang, J. WRKY transcription factors in plant responses to stresses. J. Integr. Plant Biol. 2017, 59, 86–101. [Google Scholar] [CrossRef]
- Ji, N.; Li, Y.; Wang, J.; Zuo, X.; Li, M.; Jin, P.; Zheng, Y. Interaction of PpWRKY46 and PpWRKY53 regulates energy metabolism in MeJA primed disease resistance of peach fruit. Plant Physiol. Biochem. 2022, 171, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Taurino, M.; Abelenda, J.A.; Río-Alvarez, I.; Navarro, C.; Vicedo, B.; Farmaki, T.; Jiménez, P.; García-Agustín, P.; López-Solanilla, E.; Prat, S.; et al. Jasmonate-dependent modifications of the pectin matrix during potato development function as a defense mechanism targeted by Dickeya dadantii virulence factors. Plant J. 2014, 77, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Q.; Zhai, H.; Gao, S.; Yang, L.; Wang, Z.; Xu, Y.; Huo, J.; Ren, Z.; Zhao, N.; et al. IbBBX24 Promotes the Jasmonic Acid Pathway and Enhances Fusarium Wilt Resistance in Sweet Potato. Plant Cell 2020, 32, 1102–1123. [Google Scholar] [CrossRef] [PubMed]
- Montero-Vargas, J.M.; Casarrubias-Castillo, K.; Martínez-Gallardo, N.; Ordaz-Ortiz, J.J.; Délano-Frier, J.P.; Winkler, R. Modulation of steroidal glycoalkaloid biosynthesis in tomato (Solanum lycopersicum) by jasmonic acid. Plant Sci. 2018, 277, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Sripontan, Y.; Hwang, S.-Y. Jasmonate-induced defense in tomato and cabbage deterred Spodoptera litura (Noctuidae) growth. J. Asia-Pac. Entomol. 2016, 19, 1125–1129. [Google Scholar] [CrossRef]
- Erazo-Garcia, M.P.; Sotelo-Proaño, A.R.; Ramirez-Villacis, D.X.; Garcés-Carrera, S.; Leon-Reyes, A. Methyl jasmonate-induced resistance to Delia platura (Diptera: Anthomyiidae) in Lupinus mutabilis. Pest Manag. Sci. 2021, 77, 5382–5395. [Google Scholar] [CrossRef]
- Xu, D.; Zuo, J.; Li, P.; Yan, Z.; Gao, L.; Wang, Q.; Jiang, A. Effect of methyl jasmonate on the quality of harvested broccoli after simulated transport. Food Chem. 2020, 319, 126561. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ma, J.; Luo, D.; Hou, X.; Ma, F.; Zhang, Y.; Meng, Y.; Zhang, H.; Guo, W. CaMADS, a MADS-box transcription factor from pepper, plays an important role in the response to cold, salt, and osmotic stress. Plant Sci. 2019, 280, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, G.; Zhang, J.; Zhang, Y.; Xie, Q.; Zhao, Z.; Pan, Y.; Hu, Z. The abiotic stress-responsive NAC-type transcription factor SlNAC4 regulates salt and drought tolerance and stress-related genes in tomato (Solanum lycopersicum). Plant Cell Rep. 2014, 33, 1851–1863. [Google Scholar] [CrossRef]
- Shang, C.; Liu, X.; Chen, G.; Zheng, H.; Khan, A.; Li, G.; Hu, X. SlWRKY80-mediated jasmonic acid pathway positively regulates tomato resistance to saline-alkali stress by enhancing spermidine content and stabilizing Na+/K+ homeostasis. Hortic. Res. 2024, 11, uhae028. [Google Scholar] [CrossRef] [PubMed]
- Efimova, M.V.; Mukhamatdinova, E.A.; Kovtun, I.S.; Kabil, F.F.; Medvedeva, Y.V.; Kuznetsov, V.V. Jasmonic Acid Enhances the Potato Plant Resistance to the Salt Stress in Vitro. Dokl. Biol. Sci. 2019, 488, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, M.M.; Bafeel, S.O.; El-Zohri, M. Gibberellic Acid and Jasmonic Acid Improve Salt Tolerance in Summer Squash by Modulating Some Physiological Parameters Symptomatic for Oxidative Stress and Mineral Nutrition. Plants 2021, 10, 2768. [Google Scholar] [CrossRef] [PubMed]
- Balfagón, D.; Zandalinas, S.I.; Mittler, R.; Gómez-Cadenas, A. High temperatures modify plant responses to abiotic stress conditions. Physiol. Plant 2020, 170, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Karimi, R.; Gavili-Kilaneh, K.; Khadivi, A. Methyl jasmonate promotes salinity adaptation responses in two grapevine (Vitis vinifera L.) cultivars differing in salt tolerance. Food Chem. 2022, 375, 131667. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Turkyilmaz Unal, B.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant 2021, 172, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- González, E.M. Drought Stress Tolerance in Plants. Int. J. Mol. Sci. 2023, 24, 6562. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.; Li, J.; Gong, B.; Lu, Y.; Wu, X.; Lü, G.; Gao, H. Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones. Int. J. Mol. Sci. 2023, 24, 3876. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cheng, Z.; Li, Z.; Dong, S.; Yu, X.; Zhao, P.; Liao, W.; Yu, X.; Peng, M. MeSPL9 attenuates drought resistance by regulating JA signaling and protectant metabolite contents in cassava. Theor. Appl. Genet. 2022, 135, 817–832. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, M. Physiological responses and tolerance mechanisms to low temperature stress in plants. Int. J. Adv. Res. 2015, 3, 637–646. [Google Scholar]
- Sadura, I.; Janeczko, A. Brassinosteroids and the Tolerance of Cereals to Low and High Temperature Stress: Photosynthesis and the Physicochemical Properties of Cell Membranes. Int. J. Mol. Sci. 2021, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Ghanizadeh, H.; Zhang, X.; Yang, H.; Zhou, Y.; Huang, L.; Zhang, X.; Jiang, Y.; Nie, G. Exogenous Methyl Jasmonate Mediated MiRNA-mRNA Network Improves Heat Tolerance of Perennial Ryegrass. Int. J. Mol. Sci. 2023, 24, 1085. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Zhou, J.; Jiang, Y.; He, J.; Wang, Y.; Liao, Z.; Appiah, C.; Li, D.; Feng, G.; Huang, L.; et al. Transcriptome characterization of candidate genes for heat tolerance in perennial ryegrass after exogenous methyl Jasmonate application. BMC Plant Biol. 2022, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Gul, N.; Masoodi, K.Z.; Ramazan, S.; Mir, J.I.; Aslam, S. Study on the impact of exogenously applied methyl jasmonate concentrations on Solanum lycopersicum under low temperature stress. BMC Plant Biol. 2023, 23, 437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Song, C.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid and salicylic acid induce the accumulation of sucrose and increase resistance to chilling injury in peach fruit. J. Sci. Food Agric. 2021, 101, 4250–4255. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Chen, Q.; Yin, F.; Song, M.; Cai, W.; Shuai, L. Methyl jasmonate treatment alleviates chilling injury and improves antioxidant system of okra pod during cold storage. Food Sci. Nutr. 2023, 11, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Ghuge, S.A.; Nikalje, G.C.; Kadam, U.S.; Suprasanna, P.; Hong, J.C. Comprehensive mechanisms of heavy metal toxicity in plants, detoxification, and remediation. J. Hazard Mater. 2023, 450, 131039. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kumar, D.; Tikoria, R.; Sharma, R.; Parkirti, P.; Vikram, V.; Kaushal, K.; Ohri, P. Exploring the potential role of hydrogen sulfide and jasmonic acid in plants during heavy metal stress. Nitric Oxide 2023, 140–141, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Chen, J.; Li, X. Methyl jasmonate as modulator of Cd toxicity in Capsicum frutescens var. fasciculatum seedlings. Ecotoxicol. Environ. Saf. 2013, 98, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Ranjan, A.; Singh, A.K.; Sirhindi, G. Methyl jasmonate reduces cadmium toxicity by enhancing phenol and flavonoid metabolism and activating the antioxidant defense system in pigeon pea (Cajanus cajan). Chemosphere 2024, 346, 140681. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Wang, D.; Alhaithloul, H.A.S.; Alghanem, S.M.; Aftab, T.; Xie, K.; Lu, Y.; Shi, C.; Sun, J.; Gu, W.; et al. Jasmonic acid-mediated enhanced regulation of oxidative, glyoxalase defense system and reduced chromium uptake contributes to alleviation of chromium (VI) toxicity in choysum (Brassica parachinensis L.). Ecotoxicol. Environ. Saf. 2021, 208, 111758. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Kęsy, J.; Glazińska, P.; Glinkowski, W.; Narożna, D.; Bocianowski, J.; Rucińska-Sobkowiak, R.; Mai, V.C.; Krzesiński, W.; Samardakiewicz, S.; et al. The Influence of Lead and Acyrthosiphon pisum (Harris) on Generation of Pisum sativum Defense Signaling Molecules and Expression of Genes Involved in Their Biosynthesis. Int. J. Mol. Sci. 2023, 24, 671. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Xie, J.; Yu, J.; Li, J.; Lv, J.; Gao, Y.; Niu, T.; Patience, B.E. Effects of Preharvest Methyl Jasmonate and Salicylic Acid Treatments on Growth, Quality, Volatile Components, and Antioxidant Systems of Chinese Chives. Front. Plant Sci. 2021, 12, 767335. [Google Scholar] [CrossRef] [PubMed]
- Suza, W.P.; Avila, C.A.; Carruthers, K.; Kulkarni, S.; Goggin, F.L.; Lorence, A. Exploring the impact of wounding and jasmonates on ascorbate metabolism. Plant Physiol. Biochem. 2010, 48, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Wolucka, B.A.; Goossens, A.; Inzé, D. Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J. Exp. Bot. 2005, 56, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Sekimoto, Y.; Taki, N.; Obayashi, T.; Aono, M.; Matsumoto, F.; Sakurai, N.; Suzuki, H.; Hirai, M.Y.; Noji, M.; Saito, K.; et al. Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J. 2005, 44, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, A.; Telef, N.; Saigne, C.; Cluzet, S.; Barrieu, F.; Hamdi, S.; Mérillon, J.M. Effect of methyl jasmonate in combination with carbohydrates on gene expression of PR proteins, stilbene and anthocyanin accumulation in grapevine cell cultures. Plant Physiol. Biochem. 2008, 46, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Togawa, S.; Hikawa, T.; Matsuura, H.; Masuta, C.; Inukai, T. Ascorbic acid accumulates as a defense response to Turnip mosaic virus in resistant Brassica rapa cultivars. J. Exp. Bot. 2016, 67, 4391–4402. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Wang, R.; Xian, B.; Ren, C.; Liu, Q.; Wu, Q.; Pei, J. Integrated metabolomics and transcriptome analysis on flavonoid biosynthesis in safflower (Carthamus tinctorius L.) under MeJA treatment. BMC Plant Biol. 2020, 20, 353. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.A.; Duan, S.; Jeong, H.Y.; Lee, C.; Kang, I.K.; Eom, S.H. Pigmentation and Flavonoid Metabolite Diversity in Immature ‘Fuji’ Apple Fruits in Response to Lights and Methyl Jasmonate. Int. J. Mol. Sci. 2022, 23, 1722. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, Y.; Li, D.; Shi, S.; Zhao, N.; Liao, J.; Liu, Y.; Chen, H. Eggplant transcription factor SmMYB5 integrates jasmonate and light signaling during anthocyanin biosynthesis. Plant Physiol. 2024, 194, 1139–1165. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. Physiological role of reactive oxygen species, ethylene, and jasmonic acid on UV light induced phenolic biosynthesis in wounded carrot tissue. Postharvest Biol. Technol. 2021, 172, 111388. [Google Scholar] [CrossRef]
- Abouelsaad, I.; Renault, S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J. Plant Physiol. 2018, 226, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Hernández, E.; Antunes-Ricardo, M.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Selenium, Sulfur, and Methyl Jasmonate Treatments Improve the Accumulation of Lutein and Glucosinolates in Kale Sprouts. Plants 2022, 11, 1271. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C. Effect of methyl jasmonate on phenolic compounds and carotenoids of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2007, 55, 10366–10372. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yao, L.; Pecoraro, L.; Liu, C.; Wang, J.; Huang, L.; Gao, W. Cold stress regulates accumulation of flavonoids and terpenoids in plants by phytohormone, transcription process, functional enzyme, and epigenetics. Crit. Rev. Biotechnol. 2023, 43, 680–697. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, X.; Wu, L.; Wang, M.; Yang, L.; Liu, X.; Chen, S.; Shi, Y. Integrated Analysis of Basic Helix Loop Helix Transcription Factor Family and Targeted Terpenoids Reveals Candidate AarbHLH Genes Involved in Terpenoid Biosynthesis in Artemisia argyi. Front. Plant Sci. 2021, 12, 811166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Denness, L.; McKenna, J.F.; Segonzac, C.; Wormit, A.; Madhou, P.; Bennett, M.; Mansfield, J.; Zipfel, C.; Hamann, T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011, 156, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, G.M.; Figueroa, N.E.; Poblete, L.A.; Cherian, S.; Figueroa, C.R. Effects of preharvest applications of methyl jasmonate and chitosan on postharvest decay, quality and chemical attributes of Fragaria chiloensis fruit. Food Chem. 2016, 190, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, Y.; Jin, Y.; Wang, C.; Yang, J.; Qi, H. Drought-induced ABA, H(2)O(2) and JA positively regulate CmCAD genes and lignin synthesis in melon stems. BMC Plant Biol. 2021, 21, 83. [Google Scholar] [CrossRef] [PubMed]
- Gamuyao, R.; Nagai, K.; Ayano, M.; Mori, Y.; Minami, A.; Kojima, M.; Suzuki, T.; Sakakibara, H.; Higashiyama, T.; Ashikari, M.; et al. Hormone Distribution and Transcriptome Profiles in Bamboo Shoots Provide Insights on Bamboo Stem Emergence and Growth. Plant Cell Physiol. 2017, 58, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Pokorna, E.; Dobrev, P.I.; Motyka, V.; Guignard, C.; Lutts, S.; Hausman, J.F.; Guerriero, G. Impact of jasmonic acid on lignification in the hemp hypocotyl. Plant Signal. Behav. 2019, 14, 1592641. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Chen, Y.; Xu, Y.; An, Y.; Hu, Z.; Xiong, A.; Wang, G. Effects of Jasmonic Acid on Stress Response and Quality Formation in Vegetable Crops and Their Underlying Molecular Mechanisms. Plants 2024, 13, 1557. https://doi.org/10.3390/plants13111557
Wu J, Chen Y, Xu Y, An Y, Hu Z, Xiong A, Wang G. Effects of Jasmonic Acid on Stress Response and Quality Formation in Vegetable Crops and Their Underlying Molecular Mechanisms. Plants. 2024; 13(11):1557. https://doi.org/10.3390/plants13111557
Chicago/Turabian StyleWu, Jiaqi, Yangyang Chen, Yujie Xu, Yahong An, Zhenzhu Hu, Aisheng Xiong, and Guanglong Wang. 2024. "Effects of Jasmonic Acid on Stress Response and Quality Formation in Vegetable Crops and Their Underlying Molecular Mechanisms" Plants 13, no. 11: 1557. https://doi.org/10.3390/plants13111557
APA StyleWu, J., Chen, Y., Xu, Y., An, Y., Hu, Z., Xiong, A., & Wang, G. (2024). Effects of Jasmonic Acid on Stress Response and Quality Formation in Vegetable Crops and Their Underlying Molecular Mechanisms. Plants, 13(11), 1557. https://doi.org/10.3390/plants13111557







