Asymmetric Introgression and Cryptic Natural Hybridization between Two Species of Teucrium Section Polium (Lamiaceae) on the Balkan Peninsula
Abstract
:1. Introduction
2. Results
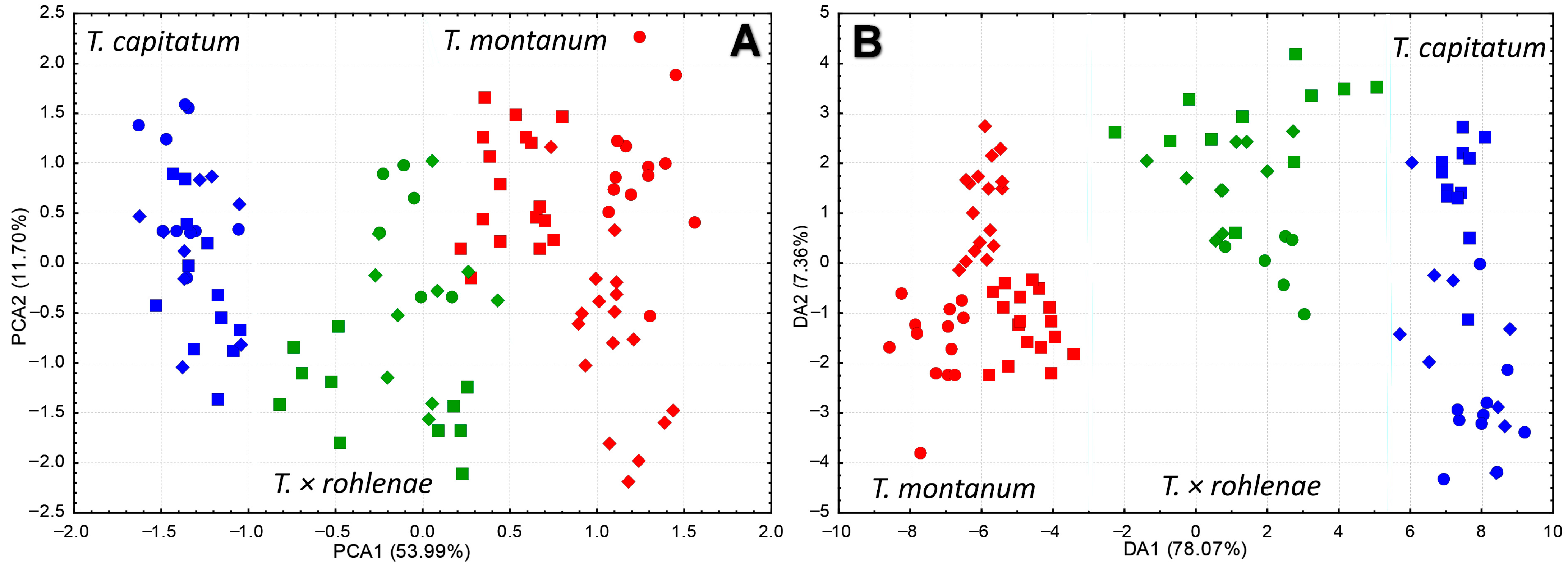
2.1. Morphological Diversification of Syntopic Populations of T. montanum, T. capitatum and T. × rohlenae
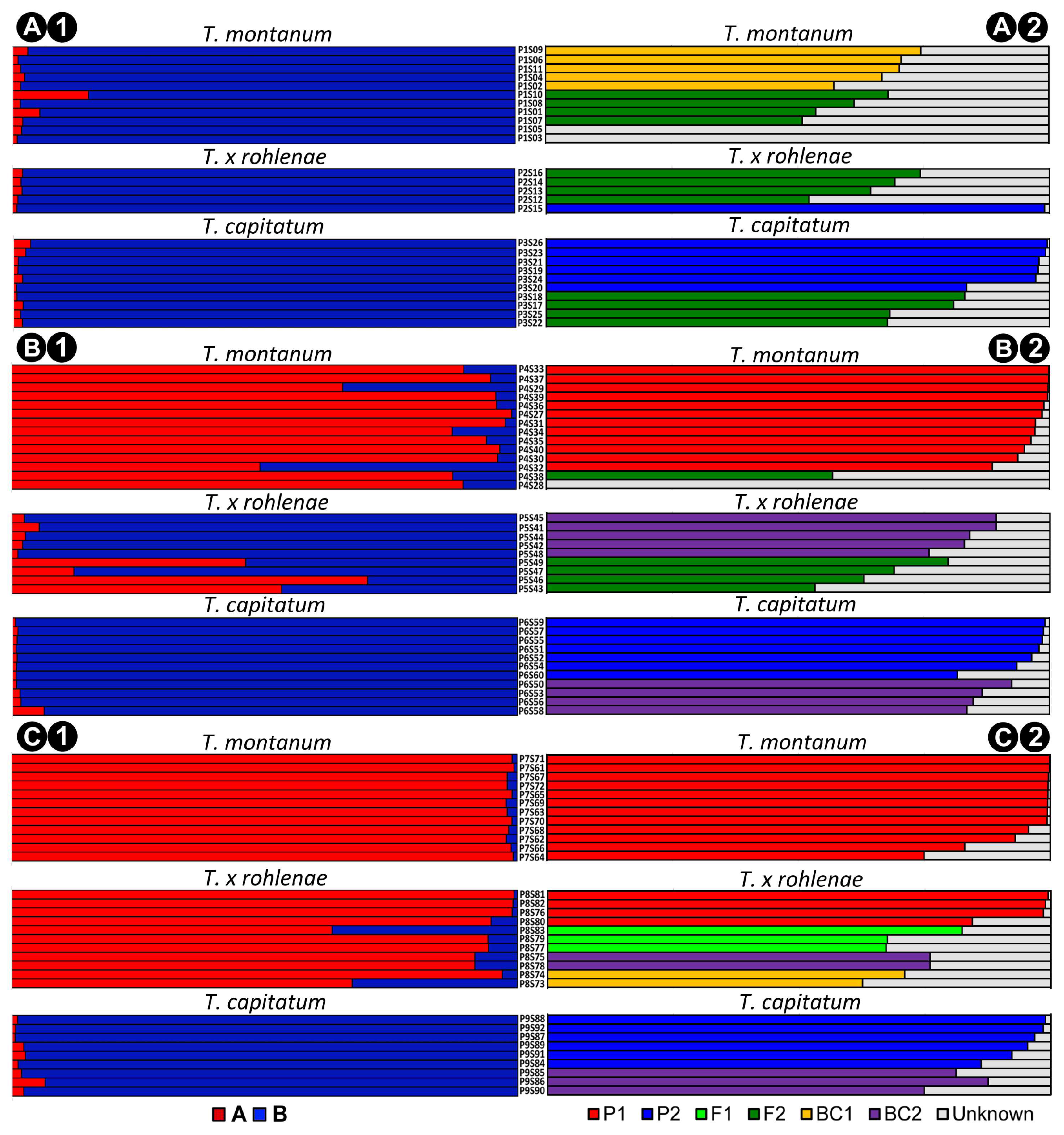
2.2. Genetic Structure and Gene Flow between the Species T. montanum and T. capitatum
3. Discussion
4. Materials and Methods
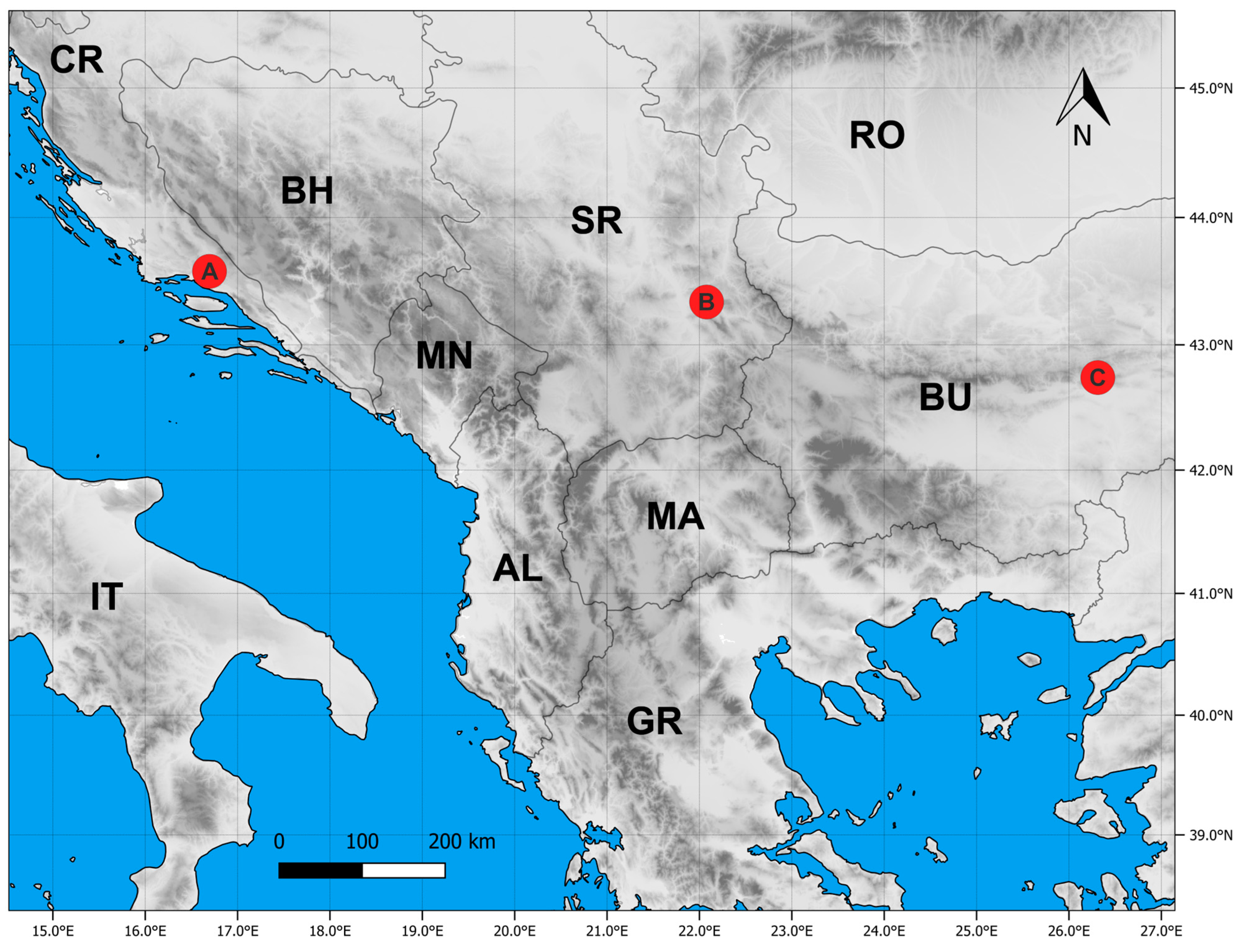
4.1. Plant Material
4.2. Taxonomy and Nomenclature
4.3. Morpho-Anatomical Analyses
4.4. Statistical Analyses
4.5. Microsatellite Analysis
4.6. Intrapopulation Genetic Diversity
4.7. Population Genetic Differentiation and Structure
4.8. Hybrid Assignment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riseberg, L.H.; Wendel, J.F. Introgression and Its Consequences. In Hybrid Zones and the Evolutionary Process; Oxford University Press: New York, NY, USA, 1993; pp. 70–109. [Google Scholar]
- Rhymer, J.M.; Simberloff, D. Extinction by Hybridization and Introgression. Annu. Rev. Ecol. Syst. 1996, 27, 83–109. [Google Scholar] [CrossRef]
- Yan, L.-J.; Burgess, K.S.; Milne, R.; Fu, C.-N.; Li, D.-Z.; Gao, L.-M. Asymmetrical Natural Hybridization Varies among Hybrid Swarms between Two Diploid Rhododendron Species. Ann. Bot. 2017, 120, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Grant, V. Factors Promoting Polyploidy. In Plant Speciation; Columbia University Press: New York, NY, USA, 1981; pp. 307–323. [Google Scholar]
- Tiffin, P.; Olson, S.; Moyle, L.C. Asymmetrical Crossing Barriers in Angiosperms. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 861–867. [Google Scholar] [CrossRef]
- Harley, R.M.; Atkins, S.; Budantsev, A.L.; Cantino, P.D.; Conn, B.J.; Grayer, R.; Harley, M.M.; De Kok, R.; Krestovskaja, T.; Morales, R.; et al. Labiatae. In Flowering Plants Dicotyledons; Kadereit, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–275. [Google Scholar] [CrossRef]
- Kovacevic, N.N.; Lakusic, B.S.; Ristic, M.S. Composition of the Essential Oils of Seven Teucrium Species from Serbia and Montenegro. J. Essent. Oil Res. 2001, 13, 163–165. [Google Scholar] [CrossRef]
- Grubešić, R.; Kremer, D.; Vladimir-Knežević, S.; Rodríguez, J. Analysis of Polyphenols, Phytosterols, and Bitter Principles in Teucrium L. Species. Open Life Sci. 2012, 7, 542–550. [Google Scholar] [CrossRef]
- Radulović, N.; Dekić, M.; Joksović, M.; Vukićević, R. Chemotaxonomy of Serbian Teucrium Species Inferred from Essential Oil Chemical Composition: The Case of Teucrium scordium L. ssp. Scordioides. Chem. Biodivers. 2012, 9, 106–122. [Google Scholar] [CrossRef]
- Mitreski, I.; Stanoeva, J.P.; Stefova, M.; Stefkov, G.; Kulevanova, S. Polyphenols in Representative Teucrium Species in the Flora of R. Macedonia: LC/DAD/ESI-MS n Profile and Content. Nat. Prod. Commun. 2014, 9, 175–180. [Google Scholar] [CrossRef]
- Boulila, A.; Béjaoui, A.; Messaoud, C.; Boussaid, M. Variation of Volatiles in Tunisian Populations of Teucrium polium L. (Lamiaceae). Chem. Biodivers. 2008, 5, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, S.F.; Barrek, S.; Skanji, T.; Zarrouk, H.; Ghrabi, Z.G. Fatty Acid, Tocopherol, and Sterol Content of Three Teucrium Species from Tunisia. Chem. Nat. Compd. 2009, 45, 304–308. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Niciforovic, N.; Topuzovic, M.; Solujic, S. Total Phenolic Content, Flavonoid Concentrations and Antioxidant Activity, of the Whole Plant and Plant Parts Extracts from Teucrium Montanum L. Var. Montanum, f. Supinum (L.) Reichenb. Biotechnol. Biotechnol. Equip. 2011, 25, 2222–2227. [Google Scholar] [CrossRef]
- Šeremet, D.; Cebin, A.V.; Mandura, A.; Komes, D. Valorisation of Teucrium Montanum as a Source of Valuable Natural Compounds: Bioactive Content, Antimicrobial and Biological Activity—A Review. Pharmacogn. Rev. 2021, 15, 191–198. [Google Scholar] [CrossRef]
- Aćimović, M.; Stanković Jeremić, J.; Miljković, A.; Rat, M.; Lončar, B. Screening of Volatile Compounds, Traditional and Modern Phytotherapy Approaches of Selected Non-Aromatic Medicinal Plants (Lamiaceae, Lamioideae) from Rtanj Mountain, Eastern Serbia. Molecules 2023, 28, 4611. [Google Scholar] [CrossRef] [PubMed]
- Meusel, H.; Jäger, E.J.; Rauschert, S.; Weinert, E. (Eds.) Vergleichende Chorologie der Zentraleuropäischen Flora: Karte; VEB Gustav Fischer Verlag: Jena, Germany, 1978. [Google Scholar]
- Navarro, T.; El Oualidi, J. Synopsis of Teucrium L. (Labiatae) in the Mediterranean Region and Surrounding Areas. Fl. Medit. 2000, 10, 349–363. [Google Scholar]
- Salmaki, Y.; Kattari, S.; Heubl, G.; Bräuchler, C. Phylogeny of Non-monophyletic Teucrium (Lamiaceae: Ajugoideae): Implications for Character Evolution and Taxonomy. Taxon 2016, 65, 805–822. [Google Scholar] [CrossRef]
- Tutin, T.G.; Wood, D. Teucrium. In Flora Europaea, 1st ed.; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D., Valentine, D., Walters, S., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 129–135. [Google Scholar]
- Baden, C. Teucrium L. In Mountain Flora of Greece, 2nd ed.; Strid, A., Tan, K., Eds.; Edinburgh University Press: Edinburgh, UK, 1991; pp. 69–75. [Google Scholar]
- Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 13 March 2024).
- Zbiljić, M. Morfoanatomska i Genotipska Diverzifikacija Teucrium Montanum Sensu Lato Na Prostoru Balkanskog Poluostrva. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2023. [Google Scholar]
- Zbiljić, M.; Lakušić, B.; Marčetić, M.; Bogdanović, S.; Lakušić, D. Morphological and Chemical Evidence of Teucrium × Rohlenae K. Malý (Lamiaceae), a New Hybrid in Croatia. Acta Bot. Croat. 2021, 80, 48–55. [Google Scholar] [CrossRef]
- Niketić, M.; Tomović, G.; Anačkov, G.; Đorđević, V.; Đurović, S.; Duraki, Š.; Kabaš, E.; Lakušić, D.; Petkovski, G.; Petrović, S. Material on the Annotated Checklist of Vascular Flora of Serbia: Nomenclatural, Taxonomic and Floristic Notes IV. Bull. Nat. Hist. Mus. 2022, 15, 27–96. [Google Scholar] [CrossRef]
- Varga, F.; Liber, Z.; Turudić, A.; Jakše, J.; Juzbašić, L.; Jeran, N.; Grdiša, M.; Zbiljić, M.; Šatović, Z. Joint Identification and Application of Microsatellite Markers in Genetic Diversity Study of Closely Related Species Teucrium montanum, T. capitatum and Their Natural Hybrid. Diversity 2024, 16, 206. [Google Scholar] [CrossRef]
- Turudić, A.; Liber, Z.; Grdiša, M.; Jakše, J.; Varga, F.; Poljak, I.; Šatović, Z. Dig-up Primers: A Pipeline for Identification of Polymorphic Microsatellites Loci within Assemblies of Related Species. Int. J. Mol. Sci. 2024, 25, 3169. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.C.; Thompson, E. A Model-Based Method for Identifying Species Hybrids Using Multilocus Genetic Data. Genetics 2002, 160, 1217–1229. [Google Scholar] [CrossRef]
- Lexer, C.; Buerkle, C.A.; Joseph, J.A.; Heinze, B.; Fay, M.F. Admixture in European Populus Hybrid Zones Makes Feasible the Mapping of Loci That Contribute to Reproductive Isolation and Trait Differences. Heredity 2007, 98, 74–84. [Google Scholar] [CrossRef]
- Minder, A.M.; Rothenbuehler, C.; Widmer, A. Genetic Structure of Hybrid Zones between Silene latifolia and Silene dioica (Caryophyllaceae): Evidence for Introgressive Hybridization. Mol. Ecol. 2007, 16, 2504–2516. [Google Scholar] [CrossRef]
- Petit, R.J.; Bodénès, C.; Ducousso, A.; Roussel, G.; Kremer, A. Hybridization as a Mechanism of Invasion in Oaks. New Phytol. 2004, 161, 151–164. [Google Scholar] [CrossRef]
- Burgess, K.S.; Morgan, M.; Deverno, L.; Husband, B.C. Asymmetrical Introgression between Two Morus Species (M. alba, M. rubra) That Differ in Abundance. Mol. Ecol. 2005, 14, 3471–3483. [Google Scholar] [CrossRef] [PubMed]
- Godbout, J.; Yeh, F.C.; Bousquet, J. Large-scale Asymmetric Introgression of Cytoplasmic DNA Reveals Holocene Range Displacement in a North American Boreal Pine Complex. Ecol. Evol. 2012, 2, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Du, F.K.; Peng, X.L.; Liu, J.Q.; Lascoux, M.; Hu, F.S.; Petit, R.J. Direction and Extent of Organelle DNA Introgression between Two Spruce Species in the Qinghai-Tibetan Plateau. New Phytol. 2011, 192, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Petrova, E.A.; Zhuk, E.A.; Popov, A.G.; Bondar, A.A.; Belokon, M.M.; Goroshkevich, S.N.; Vasilyeva, G.V. Asymmetric Introgression between Pinus sibirica and Pinus pumila in the Aldan Plateau (Eastern Siberia). Silvae Genet. 2018, 67, 66–71. [Google Scholar] [CrossRef]
- Thórsson, Æ.T.; Salmela, E.; Anamthawat-Jónsson, K. Morphological, Cytogenetic, and Molecular Evidence for Introgressive Hybridization in Birch. J. Hered. 2001, 92, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Radosavljević, I.; Bogdanović, S.; Celep, F.; Filipović, M.; Satovic, Z.; Surina, B.; Liber, Z. Morphological, Genetic and Epigenetic Aspects of Homoploid Hybridization between Salvia officinalis L. and Salvia fruticosa Mill. Sci. Rep. 2019, 9, 3276. [Google Scholar] [CrossRef] [PubMed]
- Lakušić, B. Morfološka Varijabilnost i Ekološka Diferencijacija Vrsta Roda Teucrium L. (Lamiaceae) u Jugoslaviji. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2000. [Google Scholar]
- Lakušić, B.; Stevanović, B.; Jančić, R.; Lakušić, D. Habitat-Related Adaptations in Morphology and Anatomy of Teucrium (Lamiaceae) Species from the Balkan peninsula (Serbia and Montenegro). Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 633–646. [Google Scholar] [CrossRef]
- Lihová, J.; Kučera, J.; Perný, M.; Marhold, K. Hybridization between Two Polyploid Cardamine (Brassicaceae) Species in North-Western Spain: Discordance between Morphological and Genetic Variation Patterns. Ann. Bot. 2007, 99, 1083–1096. [Google Scholar] [CrossRef]
- Čertner, M.; Kolář, F.; Schönswetter, P.; Frajman, B. Does Hybridization with a Widespread Congener Threaten the Long-term Persistence of the Eastern Alpine Rare Local Endemic Knautia carinthiaca? Ecol. Evol. 2015, 5, 4263–4276. [Google Scholar] [CrossRef] [PubMed]
- Tendal, K.; Ørgaard, M.; Larsen, B.; Pedersen, C. Recurrent Hybridisation Events between Primula vulgaris, P. veris and P. elatior (Primulaceae, Ericales) Challenge the Species Boundaries: Using Molecular Markers to Re-evaluate Morphological Identifications. Nord. J. Bot. 2018, 36, e01778. [Google Scholar] [CrossRef]
- Beirinckx, L.; Vanschoenwinkel, B.; Triest, L. Hidden Hybridization and Habitat Differentiation in a Mediterranean Macrophyte, the Euryhaline Genus Ruppia. Front. Plant Sci. 2020, 11, 516856. [Google Scholar] [CrossRef] [PubMed]
- Lazarević, M.; Siljak-Yakovlev, S.; Sanino, A.; Lamy, F.; Hinsinger, D.D. Genetic Variability in Balkan Paleoendemic Resurrection Plants Ramonda serbica and R. nathaliae across Their Range and in the Zone of Sympatry. Front. Plant Sci. 2022, 13, 873471. [Google Scholar] [CrossRef] [PubMed]
- Zlatković, B. Flora Sićevačke Klisure. Bachelor’s Thesis, Prirodno Matematički Fakultet, Department za Biologiju, University of Novi Sad, Novi Sad, Serbia, 1999. [Google Scholar]
- Slatkin, M. Gene Flow in Natural Populations. Annu. Rev. Ecol. Syst. 1985, 16, 393–430. [Google Scholar] [CrossRef]
- Slatkin, M. Gene Flow and the Geographic Structure of Natural Populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Soltis, D.E. The Role of Hybridization in Plant Speciation. Annu. Rev. Plant Biol. 2009, 60, 561–588. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. The New York Botanical Garden. 2024. Available online: http://sweetgum.nybg.org/ih/ (accessed on 13 March 2024).
- MedCalc Software. Digimizer Image Analysis Software; Version 4.6.1.0; MedCalc Software: Ostend, Belgium, 2005–2011; Available online: http://www.digimizer.com/ (accessed on 14 April 2016).
- Zbiljić, M.; Lakušić, B.; Kuzmanović, N.; Stojanović, D.; Lakušić, D. Morphological Diversification of Teucrium montanum sensu lato on the Balkan Peninsula. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2023, 157, 670–687. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System); Version 7.0; StatSoft Inc.: Tulsa, OK, USA, 2007. [Google Scholar]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising How the Computer Program CERVUS Accommodates Genotyping Error Increases Success in Paternity Assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.; Shipley, P. MICRO-CHECKER: Software for Identifying and Correcting Genotyping Errors in Microsatellite Data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Chapuis, M.-P.; Estoup, A. Microsatellite Null Alleles and Estimation of Population Differentiation. Mol. Biol. Evol. 2007, 24, 621–631. [Google Scholar] [CrossRef]
- Rousset, F. Genepop’007: A Complete Re-implementation of the Genepop Software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- SAS Institute Inc. Base SAS 9.4 Procedures Guide: Statistical Procedures; SAS Institute: Cary, NC, USA, 2011; ISBN 978-1-60764-944-1. [Google Scholar]
- Kalinowski, S.T. Hp-rare 1.0: A Computer Program for Performing Rarefaction on Measures of Allelic Richness. Mol. Ecol. Notes 2005, 5, 187–189. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT (Version 1.2): A Computer Program to Calculate F-Statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Belkhir, K. GENETIX 4.05, Logiciel Sous Windows TM Pour La Génétique Des Populations. 2004. Available online: http://www.genetix.univ-montp2.fr/genetix/genetix.htm (accessed on 1 March 2023).
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the Number of Clusters of Individuals Using the Software STRUCTURE: A Simulation Study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Earl, D.A.; VonHoldt, B.M. STRUCTURE HARVESTER: A Website and Program for Visualizing STRUCTURE Output and Implementing the Evanno Method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Kopelman, N.M.; Mayzel, J.; Jakobsson, M.; Rosenberg, N.A.; Mayrose, I. Clumpak: A Program for Identifying Clustering Modes and Packaging Population Structure Inferences across K. Mol. Ecol. Resour. 2015, 15, 1179–1191. [Google Scholar] [CrossRef] [PubMed]


| Characters | Acro | Wilks’ Lambda | Partial Lambda | F-remove (8.82) | P-Level |
|---|---|---|---|---|---|
| Central nerve—radius (µm) | R_CN | 0.000127 | 0.786839 | 2.77681 | 0.009071 |
| Indumentum adaxial (percentage of coverage) | C_In-ad | 0.000147 | 0.680441 | 4.81376 | 0.000070 |
| Number of capitate hairs | No_CH | 0.000135 | 0.740342 | 3.59495 | 0.001267 |
| Thickness of adaxial epidermal cells (µm) | T_Epi-ad | 0.000118 | 0.848545 | 1.82950 | 0.083127 |
| Thickness of cuticle (µm) | T_Cut | 0.000254 | 0.392434 | 15.86904 | 0.000000 |
| Leaf surface (mm²) | L_S | 0.000143 | 0.696917 | 4.45764 | 0.000162 |
| Leaf base width | L_B_L | 0.000113 | 0.883877 | 1.34664 | 0.232638 |
| Number of teeth on leaf margin | L_T | 0.000219 | 0.454552 | 12.29969 | 0.000000 |
| Bract length | B_L | 0.000122 | 0.817758 | 2.28428 | 0.029235 |
| Frequency of stipules on stem’s nodes | Frequ_S | 0.000128 | 0.779433 | 2.90059 | 0.006739 |
| Average length of first three internodes | Avg_L_F_I | 0.000128 | 0.776624 | 2.94815 | 0.006011 |
| Distance between calyx base and tooth base | D_Cal_b_T_b | 0.000141 | 0.707617 | 4.23524 | 0.000274 |
| Length of the narrow part of tooth | L_n_T | 0.000216 | 0.461037 | 11.98250 | 0.000000 |
| Number of flowers in terminal inflorescence | No_F_I | 0.000128 | 0.782079 | 2.85609 | 0.007499 |
| Number of terminal inflorescences | No_I | 0.000127 | 0.785021 | 2.80698 | 0.008437 |
| Configuration | Locality | All Localities | ||
|---|---|---|---|---|
| Trilj | Sićevo | Sliven | ||
| Na | Na | Na | Na | |
| OnlyTm | 43 | 38 | 21 | 29 |
| OnlyTx | 16 | 19 | 10 | 14 |
| OnlyTc | 27 | 21 | 38 | 22 |
| TmTx | 14 | 21 | 24 | 29 |
| TmTc | 27 | 15 | 5 | 24 |
| TxTc | 6 | 18 | 15 | 17 |
| All | 22 | 12 | 15 | 78 |
| Total | 155 | 144 | 128 | 213 |
| Locality | Species | n | Na | Nar | Npr | Npar | HO | HE | FIS | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Trilj | T. montanum | 11 | 11.778 | 7.192 | 8 | 1.026 | 0.889 | 0.910 | 0.023 | ns |
| T. × rohlenae | 5 | 6.444 | 6.444 | 4 | 1.015 | 0.778 | 0.867 | 0.103 | ns | |
| T. capitatum | 10 | 9.111 | 6.067 | 7 | 0.908 | 0.667 | 0.819 | 0.186 | *** | |
| Sićevo | T. montanum | 14 | 9.556 | 5.743 | 9 | 0.754 | 0.746 | 0.799 | 0.066 | ns |
| T. × rohlenae | 9 | 7.778 | 5.841 | 9 | 0.988 | 0.877 | 0.852 | −0.029 | ns | |
| T. capitatum | 11 | 7.333 | 5.242 | 6 | 0.567 | 0.768 | 0.796 | 0.036 | ns | |
| Sliven | T. montanum | 12 | 7.222 | 5.029 | 2 | 0.361 | 0.713 | 0.766 | 0.069 | ns |
| T. × rohlenae | 11 | 7.111 | 5.265 | 1 | 0.353 | 0.808 | 0.818 | 0.012 | ns | |
| T. capitatum | 9 | 8.111 | 5.918 | 6 | 0.640 | 0.840 | 0.817 | −0.027 | ns |
| Pop | Locality | Species | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | Trilj | T. montanum | ns | *** | *** | ** | *** | *** | *** | *** | |
| P2 | Trilj | T. × rohlenae | 0.040 | * | ** | * | * | ** | ** | * | |
| P3 | Trilj | T. capitatum | 0.088 | 0.033 | *** | *** | *** | *** | ** | ** | |
| P4 | Sićevo | T. montanum | 0.095 | 0.139 | 0.154 | *** | *** | *** | *** | *** | |
| P5 | Sićevo | T. × rohlenae | 0.065 | 0.070 | 0.087 | 0.086 | *** | *** | *** | ** | |
| P6 | Sićevo | T. capitatum | 0.087 | 0.106 | 0.117 | 0.174 | 0.071 | *** | ** | ** | |
| P7 | Sliven | T. montanum | 0.108 | 0.160 | 0.179 | 0.097 | 0.095 | 0.193 | ** | ** | |
| P8 | Sliven | T. × rohlenae | 0.093 | 0.096 | 0.092 | 0.127 | 0.078 | 0.128 | 0.058 | ** | |
| P9 | Sliven | T. capitatum | 0.084 | 0.062 | 0.050 | 0.159 | 0.068 | 0.077 | 0.169 | 0.079 |
| State Code | Locality | Alt | Lat | Long | Substrate | Habitat |
|---|---|---|---|---|---|---|
| CR | Trilj | 340 | 43.579 | 16.696 | Limestone | Sub-Mediterranean rocky grasslands (Thero-Brachypodietea) |
| SR | Sićevo | 429 | 43.338 | 22.078 | Limestone | Continental rocky grasslands(Festuco-Brometea) |
| BU | Sliven | 953 | 42.74 | 26.313 | Limestone | Mountain rocky grasslands (Festuco-Seslerietea) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakušić, D.; Zbiljić, M.; Šatović, Z.; Kuzmanović, N.; Liber, Z. Asymmetric Introgression and Cryptic Natural Hybridization between Two Species of Teucrium Section Polium (Lamiaceae) on the Balkan Peninsula. Plants 2024, 13, 1617. https://doi.org/10.3390/plants13121617
Lakušić D, Zbiljić M, Šatović Z, Kuzmanović N, Liber Z. Asymmetric Introgression and Cryptic Natural Hybridization between Two Species of Teucrium Section Polium (Lamiaceae) on the Balkan Peninsula. Plants. 2024; 13(12):1617. https://doi.org/10.3390/plants13121617
Chicago/Turabian StyleLakušić, Dmitar, Miloš Zbiljić, Zlatko Šatović, Nevena Kuzmanović, and Zlatko Liber. 2024. "Asymmetric Introgression and Cryptic Natural Hybridization between Two Species of Teucrium Section Polium (Lamiaceae) on the Balkan Peninsula" Plants 13, no. 12: 1617. https://doi.org/10.3390/plants13121617
APA StyleLakušić, D., Zbiljić, M., Šatović, Z., Kuzmanović, N., & Liber, Z. (2024). Asymmetric Introgression and Cryptic Natural Hybridization between Two Species of Teucrium Section Polium (Lamiaceae) on the Balkan Peninsula. Plants, 13(12), 1617. https://doi.org/10.3390/plants13121617







