Transcriptomic Insights into Molecular Response of Butter Lettuce to Different Light Wavelengths
Abstract
1. Introduction
2. Results
2.1. Morphological Response of Butter Lettuce on Exposure to Various Light Wavelengths
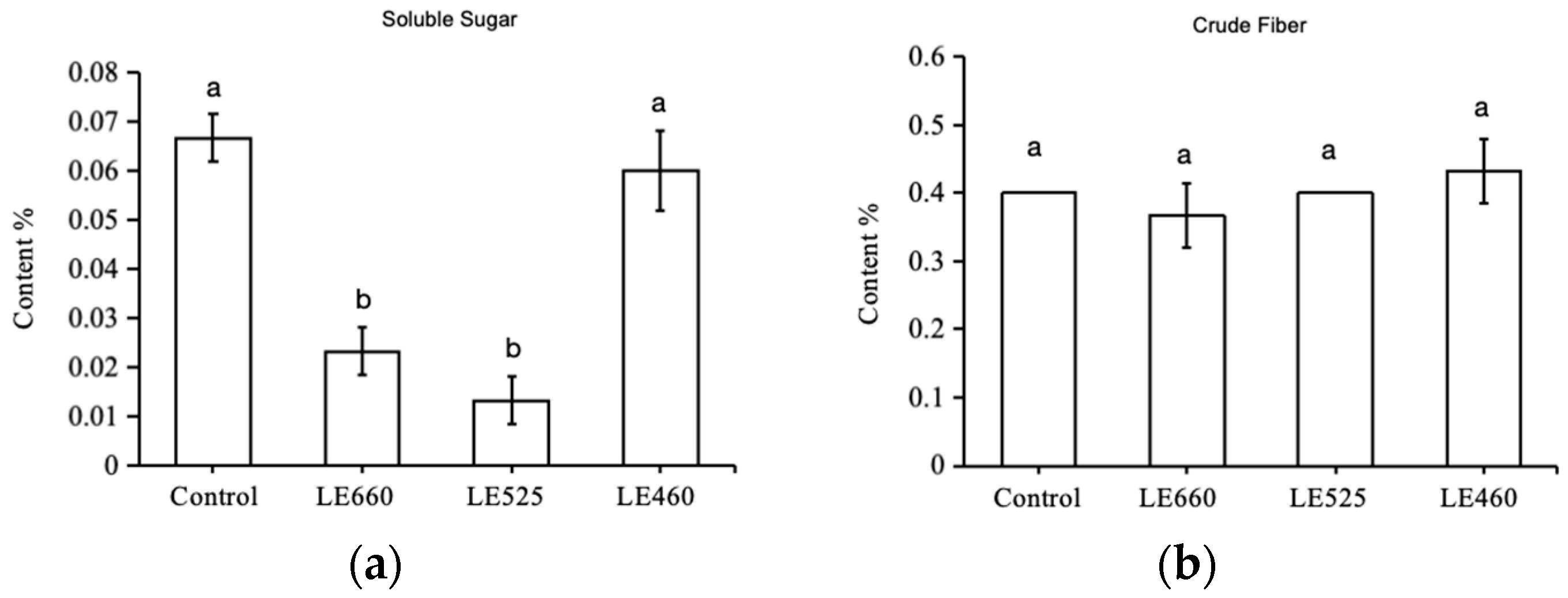
2.2. Light Wavelength Impacts the Crude Fiber and Soluble Sugar Levels in Butter Lettuce
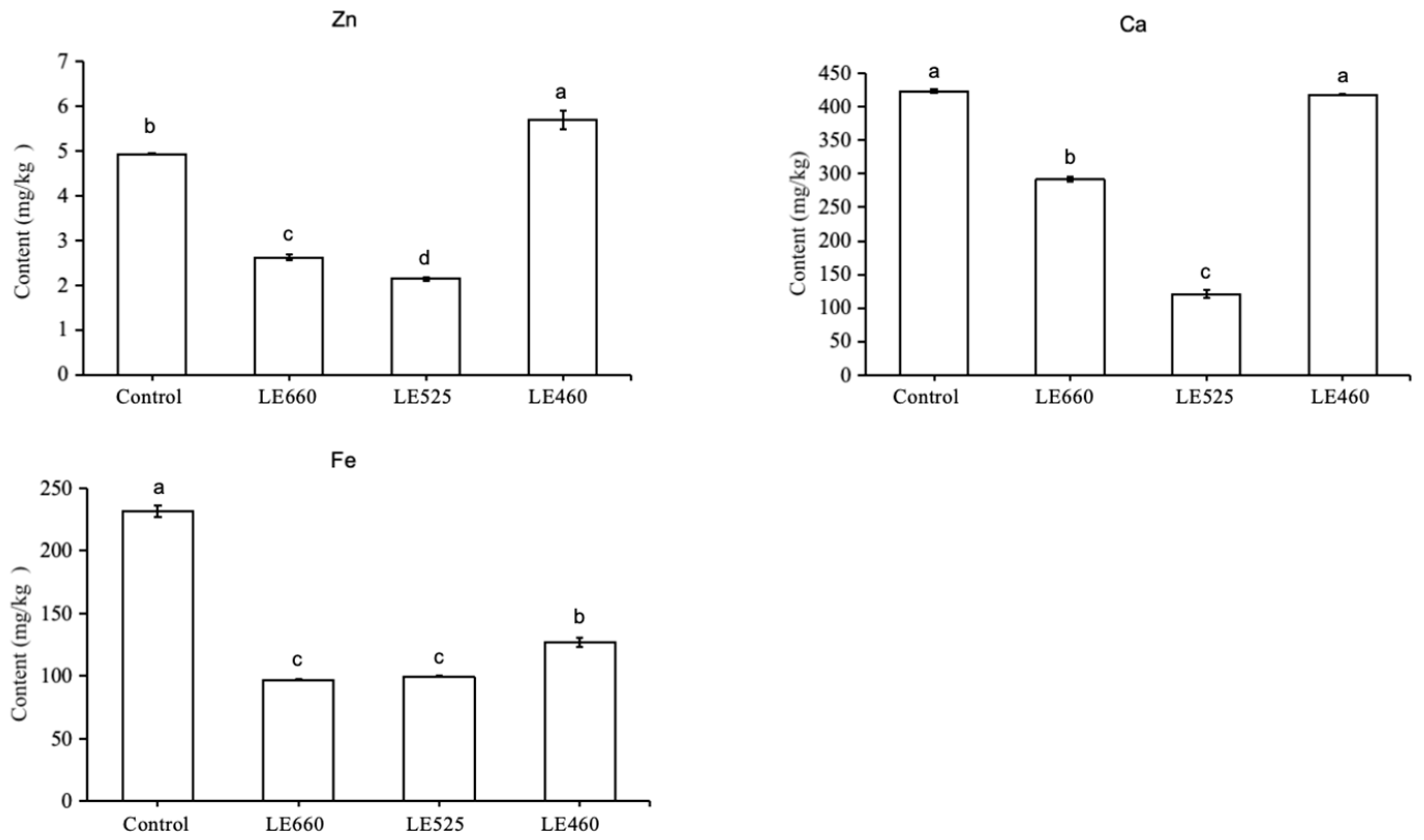
2.3. Mineral Analysis of Butter Lettuce upon Exposure to Different Light Wavelengths
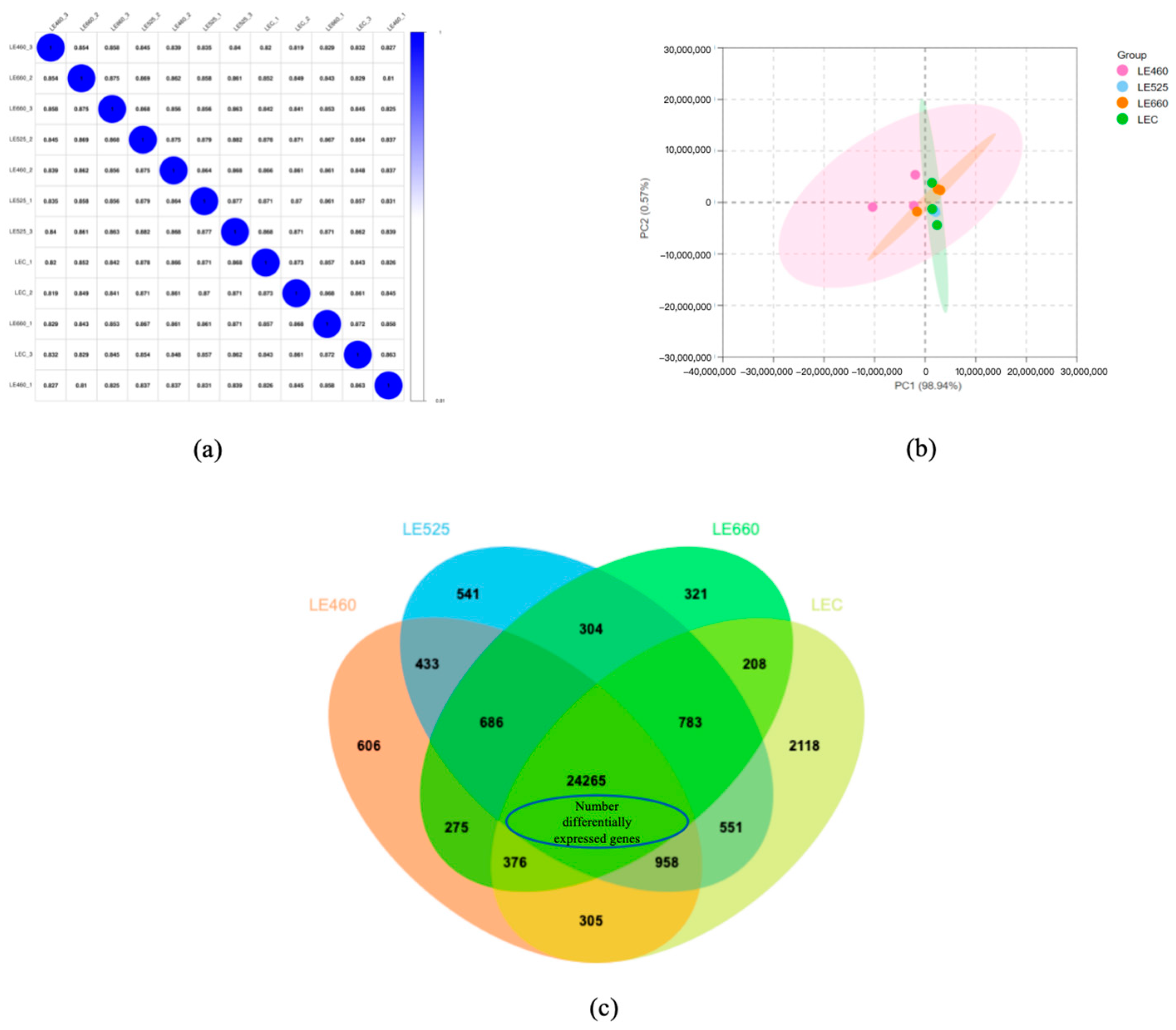
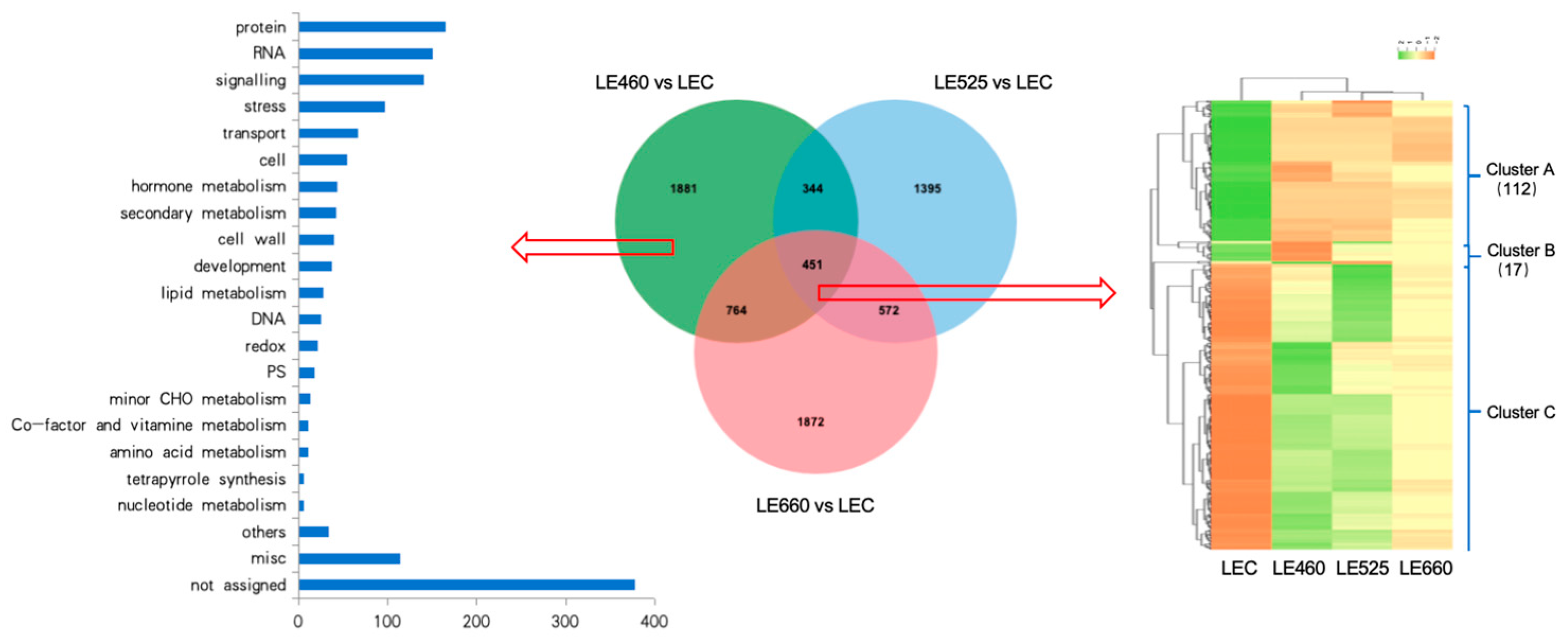
2.4. Light-Mediated Variations in Differentially Expressed Genes of Butter Lettuce Leaves
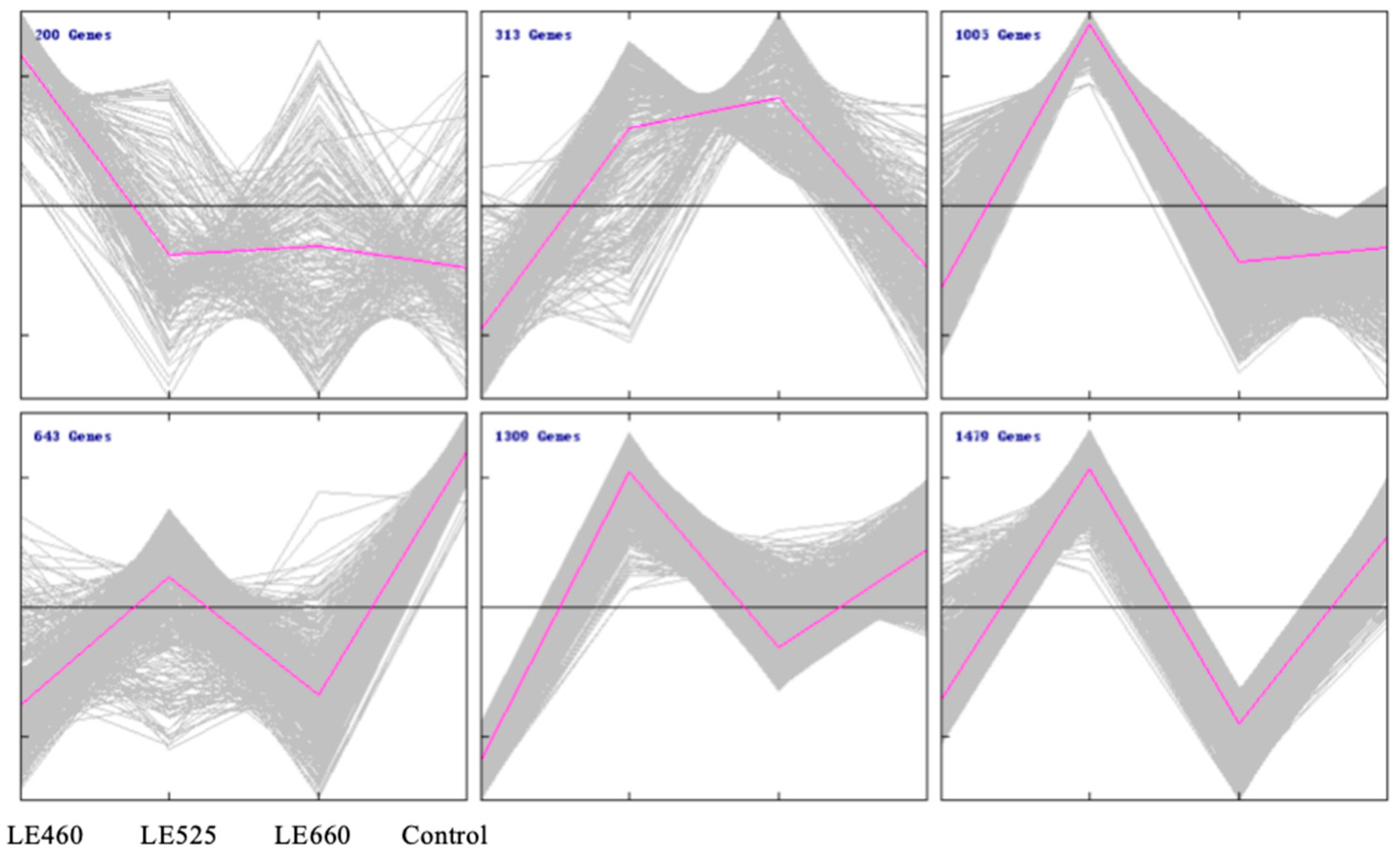
2.5. Cluster and Gene Ontology Analyses of Differentially Expressed Genes in Lettuce Leaves upon Exposure to Different Light Wavelengths
2.6. Gene Ontology Analysis of Common DEGs in Lettuce Leaves Exposed to Different Light Wavelengths
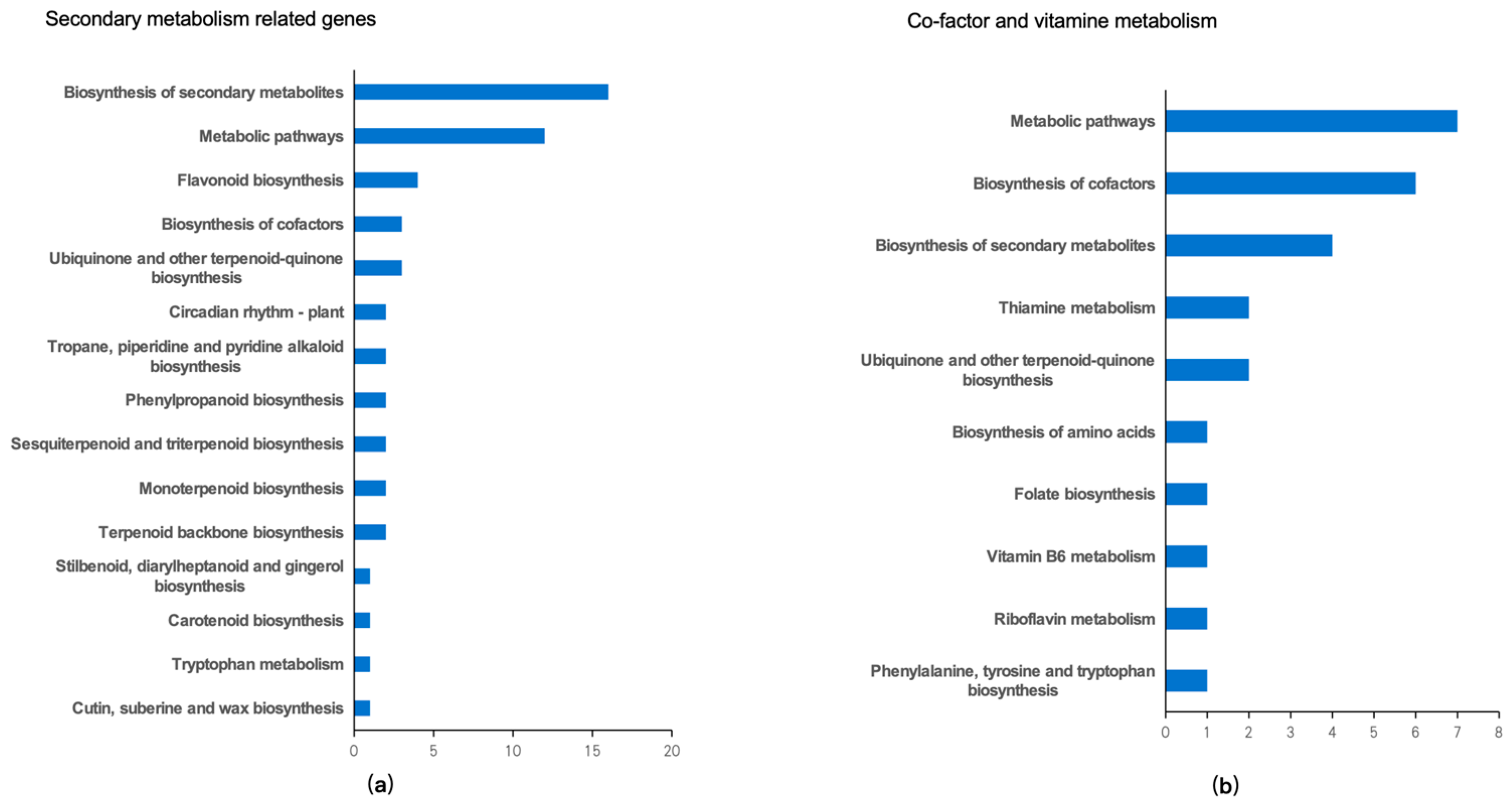
2.7. Pathway Analysis of Common Differentially Expressed Genes in Lettuce Leaves Exposed to Different Light Wavelengths
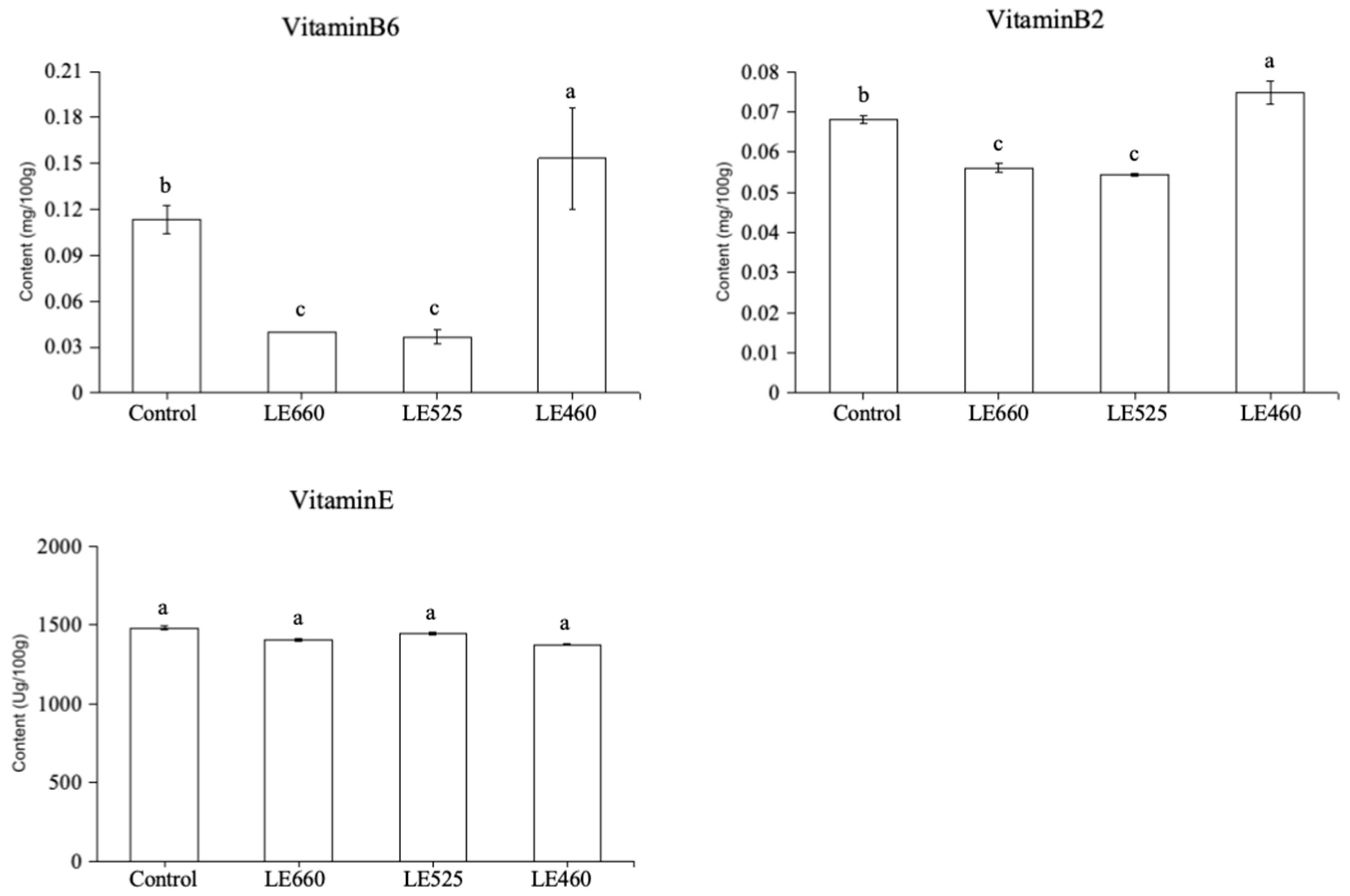
2.8. Vitamin Content of Butter Lettuce upon Exposure to Different Light Wavelengths
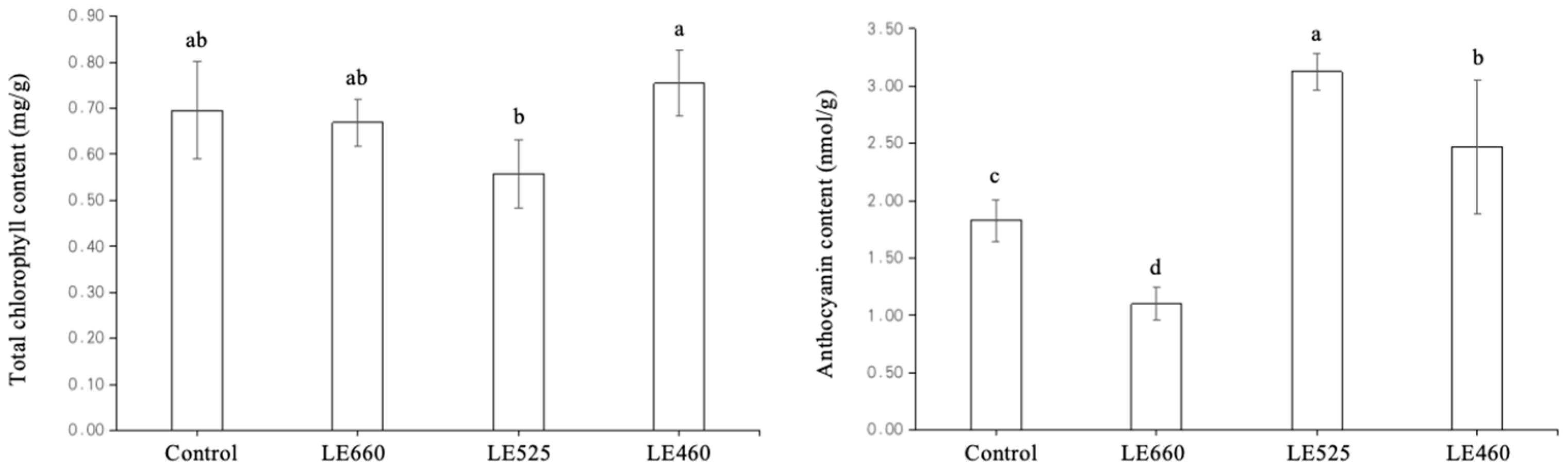
2.9. Chlorophyll and Anthocyanin Content of Lettuce Exposed to Different Light Wavelengths
3. Discussion
3.1. Regulation of Hypoxia in Lettuce upon Exposure to Different Light Wavelengths
3.2. Vitamin Regulation in Lettuce Exposed to Different Light Wavelengths
3.3. Regulation of Secondary Metabolism in Lettuce upon Exposure to Different Light Wavelengths
4. Materials and Methods
4.1. Plant Treatment
4.2. RNA Sequencing
4.3. Reads Mapping and Transcript Assembly
4.4. Differential Expression Analysis
4.5. Function Annotation and Enrichment
4.6. Determination of Metal and Non-Metal Elements
4.7. Pigment Content Determination
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yudina, L.; Sukhova, E.; Gromova, E.; Mudrilov, M.; Zolin, Y.; Popova, A.; Nerush, V.; Pecherina, A.; Grishin, A.A.; Dorokhov, A.A.; et al. Effect of duration of LED lighting on growth, photosynthesis and respiration in lettuce. Plants 2023, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Mohamed, S.J.; Rihan, H.Z.; Aljafer, N.; Fuller, M.P. The Impact of Light Spectrum and Intensity on the Growth, Physiology, and Antioxidant Activity of Lettuce (Lactuca sativa L.). Plants 2021, 10, 2162. [Google Scholar] [CrossRef] [PubMed]
- Kornpointner, C.; Martinez, A.S.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schröder, K.; Löfke, C.; Halbwirth, H. Chemical composition and antioxidant potential of Cannabis sativa L. roots. Ind. Crops Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical Farming: Moving from Genetic to Environmental Modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Siipola, S.M.; Kotilainen, T.; Sipari, N.; Morales, L.O.; Lindfors, A.V.; Robson, T.M.; Aphalo, P.J. Epidermal UV-A absorbance and whole-leaf flavonoid composition in pea respond more to solar blue light than to solar UV radiation. Plant Cell Environ. 2015, 38, 941–952. [Google Scholar] [CrossRef]
- Ji, Y.; Nuñez Ocaña, D.; Choe, D.; Larsen, D.H.; Marcelis, L.F.M.; Heuvelink, E. Far-red radiation stimulates dry mass partitioning to fruits by increasing fruit sink strength in tomato. New Phytol. 2020, 228, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Nemali, K. Blue and Far-Red Light Affect Area and Number of Individual Leaves to Influence Vegetative Growth and Pigment Synthesis in Lettuce. Front. Plant Sci. 2021, 12, 667407. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Fanourakis, D.; Tsaniklidis, G.; Woltering, E.J.; Cheng, R.; Li, T. Far-red radiation during indoor cultivation reduces lettuce nutraceutical quality and shortens the shelf-life when stored at supra optimal temperatures. Postharvest Biol. Technol. 2023, 198, 112269. [Google Scholar] [CrossRef]
- Hmelak Gorenjak, A.; Cencič, A. Nitrate in vegetables and their impact on human health. A review. Acta Aliment. 2013, 42, 158–172. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Y.; Yang, X.; Xiao, J.; Zhang, H.; Zhang, Z.; Wang, Y.; Jiang, G. Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria × ananassa) fruit. Food Chem. 2016, 207, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, Z.; Xu, Z.; Wang, Y.; Xie, Z. Transcriptome analysis and physiological responses of the potato plantlets in vitro under red, blue, and white light conditions. 3 Biotech 2018, 8, 394. [Google Scholar] [CrossRef] [PubMed]
- Noumedem, J.A.K.; Djeussi, D.E.; Hritcu, L.; Mihasan, M.; Kuete, V. Lactuca sativa. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 437–449. [Google Scholar]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C. Effect of methyl jasmonate on phenolic compounds and carotenoids of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2007, 55, 10366–10372. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar]
- Shimizu, H.; Saito, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Light environment optimization for lettuce growth in plant factory. IFAC Proc. Vol. 2011, 44, 605–609. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Zhen, S.; Bugbee, B. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 2020, 43, 1259–1272. [Google Scholar] [CrossRef]
- Legendre, R.; van Iersel, M.W. Supplemental Far-Red Light Stimulates Lettuce Growth: Disentangling Morphological and Physiological Effects. Plants 2021, 10, 166. [Google Scholar] [CrossRef]
- Kusuma, P.; Bugbee, B. On the contrasting morphological response to far-red at high and low photon fluxes. Front. Plant Sci. 2023, 14, 1185622. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, K.H.; Oh, M.M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Mudrilov, M.; Nerush, V.; Pecherina, A.; Smirnov, A.A.; Dorokhov, A.S.; Chilingaryan, N.O.; Vodeneev, V.; Sukhov, V. Ratio of intensities of blue and red light at cultivation influences photosynthetic light reactions, respiration, growth, and reflectance indices in lettuce. Biology 2022, 11, 60. [Google Scholar] [CrossRef]
- Kumar, V.; Sugumaran, K.; Al-Roumi, A.; Shajan, A. De-novo transcriptome assembly and analysis of lettuce plants grown under red, blue or white light. Sci. Rep. 2022, 12, 22477. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Aoki, K.; Nagano, A.J.; Honjo, M.N.; Fukuda, H. Circadian oscillation of the lettuce transcriptome under constant light and light-dark conditions. Front. Plant Sci. 2016, 7, 209473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, S.; Cheng, Y.; Peng, Z.; Han, J. Transcriptome profiling of anthocyanin-related genes reveals effects of light intensity on anthocyanin biosynthesis in red leaf lettuce. PeerJ 2018, 6, e4607. [Google Scholar] [CrossRef]
- Jishi, T.; Matsuda, R.; Fujiwara, K. Blue light monochromatic irradiation for 12 hours in lighting pattern with combinations of blue and red light elongates young cos lettuce leaves and promotes growth under high daily light integral. HortScience 2021, 56, 940–945. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. HortScience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Wang, L.C.; Li, T.; Yang, Q.C.; Guo, W.Z. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef]
- Shimokawa, A.; Tonooka, Y.; Matsumoto, M.; Ara, H.; Suzuki, H.; Yamauchi, N.; Shigyo, M. Effect of alternating red and blue light irradiation generated by light emitting diodes on the growth of leaf lettuce. BioRxiv 2014. [Google Scholar] [CrossRef]
- Dougher, T.A.; Bugbee, B. Differences in the response of wheat, soybean and lettuce to reduced blue radiation. Photochem. Photobiol. 2001, 73, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Zha, L.; Liu, W.; Zhang, Y.; Zhou, C.; Shao, M. Morphological and physiological stress responses of lettuce to different intensities of continuous light. Front. Plant Sci. 2019, 10, 1440. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Serres, J.; Lee, S.C.; Brinton, E. Waterproofing crops: Effective flooding survival strategies. Plant Physiol. 2012, 160, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.T.; Licausi, F. Oxygen sensing and signaling. Annu. Rev. Plant Biol. 2015, 66, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Considine, M.J.; Diaz-Vivancos, P.; Kerchev, P.; Signorelli, S.; Agudelo-Romero, P.; Gibbs, D.J.; Foyer, C.H. Learning to breathe: Developmental phase transitions in oxygen status. Trends Plant Sci. 2017, 22, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, C.; Yoo, J.; Kim, J.R.; Kim, Y.H.; Lee, S.E. Phosphine gas in the dark induces severe phytotoxicity in Arabidopsis thaliana by increasing a hypoxia stress response and disrupting the energy metabolism: Transcriptomic approaches. J. Hazard. Mater. 2023, 443, 130141. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt-Derzsó, E.; Sauter, M. Hypoxia and the group VII ethylene response transcription factor HRE2 promote adventitious root elongation in Arabidopsis. Plant Biol. 2019, 21, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Eysholdt-Derzsó, E.; Renziehausen, T.; Frings, S.; Frohn, S.; von Bongartz, K.; Igisch, C.P.; Mann, J.; Häger, L.; Macholl, J.; Leisse, D.; et al. Endoplasmic reticulum–bound ANAC013 factor is cleaved by RHOMBOID-LIKE 2 during the initial response to hypoxia in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2023, 120, e2221308120. [Google Scholar] [CrossRef]
- Riga, P.; Benedicto, L.; Gil-Izquierdo, Á.; Collado-González, J.; Ferreres, F.; Medina, S. Diffuse light affects the contents of vitamin C, phenolic compounds and free amino acids in lettuce plants. Food Chem. 2019, 272, 227–234. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, Y.L.; Wang, L.C.; Yang, Q.C.; Guo, W.Z. Responses of butter leaf lettuce to mixed red and blue light with extended light/dark cycle period. Sci. Rep. 2022, 12, 6924. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, Y.L.; Wang, L.C.; Guo, W.Z. Red and blue wavelengths affect the morphology, energy use efficiency and nutritional content of lettuce (Lactuca sativa L.). Sci. Rep. 2021, 11, 8374. [Google Scholar] [CrossRef] [PubMed]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Percudani, R.; Peracchi, A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep. 2003, 4, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Ksas, B.; Szewczyk, A.; Rumeau, D.; Franck, F.; Caffarri, S.; Triantaphylidès, C. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 2009, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Lim, G. Pyridoxine and pyridoxamine inhibits superoxide radicals and prevents lipid peroxidation, protein glycosylation, and (Na++ K+)-ATPase activity reduction in high glucose-treated human erythrocytes. Free Radic. Biol. Med. 2001, 30, 232–237. [Google Scholar] [CrossRef]
- Stocker, P.; Lesgards, J.F.; Vidal, N.; Chalier, F.; Prost, M. ESR study of a biological assay on whole blood: Antioxidant efficiency of various vitamins. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2003, 1621, 1–8. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Effect of vitamin B6 on oxygen radicals, mitochondrial membrane potential, and lipid peroxidation in H2O2-treated U937 monocytes. Free Radic. Biol. Med. 2004, 36, 423–428. [Google Scholar] [CrossRef]
- Endo, N.; Nishiyama, K.; Okabe, M.; Matsumoto, M.; Kanouchi, H.; Oka, T. Vitamin B6 suppresses apoptosis of NM-1 bovine endothelial cells induced by homocysteine and copper. Biochim. Biophys. Acta 2007, 1770, 571–577. [Google Scholar] [CrossRef]
- Danon, A.; Miersch, O.; Felix, G.; op den Camp, R.G.; Apel, K. Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J. 2005, 41, 68–80. [Google Scholar] [CrossRef]
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant responses to simultaneous biotic and abiotic stress: Molecular mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.F.; Takaki, M.; Azevedo, R.A. Plant pigments: The many faces of light perception. Acta Physiol. Plant 2011, 33, 241–248. [Google Scholar] [CrossRef]
- Zoratti, L.; Karppinen, K.; Luengo Escobar, A.; Häggman, H.; Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front. Plant Sci. 2014, 5, 109012. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.L.; Zhu, P. De novo transcriptome sequencing of Rhododendron molle and identification of genes involved in the biosynthesis of secondary metabolites. BMC Plant Biol. 2020, 20, 414. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Mori, N.; Tomari, Y.; Mitsugi, N.; Deguchi, A.; Kashima, M.; Tezuka, A.; Nagano, A.J.; Usami, H.; Tanabata, T.; et al. Effect of differences in light source environment on transcriptome of leaf lettuce (Lactuca sativa L.) to optimize cultivation conditions. PLoS ONE 2022, 17, e0265994. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Pimentel, H.; Trapnell, C.; Pachter, L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics 2011, 27, 2325–2329. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 1–21. [Google Scholar] [CrossRef]
- Lohse, M.; Nagel, A.; Herter, T.; May, P.; Schroda, M.; Zrenner, R.; Tohge, T.; Fernie, A.R.; Stitt, M.; Usadel, B. Mercator: A fast and simple web server for genome scale functional annotation of plant sequence data. Plant Cell Environ. 2014, 37, 1250–1258. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Thimm, O.; Bläsing, O.; Gibon, Y.; Nagel, A.; Meyer, S.; Krüger, P.; Selbig, J.; Müller, L.A.; Rhee, S.Y.; Stitt, M. MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004, 37, 914–939. [Google Scholar] [CrossRef] [PubMed]
- State Administration for Market Regulation. Available online: https://www.samr.gov.cn (accessed on 2 April 2023).
- Chen, X.; Yang, B.; Huang, W.; Wang, T.; Li, Y.; Zhong, Z.; Yang, L.; Li, S.; Tian, J. Comparative proteomic analysis reveals elevated capacity for photosynthesis in polyphenol oxidase expression-silenced Clematis terniflora DC. leaves. Int. J. Mol. Sci. 2018, 19, 3897. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, W.; Ran, L.; Dou, L.; Yao, S.; Hu, J.; Fan, D.; Li, C.; Luo, K. R2R3-MYB transcription factor MYB 6 promotes anthocyanin and proanthocyanidin biosynthesis but inhibits secondary cell wall formation in Populus tomentosa. Plant J. 2019, 99, 733–751. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Weng, X.; Ling, H.; Mustafa, G.; Yang, B.; Lu, N. Transcriptomic Insights into Molecular Response of Butter Lettuce to Different Light Wavelengths. Plants 2024, 13, 1582. https://doi.org/10.3390/plants13121582
Liang Y, Weng X, Ling H, Mustafa G, Yang B, Lu N. Transcriptomic Insights into Molecular Response of Butter Lettuce to Different Light Wavelengths. Plants. 2024; 13(12):1582. https://doi.org/10.3390/plants13121582
Chicago/Turabian StyleLiang, Yongqi, Xinying Weng, Hao Ling, Ghazala Mustafa, Bingxian Yang, and Na Lu. 2024. "Transcriptomic Insights into Molecular Response of Butter Lettuce to Different Light Wavelengths" Plants 13, no. 12: 1582. https://doi.org/10.3390/plants13121582
APA StyleLiang, Y., Weng, X., Ling, H., Mustafa, G., Yang, B., & Lu, N. (2024). Transcriptomic Insights into Molecular Response of Butter Lettuce to Different Light Wavelengths. Plants, 13(12), 1582. https://doi.org/10.3390/plants13121582





