Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders
Abstract
:1. Introduction
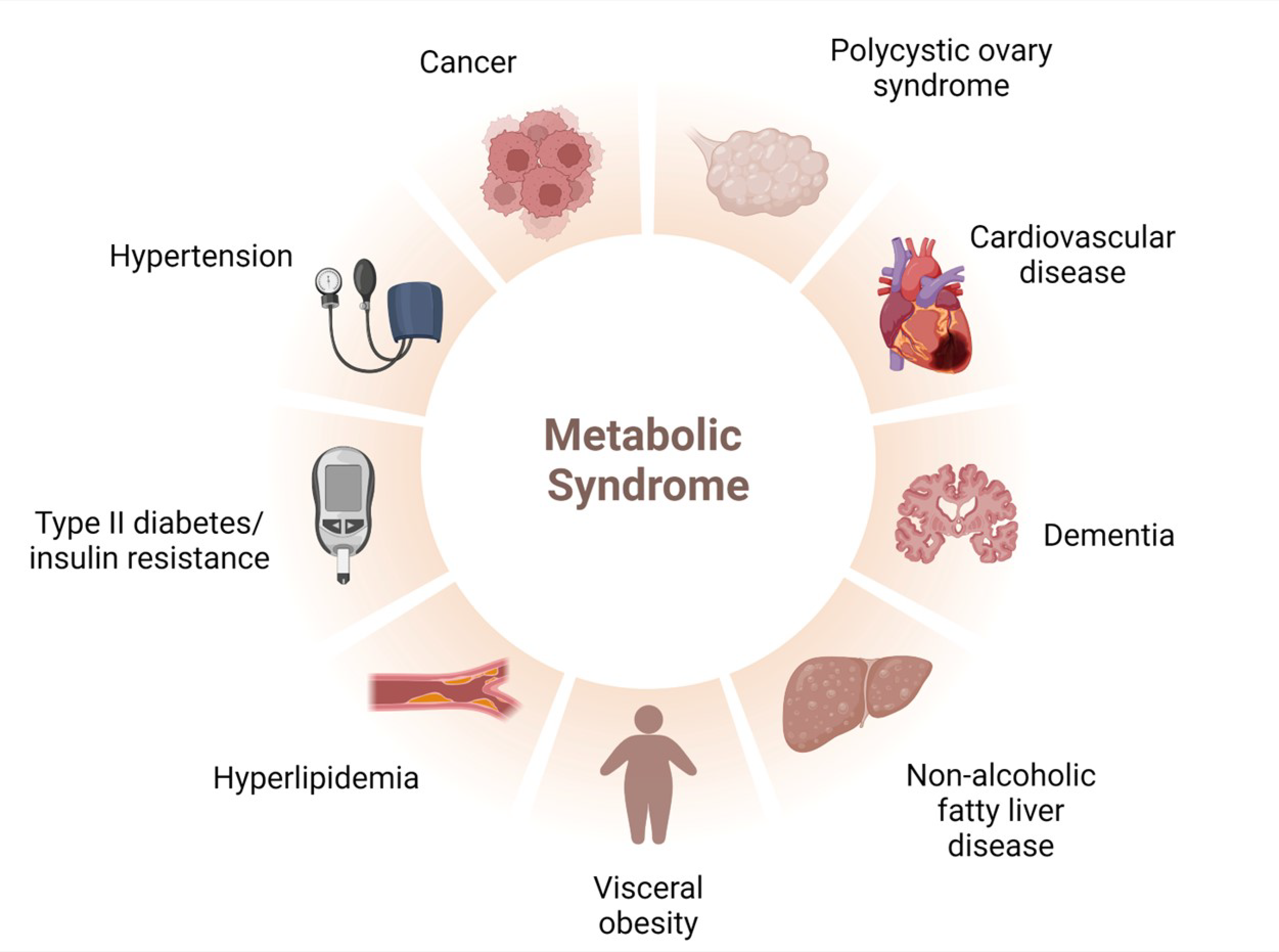
2. MetS: An Overview about Its Epidemiology, Oxidative Stress, and Treatment
2.1. Epidemiology
2.2. Risk Factors
2.3. Treatment and Limitations
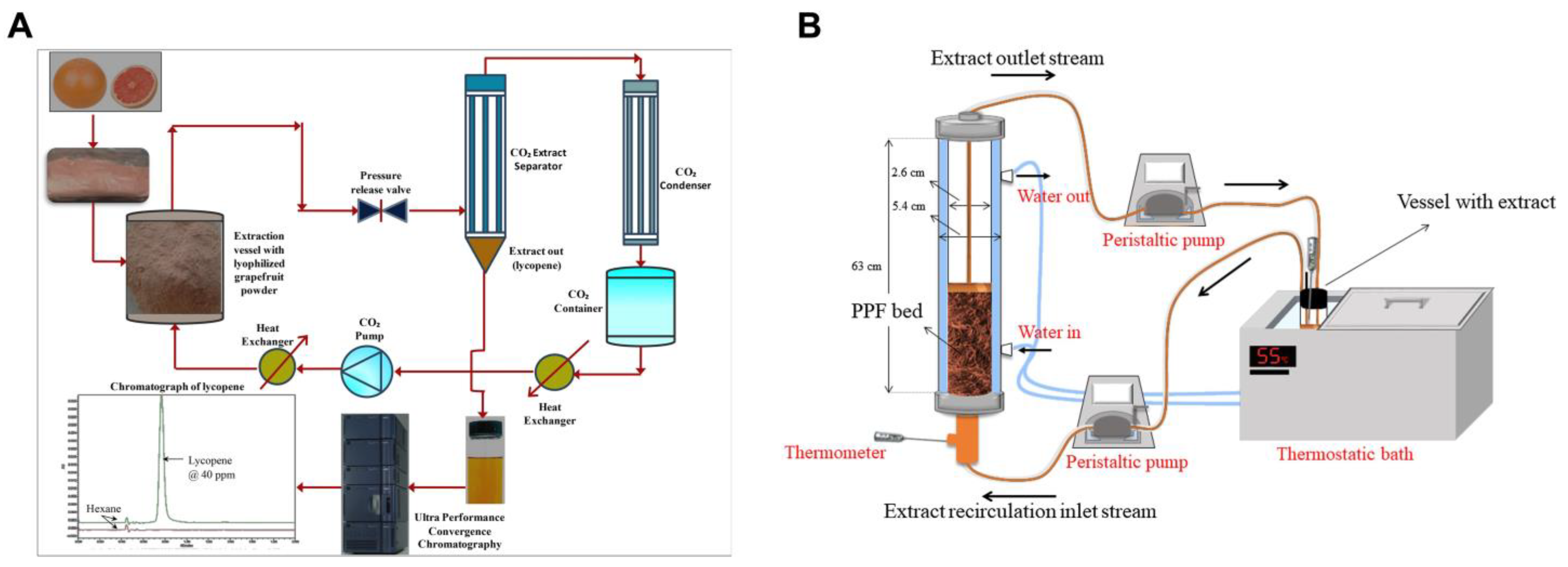
3. Carotenoids: Sources, Extraction, Characterization, and Activities against MetS
3.1. Sources, Extraction, and Characterization
3.2. Activities against MetS
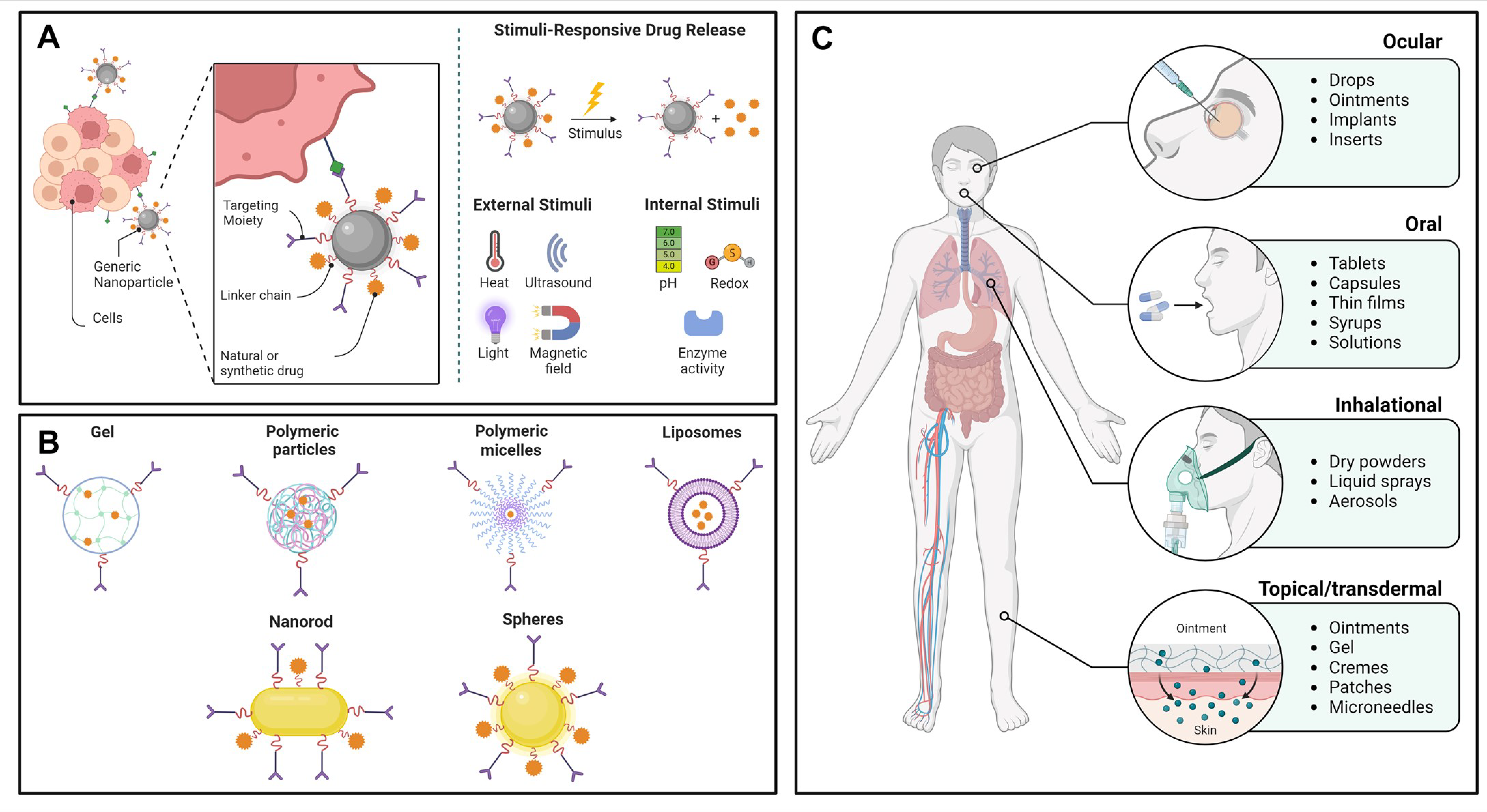
4. Encapsulation of Carotenoids
4.1. Astaxanthin
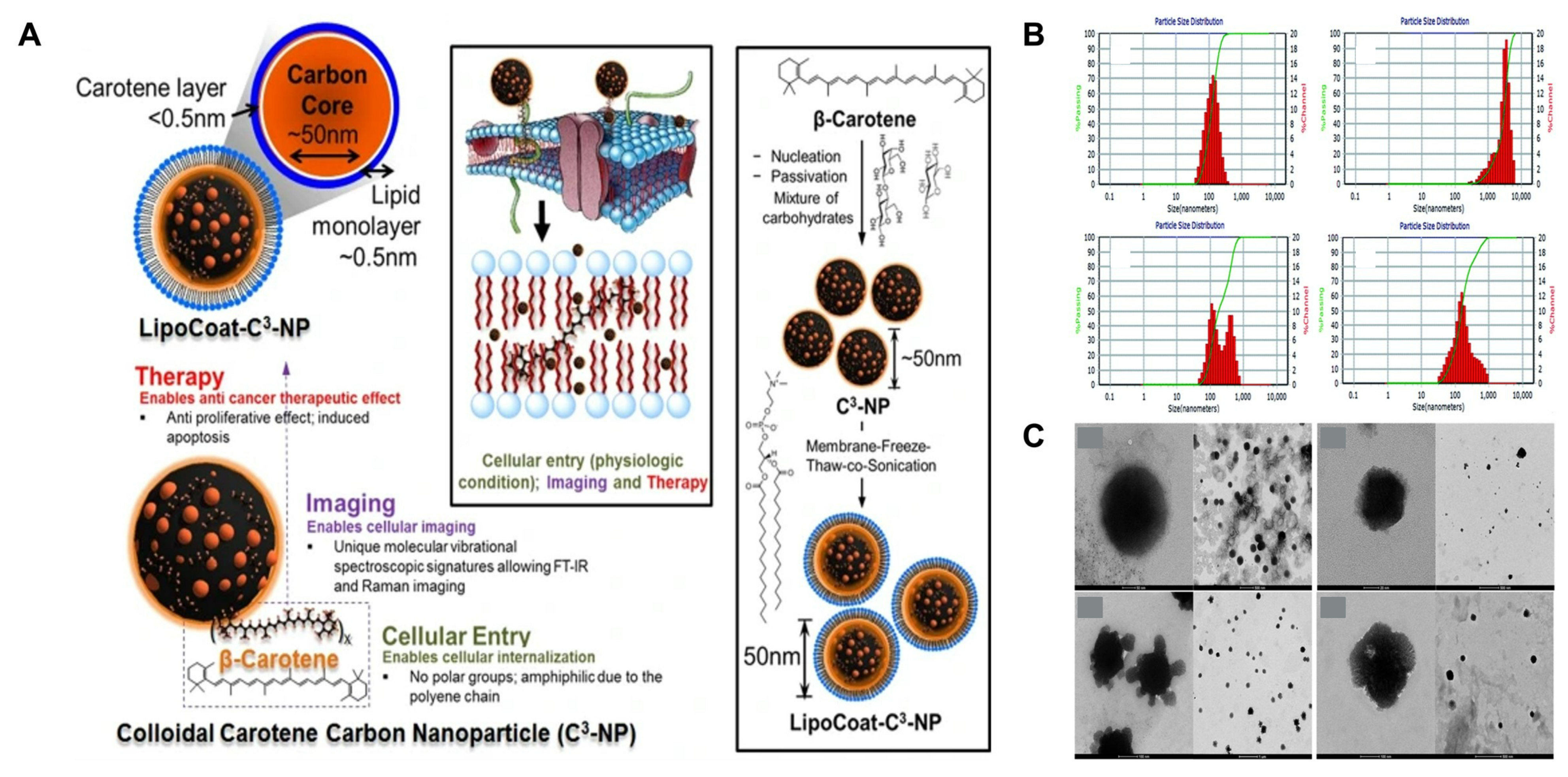
4.2. β-Carotene
4.3. Crocin
4.4. Fucoxanthin
4.5. Lycopene
4.6. Lutein
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic Syndrome: Risk Factors, Diagnosis, Pathogenesis, and Management with Natural Approaches. Food Chem. Adv. 2023, 3, 100335. [Google Scholar] [CrossRef]
- Al Shehri, H.A.; Al Asmari, A.K.; Khan, H.A.; Al Omani, S.; Kadasah, S.G.; Horaib, G.B.; Al Buraidi, A.; Al Sharif, A.A.; Mohammed, F.S.; Abbasmanthiri, R.; et al. Association between Preventable Risk Factors and Metabolic Syndrome. Open Med. 2022, 17, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Or, B.; Tsoi, M.F.; Cheung, C.L.; Cheung, B.M.Y. Prevalence of Metabolic Syndrome in the United States National Health and Nutrition Examination Survey 2011–18. Postgrad. Med. J. 2023, 99, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Manaf, M.R.A.; Nawi, A.M.; Tauhid, N.M.; Othman, H.; Rahman, M.R.A.; Yusoff, H.M.; Safian, N.; Ng, P.Y.; Manaf, Z.A.; Kadir, N.B.A.; et al. Prevalence of Metabolic Syndrome and Its Associated Risk Factors among Staffs in a Malaysian Public University. Sci. Rep. 2021, 11, 8132. [Google Scholar] [CrossRef] [PubMed]
- Belete, R.; Ataro, Z.; Abdu, A.; Sheleme, M. Global Prevalence of Metabolic Syndrome among Patients with Type I Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2021, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Rożniata, M.; Zujko, K. Individual Diet Modification Reduces the Metabolic Syndrome in Patients before Pharmacological Treatment. Nutrients 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Salinas, C.A.; Viveros-Ruiz, T. Recent Advances in Managing/Understanding the Metabolic Syndrome. F1000Research 2019, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- von Lintig, J.; Moon, J.; Lee, J.; Ramkumar, S. Carotenoid Metabolism at the Intestinal Barrier. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158580. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the Potential Beneficial Effects of Carotenoids on Consumer Health and Well-Being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef]
- Zheng, W.; Yu, S.; Zhang, W.; Zhang, S.; Fu, J.; Ying, H.; Pingcuo, G.; Liu, S.; Zhao, F.; Wu, Q.; et al. The Content and Diversity of Carotenoids Associated with High-Altitude Adaptation in Tibetan Peach Fruit. Food Chem. 2023, 398, 133909. [Google Scholar] [CrossRef]
- Dzomeku, B.M.; Wald, J.P.; Wünsche, J.N.; Nohr, D.; Biesalski, H.K. Climate Change Enhanced Carotenoid Pro-Vitamin A Levels of Selected Plantain Cultivars. Plants 2020, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global Epidemiology, Health Burden and Effective Interventions for Elevated Blood Pressure and Hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Šimat, V.; Rathod, N.B.; Hamed, I.; Čagalj, M. Algal Carotenoids: Chemistry, Sources, and Application. Foods 2023, 12, 2768. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Mapelli-Brahm, P.; Hornero-Méndez, D.; Vicario, I.M. Structures, Nomenclature and General Chemistry of Carotenoids and Their Esters. In Foods: Physical, Chemical and Biological Properties; Mercadante, A.Z., Ed.; Royal Society of Chemistry: London, UK, 2019. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-Ul-Haq, M.; Dou, D. Chemistry of Carotenoids. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 43–76. ISBN 978-3-030-46459-2. [Google Scholar]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J.; Keum, Y.-S.; Lee, J.-H. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef]
- Sereti, F.; Alexandri, M.; Papadaki, A.; Papapostolou, H.; Kopsahelis, N. Carotenoids Production by Rhodosporidium paludigenum Yeasts: Characterization of Chemical Composition, Antioxidant and Antimicrobial Properties. J. Biotechnol. 2024, 386, 52–63. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Focsan, A.L.; Gao, Y.; Kispert, L.D. The Endless World of Carotenoids—Structural, Chemical and Biological Aspects of Some Rare Carotenoids. Int. J. Mol. Sci. 2023, 24, 9885. [Google Scholar] [CrossRef] [PubMed]
- Metibemu, D.S.; Ogungbe, I.V. Carotenoids in Drug Discovery and Medicine: Pathways and Molecular Targets Implicated in Human Diseases. Molecules 2022, 27, 6005. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.M.; Kozlovskaya, E.P.; Klimovich, A.A.; Rutckova, T.A.; Vakhrushev, A.I.; Hushpulian, D.M.; Gazaryan, I.G.; Makhankov, V.V.; Son, O.M.; Tekutyeva, L.A. Carotenoids from Starfish Patiria Pectinifera: Therapeutic Activity in Models of Inflammatory Diseases. Mar. Drugs 2023, 21, 470. [Google Scholar] [CrossRef]
- Ferraz, C.A.A.; Grougnet, R.; Nicolau, E.; Picot, L.; de Oliveira, R.G., Jr. Carotenoids from Marine Microalgae as Antimelanoma Agents. Mar. Drugs 2022, 20, 618. [Google Scholar] [CrossRef]
- Malhão, F.; Macedo, A.C.; Costa, C.; Rocha, E.; Ramos, A.A. Fucoxanthin Holds Potential to Become a Drug Adjuvant in Breast Cancer Treatment: Evidence from 2D and 3D Cell Cultures. Molecules 2021, 26, 4288. [Google Scholar] [CrossRef]
- Robles-Rivera, R.R.; Castellanos-González, J.A.; Olvera-Montaño, C.; Flores-Martin, R.A.; López-Contreras, A.K.; Arevalo-Simental, D.E.; Cardona-Muñoz, E.G.; Roman-Pintos, L.M.; Rodríguez-Carrizalez, A.D. Adjuvant Therapies in Diabetic Retinopathy as an Early Approach to Delay Its Progression: The Importance of Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2020, 2020, e3096470. [Google Scholar] [CrossRef]
- Islam, F.; Khan, J.; Zehravi, M.; Das, R.; Haque, M.A.; Banu, A.; Parwaiz, S.; Nainu, F.; Nafady, M.H.; Shahriar, S.M.S.; et al. Synergistic Effects of Carotenoids: Therapeutic Benefits on Human Health. Process Biochem. 2024, 136, 254–272. [Google Scholar] [CrossRef]
- Lim, M.; Kim, J. Association between Fruit and Vegetable Consumption and Risk of Metabolic Syndrome Determined Using the Korean Genome and Epidemiology Study (KoGES). Eur. J. Nutr. 2020, 59, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cheang, I.; Tang, Y.; Shi, M.; Zhu, Q.; Gao, R.; Liao, S.; Yao, W.; Zhou, Y.; Zhang, H.; et al. Associations of Serum Carotenoids with Risk of All-Cause and Cardiovascular Mortality in Hypertensive Adults. J. Am. Heart Assoc. 2023, 12, e027568. [Google Scholar] [CrossRef]
- Gopal, S.S.; Sukhdeo, S.V.; Vallikannan, B.; Ponesakki, G. Lutein Ameliorates High-Fat Diet-Induced Obesity, Fatty Liver, and Glucose Intolerance in C57BL/6J Mice. Phytother. Res. 2023, 37, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Narumi, K.; Yamagishi, N.; Nishi, T.; Ito, T.; Iseki, K.; Kobayashi, M.; Kanai, Y. Oral Administration of Linoleic Acid Immediately before Glucose Load Ameliorates Postprandial Hyperglycemia. Front. Pharmacol. 2023, 14, 1197743. [Google Scholar] [CrossRef]
- Boonlao, N.; Ruktanonchai, U.R.; Anal, A.K. Enhancing Bioaccessibility and Bioavailability of Carotenoids Using Emulsion-Based Delivery Systems. Colloids Surf. B Biointerfaces 2022, 209, 112211. [Google Scholar] [CrossRef]
- Carotenoid-Loaded Nanocarriers: A Comprehensive Review—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0001868619302738?via%3Dihub (accessed on 15 April 2024).
- González-Peña, M.A.; Lozada-Ramírez, J.D.; Ortega-Regules, A.E. Antioxidant Activities of Spray-Dried Carotenoids Using Maltodextrin-Arabic Gum as Wall Materials. Bull. Natl. Res. Cent. 2021, 45, 58. [Google Scholar] [CrossRef]
- Tamtürk, F.; Gürbüz, B.; Toker, Ö.S.; Dalabasmaz, S.; Malakjani, N.; Durmaz, Y.; Konar, N. Optimization of Chlorella vulgaris Spray Drying Using Various Innovative Wall Materials. Algal Res. 2023, 72, 103115. [Google Scholar] [CrossRef]
- Cabezas-Terán, K.; Grootaert, C.; Ortiz, J.; Donoso, S.; Ruales, J.; Van Bockstaele, F.; Van Camp, J.; Van de Wiele, T. In Vitro Bioaccessibility and Uptake of β-Carotene from Encapsulated Carotenoids from Mango by-Products in a Coupled Gastrointestinal Digestion/Caco-2 Cell Model. Food Res. Int. 2023, 164, 112301. [Google Scholar] [CrossRef]
- Honda, M.; Zhang, Y.; Kageyama, H.; Hibino, T.; Goto, M.; Nishida, Y. Formation and Characterization of Z-Isomer-Enriched Carotenoid-Loaded Microparticles with Poly(Vinylpyrrolidone) Using a Spray Drying Technique. Ind. Eng. Chem. Res. 2024, 63, 383–393. [Google Scholar] [CrossRef]
- Sørensen, T.I.A.; Martinez, A.R.; Jørgensen, T.S.H. Epidemiology of Obesity. In From Obesity to Diabetes; Eckel, J., Clément, K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–27. ISBN 978-3-030-99995-7. [Google Scholar]
- Shi, M.; Zhang, X.; Wang, H. The Prevalence of Diabetes, Prediabetes and Associated Risk Factors in Hangzhou, Zhejiang Province: A Community-Based Cross-Sectional Study. Diabetes Metab. Syndr. Obes. 2022, 15, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Åberg, F.; Byrne, C.D.; Pirola, C.J.; Männistö, V.; Sookoian, S. Alcohol Consumption and Metabolic Syndrome: Clinical and Epidemiological Impact on Liver Disease. J. Hepatol. 2023, 78, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Fracchiolla, G.; Muraglia, M.; Tardugno, R.; Dibenedetto, R.S.; D’Alessandro, A.G. Metabolic Syndrome: A Narrative Review from the Oxidative Stress to the Management of Related Diseases. Antioxidants 2023, 12, 2091. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.K.; Khullar, M. Oxidative Stress in Metabolic Diseases: Current Scenario and Therapeutic Relevance. Mol. Cell. Biochem. 2023, 478, 185–196. [Google Scholar] [CrossRef]
- Kumar, A.; Prajapati, P.; Singh, G.; Kumar, D.; Mishra, V.; Kim, S.-C.; Raorane, C.J.; Raj, V.; Kushwaha, S. Salbutamol Attenuates Diabetic Skeletal Muscle Atrophy by Reducing Oxidative Stress, Myostatin/GDF-8, and Pro-Inflammatory Cytokines in Rats. Pharmaceutics 2023, 15, 2101. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, K.-A.; Luchian, I.; Goriuc, A.; Antoci, L.-M.; Ciobanu, C.-G.; Popescu, R.; Vlad, C.-E.; Blaj, M.; Foia, L.G. Mitochondrial Dysfunction, Oxidative Stress, and Therapeutic Strategies in Diabetes, Obesity, and Cardiovascular Disease. Antioxidants 2023, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kyriakides, T.R. The Role of Extracellular Matrix in the Pathophysiology of Diabetic Wounds. Matrix Biol. Plus 2020, 6–7, 100037. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.S.R.; Martin-Pastor, M.; Tavares Júnior, A.G.; Queiroz, K.A.; da Silva Sólon, L.G.; Sousa, F.F.O.d. Metabolomic Profile and Its Correlation with the Plasmatic Levels of Losartan, EXP3174 and Blood Pressure Control in Hypertensive and Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2023, 24, 9832. [Google Scholar] [CrossRef]
- Fishel Bartal, M.; Blackwell, S.C.; Pedroza, C.; Lawal, D.; Amro, F.; Samuel, J.; Chauhan, S.P.; Sibai, B.M. Oral Combined Hydrochlorothiazide and Lisinopril vs Nifedipine for Postpartum Hypertension: A Comparative-Effectiveness Pilot Randomized Controlled Trial. Am. J. Obstet. Gynecol. 2023, 228, 571.e1–571.e10. [Google Scholar] [CrossRef]
- Li, L.; Tong, X.; Ma, Z.; Lv, L.; Liu, H.; Chen, G.L. Folic Acid Enhances the Cardiovascular Protective Effect of Amlodipine in Renal Hypertensive Rats with Elevated Homocysteine. Clin. Exp. Hypertens. 2023, 45, 2205058. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.; Sarkar, S. Glipizide Ameliorates Human Poly(Q) Mediated Neurotoxicity by Upregulating Insulin Signalling in Drosophila Disease Models. Biochem. Biophys. Res. Commun. 2023, 645, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. Efficacy of Metformin Combined with Liraglutide on the Glucose and Lipid Metabolism, Vascular Endothelial Function, and Oxidative Stress of Patients with T2DM and Metabolic Syndrome. Pak. J. Med. Sci. 2024, 40, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.-J.; Wang, G.-Z. Clinical Efficacy of Dapagliflozin in the Treatment of Patients with Diabetic Nephropathy and Its Effect on Proteinuria Level. Diabetes Metab. Syndr. Obes. 2023, 16, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lin, X.; Tao, M.; Chen, Q.; Sun, H.; Han, Y.; Yang, S.; Gao, Y.; Qu, S.; Chen, H. Efficacy and Safety of Orlistat in Male Patients with Overweight/Obesity and Hyperuricemia: Results of a Randomized, Double-Blind, Placebo-Controlled Trial. Lipids Health Dis. 2024, 23, 77. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Hsia, D.S.; Nguyen, L.T.; Peterson, C.A.; Varghese, S.T. Effects of Phentermine/Topiramate Extended-Release, Phentermine, and Placebo on Ambulatory Blood Pressure Monitoring in Adults with Overweight or Obesity: A Randomized, Multicenter, Double-Blind Study. Obes. Pillars 2024, 9, 100099. [Google Scholar] [CrossRef] [PubMed]
- Murvelashvili, N.; Xie, L.; Schellinger, J.N.; Mathew, M.S.; Marroquin, E.M.; Lingvay, I.; Messiah, S.E.; Almandoz, J.P. Effectiveness of Semaglutide versus Liraglutide for Treating Post-Metabolic and Bariatric Surgery Weight Recurrence. Obesity 2023, 31, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Brierley, D.I.; Leeson-Payne, A.; Jiang, W.; Chianese, R.; Lam, B.Y.H.; Dowsett, G.K.C.; Cristiano, C.; Lyons, D.; Reimann, F.; et al. Obesity Medication Lorcaserin Activates Brainstem GLP-1 Neurons to Reduce Food Intake and Augments GLP-1 Receptor Agonist Induced Appetite Suppression. Mol. Metab. 2023, 68, 101665. [Google Scholar] [CrossRef] [PubMed]
- Insani, W.N.; Whittlesea, C.; Ju, C.; Man, K.K.; Adesuyan, M.; Chapman, S.; Wei, L. Impact of ACEIs and ARBs-Related Adverse Drug Reaction on Patients’ Clinical Outcomes: A Cohort Study in UK Primary Care. Br. J. Gen. Pr. 2023, 73, e832–e842. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Wang, L.; Chen, C.; Chen, L. Association between Different GLP-1 Receptor Agonists and Gastrointestinal Adverse Reactions: A Real-World Disproportionality Study Based on FDA Adverse Event Reporting System Database. Front. Endocrinol. 2022, 13, 1043789. [Google Scholar] [CrossRef]
- Koina, I.M.; Sarigiannis, Y.; Hapeshi, E. Green Extraction Techniques for the Determination of Active Ingredients in Tea: Current State, Challenges, and Future Perspectives. Separations 2023, 10, 121. [Google Scholar] [CrossRef]
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Majid, I.; Khan, S.; Aladel, A.; Dar, A.H.; Adnan, M.; Khan, M.I.; Mahgoub Awadelkareem, A.; Ashraf, S.A. Recent Insights into Green Extraction Techniques as Efficient Methods for the Extraction of Bioactive Components and Essential Oils from Foods. CyTA J. Food 2023, 21, 101–114. [Google Scholar] [CrossRef]
- Menezes Silva, J.V.; Silva Santos, A.; Araujo Pereira, G.; Campos Chisté, R. Ultrasound-Assisted Extraction Using Ethanol Efficiently Extracted Carotenoids from Peels of Peach Palm Fruits (Bactris Gasipaes Kunth) without Altering Qualitative Carotenoid Profile. Heliyon 2023, 9, e14933. [Google Scholar] [CrossRef] [PubMed]
- Savic Gajic, I.M.; Savic, I.M.; Gajic, D.G.; Dosic, A. Ultrasound-Assisted Extraction of Carotenoids from Orange Peel Using Olive Oil and Its Encapsulation in Ca-Alginate Beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Vo, T.P.; Tran, H.K.L.; Ta, T.M.N.; Nguyen, H.T.V.; Phan, T.H.; Nguyen, T.H.P.; Nguyen, V.K.; Dang, T.C.T.; Nguyen, L.G.K.; Chung, T.Q.; et al. Extraction and Emulsification of Carotenoids from Carrot Pomaces Using Oleic Acid. ACS Omega 2023, 8, 39523–39534. [Google Scholar] [CrossRef]
- Larocca, V.; Martino, M.; Trupo, M.; Magarelli, R.A.; Spagnoletta, A.; Ambrico, A. Evaluation of Carbon Dioxide Supercritical Fluid Extraction (CO2-SFE) on Carotenoids Recovery from Red Yeast Cells. Biomass Conv. Bioref. 2023. [Google Scholar] [CrossRef]
- Priyadarsani, S.; Patel, A.S.; Kar, A.; Dash, S. Process Optimization for the Supercritical Carbondioxide Extraction of Lycopene from Ripe Grapefruit (Citrus paradisi) Endocarp. Sci. Rep. 2021, 11, 10273. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, G.L.; Cuevas, M.S.; Capellini, M.C.; Crevellin, E.J.; de Moraes, L.A.B.; Rodrigues, C.E.d.C. Extraction of Carotenoid-Rich Palm Pressed Fiber Oil Using Mixtures of Hydrocarbons and Short Chain Alcohols. Food Res. Int. 2020, 128, 108810. [Google Scholar] [CrossRef]
- Naveira-Pazos, C.; Veiga, M.C.; Mussagy, C.U.; Farias, F.O.; Kennes, C.; Pereira, J.F.B. Carotenoids Production and Extraction from Yarrowia lipolytica Cells: A Biocompatible Approach Using Biosolvents. Sep. Purif. Technol. 2024, 343, 127136. [Google Scholar] [CrossRef]
- Radice, R.P.; Padula, M.C.; Liguori, A.; D’Arienzo, G.; Martelli, G. Genetic Improvement to Obtain Specialized Haematococcus Pluvialis Genotypes for the Production of Carotenoids, with Particular Reference to Astaxanthin. Int. J. Plant Biol. 2023, 14, 276–285. [Google Scholar] [CrossRef]
- Chekanov, K. Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Mar. Drugs 2023, 21, 108. [Google Scholar] [CrossRef]
- Centini, M.; Martinez-Sañudo, I.; Biagi, M.; Dreassi, E.; Mazzon, L.; Marri, L. Brevundimonas Aurantiaca M3d10, Isolated from the Olive Fly, Produces Hydroxylated Astaxanthin. Cosmetics 2023, 10, 103. [Google Scholar] [CrossRef]
- Asker, D. Isolation and Characterization of a Novel, Highly Selective Astaxanthin-Producing Marine Bacterium. J. Agric. Food Chem. 2017, 65, 9101–9109. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, Y.; Ye, D.; He, M.; Wang, H.; Zhu, Z.; Sun, Y. Production of Astaxanthin by Animal Cells via Introduction of an Entire Astaxanthin Biosynthetic Pathway. Bioengineering 2023, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- LaCourse, W.R.; LaCourse, M.E. General Instrumentation in HPLC. In Liquid Chromatography, 3rd ed.; Handbooks in Separation Science; Fanali, S., Chankvetadze, B., Haddad, P.R., Poole, C.F., Riekkola, M.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, pp. 61–73. ISBN 978-0-323-99968-7. [Google Scholar]
- Nahar, L.; Onder, A.; Sarker, S.D. A Review on the Recent Advances in HPLC, UHPLC and UPLC Analyses of Naturally Occurring Cannabinoids (2010–2019). Phytochem. Anal. 2020, 31, 413–457. [Google Scholar] [CrossRef] [PubMed]
- Queral-Beltran, A.; Marín-García, M.; Lacorte, S.; Tauler, R. UV-Vis Absorption Spectrophotometry and LC-DAD-MS-ESI(+)-ESI(−) Coupled to Chemometrics Analysis of the Monitoring of Sulfamethoxazole Degradation by Chlorination, Photodegradation, and Chlorination/Photodegradation. Anal. Chim. Acta 2023, 1276, 341563. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Wu, Q.; Li, S.; Yi, H.; Xie, F. Development and Validation of a UPLC-PDA Method for Quantifying Ceftazidime in Dried Blood Spots. J. Pharm. Biomed. Anal. 2024, 239, 115928. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Liu, L.; Wang, Y.; Peng, Y.; Li, S. An UPLC-PDA Assay for Simultaneous Determination of Seven Antibiotics in Human Plasma. J. Pharm. Biomed. Anal. 2022, 210, 114558. [Google Scholar] [CrossRef]
- Muchiri, R.N.; van Breemen, R.B. Drug Discovery from Natural Products Using Affinity Selection-Mass Spectrometry. Drug Discov. Today Technol. 2021, 40, 59–63. [Google Scholar] [CrossRef]
- Zeng, Q.; Xia, M.-C.; Yin, X.; Cheng, S.; Xue, Z.; Tan, S.; Gong, X.; Ye, Z. Recent Developments in Ionization Techniques for Single-Cell Mass Spectrometry. Front. Chem. 2023, 11, 1293533. [Google Scholar] [CrossRef]
- Abdullahi, A.D.; Unban, K.; Saenjum, C.; Kodchasee, P.; Kangwan, N.; Thananchai, H.; Shetty, K.; Khanongnuch, C. Antibacterial Activities of Miang Extracts against Selected Pathogens and the Potential of the Tannin-Free Extracts in the Growth Inhibition of Streptococcus Mutans. PLoS ONE 2024, 19, e0302717. [Google Scholar] [CrossRef] [PubMed]
- Misiurek, J.; Plech, T.; Kaproń, B.; Makuch-Kocka, A.; Szultka-Młyńska, M.; Buszewski, B.; Petruczynik, A. Determination of Some Isoquinoline Alkaloids in Extracts Obtained from Selected Plants of the Ranunculaceae, Papaveraceae and Fumarioideae Families by Liquid Chromatography and In Vitro and In Vivo Investigations of Their Cytotoxic Activity. Molecules 2023, 28, 3503. [Google Scholar] [CrossRef]
- Baranyika, J.B.; Bakire, S.; Shoucheng, P.; Meihao, S.; Hirwa, H. Application of the Selected Macroporous Resin for the Separation and Identification of Flavonoids from Chinese Radix Pueraria lobata by HPLC-Q-TOF-MS. Microchem. J. 2024, 196, 109662. [Google Scholar] [CrossRef]
- Ligor, M.; Kováčová, J.; Gadzała-Kopciuch, R.M.; Studzińska, S.; Bocian, S.; Lehotay, J.; Buszewski, B. Study of RP HPLC Retention Behaviours in Analysis of Carotenoids. Chromatographia 2014, 77, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Chaudhary, C.; Singh, L.; Sidhu, C.; Siddhardha, B.; Prasad, S.E.; Thakur, K.G. Isolation and Taxonomic Characterization of Novel Haloarchaeal Isolates from Indian Solar Saltern: A Brief Review on Distribution of Bacteriorhodopsins and V-Type ATPases in Haloarchaea. Front. Microbiol. 2020, 11, 554927. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.L.; Li, M.; McQuinn, R.P.; Chan, K.X.; McFarlane, H.E.; Ermakova, M.; Furbank, R.T.; Mares, D.; Dong, C.; Chalmers, K.J.; et al. A GDSL Esterase/Lipase Catalyzes the Esterification of Lutein in Bread Wheat. Plant Cell 2019, 31, 3092–3112. [Google Scholar] [CrossRef]
- Sinha, S.; Das, S.; Saha, B.; Paul, D.; Basu, B. Anti-Microbial, Anti-Oxidant, and Anti-Breast Cancer Properties Unraveled in Yeast Carotenoids Produced via Cost-Effective Fermentation Technique Utilizing Waste Hydrolysate. Front. Microbiol. 2022, 13, 1088477. [Google Scholar] [CrossRef]
- de Barros-Santos, R.G.; Pimentel, T.C.; Amorim, T.A.; da Silva Nogueira, E.T.; de Oliveira Vilar, S.B.; de Souza, M.E.A.O.; de Brito Araújo Carvalho, A.J.; Magnani, M.; dos Santos Lima, M. Ultra-Fast Determination of Free Carotenoids in Fruit Juices by Rapid Resolution Liquid Chromatography (RRLC): Method Validation and Characterization of Brazilian Whole Fruit Juices. Food Anal. Methods 2023, 16, 808–818. [Google Scholar] [CrossRef]
- Osakabe, M.; Shimano, S. The Flashy Red Color of the Red Velvet Mite Balaustium Murorum (Prostigmata: Erythraeidae) Is Caused by High Abundance of the Keto-Carotenoids, Astaxanthin and 3-Hydroxyechinenone. Exp. Appl. Acarol. 2023, 89, 1–14. [Google Scholar] [CrossRef]
- Erdoğan, A.; Karataş, A.B.; Demir, D.; Demirel, Z.; Aktürk, M.; Çopur, Ö.; Conk-Dalay, M. Manipulation in Culture Conditions of Nanofrustulum Shiloi for Enhanced Fucoxanthin Production and Isolation by Preparative Chromatography. Molecules 2023, 28, 1988. [Google Scholar] [CrossRef] [PubMed]
- Nemani, N.; Dehnavi, S.M.; Pazuki, G. Extraction and Separation of Astaxanthin with the Help of Pre-Treatment of Haematococcus Pluvialis Microalgae Biomass Using Aqueous Two-Phase Systems Based on Deep Eutectic Solvents. Sci. Rep. 2024, 14, 5420. [Google Scholar] [CrossRef] [PubMed]
- Seeger, J.; Wendisch, V.F.; Henke, N.A. Extraction and Purification of Highly Active Astaxanthin from Corynebacterium Glutamicum Fermentation Broth. Mar. Drugs 2023, 21, 530. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.G.P.; Mussagy, C.U.; Lima, C.d.A.; Santos-Ebinuma, V.C.; Burkert, J.F.d.M.; Santos, L.O. Sustainable Approach to Recover β-Carotene and Astaxanthin from Phaffia rhodozyma Grown in a Stirred-Tank Bioreactor under the Influence of Magnetic Fields. Bioresour. Technol. 2023, 390, 129906. [Google Scholar] [CrossRef] [PubMed]
- Pajot, A.; Chollet, S.; Nicolau, E.; Marchal, L. Improving the Extraction and the Purification of Fucoxanthin from Tisochrysis lutea Using Centrifugal Partition Chromatography. Algal Res. 2023, 74, 103174. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Silva, A.; Garcia-Oliveira, P.; Soria-Lopez, A.; Echave, J.; Grosso, C.; Cassani, L.; Barroso, M.F.; Simal-Gandara, J.; Fraga-Corral, M.; et al. Kinetic Extraction of Fucoxanthin from Undaria Pinnatifida Using Ethanol as a Solvent. Mar. Drugs 2023, 21, 414. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.T.N.; Nguyen, H.V.H. Optimization of Enzyme-Assisted Lycopene Extraction from Tomato (Lycopersicon esculentum) Peel Using Rice Bran Oil. Food Meas. 2023, 17, 5154–5162. [Google Scholar] [CrossRef]
- Ge, B.; Wang, W.; Gao, Y.; Chen, X. Optimization of Extraction of Lycopene from Carrot and Determination of Its Antioxidant Activity. Food Meas. 2023, 17, 5497–5505. [Google Scholar] [CrossRef]
- Surmanidze, N.; Vanidze, M.; Djafaridze, I.; Davitadze, R.; Qarcivadze, I.; Khakhutaishvili, M.; Kalandia, A. Optimization of the Method of Ultrasonic Extraction of Lycopene with a Green Extract from the Fruit of Elaeagnus Umbellata, Common in Western Georgia. Food Sci. Nutr. 2024, 12, 3593–3601. [Google Scholar] [CrossRef]
- Dhakane-Lad, J.; Kar, A.; Patel, A.S. SC-CO2 Extraction of Lycopene from Red Papaya Using Rice Bran Oil as a Co-Solvent Lessens Its Degradation during Storage. Sep. Sci. Technol. 2023, 58, 2357–2368. [Google Scholar] [CrossRef]
- Ahmadi, R.; Honarvar, M.; Ghavami, M.; Daali, Y. Optimization of Lutein Extraction from Pistachio Waste Using Experimental Design and Ultrasonic Method. Waste Biomass Valor. 2023, 15, 3593–3601. [Google Scholar] [CrossRef]
- Maheshwari, N.; Arya, R.K.; Verros, G.D.; Dhamole, P.B.; Kannan, A. Surfactant-Enhanced Extraction of Lutein from Marigold Petals Using an Aqueous Two-Phase System. Separations 2023, 10, 133. [Google Scholar] [CrossRef]
- Sun, X.; Ma, L.; Muhire, J.; Zhang, F.-X.; Huang, X.-Y.; Liu, J.-F.; Pei, D.; Di, D.-L. An Integrated Strategy for Combining Three-Phase Liquid-Liquid Extraction with Continuous High-Speed Countercurrent Chromatography: Highly Efficient in Isolating and Purifying Zeaxanthin from the Industrial Crop Lycium barbarum L. Ind. Crops Prod. 2023, 206, 117641. [Google Scholar] [CrossRef]
- Yang, L.; Zi, C.; Li, Y.; Huang, J.; Gu, Z.; Wang, C.; Hu, J.-M.; Jiang, Z.; Zhang, W. An In-Depth Investigation of Molecular Interaction in Zeaxanthin/Corn Silk Glycan Complexes and Its Positive Role in Hypoglycemic Activity. Food Chem. 2024, 438, 137986. [Google Scholar] [CrossRef]
- Wang, M.; Morón-Ortiz, Á.; Zhou, J.; Benítez-González, A.; Mapelli-Brahm, P.; Meléndez-Martínez, A.J.; Barba, F.J. Effects of Pressurized Liquid Extraction with Dimethyl Sulfoxide on the Recovery of Carotenoids and Other Dietary Valuable Compounds from the Microalgae Spirulina, Chlorella and Phaeodactylum tricornutum. Food Chem. 2023, 405, 134885. [Google Scholar] [CrossRef]
- Macedo, M.C.C.; Correia, V.T.d.V.; Silva, V.D.M.; Pereira, D.T.V.; Augusti, R.; Melo, J.O.F.; Pires, C.V.; de Paula, A.C.C.F.F.; Fante, C.A. Development and Characterization of Yellow Passion Fruit Peel Flour (Passiflora edulis f. Flavicarpa). Metabolites 2023, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wan, P.; Liu, J.; Yao, J.; Chen, D.-W. Investigation on the Changes of Carotenoids and Capsaicinoids in Chili Oil at Different Frying Temperature by Using 1H NMR. Curr. Res. Food Sci. 2023, 6, 100411. [Google Scholar] [CrossRef]
- Ahn, S.; Ahn, S.; Jang, H.; Eom, K.; Kim, Y.J.; Hwang, J.-E.; Chung, J.I.; Park, J.-Y.; Nam, S.; Choi, Y.-H.; et al. Validation of Resonance Raman Spectroscopy-Measured Skin Carotenoid Status as a Biomarker for Fruit and Vegetable Intake in Korean Adults. Br. J. Nutr. 2023, 130, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Casperson, S.L.; Roemmich, J.N.; Larson, K.J.; Hess, J.M.; Palmer, D.G.; Jahns, L. Sensitivity of Pressure-Mediated Reflection Spectroscopy to Detect Changes in Skin Carotenoids in Adults without Obesity in Response to Increased Carotenoid Intake: A Randomized Controlled Trial. J. Nutr. 2023, 153, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.; Lobakova, E.; Semenov, A.; Gorelova, O.; Fedorenko, T.; Chivkunova, O.; Parshina, E.; Maksimov, G.; Sluchanko, N.N.; Maksimov, E. Multimodal Non-Invasive Probing of Stress-Induced Carotenogenesis in the Cells of Microalga Bracteacoccus Aggregatus. Protoplasma 2024. [Google Scholar] [CrossRef]
- Ba, W.; Xu, W.; Deng, Z.; Zhang, B.; Zheng, L.; Li, H. The Antioxidant and Anti-Inflammatory Effects of the Main Carotenoids from Tomatoes via Nrf2 and NF-κB Signaling Pathways. Nutrients 2023, 15, 4652. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, J.; Gu, Y.; Li, Y.; Zhang, W.; Lv, N.; Dang, A. Comparing Lycopene’s Impact on Mortality in Adults with or without Obesity. Food Funct. 2024, 15, 3340–3352. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.G.; César, N.R.; Melo, D.S.; Figueiró, M.T.O.; dos Santos, E.C.; Evangelista-Silva, P.H.; de Sousa Santos, C.; Costa, K.B.; Rocha-Vieira, E.; Dias-Peixoto, M.F.; et al. A MUFA/Carotenoid-Rich Oil Ameliorated Insulin Resistance by Improving Inflammation and Oxidative Stress in Obese Rats. Mol. Cell. Endocrinol. 2024, 581, 112110. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, J.-S.; Lee, H.; Jung, C.H.; Ahn, J. Whole Red Paprika (Capsicum annuum L.) and Its Orange-Red Pigment Capsanthin Ameliorate Obesity-Induced Skeletal Muscle Atrophy in Mice. J. Funct. Foods 2023, 107, 105624. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; Boeder, S.C.; Mudaliar, S.R.; Giovannetti, E.R.; Henry, R.R.; Pettus, J.H. Astaxanthin, a Natural Antioxidant, Lowers Cholesterol and Markers of Cardiovascular Risk in Individuals with Prediabetes and Dyslipidaemia. Diabetes Obes. Metab. 2023, 25, 1985–1994. [Google Scholar] [CrossRef] [PubMed]
- López-Ramos, A.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G. Effect of Fucoxanthin on Metabolic Syndrome, Insulin Sensitivity, and Insulin Secretion. J. Med. Food 2023, 26, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zeleznik, O.A.; Shutta, K.H.; Rosner, B.A.; Kraft, P.; Clish, C.B.; Stampfer, M.J.; Willett, W.C.; Tamimi, R.M.; Eliassen, A.H. A Metabolomics Analysis of Circulating Carotenoids and Breast Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2022, 31, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Sun, W.; Qin, Y.; Xu, H. Association of Dietary α-Carotene and β-Carotene Intake with Low Cognitive Performance in Older Adults: A Cross-Sectional Study from the National Health and Nutrition Examination Survey. Nutrients 2023, 15, 239. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, X.; Liu, M.; Zhao, H.; Sun, Y. Lycopene Prevents Non-Alcoholic Fatty Liver Disease through Regulating Hepatic NF-κB/NLRP3 Inflammasome Pathway and Intestinal Microbiota in Mice Fed with High-Fat and High-Fructose Diet. Front. Nutr. 2023, 10, 1120254. [Google Scholar] [CrossRef]
- Liu, H.; Yan, J.; Guan, F.; Jin, Z.; Xie, J.; Wang, C.; Liu, M.; Liu, J. Zeaxanthin Prevents Ferroptosis by Promoting Mitochondrial Function and Inhibiting the P53 Pathway in Free Fatty Acid-Induced HepG2 Cells. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2023, 1868, 159287. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Sun, C.; Wei, X.; Liu, S.; Jiang, Y. Gastrointestinal pH-Sensitive Pickering Emulsions Stabilized by Zein Nanoparticles Coated with Bioactive Glycyrrhizic Acid for Improving Oral Bioaccessibility of Curcumin. ACS Appl. Mater. Interfaces 2023, 15, 14678–14689. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the Therapeutic Efficacy of Nanoparticles for Cancer Treatment Using Versatile Targeted Strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Vera-Vázquez, F.; Ramírez-Bribiesca, J.E.; Cruz-Monterrosa, R.G.; Crosby-Galvan, M.M.; Barcena-Gama, J.R.; Ramírez, D.T.; Mejía-Méndez, J.L.; Vallejo-Hernández, L.H.; López-Mena, E.R. Enhancing Pectin Particles with Polymer Additives: Mitigating Rumen Degradation and Minimizing Yellowish Milk Color in Grazed Cows. Polymers 2024, 16, 106. [Google Scholar] [CrossRef]
- Murueva, A.V.; Shershneva, A.M.; Shishatskaya, E.I.; Volova, T.G. Characteristics of Microparticles Based on Resorbable Polyhydroxyalkanoates Loaded with Antibacterial and Cytostatic Drugs. Int. J. Mol. Sci. 2023, 24, 14983. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Klier, J.; Beltramo, P.J. Encapsulation of Inorganic Nanoparticles by Anionic Emulsion Polymerization of Diethyl Methylene Malonate for Developing Hybrid Microparticles with Tailorable Composition. Colloids Interfaces 2024, 8, 10. [Google Scholar] [CrossRef]
- da Silva, R.Y.P.; Menezes, D.L.B.d.; Oliveira, V.d.S.; Converti, A.; Lima, Á.A.N.d. Microparticles in the Development and Improvement of Pharmaceutical Formulations: An Analysis of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2023, 24, 5441. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Boomija, R.V.; Muthukumar, S.; Gandhi, M.; Salma, S.; Prinsha, T.K.; Rengasamy, B. Green Synthesis of Chitosan Nanoparticles Using Tea Extract and Its Antimicrobial Activity against Economically Important Phytopathogens of Rice. Sci. Rep. 2024, 14, 7381. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, A.L.; Perfecto-Avalos, Y.; Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Sepulveda-Villegas, M.; Rincón-Enríquez, G.; Rodríguez-González, V.; Garcia-Varela, R.; Lozano, L.M.; Eloyr Navarro-López, D.; et al. Influence of Erbium Doping on Zinc Oxide Nanoparticles: Structural, Optical and Antimicrobial Activity. Appl. Surf. Sci. 2022, 575, 151764. [Google Scholar] [CrossRef]
- Zhang, X.; Centurion, F.; Misra, A.; Patel, S.; Gu, Z. Molecularly Targeted Nanomedicine Enabled by Inorganic Nanoparticles for Atherosclerosis Diagnosis and Treatment. Adv. Drug Deliv. Rev. 2023, 194, 114709. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Alkhursani, S.A.; Alqahtani, H.A.; El-damhougy, T.K.; Madani, M. Gold Nanoparticles in Microelectronics Advancements and Biomedical Applications. Mater. Sci. Eng. B 2024, 301, 117191. [Google Scholar] [CrossRef]
- Misra, S.K.; Mukherjee, P.; Chang, H.-H.; Tiwari, S.; Gryka, M.; Bhargava, R.; Pan, D. Multi-Functionality Redefined with Colloidal Carotene Carbon Nanoparticles for Synchronized Chemical Imaging, Enriched Cellular Uptake and Therapy. Sci. Rep. 2016, 6, 29299. [Google Scholar] [CrossRef]
- Elkholy, N.S.; Shafaa, M.W.; Mohammed, H.S. Biophysical Characterization of Lutein or Beta Carotene-Loaded Cationic Liposomes. RSC Adv. 2020, 10, 32409–32422. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Wulandari, S.; Choi, J.; Kurniawan, R.G.; Sugiarto, J.R.; Myint, A.A.; Kwak, S.K.; Kim, J. Synthesis of Highly Stable Encapsulated Astaxanthin/β-Cyclodextrin Microparticles Using Supercritical CO2 as an Antisolvent. J. CO2 Util. 2023, 75, 102575. [Google Scholar] [CrossRef]
- Vakarelova, M.; Zanoni, F.; Donà, G.; Fierri, I.; Chignola, R.; Gorrieri, S.; Zoccatelli, G. Microencapsulation of Astaxanthin by Ionic Gelation: Effect of Different Gelling Polymers on the Carotenoid Load, Stability and Bioaccessibility. Int. J. Food Sci. Technol. 2023, 58, 2489–2497. [Google Scholar] [CrossRef]
- Meira, A.C.F.D.O.; Morais, L.C.D.; Figueiredo, J.D.A.; Veríssimo, L.A.A.; Botrel, D.A.; Resende, J.V.D. Microencapsulation of β-Carotene Using Barley Residue Proteins from Beer Waste as Coating Material. J. Microencapsul. 2023, 40, 171–185. [Google Scholar] [CrossRef]
- Sánchez, C.A.O.; Zavaleta, E.B.; García, G.R.U.; Solano, G.L.; Díaz, M.P.R. Krill Oil Microencapsulation: Antioxidant Activity, Astaxanthin Retention, Encapsulation Efficiency, Fatty Acids Profile, in Vitro Bioaccessibility and Storage Stability. LWT 2021, 147, 111476. [Google Scholar] [CrossRef]
- Morales, E.; Burgos-Díaz, C.; Zúñiga, R.N.; Jorkowski, J.; Quilaqueo, M.; Rubilar, M. Influence of O/W Emulsion Interfacial Ionic Membranes on the Encapsulation Efficiency and Storage Stability of Powder Microencapsulated Astaxanthin. Food Bioprod. Process. 2021, 126, 143–154. [Google Scholar] [CrossRef]
- Foo, S.C.; Khong, N.M.H.; Yusoff, F.M. Physicochemical, Microstructure and Antioxidant Properties of Microalgae-Derived Fucoxanthin Rich Microcapsules. Algal Res. 2020, 51, 102061. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Fazaeli, M. Double Encapsulation of Fucoxanthin Using Porous Starch through Sequential Coating Modification with Maltodextrin and Gum Arabic. Food Sci. Nutr. 2020, 8, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dou, X.; Pang, J.; Liang, M.; Feng, C.; Kong, M.; Liu, Y.; Cheng, X.; Wang, Y.; Chen, X. Improvement of Fucoxanthin Oral Efficacy via Vehicles Based on Gum Arabic, Gelatin and Alginate Hydrogel: Delivery System for Oral Efficacy Enhancement of Functional Food Ingredients. J. Funct. Foods 2019, 63, 103573. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, S.C.; Duarte, D.F.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of Tomato (Solanum lycopersicum L.) Pomace Ethanolic Extract by Spray Drying: Optimization of Process Conditions. Appl. Sci. 2019, 9, 612. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Santos, D.I.; Brito, L.; Moldão-Martins, M.; Alves, V.D. Storage Stability and In Vitro Bioaccessibility of Microencapsulated Tomato (Solanum lycopersicum L.) Pomace Extract. Bioengineering 2022, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Microspheres as Carriers of Sea Buckthorn Carotenoids and Tocols with Antidiabetic Potential: Effect of Biopolymers, Cross-Linking and Storage. Food Biosci. 2024, 59, 103995. [Google Scholar] [CrossRef]
- Gheonea (Dima), I.; Aprodu, I.; Cîrciumaru, A.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Microencapsulation of Lycopene from Tomatoes Peels by Complex Coacervation and Freeze-Drying: Evidences on Phytochemical Profile, Stability and Food Applications. J. Food Eng. 2021, 288, 110166. [Google Scholar] [CrossRef]
- Ding, Z.; Tao, T.; Yin, X.; Prakash, S.; Wang, X.; Zhao, Y.; Han, J.; Wang, Z. Improved Encapsulation Efficiency and Storage Stability of Spray Dried Microencapsulated Lutein with Carbohydrates Combinations as Encapsulating Material. LWT 2020, 124, 109139. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, B.; Liang, W.; Liu, X.; Zheng, J.; Ge, X.; Shen, H.; Lu, Y.; Zhang, X.; Sun, Z.; et al. Lutein Encapsulated in Whey Protein and Citric Acid Potato Starch Ester: Construction and Characterization of Microcapsules. Int. J. Biol. Macromol. 2022, 220, 1–12. [Google Scholar] [CrossRef]
- Hao, J.; Xu, J.; Zhang, W.; Li, X.; Liang, D.; Xu, D.; Cao, Y.; Sun, B. The Improvement of the Physicochemical Properties and Bioaccessibility of Lutein Microparticles by Electrostatic Complexation. Food Hydrocoll. 2022, 125, 107381. [Google Scholar] [CrossRef]
- Ganjali, E.; Elhamirad, A.H.; Hosseini, F.; Mahfoozi, M. The Improvement of Crocin Stability in Rock Candy (Nabat) By Microencapsulation. J. Nutr. Fasting Health 2020, 8, 280–287. [Google Scholar] [CrossRef]
- Essifi, K.; Brahmi, M.; Berraaouan, D.; Ed-Daoui, A.; El Bachiri, A.; Fauconnier, M.-L.; Tahani, A. Influence of Sodium Alginate Concentration on Microcapsules Properties Foreseeing the Protection and Controlled Release of Bioactive Substances. J. Chem. 2021, 2021, e5531479. [Google Scholar] [CrossRef]
- Merati, F.; Mehryab, F.; Mortazavi, S.A.; Haeri, A. An Experimental Design Approach for Development of Crocin-Loaded Microparticles Embedded in Gelatin/Oxidized Alginate-Based Hydrogel. J. Pharm. Innov. 2023, 18, 1812–1826. [Google Scholar] [CrossRef]
- Dłużewska, E.; Florowska, A.; Domian, E.; Wojciechowska, M.; Maszewska, M. The Influence of the Agglomeration Process on Stability of Microencapsulated β-Carotene. Int. J. Food Eng. 2020, 16, 20180310. [Google Scholar] [CrossRef]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Microencapsulation of Beta-Carotene by Complex Coacervation Using Amaranth Carboxymethyl Starch and Lactoferrin for Application in Gummy Candies. Food Hydrocoll. 2023, 139, 108488. [Google Scholar] [CrossRef]
- Perrechil, F.A.; Maximo, G.J.; Sato, A.C.K.; Cunha, R.L. Microbeads of Sodium Caseinate and κ-Carrageenan as a β-Carotene Carrier in Aqueous Systems. Food Bioprocess. Technol. 2020, 13, 661–669. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Wu, X.; Ma, D.; Huang, Y.; Zhao, Q.; Zhang, S.; Li, Y. Co-Delivery of Vitamin C and β-Carotene in W/O/W Emulsions Stabilized by Modified Aggregated Insoluble Soybean Protein Hydrolysate-Xanthan Gum Complexes. Int. J. Biol. Macromol. 2024, 261, 129855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, Q.; Zheng, J.; Xiong, H.; Zhao, L.; Xu, Y.; Bai, C. Fabricating Pectin and Chitosan Double Layer Coated Liposomes to Improve Physicochemical Stability of Beta-Carotene and Alter Its Gastrointestinal Fate. Int. J. Biol. Macromol. 2023, 247, 125780. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Pais, T.; Luchiari, A.C.; de Souza, A.M.; Medeiros, I.; Silva, M.G.F.R.; dos Santos, Y.L.; Silva-Maia, J.K.; Passos, T.S.; Morais, A.H.d.A. Assessment of Acute Toxicity of Crude Extract Rich in Carotenoids from Cantaloupe Melon (Cucumis melo L.) and the Gelatin-Based Nanoparticles Using the Zebrafish (Danio rerio) Model. Food Chem. Toxicol. 2023, 181, 114091. [Google Scholar] [CrossRef]
- Kim, D.; Jung, Y.; Rho, S.-J.; Kim, Y.-R. Improved Stability and in Vitro Bioavailability of β-Carotene in Filled Hydrogel Prepared from Starch Blends with Different Granule Sizes. Food Hydrocoll. 2023, 139, 108546. [Google Scholar] [CrossRef]
- Guo, M.; Cui, W.; Li, Y.; Fei, S.; Sun, C.; Tan, M.; Su, W. Microfluidic Fabrication of Size-Controlled Nanocarriers with Improved Stability and Biocompatibility for Astaxanthin Delivery. Food Res. Int. 2023, 170, 112958. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Baek, Y.; Yoo, H.-J.; Lee, J.-S.; Lee, H.G. Chitosan-Tripolyphosphate Nanoparticles Prepared by Ionic Gelation Improve the Antioxidant Activities of Astaxanthin in the In Vitro and In Vivo Model. Antioxidants 2022, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhou, Y.; Li, S.; Yan, J.; Chen, H.; Qin, W.; Zhang, Q. Astaxanthin Encapsulation in Soybean Protein Isolate–Sodium Alginate Complexes-Stabilized Nanoemulsions: Antioxidant Activities, Environmental Stability, and in Vitro Digestibility. J. Sci. Food Agric. 2024, 104, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, K.T.; Hwang, S.; Choi, K.Y.; Park, Y.J.; Choi, J.-H.; Truong, T.Q.; Kim, S.M. Nanoencapsulation Enhances the Bioavailability of Fucoxanthin in Microalga Phaeodactylum tricornutum Extract. Food Chem. 2023, 403, 134348. [Google Scholar] [CrossRef] [PubMed]
- İnanç Horuz, T.; Belibağlı, K.B. Nanoencapsulation by Electrospinning to Improve Stability and Water Solubility of Carotenoids Extracted from Tomato Peels. Food Chem. 2018, 268, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Shwetha, H.J.; Arathi, B.P.; Beto Mukherjee, M.; Ambedkar, R.; Shivaprasad, S.; Raichur, A.M.; Lakshminarayana, R. Zein-Alginate-Phosphatidylcholine Nanocomplex Efficiently Delivers Lycopene and Lutein over Dietary-Derived Carotenoid Mixed Micelles in Caco-2 Cells. J. Agric. Food Chem. 2022, 70, 15474–15486. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C.; et al. Cytotoxic Activity of Poly-ɛ-Caprolactone Lipid-Core Nanocapsules Loaded with Lycopene-Rich Extract from Red Guava (Psidium guajava L.) on Breast Cancer Cells. Food Res. Int. 2020, 136, 109548. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Song, J.; Chen, Y.; Feng, L.; Xu, Y.; Li, D.; Wu, C.; Zhang, Z.; Liu, J. Study on the Bioavailability of Stevioside-Encapsulized Lutein and Its Mechanism. Food Chem. 2021, 354, 129528. [Google Scholar] [CrossRef] [PubMed]
- Toragall, V.; Srirangam, P.; Jayapala, N.; Baskaran, V. Lutein Encapsulated Oleic—Linoleic Acid Nanoemulsion Boosts Oral Bioavailability of the Eye Protective Carotenoid Lutein in Rat Model. Mater. Today Commun. 2021, 28, 102522. [Google Scholar] [CrossRef]
- Saroglu, O.; Atalı, B.; Yıldırım, R.M.; Karadag, A. Characterization of Nanoliposomes Loaded with Saffron Extract: In Vitro Digestion and Release of Crocin. Food Meas. 2022, 16, 4402–4415. [Google Scholar] [CrossRef]
- Nasrpour, S.; Yousefi, G.; Niakosari, M.; Aminlari, M. Nanoencapsulation of Saffron Crocin into Chitosan/Alginate Interpolyelectrolyte Complexes for Oral Delivery: A Taguchi Approach to Design Optimization. J. Food Sci. 2022, 87, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Guo, M.; Tan, M.; Su, W. Lipid-Lowering and Antioxidant Effects of Self-Assembled Astaxanthin–Anthocyanin Nanoparticles on High-Fat Caenorhabditis Elegans. Foods 2024, 13, 514. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yuan, S.; Chen, Y.; Liu, F.; Wei, Z.; Cao, W.; Li, R.W.; Xu, J.; Xue, C.; Tang, Q. The Improvement Effect of Astaxanthin-Loaded Emulsions on Obesity Is Better than That of Astaxanthin in the Oil Phase. Food Funct. 2022, 13, 3720–3731. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Song, Y.; Su, W.; Xing, S.; Wang, H.; Tan, M. Hepatic Parenchymal Cell and Mitochondrial-Targeted Astaxanthin Nanocarriers for Relief of High Fat Diet-Induced Nonalcoholic Fatty Liver Disease. Food Funct. 2023, 14, 2908–2920. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Banik, S.; Yamada, K.; Misaka, S.; Prud’homme, R.K.; Sato, H.; Onoue, S. Stabilized Astaxanthin Nanoparticles Developed Using Flash Nanoprecipitation to Improve Oral Bioavailability and Hepatoprotective Effects. Pharmaceutics 2023, 15, 2562. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Pereira, J.M.; Marques-Oliveira, R.; Costa, I.; Gil-Martins, E.; Silva, R.; Remião, F.; Peixoto, A.F.; Sousa Lobo, J.M.; Silva, A.C. An In Vitro Evaluation of the Potential Neuroprotective Effects of Intranasal Lipid Nanoparticles Containing Astaxanthin Obtained from Different Sources: Comparative Studies. Pharmaceutics 2023, 15, 1035. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Gomes, C.; Lima, M.S.R.; de Oliveira, G.L.R.; Medeiros, I.; Xavier, H.S.T.; dos Santos Pais, T.; de Sousa Costa, I.; de Carvalho, F.M.C.; Serquiz, A.C.; de Souza Lima, M.C.J.; et al. Nanoparticles Loaded with a Carotenoid-Rich Extract from Cantaloupe Melon Improved Hepatic Retinol Levels in a Diet-Induced Obesity Preclinical Model. ACS Omega 2023, 8, 28475–28486. [Google Scholar] [CrossRef] [PubMed]
- Mahadev, M.; Dubey, A.; Shetty, A. Ultrasonically Fabricated Beta-Carotene Nanoemulsion: Optimization, Characterization and Evaluation of Combinatorial Effect with Quercetin on Streptozotocin-Induced Diabetic Rat Model. Pharmaceutics 2023, 15, 574. [Google Scholar] [CrossRef] [PubMed]
- Teixé-Roig, J.; Oms-Oliu, G.; Odriozola-Serrano, I.; Martín-Belloso, O. Enhancing in Vivo Retinol Bioavailability by Incorporating β-Carotene from Alga Dunaliella salina into Nanoemulsions Containing Natural-Based Emulsifiers. Food Res. Int. 2023, 164, 112359. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Yin, H.; Chen, L.; He, Y.; Qin, Q.; Zhou, Y.; Fan, M.; Wang, D. Exploiting Fucoxanthin Mono-Carrier Nanoparticles to Modulate Digestion and Metabolic Regulation in an Obesity Model. Food Biosci. 2024, 57, 103466. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, M.; Luo, K.; Xu, W.; Dong, J.; Wang, D.; Chen, L.; Yu, J. In Vivo Assessment of the Effects of Mono-Carrier Encapsulated Fucoxanthin Nanoparticles on Type 2 Diabetic C57 Mice and Their Oxidative Stress. Antioxidants 2022, 11, 1976. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bai, Y.; Yin, W.; Qiu, B.; Jiang, P.; Dong, X.; Qi, H. Nanoencapsulation Motivates the High Inhibitive Ability of Fucoxanthin on H2O2-Induced Human Hepatocyte Cell Line (L02) Apoptosis via Regulating Lipid Metabolism Homeostasis. J. Agric. Food Chem. 2023, 71, 6087–6098. [Google Scholar] [CrossRef] [PubMed]
- Raji, V.; Loganathan, C.; Ramesh, T.; Thayumanavan, P. Dual Antidiabetic and Antihypertensive Activity of Fucoxanthin Isolated from Sargassum wightii Greville in in Vivo Rat Model. Food Sci. Hum. Wellness 2023, 12, 1693–1700. [Google Scholar] [CrossRef]
- Fang, Y.; Nie, T.; Li, G.; Wang, L.; Du, J.; Wu, J. Multifunctional Antibiotic Hydrogel Doped with Antioxidative Lycopene-Based Liposome for Accelerative Diabetic Wound Healing. Chem. Eng. J. 2024, 480, 147930. [Google Scholar] [CrossRef]
- Ge, J.; Ye, L.; Cheng, M.; Xu, W.; Chen, Z.; Guan, F. Preparation of Microgels Loaded with Lycopene/NMN and Their Protective Mechanism against Acute Liver Injury. Food Funct. 2024, 15, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, H.; Xu, X.; Wang, Z.; Zhao, G.; Du, M. Improving Mitochondrial Function for Alleviating Memory Decline of Aging Mice via Dual-Delivering Lycopene Nanoparticles. Appl. Mater. Today 2024, 37, 102132. [Google Scholar] [CrossRef]
- Xia, X.; Li, H.; Xu, X.; Zhao, G.; Du, M. Facilitating Pro-Survival Mitophagy for Alleviating Parkinson’s Disease via Sequence-Targeted Lycopene Nanodots. ACS Nano 2023, 17, 17979–17995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, M.; Pei, Y.; Qian, K.; Xie, J.; Huang, Q.; Liu, S.; Xue, N.; Zu, Y.; Wang, H. Enhancing Stability of Liposomes Using High Molecular Weight Chitosan to Promote Antioxidative Stress Effects and Lipid-Lowering Activity of Encapsulated Lutein in Vivo and in Vitro. Int. J. Biol. Macromol. 2023, 253, 126564. [Google Scholar] [CrossRef]
- Guo, T.; Chen, L.; Li, F.; Cao, Y.; Li, D.; Xiong, Q.; Ling, Z. Biomimetic Nanoparticles Loaded Lutein Functionalized by Macrophage Membrane for Targeted Amelioration Pressure Overload-Induced Cardiac Fibrosis. Biomed. Pharmacother. 2023, 167, 115579. [Google Scholar] [CrossRef]


| Carotenoid | Source | Extraction Technique | Separation Method | Identification Approach | References |
|---|---|---|---|---|---|
| Haematococcus pluvialis | Liquid–liquid extraction | HPLC | UV-Vis spectroscopy FTIR spectroscopy | [87] | |
| Astaxanthin | Corynebacterium glutamicum | Maceration | HPLC | N.I. | [88] |
| Phaffia rhodozyma | Solid–liquid extraction | N.I. | UV-Vis spectroscopy | [89] | |
| Tisochrysis lutea | Solid–liquid US-assisted extraction | HPLC and CPC | N.I. | [90] | |
| Fucoxanthin | Undaria pinnatifida | Heat extraction | HPLC | UV-Vis spectroscopy | [91] |
| Tomato (Solanum lycopersicum) | Enzyme-assisted extraction | UHPLC | N.I. | [92] | |
| Carrot (Daucus carota) | Organic solvent extraction | N.I. | UV-Vis spectroscopy | [93] | |
| Lycopene | Elaeagnus umbellata | UAE | UHPLC | UV-Vis spectroscopy FTIR spectroscopy MS | [94] |
| Red papaya | SC-CO2 | N.I. | N.I. | [95] | |
| Pistachio waste | Soxhlet extraction | LC-MS/MS | MS | [96] | |
| Lutein | Fruit juices | Liquid–liquid extraction | HPLC | N.I. | [84] |
| Marigold flowers | Surfactant-based ATPS extraction | N.I. | UV-Vis spectroscopy | [97] | |
| Lycium barbarum | Liquid–liquid extraction | HPLC HSCCC | N.I. | [98] | |
| Zeaxanthin | Dried corn silk | Solid–liquid extraction | HPLC CC | UV-Vis spectroscopy FTIR spectroscopy NMR spectroscopy | [99] |
| Chlorella | PLE | HPLC | UV-Vis spectroscopy | [100] |
| Carotenoid | Encapsulation Technique | Raw Materials | Observed Activities | References |
|---|---|---|---|---|
| Astaxanthin | Spray drying | Gum Arabic | Increased solubility, stability, and enhanced bioavailability in simulated GIT. | [134] |
| Multilayer O/W emulsion and spray drying | ι-carrageenan, chitosan, lupin protein isolate, and sunflower oil | Augmented astaxanthin retention, storage stability, and water solubility. | [135] | |
| Ionic gelation | Low-methoxyl pectin, chitosan, and alginate | Improved particle sphericity and limited oil oxidation during formulation. Increased thermal stability and bioavailability in simulated GIT. | [132] | |
| Fucoxanthin | Spray drying and freeze drying | Maltodextrin, soy lecithin, and gum Arabic | Microcapsules exhibited high encapsulation efficiency, increased bioavailability, and ABTS•+ scavenging activity. | [136] |
| Sequential coating modification | Maltodextrin and gum Arabic | Enhanced encapsulation efficiency and fucoxanthin stability. | [137] | |
| Complex carriers | Gum Arabic, gelatin, and alginate hydrogel | In vitro increased bioavailability and fucoxanthin protection against SGF. In vivo oral administration demonstrated lowering the blood lipid and oxidative stress levels. | [138] | |
| Lycopene | Spray drying | Gum Arabic and inulin | Microcapsules demonstrated high lycopene release in simulated gastric fluid. | [139,140] |
| Ionotropic gelation | Alginate and κ-carrageenan | Microspheres exhibited in vitro α-amylase and α-glucosidase inhibitory activity. | [141] | |
| Complex coacervation and freeze drying | Whey proteins and acacia gum | Formulation increased antioxidant activity and showed in vitro α-amylase and α-glucosidase inhibitory activity. | [142] | |
| Lutein | Spray drying | Inulin and modified starch | Microencapsulated powders significantly increased lutein stability and thermal degradation resistance. | [143] |
| Citric acid-esterified potato starch and whey protein | Increased encapsulation efficiency, embedding effect, lutein aqueous solubility, and thermal resistance. | [144] | ||
| Electrostatic complexation | Sodium caseinate and sodium alginate | Reduced lutein decomposition during storage and increased FFA release and lutein bio accessibility in a simulated GIT. | [145] | |
| Crocin | Spray drying | Gelatin | Increased encapsulation efficiency and preserved crocin physicochemical properties. | [146] |
| Ionotropic gelation | Alginate | High encapsulation efficiency and fast kinetics release. | [147] | |
| Chitosan, gelatin, and oxidized alginate | Hydrogel exhibited high encapsulation efficiency, sustained crocin release, and superior mucoadhesive strength. | [148] | ||
| β-carotene | Spray drying | Gum Arabic, maltodextrin, modified starch, and whey protein | Increased β-carotene half-life and decreased the β-carotene encapsulated fraction. | [149] |
| Complex coacervation | Amaranth carboxymethyl starch and lactoferrin | Enhanced encapsulation efficiency and thermal and photolytic stability. High intestinal release in oily matrices. | [150] | |
| Ionotropic gelation | Sodium caseinate and κ-carrageenan | High encapsulation efficiency and β-carotene retention. Low viscosity. | [151] | |
| W/O/W emulsion | Aggregated insoluble soybean protein hydrolysate and xanthan gum | Increased encapsulation efficiency and good pH, ionic, and thermal stability. Enhanced bioavailability in simulated GIT. | [152] |
| Carotenoid | Encapsulation System | Raw Materials | Observed Activities | References |
|---|---|---|---|---|
| Astaxanthin | Nanocapsules | Formaldehyde and lysine | Nanocapsules reduced the production of H2O2 and maintain mitochondrial membrane potential. Nanocapsules exhibited stability against high temperatures, pH, and UV radiation. | [156] |
| NPs | Chitosan and sodium triphosphate | NPs exhibited sustained in vitro release in simulated gastric and intestinal conditions. NPs loaded with astaxanthin executed prolonged residence, time levels, and antioxidant activities in Sprague-Dawley rats. | [157] | |
| Nanoemulsion | Soybean protein isolate and sodium alginate | Nanoemulsion presented scavenging activity against H2O2 and DPPH radicals. Nanoemulsion exhibited stability upon thermal, light, storage, and gastrointestinal digestion exposure. | [158] | |
| Fucoxanthin | NPs | Alginate, casein, and chitosan | NPs improved the release of fucoxanthin under simulated gastrointestinal digestion conditions. The membrane permeability of fucoxanthin was enhanced. NPs loaded with fucoxanthin exhibited enhanced plasma levels after oral administration. | [159] |
| Nanocomplexes | Whey protein | Nanocomplexes protected fucoxanthin against UV-B radiation, heat, and pH. Enhanced ROS accumulation, caused mitochondrial damage, and regulated apoptosis. | [156] | |
| Lycopene | Nanofibers | Gelatin | Improved water solubility. Enhanced antioxidant activity during 14-day storage. | [160] |
| NPs | Alginate phosphatidylcholine | Improved lycopene bioavailability upon cellular uptake by Caco-2 cells. Increased efficiency, stability, and dispersion of lycopene. | [161] | |
| Lipid-core nanocapsules | Tween 80, span 60, poly-ɛ-caprolactone, and coconut oil | Decreased the viability of MCF-7 cells after 24 and 72 h of exposure. Inhibited the activation of NF-κB and reduced ROS production in microglial (HMC3) cells. | [162] | |
| Lutein | NPs | Stevioside | Entrapment into NPs improved the bioavailability of lutein. Lutein-loaded NPs enter cells by clathrin-mediated endocytosis. | [163] |
| Nanoemulsion | Linoleic acid, oleic acid, sodium taurocholate, and mono-oleoyl glycerol | Enhanced the aqueous solubility of lutein. Improved the tissue distribution pattern of lutein in liver and eyes of mice models. | [164] | |
| Crocin | Nanocapsules | Lecithin and chitosan | The sustained release of crocin was promoted. Encapsulation of crocin protective its integrity under in vitro digestion conditions. | [165] |
| Nanoemulsion | Chitosan and alginate | Nanoemulsion exhibited high stability under stimulated gastric conditions (pH 2) and executed sustained release of crocin. | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Regules, A.E.; Martínez-Thomas, J.A.; Schürenkämper-Carrillo, K.; de Parrodi, C.A.; López-Mena, E.R.; Mejía-Méndez, J.L.; Lozada-Ramírez, J.D. Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders. Plants 2024, 13, 1584. https://doi.org/10.3390/plants13121584
Ortega-Regules AE, Martínez-Thomas JA, Schürenkämper-Carrillo K, de Parrodi CA, López-Mena ER, Mejía-Méndez JL, Lozada-Ramírez JD. Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders. Plants. 2024; 13(12):1584. https://doi.org/10.3390/plants13121584
Chicago/Turabian StyleOrtega-Regules, Ana E., Juan Alonso Martínez-Thomas, Karen Schürenkämper-Carrillo, Cecilia Anaya de Parrodi, Edgar R. López-Mena, Jorge L. Mejía-Méndez, and J. Daniel Lozada-Ramírez. 2024. "Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders" Plants 13, no. 12: 1584. https://doi.org/10.3390/plants13121584
APA StyleOrtega-Regules, A. E., Martínez-Thomas, J. A., Schürenkämper-Carrillo, K., de Parrodi, C. A., López-Mena, E. R., Mejía-Méndez, J. L., & Lozada-Ramírez, J. D. (2024). Recent Advances in the Therapeutic Potential of Carotenoids in Preventing and Managing Metabolic Disorders. Plants, 13(12), 1584. https://doi.org/10.3390/plants13121584






