p-Coumaric Acid Differential Alters the Ion-Omics Profile of Chia Shoots under Salt Stress
Abstract
1. Introduction
2. Results
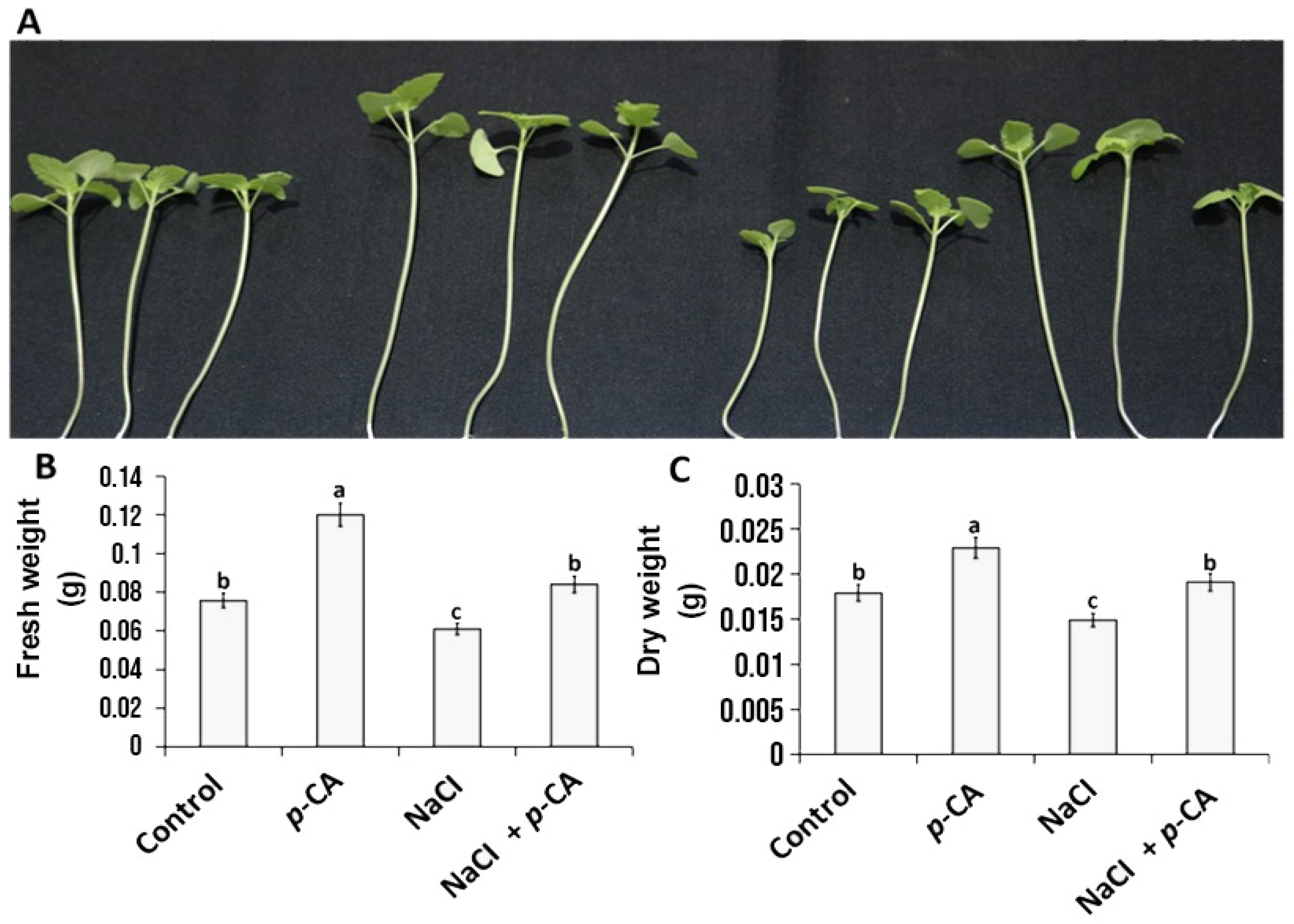
2.1. p-CA Improves Chia Seedling Growth under Salt Stress
2.2. Effects of p-CA and Salt Stress on Mineral Content
2.2.1. Na Content
2.2.2. K Content
2.2.3. P Content
2.2.4. Mg Content
2.2.5. Ca Content
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Treatment
4.2. Measurement of Plant Growth
4.3. Measurement of Inductively Coupled Plasma Optical Emission Spectroscopy Analysis
4.4. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- George, R.; Mcfarlane, D.; Nulsen, B. Salinity threatens the viability of agriculture and ecosystems in western Australia. Hydrogeol. J. 1997, 5, 6–21. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK; San Diego, CA, USA, 1995. [Google Scholar]
- Grossman, A.; Takahashi, H. Macronutrient utilization by photosynthetic eukaryotes and the fabric of interactions. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 163–210. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stress: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, M.; Gokul, A.; Keyster, M.; Klein, A. Exogenous p-coumaric acid improves Salvia hispanica L. seedling shoot growth. Plants 2019, 8, 546. [Google Scholar] [CrossRef]
- Klein, A.; Keyster, M.; Ludidi, N. Response of soybean nodules to exogenously applied caffeic acid during NaCl-induced salinity. S. Afr. J. Bot. 2015, 96, 13–18. [Google Scholar] [CrossRef]
- Jones, S.; Keyster, M.; Klein, A. Exogenous Caffeic Acid Alters Physiological and Molecular Responses in Chia (Salvia hispanica L.). S. Afr. J. Bot. 2017, 100, 339–340. [Google Scholar] [CrossRef]
- Khairy, A.I.H.; Roh, K.S. Effect of salicylic acid, benzoic acid, and p-Coumaric acid on growth, chlorophyll, proline, and vitamin C of salinity-stressed tobacco (Nicotiana tabacum). Int. J. Plant. Soil Sci. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Kaur, H.; Bhardwaj, R.D.; Grewal, S.K. Mitigation of salinity-induced oxidative damage in wheat (Triticum aestivum L.) seedlings by exogenous application of phenolic acids. Acta Physiol. Plant. 2017, 39, 221–236. [Google Scholar] [CrossRef]
- Nguyen, N.L.; Bui, V.H.; Pham, H.N.; To, H.M.; Dijoux-Franca, M.G.; Vu, C.T.; Nguyen, K.O.T. Ionomics and metabolomics analysis reveal the molecular mechanism of metal tolerance of Pteris vittata L. dominating in a mining site in Thai Nguyen province, Vietnam. ESPR 2022, 29, 87268–87280. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Blumwald, E. Developing salt-tolerant crop plants: Challenges and opportunities. Trends Plant Sci. 2005, 10, 615–620. [Google Scholar] [CrossRef]
- FAO. FAO Land and Plant Nutrition Management Service. 2009. Available online: http://www.fao.org/ag/agl/agll/spush (accessed on 10 April 2024).
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Newman, I.; Zhou, M.X.; Mendham, N.; Zhang, G.P.; Shabala, S. Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant Cell Environ. 2005, 28, 1230–1246. [Google Scholar] [CrossRef]
- Shabala, S.; Cuin, T.A.; Pang, J.; Percey, W.; Chen, Z.; Conn, S.; Eing, C.; Wegner, L.H. Xylem ionic relations and salinity tolerance in barley. Plant J. 2010, 1, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.; Bernstein, N.; Lauchli, A. Salt-induced inhibition of phosphorus transport in lettuce plants. Physiol. Plant. 1996, 97, 118–122. [Google Scholar] [CrossRef]
- Loupassaki, M.H.; Chartzoulakis, K.S.; Digalaki, N.B.; Androulakis, I.I. Effects of salt stress on concentration of nitrogen, phosphorus, potassium, calcium, magnesium, sodium in leaves, shoots, roots of six olive cultivars. J. Plant Nutr. 2002, 25, 2457–2482. [Google Scholar] [CrossRef]
- Kopittke, P.M. Interactions between Ca, Mg, Na and K: Alleviation of toxicity in saline solutions. Plant Soil 2012, 352, 353–362. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.J.; Reid, R.J.; Smith, F.A. Sodium–calcium interactions in two wheat species differing in salinity tolerance. Physiol. Plant. 1997, 99, 323–327. [Google Scholar] [CrossRef]
- Nkomo, M.; Gokul, A.; Ndimba, R.; Badiwe, M.; Keyster, M.; Klein, A. Piperonylic acid alters growth, mineral content accumulation and reactive oxygen species-scavenging capacity in chia seedlings. AoB Plants 2022, 14, plac025. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef] [PubMed]
- Khawula, S.; Gokul, A.; Niekerk, L.A.; Basson, G.; Keyster, M.; Badiwe, M.; Klein, A.; Nkomo, M. Insights into the Effects of Hydroxycinnamic Acid and Its Secondary Metabolites as Antioxidants for Oxidative Stress and Plant Growth under Environmental Stresses. Curr. Issues Mol. Biol. 2024, 46, 81–95. [Google Scholar] [CrossRef]
- Gokul, A.; Roode, E.; Klein, A.; Keyster, M. Exogenous 3, 3′-diindolylmethane increases Brassica napus L. seedling shoot growth through modulation of superoxide and hydrogen peroxide content. J. Plant Physiol. 2016, 196, 93–98. [Google Scholar] [CrossRef]
- Huang, L.; Bell, R.W.; Dell, B.; Woodward, J. Rapid nitric acid digestion of plant material with an open-vessel microwave system. Commun. Soil Sci. Plant Anal. 2004, 35, 427–440. [Google Scholar] [CrossRef]
- Vachirapatama, N.; Jirakiattikul, Y. Effect of vanadium on growth of Chinese green mustard (Brassica campestris ssp. chinensis var. parachinensis) under substrate culture. Songklanakarin J. Sci. Technol. SJST 2008, 30, 427–431. [Google Scholar]
| Minerals | Mineral Relative Content (mg·g−1 FW) | |||
|---|---|---|---|---|
| Control | p-CA | Salt | Salt + p-CA | |
| Na | 0.057 ± 0.004 | 0.241 ± 0.194 ↑ | 0.349 ± 0.015 ↑ | 0.727 ± 0.067 ↑↑ |
| K | 4.513 ± 0.068 | 4.326 ± 0.270 NS | 1.552 ± 0.062 ↓ | 4.007 ± 0.707 ↓↑ |
| P | 0.329± 0.002 | 0.376 ± 0.064 ↑ | 0.081 ± 0.004 ↓ | 0.214± 0.012 ↓↑ |
| Mg | 0.387 ± 0.006 | 0.439 ± 0.035 ↑ | 0.168 ± 0.007 ↓ | 0.471 ± 0.031 ↑↑ |
| Ca | 0.497 ± 0.002 | 0.751 ± 0.200 ↑ | 0.250 ± 0.008 ↓ | 0.773 ± 0.039 ↑↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nkomo, M.; Badiwe, M.; Niekerk, L.-A.; Gokul, A.; Keyster, M.; Klein, A. p-Coumaric Acid Differential Alters the Ion-Omics Profile of Chia Shoots under Salt Stress. Plants 2024, 13, 1564. https://doi.org/10.3390/plants13111564
Nkomo M, Badiwe M, Niekerk L-A, Gokul A, Keyster M, Klein A. p-Coumaric Acid Differential Alters the Ion-Omics Profile of Chia Shoots under Salt Stress. Plants. 2024; 13(11):1564. https://doi.org/10.3390/plants13111564
Chicago/Turabian StyleNkomo, Mbukeni, Mihlali Badiwe, Lee-Ann Niekerk, Arun Gokul, Marshal Keyster, and Ashwil Klein. 2024. "p-Coumaric Acid Differential Alters the Ion-Omics Profile of Chia Shoots under Salt Stress" Plants 13, no. 11: 1564. https://doi.org/10.3390/plants13111564
APA StyleNkomo, M., Badiwe, M., Niekerk, L.-A., Gokul, A., Keyster, M., & Klein, A. (2024). p-Coumaric Acid Differential Alters the Ion-Omics Profile of Chia Shoots under Salt Stress. Plants, 13(11), 1564. https://doi.org/10.3390/plants13111564











