Integrated Metabolomics and Transcriptomics Analysis Reveals New Insights into Triterpene Biosynthesis in Rosa rugosa
Abstract
:1. Introduction
2. Results
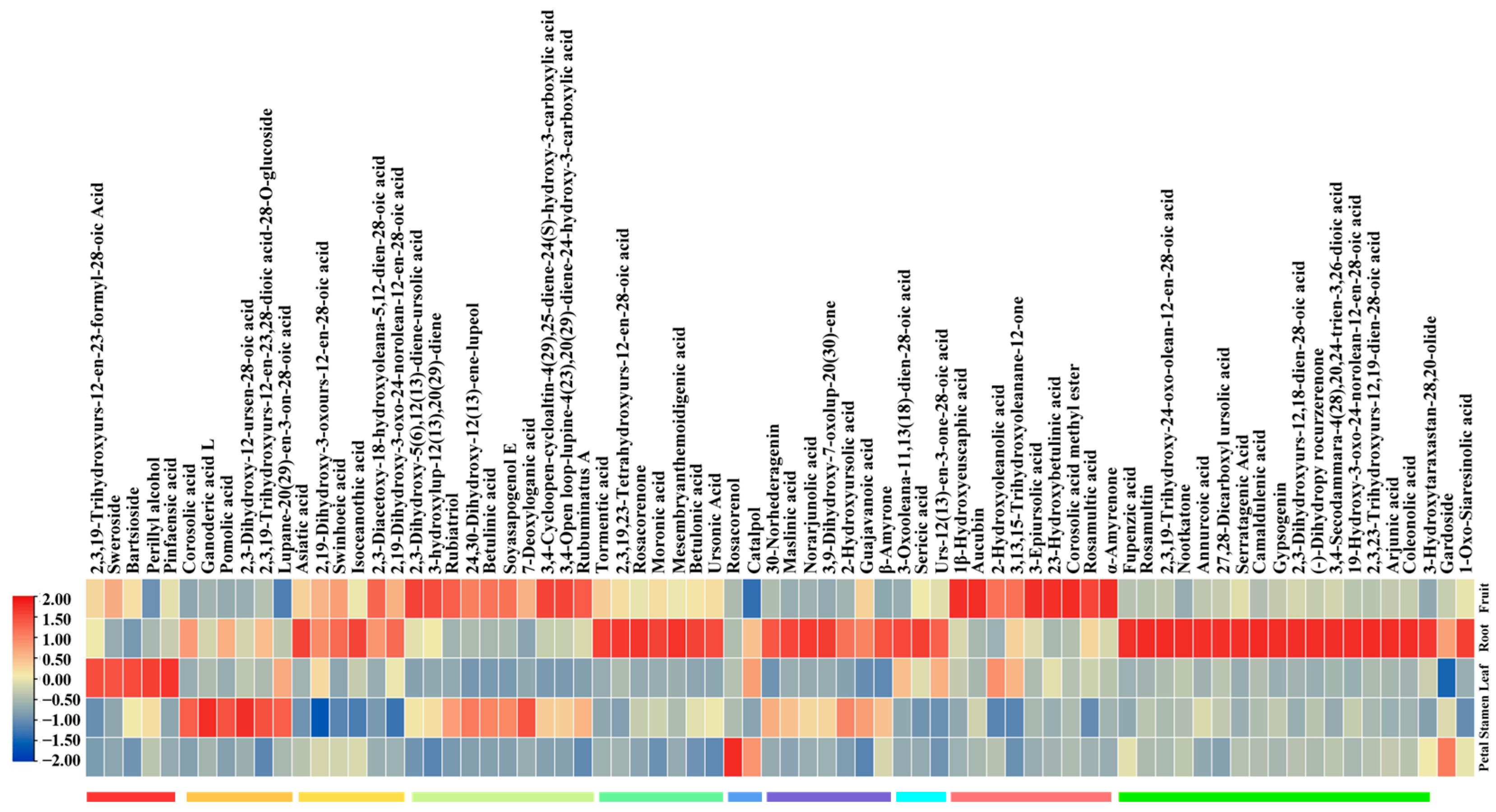
2.1. The Accumulation of Non-Volatile Terpenoids across Various Tissues of R. rugosa
2.2. Transcriptome Profiling across Different Tissues of R. rugosa
2.3. Integrative Analysis of Transcriptome and Metabolome
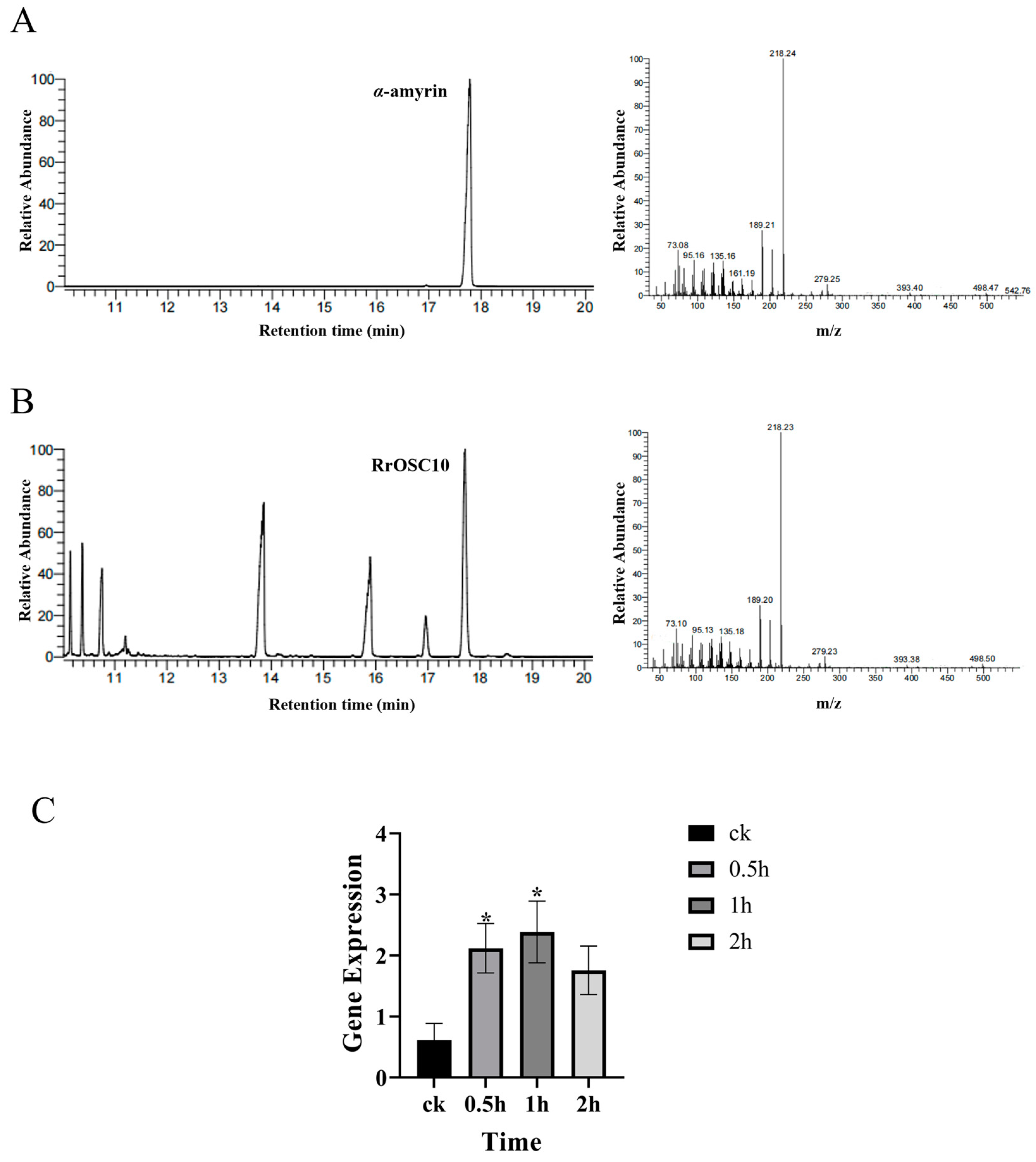
2.4. Characterization of RrOSC10
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Metabolite Extraction and Identification
4.3. Screening for Differential Metabolites
4.4. Transcriptome Analysis
4.5. Identification of Differentially Expressed Genes
4.6. Integrated Analysis of Transcriptome and Metabolome
4.7. Enzymatic Assays in Yeast
4.8. Salt Treatment and Quantitative Real-Time PCR (RT-qPCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yasuyuki, H. The phytochemistry of Rosa rugosa. Phytochemistry 1996, 43, 535–549. [Google Scholar] [CrossRef]
- Wenzig, E.M.; Widowitz, U.; Kunert, O.; Chrubasik, S.; Bucar, F.; Knauder, E.; Bauer, R. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008, 15, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Ochir, S.; Ishii, K.; Park, B.; Matsuta, T.; Nishizawa, M.; Kanazawa, T.; Funaki, M.; Yamagishi, T. Botanical origin of mei-gui hua (petal of a Rosa species). J. Nat. Med. 2010, 64, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Olech, M.; Pecio, L.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food Agric. 2014, 94, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Ścibisz, I.; Kieliszek, M.; Kolniak-Ostek, J.; Mitek, M. UPLC-PDA-Q/TOF-MS profile of polyphenolic compounds of liqueurs from rose petals (Rosa rugosa). Molecules 2017, 22, 1832. [Google Scholar] [CrossRef]
- Cheng, X.; Feng, Y.; Chen, D.; Luo, C.; Yu, X.; Huang, C. Evaluation of Rosa germplasm resources and analysis of floral fragrance components in R. rugosa. Front. Plant Sci. 2022, 13, 1026763. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Beneficial medicinal effects and material applications of rose. Heliyon 2024, 10, e23530. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Yang, Y.; Bakri, M.; Chen, Q.; Xin, X.; Aisa, H.A. A LC/QTOF-MS/MS application to investigate chemical compositions in a fraction with protein tyrosine phosphatase 1B inhibitory activity from Rosa rugosa flowers. Phytochem. Anal. PCA 2013, 24, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Magnard, J.L.; Roccia, A.; Caissard, J.C.; Vergne, P.; Sun, P.; Hecquet, R.; Dubois, A.; Hibrand-Saint Oyant, L.; Jullien, F.; Nicolè, F.; et al. PLANT VOLATILES. Biosynthesis of monoterpene scent compounds in roses. Science 2015, 349, 81–83. [Google Scholar] [CrossRef]
- Conart, C.; Bomzan, D.P.; Huang, X.Q.; Bassard, J.E.; Paramita, S.N.; Saint-Marcoux, D.; Rius-Bony, A.; Hivert, G.; Anchisi, A.; Schaller, H.; et al. A cytosolic bifunctional geranyl/farnesyl diphosphate synthase provides MVA-derived GPP for geraniol biosynthesis in rose flowers. Proc. Natl. Acad. Sci. USA 2023, 120, e2221440120. [Google Scholar] [CrossRef]
- Conart, C.; Saclier, N.; Foucher, F.; Goubert, C.; Rius-Bony, A.; Paramita, S.N.; Moja, S.; Thouroude, T.; Douady, C.; Sun, P.; et al. Duplication and specialization of NUDX1 in Rosaceae led to geraniol production in rose petals. Mol. Biol. Evol. 2022, 39, msac002. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Dégut, C.; Réty, S.; Caissard, J.C.; Hibrand-Saint Oyant, L.; Bony, A.; Paramita, S.N.; Conart, C.; Magnard, J.L.; Jeauffre, J.; et al. Functional diversification in the Nudix hydrolase gene family drives sesquiterpene biosynthesis in Rosa × wichurana. Plant J. 2020, 104, 185–199. [Google Scholar] [CrossRef]
- Jian, C.X.; Li, M.Z.; Zheng, W.Y.; He, Y.; Ren, Y.; Wu, Z.M.; Fan, Q.S.; Hu, Y.H.; Li, C.J. Tormentic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts via inhibition of TLR4-mediated NF-κB and MAPK signalling pathway. Arch. Oral Biol. 2015, 60, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, Y.J.; Jang, H.J.; Lee, S.J.; Lee, S.; Yun, B.S.; Lee, S.W.; Rho, M.C. Rugosic acid A, derived from Rosa rugosa Thunb., is novel inhibitory agent for NF-κB and IL-6/STAT3 axis in acute lung injury model. Phytother. Res. PTR 2020, 34, 3200–3210. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W. Structural and chemical biology of terpenoid cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Pichersky, E. More is better: The diversity of terpene metabolism in plants. Curr. Opin. Plant Biol. 2020, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, J.; Ma, Y.; Yuan, W.; Zhang, P.; Wang, G. Occurrence and biosynthesis of plant sesterterpenes (C25), a new addition to terpene diversity. Plant Commun. 2021, 2, 100184. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Haralampidis, K.; Trojanowska, M.; Osbourn, A.E. Biosynthesis of triterpenoid saponins in plants. Adv. Biochem. Eng. Biotechnol. 2002, 75, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.; Goss, R.J.; Field, R.A. The saponins: Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef]
- Abe, I. Enzymatic synthesis of cyclic triterpenes. Nat. Prod. Rep. 2007, 24, 1311–1331. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Duan, L.; Liu, D.; Guo, J.; Ge, S.; Dicks, J.; ÓMáille, P.; Osbourn, A.; Qi, X. Divergent evolution of oxidosqualene cyclases in plants. New Phytol. 2012, 193, 1022–1038. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, L.; Song, W.; Li, M.; Hua, X.; Wang, Y.; Yuan, J.; Xue, Z. Natural products of pentacyclic triterpenoids: From discovery to heterologous biosynthesis. Nat. Prod. Rep. 2023, 40, 1303–1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.; Yin, X.; Wang, X.; Qi, X.; Xue, Z. Diverse triterpene skeletons are derived from the expansion and divergent evolution of 2,3-oxidosqualene cyclases in plants. Crit. Rev. Biochem. Mol. Biol. 2022, 57, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Zhang, F.; Xu, J.; Wang, X.; Wu, R.; Xue, Z. A conserved mechanism affecting hydride shifting and deprotonation in the synthesis of hopane triterpenes as compositions of wax in oat. Proc. Natl. Acad. Sci. USA 2022, 119, e2118709119. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Xu, M.; Chen, Y.; Feng, L. Systematic identification and analysis of OSC gene family of Rosa rugosa Thunb. Int. J. Mol. Sci. 2022, 23, 13884. [Google Scholar] [CrossRef]
- Olech, M.; Ziemichód, W.; Nowacka-Jechalke, N. The occurrence and biological activity of tormentic acid—A review. Molecules 2021, 26, 3797. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Ohta, D. Diversification of P450 genes during land plant evolution. Annu. Rev. Plant Biol. 2010, 61, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Legay, S.; Deleruelle, A.; Nieuwenhuizen, N.; Punter, M.; Brendolise, C.; Cooney, J.M.; Lateur, M.; Hausman, J.F.; Larondelle, Y.; et al. Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids. New Phytol. 2016, 211, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Sandeep; Misra, R.C.; Chanotiya, C.S.; Mukhopadhyay, P.; Ghosh, S. Oxidosqualene cyclase and CYP716 enzymes contribute to triterpene structural diversity in the medicinal tree banaba. New Phytol. 2019, 222, 408–424. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Jing, Y.; Lin, S.; Yue, Z.; Yang, X.; Xu, J.; Wu, J.; Zhang, Z.; Xia, R.; Zhu, J.; et al. Polyploidy underlies co-option and diversification of biosynthetic triterpene pathways in the apple tribe. Proc. Natl. Acad. Sci. USA 2021, 118, e2101767118. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Chang, J.; Wu, M.; Xu, Z.; Zhang, F.; Yang, M.; Xu, H.; Wang, L.J.; Chen, X.Y.; Wu, S. Mediation of JA signalling in glandular trichomes by the woolly/SlMYC1 regulatory module improves pest resistance in tomato. Plant Biotechnol. J. 2021, 19, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Idle, J.R.; Gonzalez, F.J. Metabolomics. Cell Metab. 2007, 6, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Afendi, F.M.; Altaf-Ul-Amin, M.; Takahashi, H.; Nakamura, K.; Kanaya, S. Metabolomics of medicinal plants: The importance of multivariate analysis of analytical chemistry data. Curr. Comput. Aided. Drug Des. 2010, 6, 179–196. [Google Scholar] [CrossRef]
- Wei, G.; Tian, P.; Zhang, F.; Qin, H.; Miao, H.; Chen, Q.; Hu, Z.; Cao, L.; Wang, M.; Gu, X.; et al. Integrative analyses of nontargeted volatile profiling and transcriptome data provide molecular insight into VOC diversity in cucumber plants (Cucumis sativus). Plant Physiol. 2016, 172, 603–618. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C.; Maáñez, S.; Giner, R.M. Natural triterpenoids as anti-inflammatory agents. In Bioactive Natural Products (Part C); Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 22, pp. 93–143. [Google Scholar]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and aromatic meroterpenoids with antioxidant activity and neuroprotective effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Huang, M.Q.; Bao, J.L.; Chen, X.P.; Wang, Y.T. Terpenoids: Natural products for cancer therapy. Expert Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.L.; Han, F.; Luan, S.S.; Ai, R.; Zhang, P.; Li, H.; Chen, L.X. Triterpenoids from Ganoderma lucidum and their potential anti-inflammatory effects. J. Agric. Food Chem. 2019, 67, 5147–5158. [Google Scholar] [CrossRef] [PubMed]
- Thoppil, R.J.; Bishayee, A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef]
- Bui, T.T.; Nguyen, T.H. Natural product for the treatment of Alzheimer’s disease. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Kong, M.; Du, D.; Ai, F.; Li, J.; Li, Y. Swinhoeic acid from Potentilla fragarioides ameliorates high glucose-induced oxidative stress and accumulation of ECM in mesangial cells via Keap1-dependent activation of Nrf2. Redox. Rep. 2022, 27, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Komissarova, N.G.; Dubovitskii, S.N.; Shitikova, O.V.; Orlov, A.V. Synthesis of 2-aminoethanesulfonamides of betulinic and betulonic acids. Chem. Nat. Compd. 2021, 57, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Dashbaldan, S.; Rogowska, A.; Pączkowski, C.; Szakiel, A. Distribution of triterpenoids and steroids in developing Rugosa Rose (Rosarugosa Thunb.) accessory fruit. Molecules 2021, 26, 5158. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.; Jo, S.H.; Hung, T.M.; Cuong, N.X.; Nam, N.H.; Kwon, Y.I.; Minh, C.V.; Kim, Y.H. Triterpenoid saponins from the roots of Rosa rugosa Thunb. as rat intestinal sucrase inhibitors. Arch Pharm. Res. 2014, 37, 1280–1285. [Google Scholar] [CrossRef]
- Matsuda, F.; Hirai, M.Y.; Sasaki, E.; Akiyama, K.; Yonekura-Sakakibara, K.; Provart, N.J.; Sakurai, T.; Shimada, Y.; Saito, K. AtMetExpress development: A phytochemical atlas of Arabidopsis development. Plant Physiol. 2009, 152, 566–578. [Google Scholar] [CrossRef]
- Yu, Y.; Chang, P.; Yu, H.; Ren, H.; Hong, D.; Li, Z.; Wang, Y.; Song, H.; Huo, Y.; Li, C. Productive amyrin synthases for efficient α-amyrin synthesis in engineered Saccharomyces cerevisiae. ACS Synth. Biol. 2018, 7, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Bakht, S.; Leggett, M.; Maxwell, C.; Melton, R.; Osbourn, A. A gene cluster for secondary metabolism in oat: Implications for the evolution of metabolic diversity in plants. Proc. Natl. Acad. Sci. USA 2004, 101, 8233–8238. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Xu, X.; Zhou, Y.; Wang, X.; Zhang, Y.; Liu, D.; Zhao, B.; Duan, L.; Qi, X. Deficiency of a triterpene pathway results in humidity-sensitive genic male sterility in rice. Nat. Commun. 2018, 9, 604. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Pollier, J.; Shen, Q.; Soetaert, S.; Reed, J.; Erffelinck, M.L.; Van Nieuwerburgh, F.C.; Vanden Bossche, R.; Osbourn, A.; Thevelein, J.M.; et al. OSC2 and CYP716A14v2 catalyze the biosynthesis of triterpenoids for the cuticle of aerial organs of Artemisia annua. Plant Cell 2015, 27, 286–301. [Google Scholar] [CrossRef] [PubMed]
- Jenifer Joseph, B.; Luigi, L.; Saranya, J.; Ajay, P. Metabolomic insights into the mechanisms underlying tolerance to salinity in different halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Yano, R.; Takagi, K.; Takada, Y.; Mukaiyama, K.; Tsukamoto, C.; Sayama, T.; Kaga, A.; Anai, T.; Sawai, S.; Ohyama, K.; et al. Metabolic switching of astringent and beneficial triterpenoid saponins in soybean is achieved by a loss-of-function mutation in cytochrome P450 72A69. Plant J. 2017, 89, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Kim, J.; Mijakovic, I.; Jung, K.H.; Choi, G.; Kim, S.C.; Kim, Y.J. Triterpenoid-biosynthetic UDP-glycosyltransferases from plants. Biotechnol. Adv. 2019, 37, 107394. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, K.; Pollier, J.; Buyst, D.; Arendt, P.; Csuk, R.; Sommerwerk, S.; Moses, T.; Mertens, J.; Sonawane, P.D.; Pauwels, L.; et al. The ancient CYP716 family is a major contributor to the diversification of eudicot triterpenoid biosynthesis. Nat. Commun. 2017, 8, 14153. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Wu, J.; Han, X.; Bian, X.; Xu, Z.; Sun, C.; Wang, R.; Zhang, W.; Liang, F.; Zhang, H.; et al. Auxin homeostasis is maintained by sly-miR167-SlARF8A/B-SlGH3.4 feedback module in the development of locular and placental tissues of tomato fruits. New Phytol. 2024, 241, 1177–1192. [Google Scholar] [CrossRef]
- Wei, G.; Chen, Y.; Wang, J.; Feng, L. Molecular cloning and characterization of farnesyl diphosphate synthase from Rosa rugosa Thunb associated with salinity stress. PeerJ 2024, 12, e16929. [Google Scholar] [CrossRef]




| Sample | Raw Reads (bp) | Clean Reads (bp) | Clean Base (Gb) | Error Rate (%) | Q20 (%) | Q30 (%) | GC Content (%) |
|---|---|---|---|---|---|---|---|
| P | 83,693,494 | 81,511,490 | 12.23 | 0.03 | 97.78 | 93.58 | 46.52 |
| L | 55,959,736 | 54,116,102 | 8.12 | 0.03 | 97.90 | 93.86 | 47.46 |
| S | 66,390,516 | 64,241,230 | 9.64 | 0.03 | 97.71 | 93.43 | 47.34 |
| R | 66,390,338 | 64,211,898 | 9.63 | 0.03 | 97.85 | 93.72 | 46.91 |
| F | 63,512,398 | 60,685,890 | 9.10 | 0.03 | 97.83 | 93.73 | 47.17 |
| Average | 67,189,296 | 64,953,322 | 9.744 | 0.03 | 97.81 | 93.66 | 47.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Xu, Y.; Wang, P.; Hussain, H.; Chen, Y.; Shi, Y.; Zhu, K.; Bai, M.; Xu, Y.; Wang, J.; et al. Integrated Metabolomics and Transcriptomics Analysis Reveals New Insights into Triterpene Biosynthesis in Rosa rugosa. Plants 2024, 13, 1600. https://doi.org/10.3390/plants13121600
Wei G, Xu Y, Wang P, Hussain H, Chen Y, Shi Y, Zhu K, Bai M, Xu Y, Wang J, et al. Integrated Metabolomics and Transcriptomics Analysis Reveals New Insights into Triterpene Biosynthesis in Rosa rugosa. Plants. 2024; 13(12):1600. https://doi.org/10.3390/plants13121600
Chicago/Turabian StyleWei, Guo, Yang Xu, Pengqing Wang, Hammad Hussain, Yudie Chen, Yuqing Shi, Kaikai Zhu, Mengjuan Bai, Yong Xu, Jianwen Wang, and et al. 2024. "Integrated Metabolomics and Transcriptomics Analysis Reveals New Insights into Triterpene Biosynthesis in Rosa rugosa" Plants 13, no. 12: 1600. https://doi.org/10.3390/plants13121600






