The Physiological Response Mechanism of Peanut Leaves under Al Stress
Abstract
:1. Introduction
2. Results
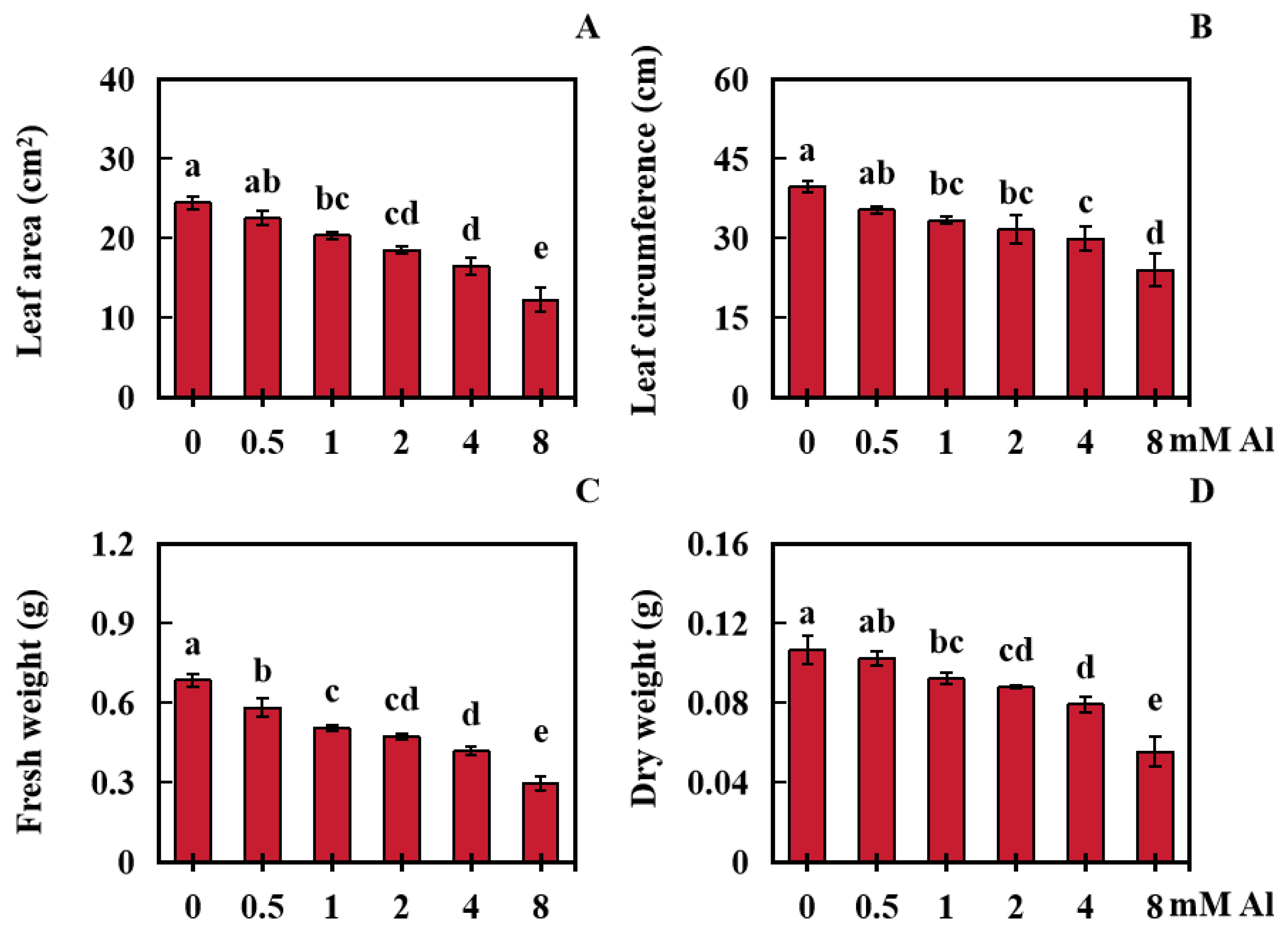
2.1. Influences of Al Toxicity Stress on Peanut Leaf Growth
2.2. Influences of Al Poisoning Stress on Chlorophyll and Total Carotenoid Contents in Peanut Leaves
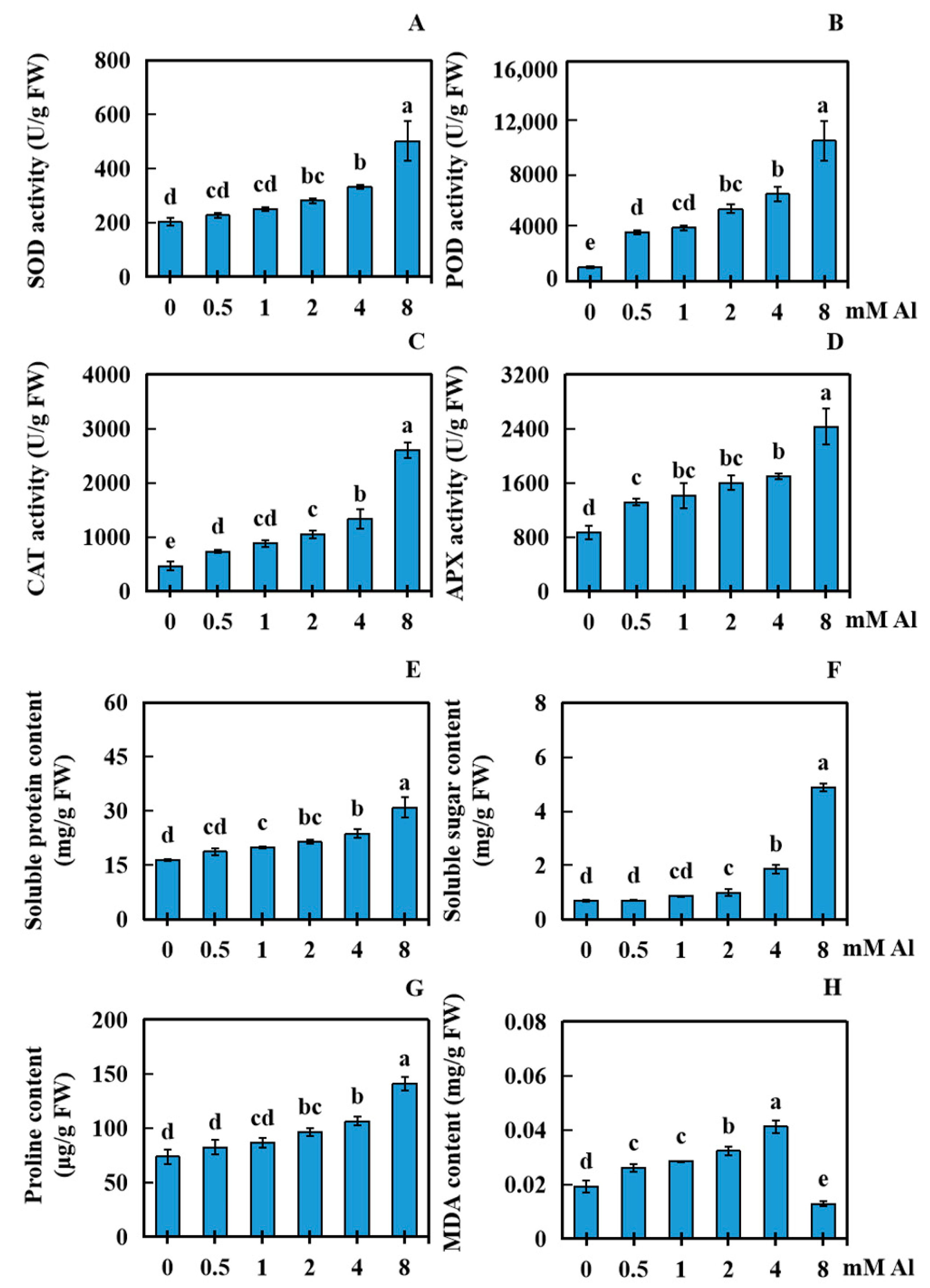
2.3. Effects of Al Poisoning Stress on Various Physiological Parameters of Peanut Leaves
2.4. Effects of Al Toxicity Stress on the Absorption of Ten Elements in Peanut Leaves
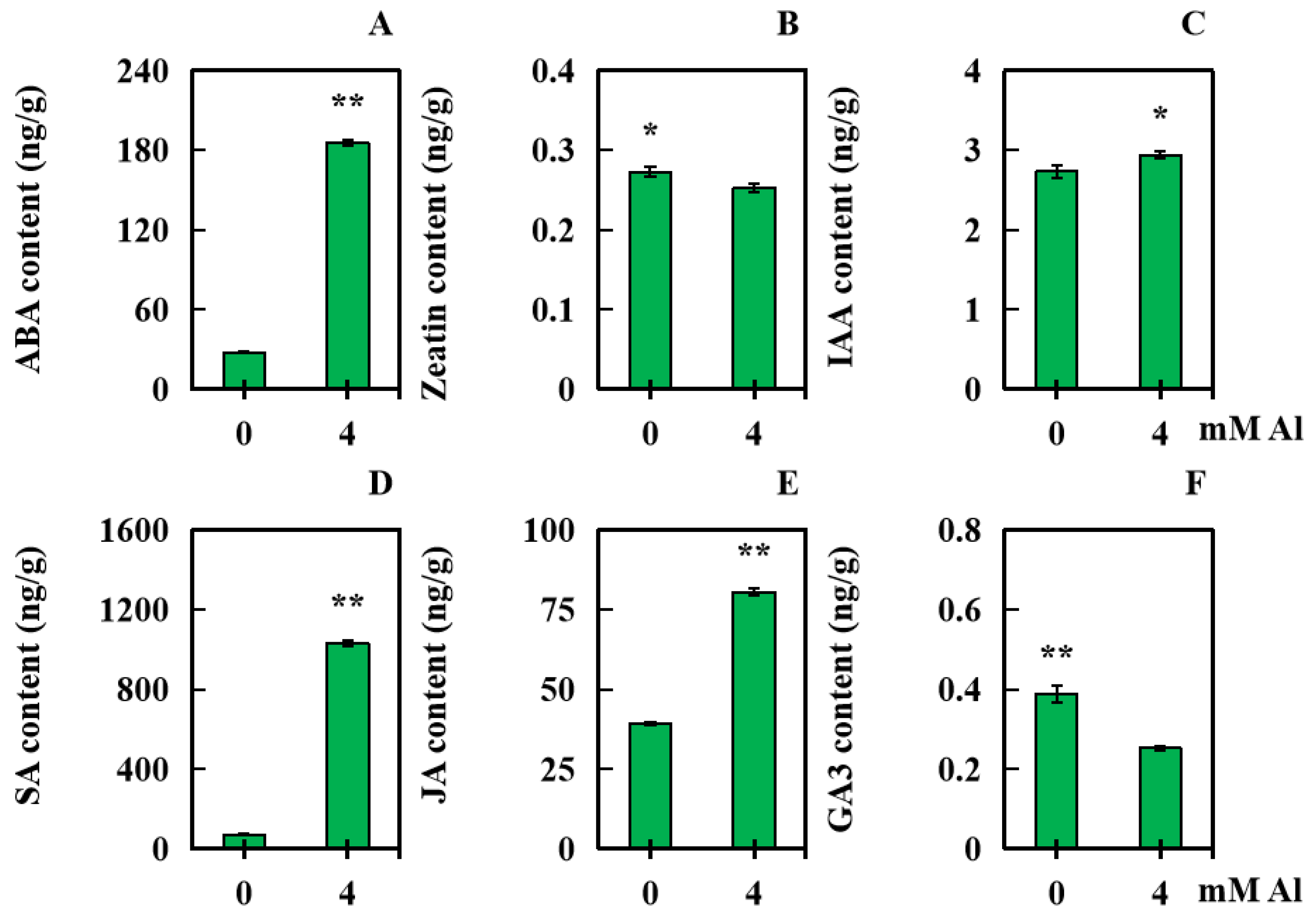
2.5. Influences of Al Poisoning Stress on Six Kinds of Hormone Levels in Peanut Leaves
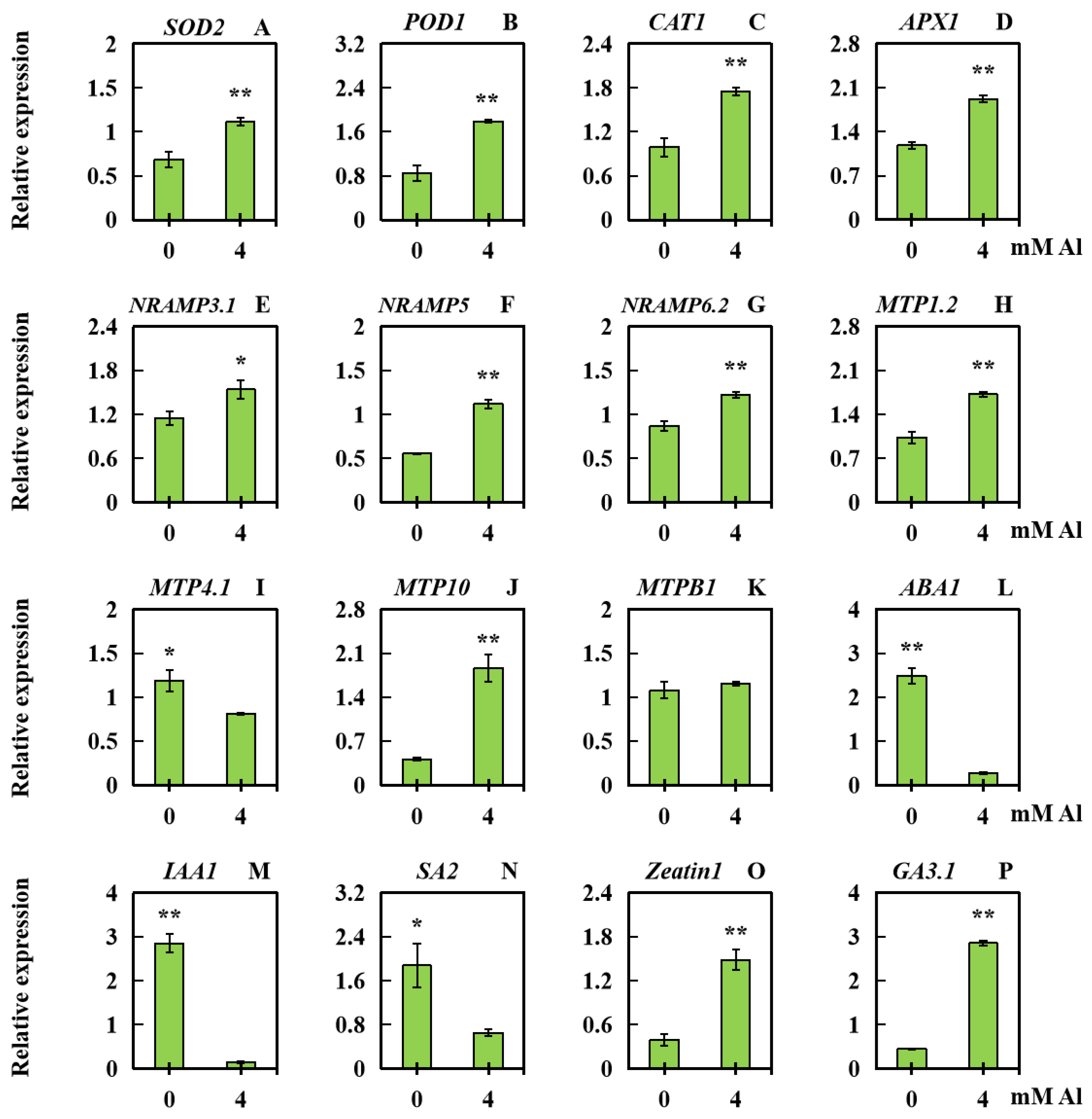
2.6. Analysis of Related Gene Expression Levels in Peanut Leaves Subjected to Al Poisoning Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Disposal
4.2. Measurement of the Dry and Fresh Weights of Peanut Leaves
4.3. Determination of the Peanut Leaf Morphology Index
4.4. Determination of Chlorophyll Content
4.5. Determination of the Physiological Response Indices of Peanut Leaves
4.6. Determination of Al, Na, K, Mg, Mn, Fe, Cu, Zn, Ca, and Se Contents in Peanut Leaves
4.7. Determination of the Levels of Six Phytohormones in Peanut Leaves
4.8. qPCR (Quantitative RT‒PCR) Fluorescence Analysis
4.9. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, S.Q.; Li, X.X.; Luo, J.; Han, R.F.; Chen, Q.Q.; Shen, D.S.; Shentu, J.L. Soil heterogeneity influence on the distribution of heavy metals in soil during acid rain infiltration: Experimental and numerical modeling. J. Environ. Manag. 2022, 322, 116144. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 475–490. [Google Scholar]
- Von Uexküll, H.R.; Mutert, E. Global extent, development and economicimpact of acid soils. Plant Soil. 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Han, T.F.; Liu, K.L.; Huang, J.; Ma, C.B.; Zheng, L.; Wang, H.Y.; Qu, X.L.; Ren, Y.; Yu, Z.K.; Zhang, H.M. Spatio-temporal evolution of soil pH and its driving factors in the main Chinese farmland during past 30 years. J. Plant Nutr. Fertil. 2020, 26, 2137–2149. [Google Scholar]
- Shen, R.F.; Zhao, X.Q. Sustainable utilization of acid soil. J. Agric. 2019, 9, 16–20. (In Chinese) [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Hou, C.C.; Chen, L.Y.; Kaskel, S.; Xu, Q. Rechargeable Al-ion batteries. EnergyChem 2021, 3, 100049. [Google Scholar] [CrossRef]
- Huang, W.J.; Yang, X.D.; Yao, S.C.; LwinOo, T.; He, H.Y.; Wang, A.Q.; Li, C.Z.; He, L.F. Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiol. Biochem. 2014, 82, 76–84. [Google Scholar] [CrossRef]
- Mohanty, S.; Das, A.B.; Das, P.; Mohanty, P. Effect of a low dose of aluminum on mitotic and meiotic activity, 4C DNA content, and pollen sterility in rice, Oryza sativa L. cv. Lalat. Ecotoxicol. Environ. Saf. 2004, 59, 70–75. [Google Scholar] [CrossRef]
- Xu, S.S.; Wu, L.H.; Li, L.Y.; Zhong, M.H.; Tang, Y.; Cao, G.Q.; Lin, K.M.; Ye, Y.Q. Aluminum-induced alterations to the cell wall and antioxidant enzymes involved in the regulation of the aluminum tolerance of Chinese Fir (Cunninghamia lanceolata). Front. Plant Sci. 2022, 13, 891117. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, M.; Zhang, F.; Feng, D.; Yang, S.; Xue, Y.; Liu, Y. Physiological mechanism through which al toxicity inhibits peanut root growth. Plants 2024, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.A.; Barros, J.A.S.; Martins, S.C.V.; Ribeiro, D.M.; DaMatta, F.M.; Arau’jo, W.L. Metabolic and physiological adjustments of maize leaves in response to aluminum stress. Theor. Exp. Plant Physiol. 2020, 32, 133–145. [Google Scholar] [CrossRef]
- Dar, F.A.; Tahir, I.; Rehman, R.U.; Alharby, H.F.; Alzahrani, Y.; Alsamadany, H.; Hakeem, K.R. Exogenous silicon alleviates aluminum phytotoxicity in Fagopyrum esculentum Moench by modulating physiological and antioxidant responses. S. Afr. J. Bot. 2024, 167, 367–384. [Google Scholar] [CrossRef]
- Donnelly, C.P.; Sousa, A.D.; Cuypers, B.; Laukens, K.; Al-Huqail, A.A.; Asard, H.; Beemster, G.T.S.; AbdElgawad, H. Malate production, sugar metabolism, and redox homeostasis in the leaf growth zone of Rye (Secale cereale) increase stress tolerance to aluminum stress: A biochemical and genome-wide transcriptional study. J. Hazard. Mater. 2023, 464, 132956. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, A.; AbdElgawad, H.; Fidalgo, F.; Teixeira, J.; Matos, M.; Tamagnini, P.; Fernandes, R.; Figueiredo, F.; Azenha, M.; Teles, L.O.; et al. Subcellular compartmentalization of aluminum reduced its hazardous impact on rye photosynthesis. Environ. Pollut. 2022, 315, 120313. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.H.; DaMatta, F.M.; Cambraia, J. Responses of thephotosynthetic apparatus to aluminum stress in two sorghum cultivars. J. Plant Nutr. 2002, 25, 821–832. [Google Scholar] [CrossRef]
- Wang, L.; Fan, X.W.; Pan, J.L.; Huang, Z.B.; Li, Y.Z. Physiological characterization of maize tolerance to low dose of aluminum, highlighted by promoted leaf growth. Planta 2015, 242, 1391–1403. [Google Scholar] [CrossRef]
- Daspute, A.A.; Sadhukhan, A.; Tokizawa, M.; Kobayashi, Y.; Panda, S.K.; Koyama, H. Transcriptional regulation of aluminum-tolerance genes in higher plants: Clarifying the underlying molecular mechanisms. Front. Plant Sci. 2017, 8, 1358. [Google Scholar] [CrossRef]
- Delhaize, E.; Ma, J.F.; Ryan, P.R. Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012, 17, 341–348. [Google Scholar] [CrossRef]
- Huang, D.; Gong, Z.; Chen, X.; Wang, H.; Tan, R.; Mao, Y. Transcriptomic responses to aluminum stress in tea plant leaves. Sci. Rep. 2021, 11, 5800. [Google Scholar] [CrossRef] [PubMed]
- Mattiello, L.; Begcy, K.; da Silva, F.R.; Jorge, R.A.; Menossi, M. Transcriptome analysis highlights changes in the leaves of maize plants cultivated in acidic soil containing toxic levels of Al(3+). Mol. Biol. Rep. 2014, 41, 8107–8116. [Google Scholar] [CrossRef]
- Liao, B.S. Current situation and potential analysis of peanut production in China. Chin. J. Oil Crop Sci. 2020, 42, 161–166. (In Chinese) [Google Scholar]
- Yan, M.L.; Ge, W.W.; Zhang, X.; Huang, Y.N.; Zhang, Z.D.; Zhang, Y. The situation analysis and development of oil industry in China countermeasures. China Oils Fats 2023, 48, 8–18. (In Chinese) [Google Scholar]
- Xiao, D.; Li, X.; Zhou, Y.Y.; Wei, L.; Keovongkod, C.; He, H.Y.; Zhan, J.; Wang, A.Q.; He, L.F. Transcriptome analysis reveals significant difference in gene expression and pathways between two peanut cultivars under Al stress. Gene 2021, 781, 145535. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, J.; Fukui, K.; Seto, Y.; Takaoka, Y.; Okamoto, M. Ligand–receptor interactions in plant hormone signaling. Plant J. 2021, 105, 290–306. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Riaz, M.; Li, S.; Cheng, J.; Jiang, C.C. Harnessing the power of exogenous factors to enhance plant resistance to aluminum toxicity; a critical review. Plant Physiol. Biochem. 2023, 203, 108064. [Google Scholar] [CrossRef]
- Zluhan-Martínez, E.; López-Ruíz, B.A.; García-Gómez, M.L.; García-Ponce, B.; Sánchez, M.D.L.P.; Álvarez-Buylla, E.R.; GarayArroyo, A. Integrative roles of phytohormones on cell proliferation, elongation and differentiation in the Arabidopsis thaliana primary root. Front. Plant Sci. 2021, 12, 659155. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, P.; Shao, W.; An, G.; Chen, J.; Yu, C.; Kuang, H. Up-regulation of LsKN1 promotes cytokinin and suppresses gibberellin biosynthesis to generate wavy leaves in lettuce. J. Exp. Bot. 2022, 73, 6615–6629. [Google Scholar] [CrossRef]
- Silva, G.S.; Gavassi, M.A.; Carvalho, B.M.D.O.; Habermann, G. High abscisic acid and low root hydraulic conductivity may explain low leaf hydration in ‘Mandarin’ lime exposed to aluminum. Tree Physiol. 2022, 43, 404–417. [Google Scholar] [CrossRef]
- Beyer, S.F.; Bel, P.S.; Flors, V.; Schultheiss, H.; Conrath, U.; Langenbach, C.J.G. Disclosure of salicylic acid and jasmonic acid-responsive genes provides a molecular tool for deciphering stress responses in soybean. Sci. Rep. 2021, 11, 20600. [Google Scholar] [CrossRef]
- Qi, T.; Yang, W.; Hassan, M.J.; Liu, J.; Yang, Y.; Zhou, Q.; Li, H.; Peng, Y. Genome-wide identification of Aux/IAA gene family in white clover (Trifolium repens L.) and functional verification of TrIAA18 under different abiotic stress. BMC Plant Biol. 2024, 24, 346. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.J.; Li, J.X.; Guan, J.H.; Wang, C.H.; Zhang, Z.; Shi, G.R. Genome-wide identification and expression analysis reveals roles of the NRAMP gene family in iron/cadmium interactions in peanut. Int. J. Mol. Sci. 2023, 24, 1713. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Han, P.P.; Chen, L.Y.; Walk, T.C.; Li, Y.S.; Hu, X.J.; Xie, L.H.; Liao, H.; Liao, X. Genome-wide identification and expression analysis of NRAMP family genes in soybean (Glycine max L.). Front. Plant Sci. 2017, 8, 1436. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Wang, C.H.; Zhang, Z.; Shi, G.R. Genome-wide identification of metal tolerance protein genes in peanut: Differential expression in the root of two contrasting cultivars under metal stresses. Front. Plant Sci. 2022, 13, 791200. [Google Scholar] [CrossRef] [PubMed]
- El-Sappah, A.H.; Abbas, M.; Rather, S.A.; Wani, S.H.; Soaud, N.; Noor, Z.; Huang, Q.L.; Eldomiaty, A.S.; Mir, R.R.; Li, J. Genomewide identification and expression analysis of metal tolerance protein (MTP) gene family in soybean (Glycine max) under heavy metal stress. Mol. Biol. Rep. 2023, 50, 2975–2990. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Zvobgo, G.; Zhang, G.P. A review: The beneficial effects and possible mechanisms of aluminum on plant growth in acidic soil. J. Integr. Agric. 2019, 18, 1518–1528. [Google Scholar] [CrossRef]
- Su, C.; Jiang, Y.; Yang, Y.; Zhang, W.; Xu, Q. Responses of duckweed (Lemna minor L.) to aluminum stress: Physiological and proteomics analyses. Ecotoxicol. Environ. Saf. 2019, 170, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.K.; Yadav, V.; Vaculík, M.; Gassmann, W.; Pike, S.; Arif, N.; Singh, V.P.; Deshmukh, R.; Sahi, S.; Tripathi, D.K. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit. Rev. Biotechnol. 2021, 41, 715–730. [Google Scholar] [CrossRef]
- Xu, Z.; Ge, Y.; Zhang, W.; Zhao, Y.; Yang, G. The walnut JrVHAG1 gene is involved in cadmium stress response through ABA-signal pathway and MYB transcription regulation. BMC Plant Biol. 2018, 18, 19. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Xu, Z.; Huang, H.; Zhou, J.; Yang, G. Morphological and physiological changes of broussonetia papyrifera seedlings in cadmium contaminated soil. Plants 2020, 9, 1698. [Google Scholar] [CrossRef] [PubMed]
- Zhenggang, X.; Li, F.; Mengxi, Z.; Yunlin, Z.; Huimin, H.; Guiyan, Y. Physiological dynamics as indicators of plant response to manganese binary effect. Front. Plant Sci. 2023, 14, 1145427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Chen, Z.; Yang, X.; Sheng, L.; Mao, H.; Zhu, S. Integrated transcriptomics and metabolomics reveal key metabolic pathway responses in Pistia stratiotes under Cd stress. J. Hazard. Mater. 2023, 452, 131214. [Google Scholar]
- Ribeiro, C.; de Marcos Lapaz, A.; de Freitas-Silva, L.; Ribeiro, K.V.G.; Yoshida, C.H.P.; Dal-Bianco, M.; Cambraia, J. Aluminum promotes changes in rice root structure and ascorbate and glutathione metabolism. Physiol. Mol. Biol. Plants. 2022, 28, 2085–2098. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Monde, R.A.; Last, R.L. Superoxide dismutase in Arabidopsis: An eclectic enzyme family with disparate regulation and protein localization. Plant Physiol. 1998, 118, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Piñeros, M.A.; Kochian, L.V. The role of aluminum sensing and signaling in plant aluminum resistance. J. Integr. Plant Biol. 2014, 56, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Pirzadah, T.B.; Malik, B.; Tahir, I.; Rehman, R.U.; Hakeem, K.R.; Alharby, H.F. Aluminium stress modulates the osmolytes and enzyme defense system in Fagopyrum species. Plant Physiol. Biochem. 2019, 144, 178–186. [Google Scholar] [CrossRef]
- Killi, D.; Raschi, A.; Bussotti, F. Lipid peroxidation and chlorophyll fluorescence of photosystem II performance during drought and heat stress is associated with the antioxidant capacities of C3 sunflower and C4 maize varieties. Int. J. Mol. Sci. 2020, 21, 4846. [Google Scholar] [CrossRef]
- Dreyer, B.H.; Schippers, J.H.M. Copper-Zinc superoxide dismutases in plants: Evolution, enzymatic properties, and beyond. Annu. Plant Rev. Online 2019, 2, 933–968. [Google Scholar]
- Su, W.; Raza, A.; Gao, A.; Jia, Z.; Zhang, Y.; Hussain, M.A.; Mehmood, S.S.; Cheng, Y.; Lv, Y.; Zou, X. Genome-wide analysis and expression profile of superoxide dismutase (SOD) gene family in rapeseed (Brassica napus L.) under different hormones and abiotic stress conditions. Antioxidants 2021, 10, 1182. [Google Scholar]
- Feng, K.; Yu, J.; Cheng, Y.; Ruan, M.; Wang, R.; Ye, Q.; Zhou, G.; Li, Z.; Yao, Z.; Yang, Y.; et al. The SOD gene family in tomato: Identification, phylogenetic relationships, and expression patterns. Front. Plant Sci. 2016, 7, 1279. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.L.; Wang, C.P.; Khan, N.; Chen, M.; Fu, W.; Guan, L.; Leng, X. Genome-wide identification of the class III POD gene family and their expression profiling in grapevine (Vitis vinifera L.). BMC Genom 2020, 21, 444. [Google Scholar]
- Shang, H.; Fang, L.; Xu, J.; Yao, W.; Zhang, M. Genome-wide identification of the class III peroxidase gene family of sugarcane and its expression profiles under stresses. Front. Plant Sci. 2023, 14, 1101665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ye, Q.; Wu, Z.; Zhang, Q.; Wang, L.; Liu, J.; Hu, X.; Guo, D.; Wang, X.; Zhang, Z.; et al. Analysis of CAT gene family and functional identification of OsCAT3 in rice. Genes 2023, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.C.; Salvi, P.; Kamble, N.U.; Joshi, P.K.; Majee, M.; Arora, S. Ectopic overexpression of cytosolic ascorbate peroxidase gene (Apx1) improves salinity stress tolerance in Brassica juncea by strengthening antioxidative defense mechanism. Acta Physiol. Plant 2020, 42, 1–14. [Google Scholar] [CrossRef]
- Ling, J.Y.; Tan, J.H.; Chen, H.; Yang, Z.Q.; Luo, Q.F.; Jia, J. Physiology, transcriptome and root exudates analysis of response to aluminum stress in Pinus massoniana. Forests 2023, 14, 1410. [Google Scholar] [CrossRef]
- Li, F.; Wu, X.J.; Wu, W. Effects of malondialdehyde-induced protein oxidation on the structural characteristics of rice protein. Int. J. Food Sci. Technol. 2020, 55, 760–768. [Google Scholar] [CrossRef]
- Dai, B.; Chen, C.; Liu, Y.; Liu, L.; Qaseem, M.F.; Wang, J.; Li, H.; Wu, A.M. Physiological, biochemical, and transcriptomic responses of neolamarckia cadamba to aluminum stress. Int. J. Mol. Sci. 2020, 21, 9624. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Lu, L.L.; Yu, Y.; Liu, L.J.; Hu, Y.; Ye, Y.Q.; Jin, C.W.; Lin, X.Y. Decreasing methylation of pectin caused by nitric oxide leads to higher aluminium binding in cell walls and greater aluminium sensitivity of wheat roots. J. Exp. Bot. 2016, 67, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Tränkner, M.; Lu, J.; Yan, J.; Huang, S.; Ren, T.; Cong, R.; Li, X. Interactive effects of nitrogen and potassium on photosynthesis and photosynthetic nitrogen allocation of rice leaves. BMC Plant Biol. 2019, 19, 1–13. [Google Scholar]
- Singh, D.; Alam, A.; Jha, S.K.; Kumar, S.; Pandey, R.; Chinnusamy, V.; Tripathi, S.; Sathee, L. Impact of single and dual deficiency of nitrogen and iron on photosynthesis and fluorescence parameters in hydroponically and field grown bread wheat. Plant Physiol. Rep. 2022, 27, 632–640. [Google Scholar] [CrossRef]
- Lysenko, E.A.; Klaus, A.A.; Kartashov, A.V.; Kusnetsov, V.V. Specificity of Cd, Cu, and Fe effects on barley growth, metal contents in leaves and chloroplasts, and activities of photosystem I and photosystem II. Plant Physiol. Biochem. 2020, 147, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Y.; He, D.D.; Bai, S.; Zeng, W.Z.; Wang, Z.; Wang, M.; Wu, L.Q.; Chen, Z.C. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 2021, 468, 1–17. [Google Scholar] [CrossRef]
- Muhammad, N.; Zvobgo, G.; Fu, L.B.; Lwalaba, J.L.W.; Zhang, G.P. Physiological mechanisms for antagonistic interaction of manganese and aluminum in barley. J. Plant Nutr. 2019, 42, 466–476. [Google Scholar] [CrossRef]
- Meng, X.; Bai, S.; Wang, S.; Pan, Y.; Chen, K.; Xie, K.; Wang, M.; Guo, S. The sensitivity of photosynthesis to magnesium deficiency differs between rice (Oryza sativa L.) and cucumber (Cucumis sativus L.). Front. Plant Sci. 2023, 14, 1164866. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Furutani, R.; Sohtome, T.; Suzuki, T.; Wada, S.; Tanaka, S.; Ifuku, K.; Ueno, D.; Miyake, C. Photosynthetic parameters show specific responses to essential mineral deficiencies. Antioxidants. 2021, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.Q.; Xu, Q.S.; Pan, L.; Kong, Y.L.; Zhu, L.F.; Cao, X.C.; Tian, W.H.; Liu, J.; Jin, Q.Y.; Xiang, X.J.; et al. Mechanism of interaction between calcium ions and hydrogen sulfide to alleviate the inhibitory effect of aluminum on root elongation of rice. Chin. J. Rice Sci. 2023, 37, 142–152. (In Chinese) [Google Scholar]
- Tian, W.; He, G.; Qin, L.; Li, D.; Meng, L.; Huang, Y.; He, T. Genome-wide analysis of the NRAMP gene family in potato (Solanum tuberosum): Identification, expression analysis and response to five heavy metals stress. Ecotox. Environ. Safe 2021, 208, 111661. [Google Scholar] [CrossRef] [PubMed]
- El-Sappah, A.H.; Elrys, A.S.; Desoky, E.S.M.; Zhao, X.; Bingwen, W.; El-Sappah, H.H.; Zhu, Y.; Zhou, W.; Zhao, X.; Li, J. Comprehensive genome wide identification and expression analysis of MTP gene family in tomato (Solanum lycopersicum) under multiple heavy metal stress. Saudi J. Biol. Sci. 2021, 28, 6946–6956. [Google Scholar] [CrossRef]
- Tao, Q.; Jupa, R.; Liu, Y.K.; Luo, J.P.; Li, J.X.; Kováč, J.; Li, T.Q. Abscisic acid-mediated modifications of radial apoplastic transport pathway play a key role in cadmium uptake in hyperaccumulator Sedum alfredii. Plant Cell Environ. 2019, 42, 1425–1440. [Google Scholar] [CrossRef]
- Sato, H.; Takasaki, H.; Takahashi, F.; Suzuki, T.; Iuchi, S.; Mitsuda, N.; Ohme-Takagi, M.; Ikeda, M.; Seo, M.; Yamaguchi-Shinozaki, K.; et al. Arabidopsis thaliana NGATHA1 transcription factor induces ABA biosynthesis by activating NCED3 gene during dehydration stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11178–E11187. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Du, K.; Kang, X.; Wei, H. The diverse roles of cytokinins in regulating leaf development. Hortic. Res. 2021, 8, 118. [Google Scholar] [CrossRef]
- Skalák, J.; Vercruyssen, L.; Claeys, H.; Hradilová, J.; Černý, M.; Novák, O.; Plačková, L.; Saiz-Fernández, I.; Skaláková, P.; Coppens, F.; et al. Multifaceted activity of cytokinin in leaf development shapes its size and structure in Arabidopsis. Plant J. 2019, 97, 805–824. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, F.; Li, M.; He, Y.; Chen, Y.; Hu, F. Functional analysis of ARF1 from Cymbidium goeringii in IAA response during leaf development. PeerJ 2022, 10, e13077. [Google Scholar] [CrossRef]
- Budimir, J.; Trefon, K.; Nair, A.; Thurow, C.; Gatz, C. Redox–active cysteines in TGACG-BINDING FACTOR 1 (TGA1) do not play a role in salicylic acid or pathogen-induced expression of TGA1-regulated target genes in Arabidopsis thaliana. New Phytol. 2021, 230, 2420–2432. [Google Scholar] [CrossRef]
- Ke, M.Y.; Ma, Z.M.; Wang, D.Y.; Sun, Y.B.; Wen, C.J.; Huang, D.Q.; Chen, Z.C.; Yang, L.; Tan, S.T.; Li, R.X.; et al. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin-dependent lipid nanodomain organisation in Arabidopsis thaliana. New Phytol. 2021, 229, 963–978. [Google Scholar] [CrossRef]
- Miao, Y.; Luo, X.; Gao, X.; Wang, W.; Li, B.; Hou, L. Exogenous salicylic acid alleviates salt stress by improving leaf photosynthesis and root system architecture in cucumber seedlings. Sci. Hortic. 2020, 272, 109577. [Google Scholar] [CrossRef]
- Li, Q.; Wang, G.; Guan, C.; Yang, D.; Wang, Y.; Zhang, Y.; Ji, J.; Jin, C.; An, T. Overexpression of LcSABP, an orthologous gene for salicylic acid binding protein 2, enhances drought stress tolerance in transgenic tobacco. Front. Plant Sci. 2019, 10, 200. [Google Scholar] [CrossRef]
- Salazar-Chavarría, V.; Sánchez-Nieto, S.; Cruz-Ortega, R. Fagopyrum esculentum at early stages copes with aluminum toxicity by increasing ABA levels and antioxidant system. Plant Physiol. Biochem. 2020, 152, 170–176. [Google Scholar] [CrossRef]
- Yang, Y.; Dai, C.; Guo, L.; Qu, Y.; Yang, X.; Liu, D.; Wang, C.; Cui, X. Salicylic acid reduces the accumulation of aluminum in Panax notoginsen root cell wall pectin via the NO signaling pathway. Plant Soil 2018, 430, 171–184. [Google Scholar]
- Ulloa-Inostroza, E.M.; Alberdi, M.; Meriño-Gergichevich, C.; Reyes-Díaz, M. Low doses of exogenous methyl jasmonate applied simultaneously with toxic aluminum improve the antioxidant performance of Vaccinium corymbosum. Plant Soil 2017, 412, 81–96. [Google Scholar] [CrossRef]
- Shkryl, Y.N.; Vasyutkina, E.A.; Gorpenchenko, T.V.; Mironova, A.A.; Rusapetova, T.V.; Velansky, P.V.; Bulgakov, V.P.; Yugay, Y.A. Salicylic acid and jasmonic acid biosynthetic pathways are simultaneously activated in transgenic Arabidopsis expressing the rolB/C gene from Ipomoea batatas. Plant Physiol. Biochem. 2024, 208, 108521. [Google Scholar] [CrossRef] [PubMed]
- Bagale, P.; Pandey, S.; Regmi, P.; Bhusal, S. Role of plant growth regulator “Gibberellins” in vegetable production: An overview. Int. J. Hortic. Sci. Technol. 2022, 9, 291–299. [Google Scholar]
- Wang, Y.Y.; Li, J.Y.; Pan, Y.H.; Chen, J.Y.; Liu, Y. Metabolic responses to manganese toxicity in soybean roots and leaves. Plants 2023, 12, 3615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, Y.B.; Xie, B.X.; Zhu, S.N.; Lu, X.; Liang, C.Y.; Tian, J. Complex gene regulation between young and old soybean leaves in responses to manganese toxicity. Plant Physiol. Biochem. 2020, 155, 231–242. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, M.; Chen, J.Y.; Yang, S.X.; Chen, J.P.; Xue, Y.B. Comparative transcriptome analysis reveals complex physiological response and gene regulation in peanut roots and leaves under manganese toxicity stress. Int. J. Mol. Sci. 2023, 24, 1161. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chen, H.; Wang, H.X.; Zhan, J.Y.; Yuan, X.X.; Cui, J.; Su, N.N. Selenium treatment promotes anthocyanin accumulation in radish sprouts (Raphanus sativus L.) by its regulation of photosynthesis and sucrose transport. Food Res. Int. 2023, 165, 112551. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Zheng, D.F.; Feng, N.J.; Zhou, H.; Mu, D.W.; Liu, L.; Zhao, L.M.; Shen, X.F.; Rao, G.H.; Li, T.Z. Effects of exogenous salicylic acid and abscisic acid on growth, photosynthesis and antioxidant system of rice. Chil. J. Agric. Res. 2022, 82, 21–32. [Google Scholar] [CrossRef]
- Rácz, A.; Hideg, É.; Czégény, G. Selective responses of class III plant peroxidase isoforms to environmentally relevant UV-B doses. J. Plant Physiol. 2018, 221, 101–106. [Google Scholar] [CrossRef]
- Ekinci, M.; Ors, S.; Yildirim, E.; Turan, M.; Sahin, U.; Dursun, A.; Kul, R. Determination of physiological indices and some antioxidant enzymes of chard exposed to nitric oxide under drought stress. Russ. J. Plant Physiol. 2020, 67, 740–749. [Google Scholar] [CrossRef]
- Li, C.G.; Li, J.X.; Du, X.H.; Zhang, J.X.; Zou, Y.R.; Liu, Y.D.; Li, Y.; Lin, H.Y.; Li, H.; Liu, D.; et al. Chloroplast thylakoidal ascorbate peroxidase, PtotAPX, has enhanced resistance to oxidative stress in Populus tomentosa. Int. J. Mol. Sci. 2022, 23, 3340. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tadayon, R.M.; Nadeem, M.; Cheema, M.; Razmjoo, J. Proline-mediated changes in antioxidant enzymatic activities and the physiology of sugar beet under drought stress. Acta Physiol. Plant 2019, 41, 23. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, Y.H.; Li, J.Y.; Chen, J.Y.; Yang, S.X.; Zhao, M.; Xue, Y.B. Transcriptome sequencing analysis of root in soybean responding to Mn poisoning. Int. J. Mol. Sci. 2023, 24, 12727. [Google Scholar] [CrossRef] [PubMed]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M.I. Role of melatonin in improving growth, yield quantity and quality of Moringa oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 18. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef]
- Kalogiouri, N.P.; Manousi, N.; Zachariadis, G.A. Determination of the toxic and nutrient element content of almonds, walnuts, hazelnuts and pistachios by ICP-AES. Separations 2021, 8, 28. [Google Scholar] [CrossRef]
- Khew, C.Y.; Mori, I.C.; Matsuura, T.; Hirayama, T.; Harikrishna, J.A.; Lau, E.T.; Mercer, Z.J.A.; Hwang, S.S. Hormonal and transcriptional analyses of fruit development and ripening in different varieties of black pepper (Piper nigrum). J. Plant Res. 2020, 133, 73–94. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.B.; Zhuang, Q.L.; Zhu, S.N.; Xiao, B.X.; Liang, C.Y.; Liao, H.; Tian, J. Genome wide transcriptome analysis reveals complex regulatory mechanisms underlying phosphate homeostasis in soybean nodules. Int. J. Mol. Sci. 2018, 19, 2924. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.L.; Xue, Y.B.; Yao, Z.F.; Zhu, S.N.; Liang, C.Y.; Liao, H.; Tian, J. Phosphate starvation responsive GmSPX5 mediates nodule growth through interaction with GmNF-YC4 in soybean (Glycine max). Plant J. 2021, 108, 1422–1438. [Google Scholar] [CrossRef]
- Li, G.J.; Song, H.Y.; Li, B.H.; Kronzucker, H.J.; Shi, W.M. Auxin resistant1 and PIN-FORMED2 protect lateral root formation in Arabidopsis under iron stress. Plant Physiol. 2015, 169, 2608–2623. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Li, J.; Pan, Y.; Zhao, M.; Zhang, R.; Xue, Y.; Liu, Y. The Physiological Response Mechanism of Peanut Leaves under Al Stress. Plants 2024, 13, 1606. https://doi.org/10.3390/plants13121606
Shi J, Li J, Pan Y, Zhao M, Zhang R, Xue Y, Liu Y. The Physiological Response Mechanism of Peanut Leaves under Al Stress. Plants. 2024; 13(12):1606. https://doi.org/10.3390/plants13121606
Chicago/Turabian StyleShi, Jianning, Jianyu Li, Yuhu Pan, Min Zhao, Rui Zhang, Yingbin Xue, and Ying Liu. 2024. "The Physiological Response Mechanism of Peanut Leaves under Al Stress" Plants 13, no. 12: 1606. https://doi.org/10.3390/plants13121606
APA StyleShi, J., Li, J., Pan, Y., Zhao, M., Zhang, R., Xue, Y., & Liu, Y. (2024). The Physiological Response Mechanism of Peanut Leaves under Al Stress. Plants, 13(12), 1606. https://doi.org/10.3390/plants13121606





