Green Synthesis of Silver Nanoparticles with Hyssopus officinalis and Salvia officinalis Extracts, Their Properties, and Antifungal Activity on Fusarium spp.
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Composition of Prepared Extracts and AgNPs
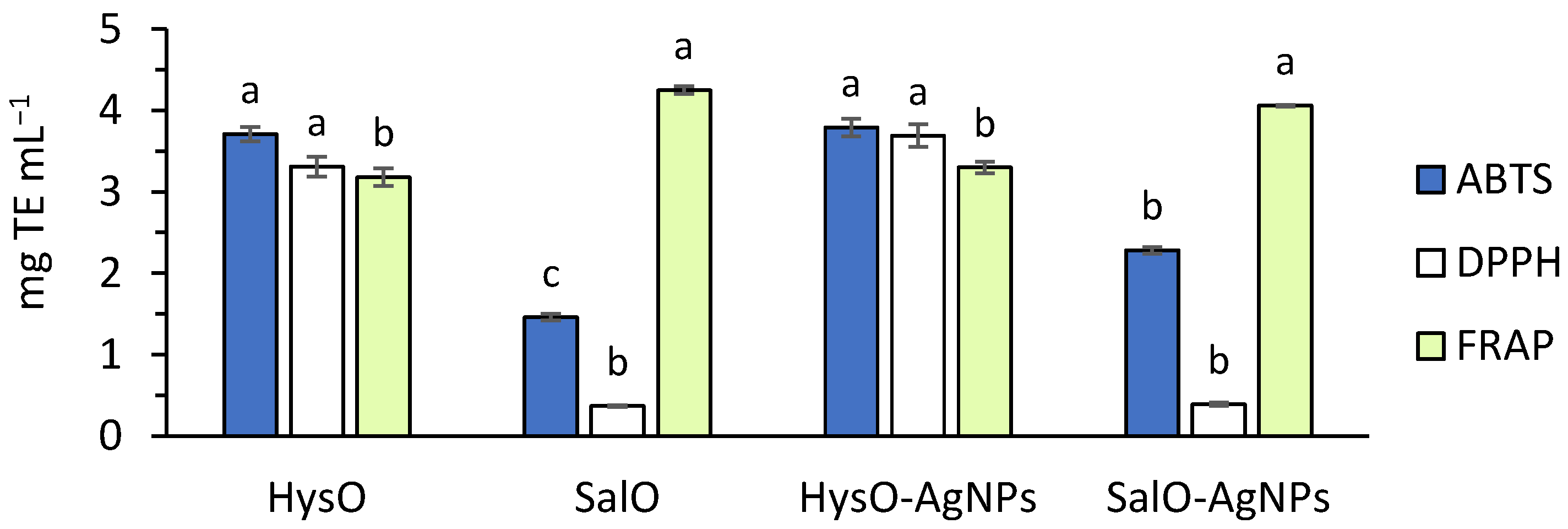
2.2. Antioxidant Capacity of the Extracts and AgNPs
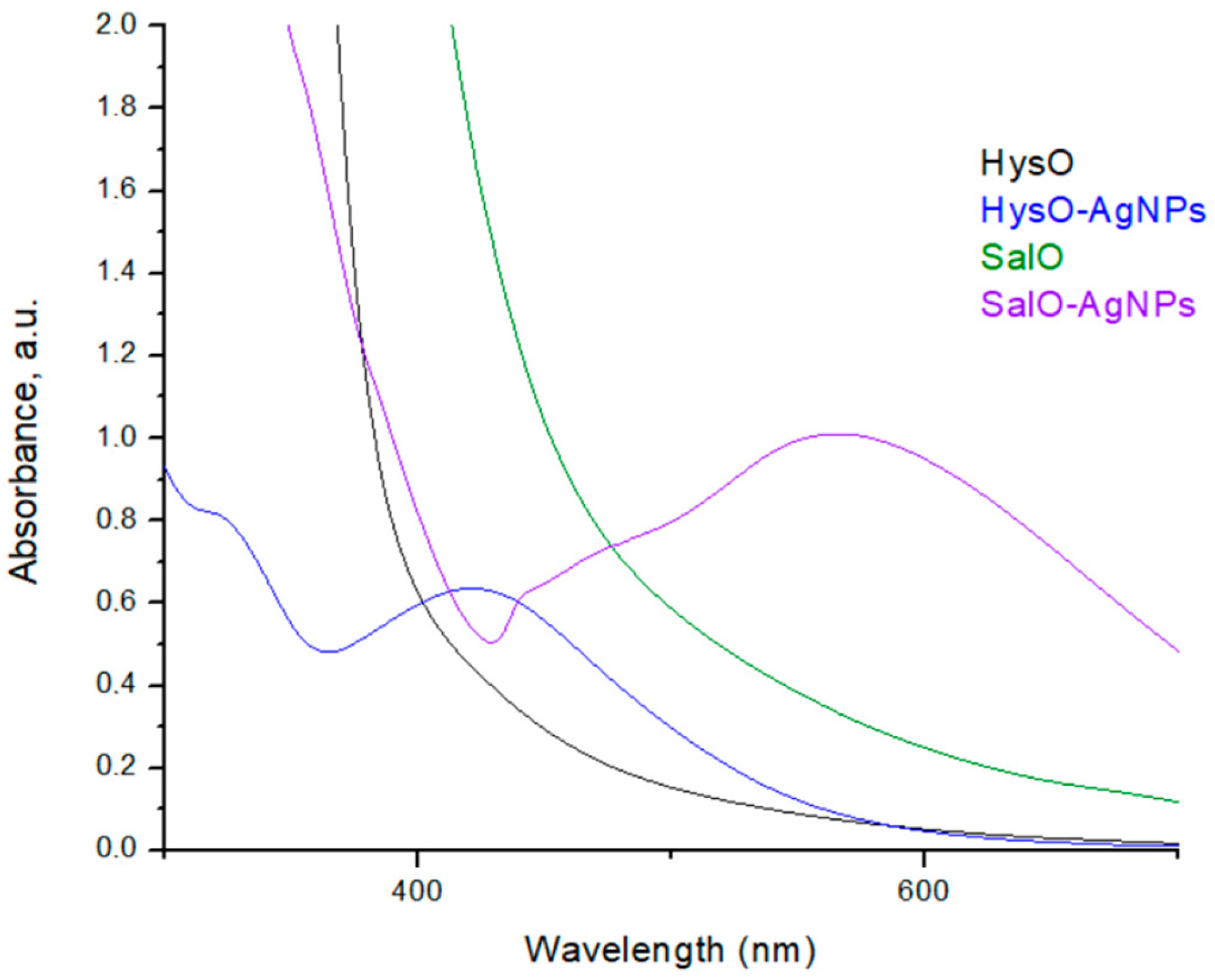
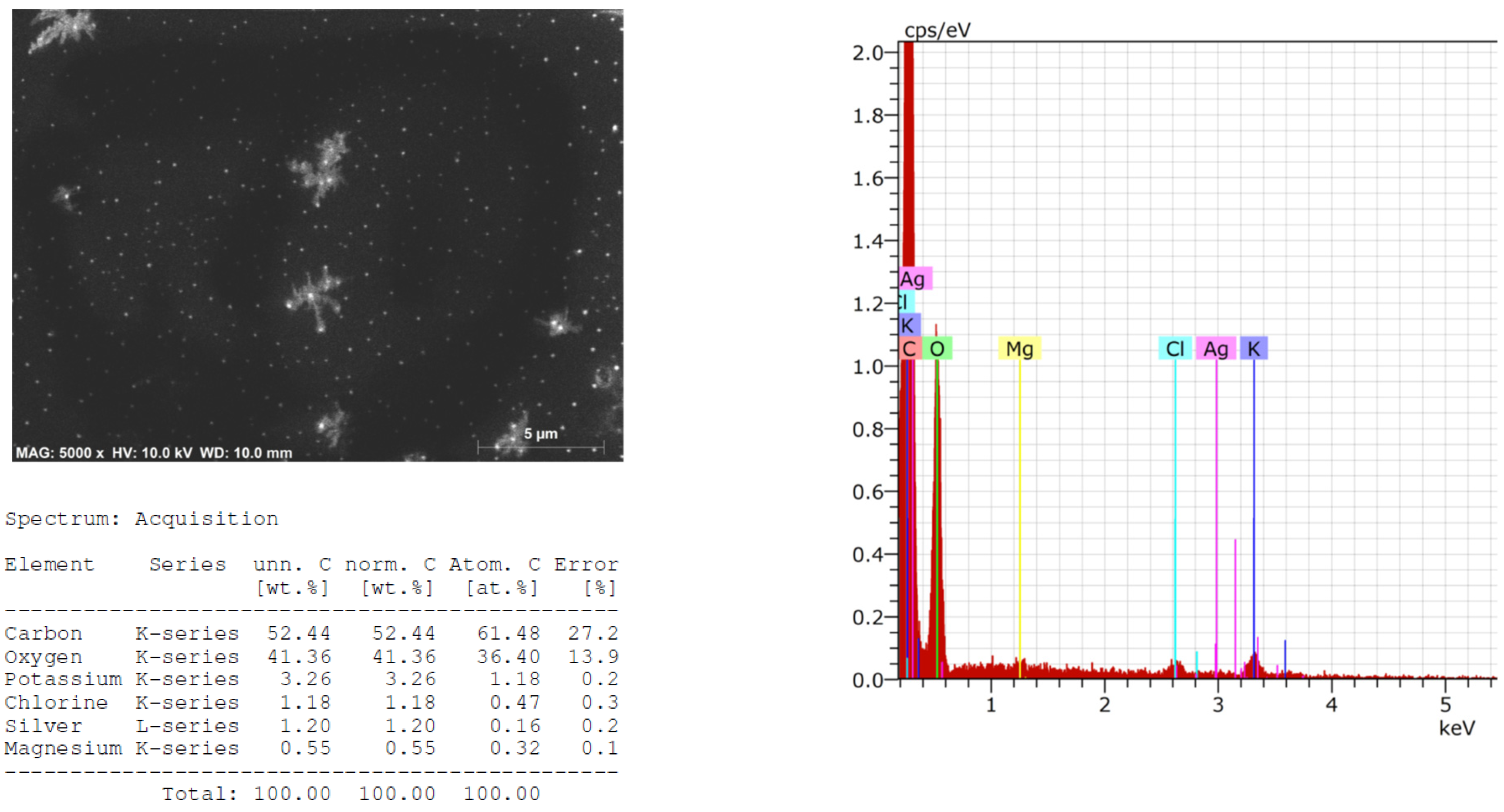
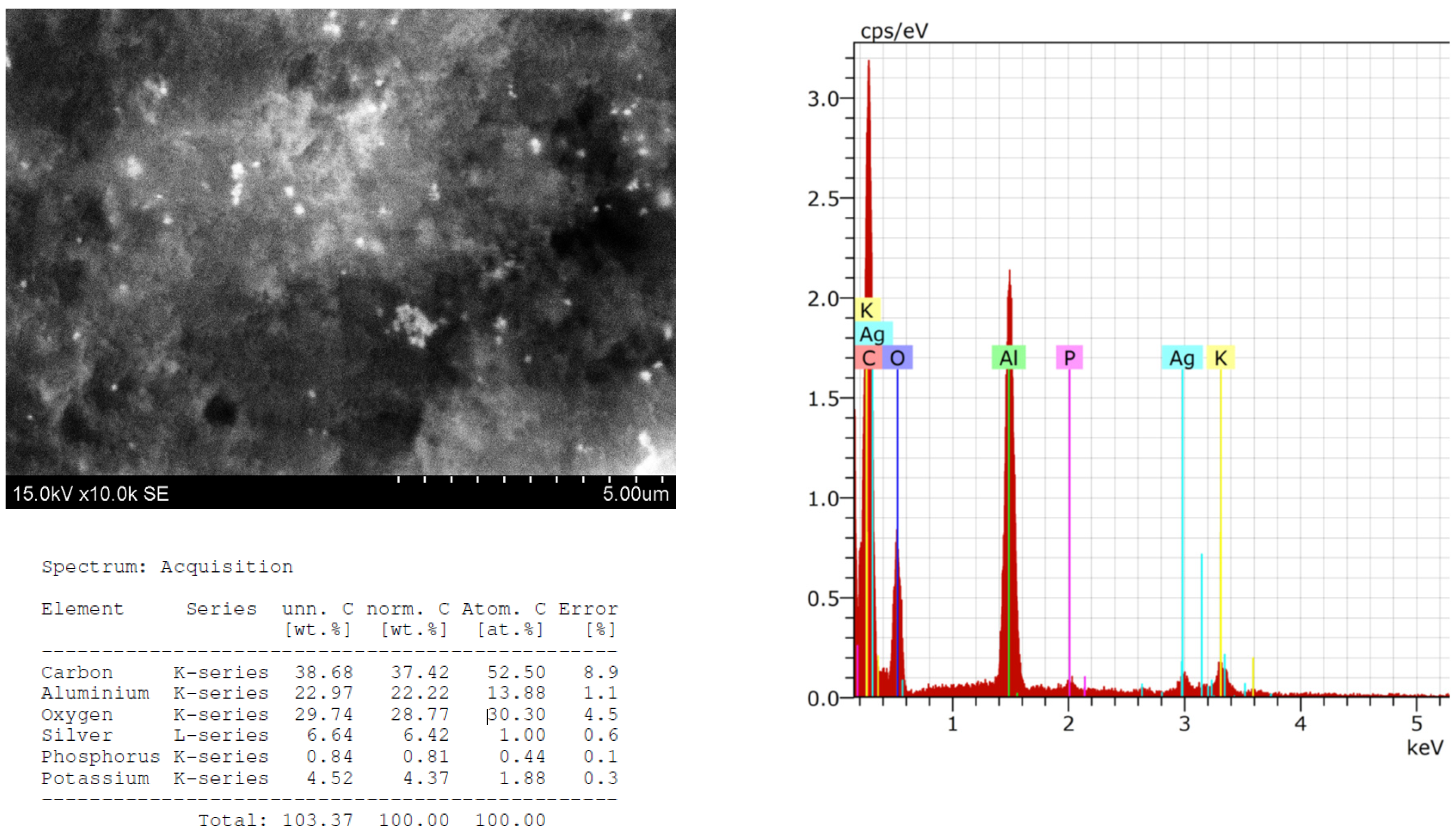
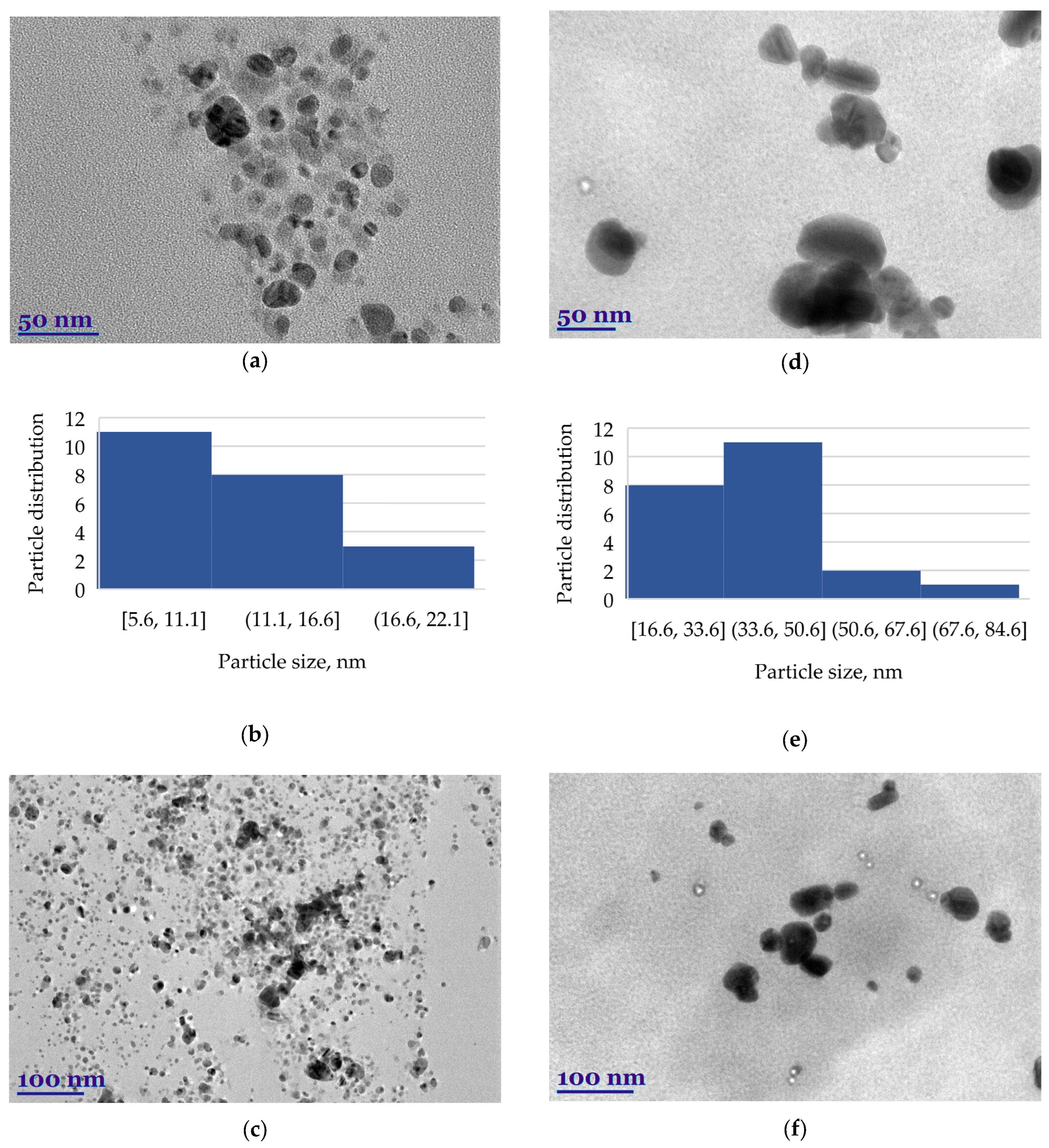
2.3. Structural and Qualitative Analysis of Silver Nanoparticles
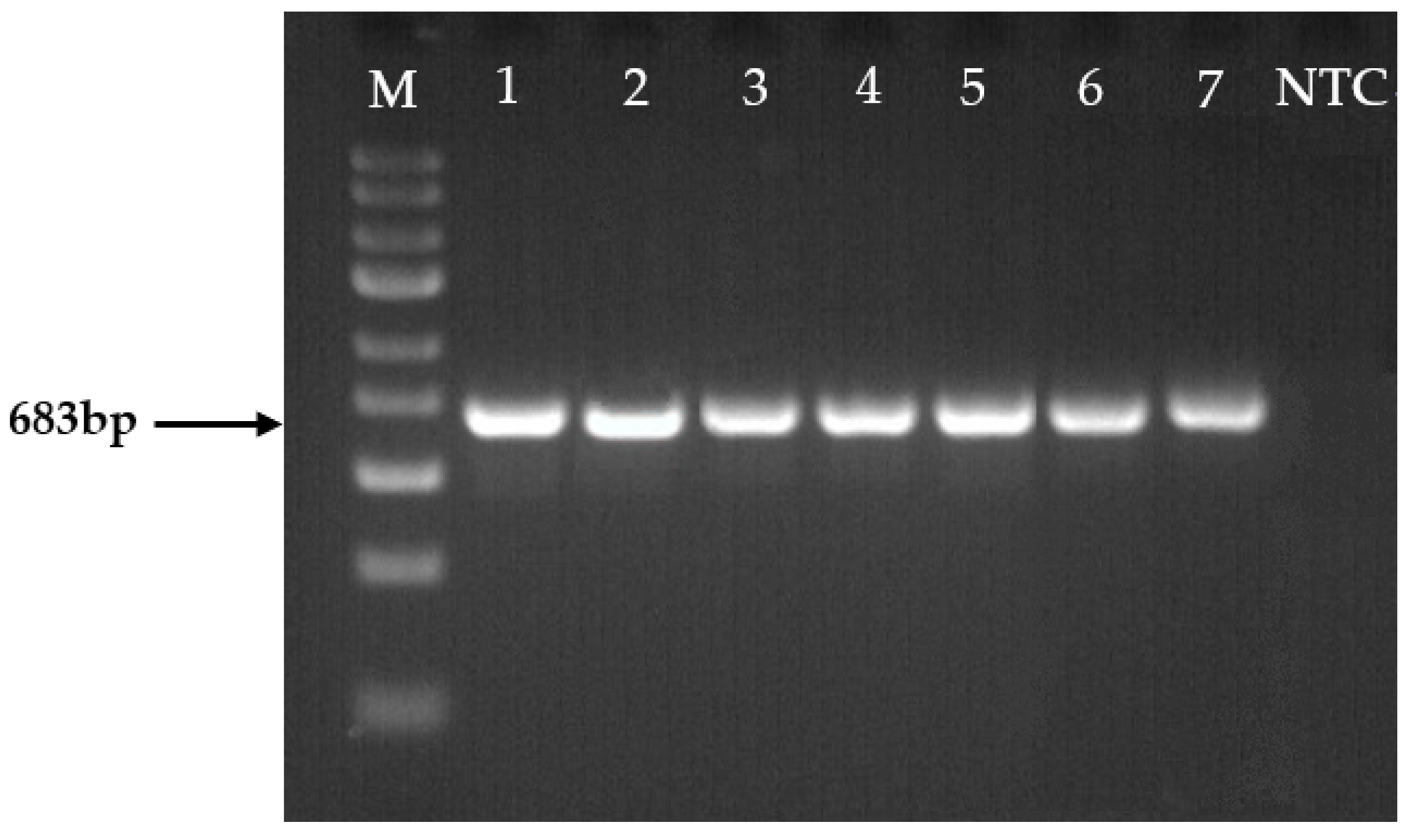
2.4. Molecular Identification of Fusarium spp.
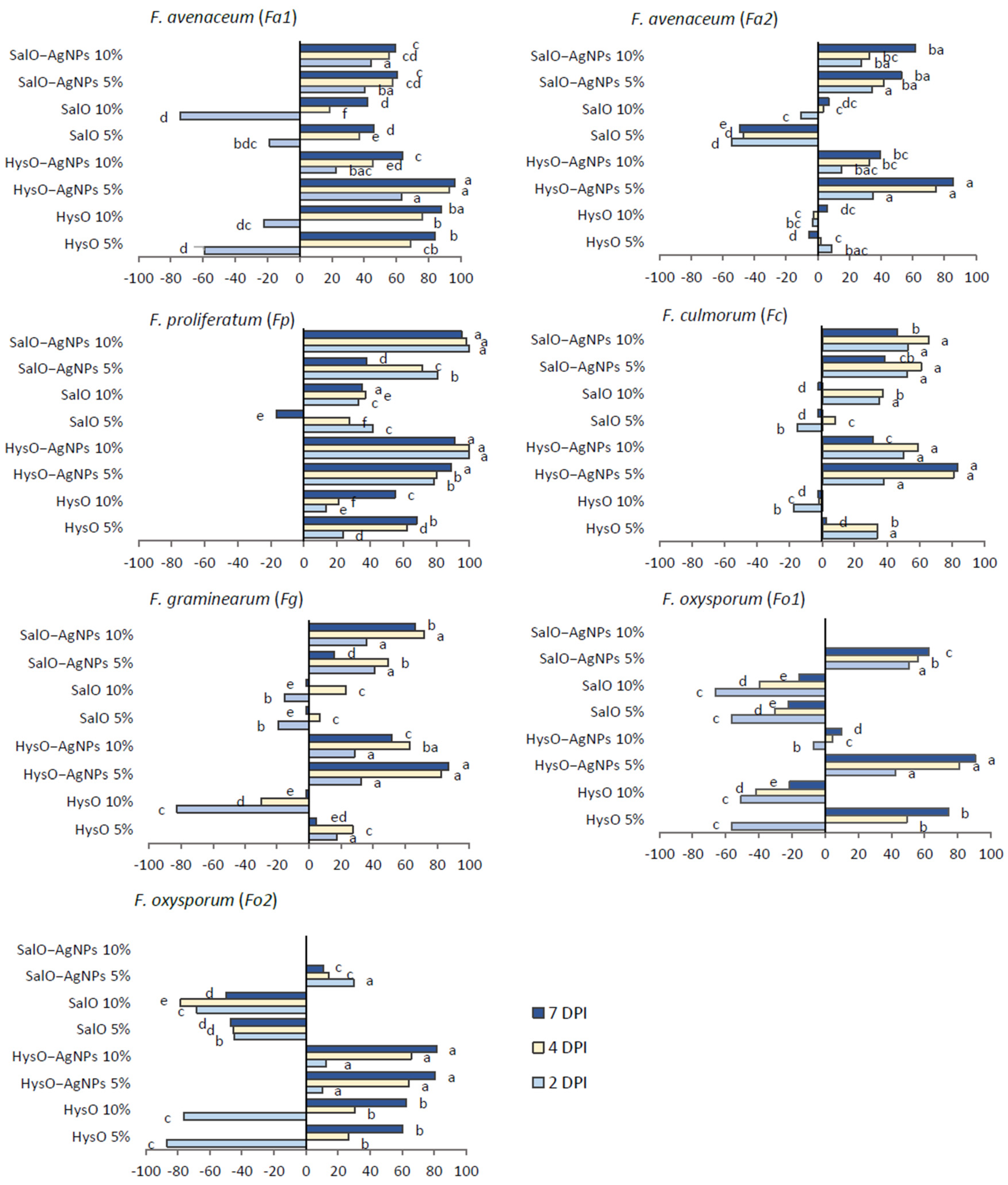
2.5. Inhibition of Fusarium spp. In Vitro
3. Discussion
4. Materials and Methods
4.1. Preparation of the Extracts and Green Synthesis of AgNPs
4.2. Phytochemical Composition of Prepared Extracts
4.3. Antioxidant Capacity
4.4. Structural and Qualitative Analysis of Silver Nanoparticles
4.5. Isolation of Fusarium spp.
4.6. DNA Amplification and Sequencing
4.7. In Vitro Experiments
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Jian, Y.; Chen, X.; Ahmed, T.; Shang, Q.; Zhang, S.; Ma, Z.; Yin, Y. Toxicity and action mechanisms of silver nanoparticles against the mycotoxin-producing fungus Fusarium graminearum. J. Adv. Res. 2022, 38, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabaan, F.A.; Shoala, T.; Ali, A.A.M.; Alaa, M.; Abd-Elsalam, K. Chemically-produced copper, zinc nanoparticles and chitosan–bimetallic nanocomposites and their antifungal activity against three phytopathogenic fungi. Int. J. Agric. Technol. 2017, 13, 753–769. [Google Scholar]
- Akpinar, I.; Unal, M.; Sar, T. Potential antifungal effects of silver nanoparticles (AgNPs) of different sizes against phytopathogenic Fusarium oxysporum f. sp. radicis-lycopersici (FORL) strains. SN Appl. Sci. 2021, 3, 506. [Google Scholar] [CrossRef]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles-formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef] [PubMed]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and green synthesis ofnanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Chrapačienė, S.; Rasiukevičiūtė, N.; Valiuškaitė, A. Control of seed-borne fungi by selected essential oils. Horticulturae 2022, 8, 220. [Google Scholar] [CrossRef]
- Chrapačienė, S.; Rasiukevičiūtė, N.; Valiuškaitė, A. Biocontrol of carrot disease-causing pathogens using essential oils. Plants 2021, 10, 2231. [Google Scholar] [CrossRef]
- Dėnė, L.; Valiuškaitė, A. Sensitivity of Botrytis cinerea Isolates Complex to Plant Extracts. Molecules 2021, 26, 4595. [Google Scholar] [CrossRef] [PubMed]
- Dėnė, L.; Laužikė, K.; Rasiukevičiūtė, N.; Chrapačienė, S.; Brazaitytė, A.; Viršilė, A.; Vaštakaitė-Kairienė, V.; Miliauskienė, J.; Sutulienė, R.; Samuolienė, G.; et al. Defense response of strawberry plants against Botrytis cinerea influenced by coriander extract and essential oil. Front. Plant Sci. 2023, 13, 1098048. [Google Scholar] [CrossRef]
- Judžentienė, A. Hyssop (Hyssopus officinalis L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 471–479. [Google Scholar] [CrossRef]
- Tahir, M.; Khushtar, M.; Fahad, M.; Rahman, M.A. Phytochemistry and pharmacological profile of traditionally used medicinal plant Hyssop (Hyssopus officinalis L.). J. Appl. Pharm. Sci. 2018, 8, 132–140. [Google Scholar] [CrossRef]
- Ahmad, H.; Matsubara, Y. Antifungal effect of Lamiaceae herb water extracts against Fusarium root rot in Asparagus. J. Plant Dis. Prot. 2020, 127, 229–236. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Maliki, I.; Es-Safi, I.; El Moussaoui, A.; Mechchate, H.; El Majdoub, Y.O.; Bouymajane, A.; Cacciola, F.; Mondello, L.; Elbadaoui, K. Salvia officinalis and Lippia triphylla: Chemical characterization and evaluation of antidepressant-like activity. J. Pharm. Biomed. Anal. 2021, 203, 114207. [Google Scholar] [CrossRef]
- Sabry, M.M.; Abdel-Rahman, R.F.; El-Shenawy, S.M.; Hassan, A.M.; El-Gayed, S.H. Estrogenic activity of Sage (Salvia officinalis L.) aerial parts and its isolated ferulic acid in immature ovariectomized female rats. J. Ethnopharmacol. 2022, 282, 114579. [Google Scholar] [CrossRef]
- Sharma, R.; Dewanjee, S.; Kole, C. Utilization of Nanoparticles for Plant Protection. In Plant Nanotechnology; Kole, C., Kumar, D.S., Khodakovskaya, M.V., Eds.; Springer: Cham, Switzerland, 2016; p. 383. ISBN 9783319421544. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: An overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- El-Seedi, H.; El-Shabasy, R.M.; Khalifa, S.A.M.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H.; et al. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- Afreen, A.; Ahmed, R.; Mehboob, S.; Tariq, M.; Alghamdi, H.A.; Zahid, A.A.; Ali, I.; Malik, K.; Hasan, A. Phytochemical-assisted biosynthesis of silver nanoparticles from Ajuga bracteosa for biomedical applications. Mater. Res. Express 2020, 7, 075404. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Bonjar, A.H.S.; Rahdar, A.; Varma, R.S.; Ajalli, N.; Pandey, S. Eco-friendly biosynthesis of silver nanoparticles using Aloysia citrodora leaf extract and evaluations of their bioactivities. Mater. Today Commun. 2022, 33, 104183. [Google Scholar] [CrossRef]
- Balčiūnaitienė, A.; Liaudanskas, M.; Puzerytė, V.; Viškelis, J.; Janulis, V.; Viškelis, P.; Griškonis, E.; Jankauskaitė, V. Eucalyptus globulus and Salvia officinalis Extracts Mediated Green Synthesis of Silver Nanoparticles and Their Application as an Antioxidant and Antimicrobial Agent. Plants 2022, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Balciunaitiene, A.; Puzeryte, V.; Radenkovs, V.; Krasnova, I.; Memvanga, P.B.; Viskelis, P.; Streimikyte, P.; Viskelis, J. Sustainable–Green Synthesis of Silver Nanoparticles Using Aqueous Hyssopus officinalis and Calendula officinalis Extracts and Their Antioxidant and Antibacterial Activities. Molecules 2022, 27, 7700. [Google Scholar] [CrossRef] [PubMed]
- Thrane, U. Fusarium. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 2, pp. 76–81. ISBN 9780123847331. [Google Scholar]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Tan, J.; De Zutter, N.; De Saeger, S.; De Boevre, M.; Tran, T.M.; van der Lee, T.; Waalwijk, C.; Willems, A.; Vandamme, P.; Ameye, M.; et al. Presence of the weakly pathogenic Fusarium poae in the Fusarium head blight disease complex hampers biocontrol and chemical control of the virulent Fusarium graminearum pathogen. Front. Plant Sci. 2021, 12, 641890. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.; Zhang, M.; Zhang, Y.; Hossain, A.; Qiu, W.; Chen, Y.; Wang, Y.; Wu, W.; Sun, G.; Li, B. Green-synthesization of silver nanoparticles using endophytic bacteria isolated from garlic and its antifungal activity against wheat Fusarium head blight pathogen Fusarium graminearum. Nanomaterials 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Ghojavand, S.; Madani, M.; Karimi, J. Green synthesis, characterization and antifungal activity of silver nanoparticles using stems and flowers of Felty germander. J. Inorg. Organomet. Polym. Mater. 2020, 30, 2987–2997. [Google Scholar] [CrossRef]
- Abd-Elbaky, A.A.; El-Nahas, S.E.M.; Hassan, M.E.M.; Desoukey, A.F. Effect of silver nanoparticles on Fusarium basal-rot of onion. Int. J. Sci. Eng. Res. 2021, 12, 481–495. [Google Scholar]
- Jeon, H.B.; Tsalu, P.V.; Ha, J.W. Shape effect on the refractive index sensitivity at localized surface plasmon resonance inflection points of single gold nanocubes with vertices. Sci. Rep. 2019, 9, 13635. [Google Scholar] [CrossRef]
- Mekuye, B. The Impact of Size on the Optical Properties of Silver Nanoparticles Based on Dielectric Function. In Nanomaterials and Nanostructures—Annual Volume 2024; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Guibal, E.; Cambe, S.; Bayle, S.; Taulemesse, J.; Vincent, T. Silver/chitosan/cellulose fibers foam composites: From synthesis to antibacterial properties. J. Colloid Interface Sci. 2013, 393, 411–420. [Google Scholar] [CrossRef]
- Kursa, W.; Jamiołkowska, A.; Wyrostek, J.; Kowalski, R. Antifungal effect of plant extracts on the growth of the cereal pathogen fusarium spp.—An in vitro study. Agronomy 2022, 12, 3204. [Google Scholar] [CrossRef]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro evaluation of antioxidant and antibacterial potential of greensynthesized silver nanoparticles using Prosopis farcta fruit extract. Iran. J. Pharm. Res. 2019, 18, 430–455. [Google Scholar]
- Dean, R.; Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Molecular 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Michielse, C.B.; Rep, M. Pathogen profile update: Fusarium oxysporum. Mol. Plant Pathol. 2009, 10, 311–324. [Google Scholar] [CrossRef]
- Edel-Hermann, V.; Gautheron, N.; Mounier, A.; Steinberg, C. Fusarium diversity in soil using a specific molecular approach and a cultural approach. J. Microbiol. Methods 2015, 111, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Zaidi, A.; Ahmed, B.; Rizvi, A.; Khan, M.S.; Musarrat, J. Effective Inhibition of Phytopathogenic Microbes by Eco-Friendly Leaf Extract Mediated Silver Nanoparticles (AgNPs). Indian J. Microbiol. 2019, 59, 273–287. [Google Scholar] [CrossRef]
- Shaikh, W.A.; Chakraborty, S.; Owens, G.; Islam, R.U. A review of the phytochemical mediated synthesis of AgNP (silver nanoparticle): The wonder particle of the past decade. Appl. Nanosci. 2021, 11, 2625–2660. [Google Scholar] [CrossRef]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef]
- Nayak, P.S.; Pradhan, S.; Arakha, M.; Kumar, D.; Saleem, M.; Mallick, B.; Jha, S. Silver nanoparticles fabricated using medicinal plant extracts show enhanced antimicrobial and selective cytotoxic propensities. IET Nanobiotechnol. 2019, 13, 193–201. [Google Scholar] [CrossRef]
- Puzerytė, V.; Viškelis, P.; Balčiūnaitienė, A.; Štreimikytė, P.; Viškelis, J.; Urbonavičienė, D. Aralia cordata Thunb. as a source of bioactive compounds: Phytochemical composition and antioxidant activity. Plants 2022, 11, 1704. [Google Scholar] [CrossRef]
- Fraisse, D.; Felgines, C.; Texier, O.; Lamaison, J.-L. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nutr. Sci. 2011, 2, 181–192. [Google Scholar] [CrossRef]
- da Silva, L.A.; Pezzini, B.R.; Soares, L. Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L. (Lamiaceae) leaves. Pharmacogn. Mag. 2015, 11, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Raudonė, L.; Liaudanskas, M.; Vilkickytė, G.; Kviklys, D.; Žvikas, V.; Viškelis, J.; Viškelis, P. Phenolic Profiles, Antioxidant Activity and Phenotypic Characterization of Lonicera caerulea L. Berries, Cultivated in Lithuania. Antioxidants 2021, 10, 115. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. (Eds.) The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing: Hoboken, NJ, USA, 2006; ISBN 9780470278376. [Google Scholar]
- O’Donnell, K.; Kistlerr, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sutton, D.A.; Rinaldi, M.G.; Sarver, B.A.J.; Balajee, S.A.; Schroers, H.J.; Summerbell, R.C.; Robert, V.A.R.G.; Crous, P.W.; Zhang, N.; et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010, 48, 3708–3718. [Google Scholar] [CrossRef] [PubMed]
- Cherkupally, R.; Kota, S.R.; Amballa, H.; Reddy, B.N. In vitro antifungal potential of plant extracts against Fusarium oxysporum, Rhizoctonia solani and Macrophomina phaseolina. Ann. Plant Sci. 2017, 6, 1676–1680. [Google Scholar] [CrossRef]
| Compounds | HysO | SalO | HysO-AgNPs | SalO-AgNPs |
|---|---|---|---|---|
| The total amount of proanthocyanidins, mg EE/g DW | 0.15 ± 0.03 | 0.18 ± 0.01 | 0.08 ± 0.01 | 0.12 ± 0.01 |
| The total amount of hydroxycinnamic acid derivatives, mg CAE/g DW | 1.09 ± 0.02 | 1.51 ± 0.04 | 0.86 ± 0.03 | 1.42 ± 0.03 |
| The total amount of phenolic compounds, mg GAE/g DW | 1.17 ± 0.00 | 0.92 ± 0.01 | 0.79 ± 0.00 | 0.73 ± 0.01 |
| The total amount of flavonoids, mg RE/g DW | 0.58 ± 0.01 | 0.31 ± 0.01 | 0.35 ± 0.02 | 0.27 ± 0.01 |
| S. No. | Host Plant | Isolate Code | Identification by TEF1α Gene | NCBI Accession No. |
|---|---|---|---|---|
| 1. | Strawberry (Fragaria × ananassa Duch.) | Fp | Fusarium proliferatum | JX118976.1 |
| 2. | Pea (Pisum sativum L.) | Fo1 | Fusarium oxysporum | MG356947.1 |
| 3. | Rapeseed (Brassica napus L.) | Fo2 | Fusarium oxysporum | MG857278.1 |
| 4. | Asparagus (Asparagus officinalis L.) | Fa2 | Fusarium avenaceum | MG857265.1 |
| 5. | Carrot (Daucus sativus (Hoffm.) Röhl.) | Fc | Fusarium culmorum | MG857178.1 |
| 6. | Strawberry (Fragaria × ananassa Duch.) | Fa1 | Fusarium avenaceum | MG857265.1 |
| 7. | Spring wheat (Triticum aestivum L.) | Fg | Fusarium graminearum | MG857515.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dėnė, L.; Chrapačienė, S.; Laurinaitytė, G.; Rudinskaitė, A.; Viškelis, J.; Viškelis, P.; Balčiūnaitienė, A. Green Synthesis of Silver Nanoparticles with Hyssopus officinalis and Salvia officinalis Extracts, Their Properties, and Antifungal Activity on Fusarium spp. Plants 2024, 13, 1611. https://doi.org/10.3390/plants13121611
Dėnė L, Chrapačienė S, Laurinaitytė G, Rudinskaitė A, Viškelis J, Viškelis P, Balčiūnaitienė A. Green Synthesis of Silver Nanoparticles with Hyssopus officinalis and Salvia officinalis Extracts, Their Properties, and Antifungal Activity on Fusarium spp. Plants. 2024; 13(12):1611. https://doi.org/10.3390/plants13121611
Chicago/Turabian StyleDėnė, Lina, Simona Chrapačienė, Greta Laurinaitytė, Aira Rudinskaitė, Jonas Viškelis, Pranas Viškelis, and Aistė Balčiūnaitienė. 2024. "Green Synthesis of Silver Nanoparticles with Hyssopus officinalis and Salvia officinalis Extracts, Their Properties, and Antifungal Activity on Fusarium spp." Plants 13, no. 12: 1611. https://doi.org/10.3390/plants13121611










