The Micromorphology and Its Taxonomic Value of the Genus Sanicula L. in China (Apiaceae)
Abstract
:1. Introduction
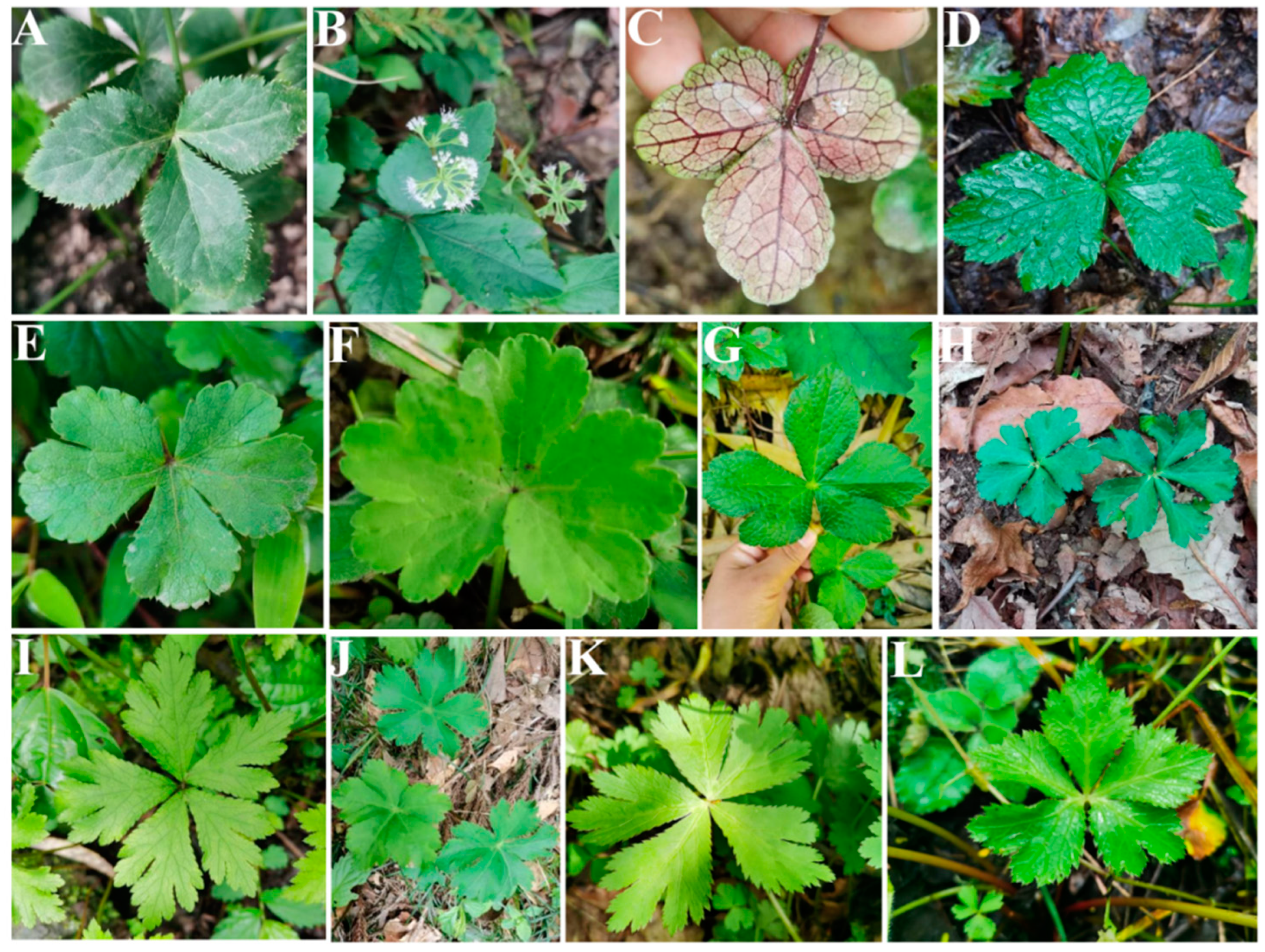
2. Material and Method
2.1. The Micromorphological Study on the Leaf Epidermis of the Genus Sanicula L.
- (1)
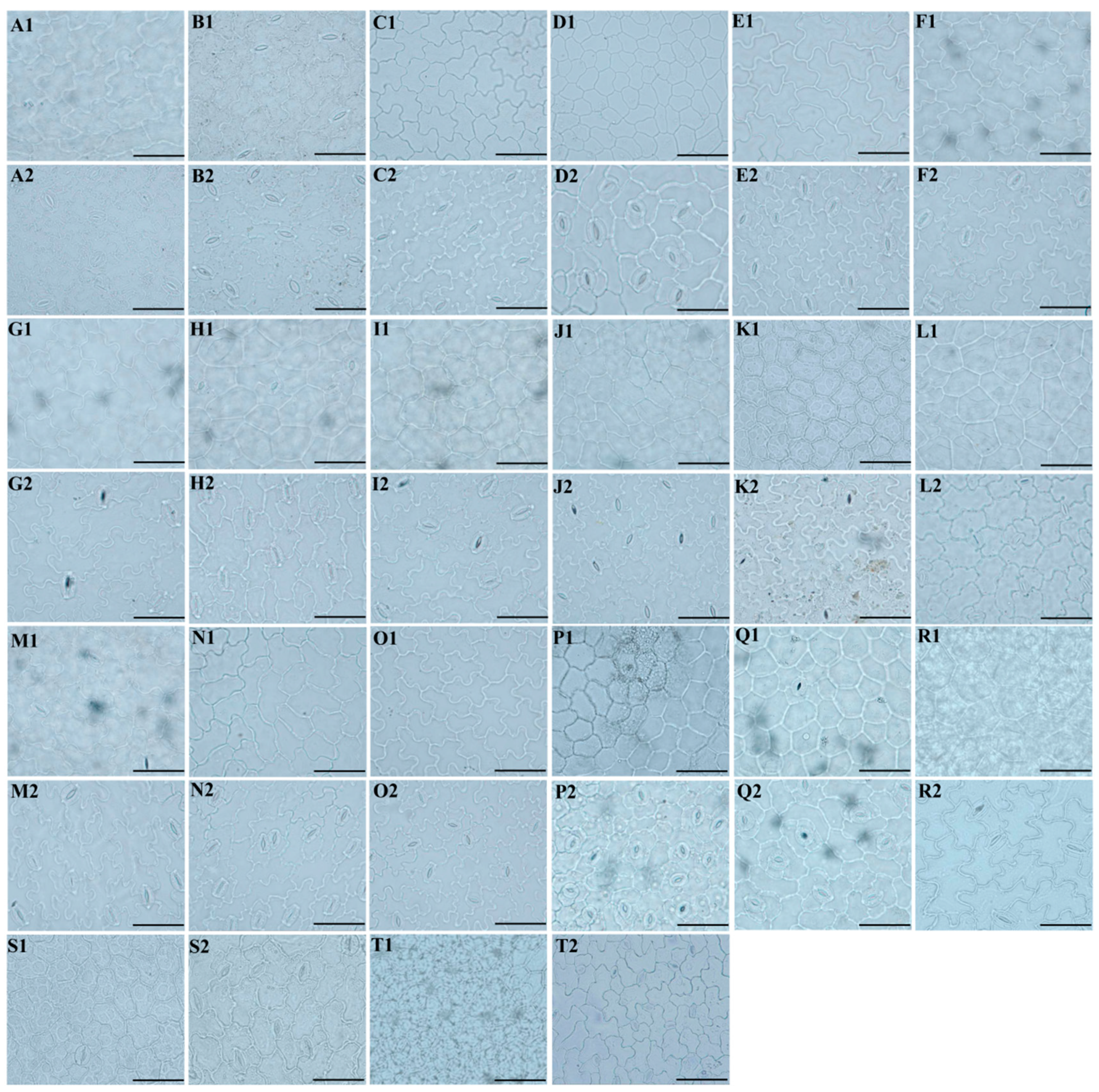
- Light microscope observation: Silica gel-dried basal leaves were placed in 30% sodium hypochlorite solution (NaClO3) in the dark treatment for 1–3 h. When the leaves faded to white and were nearly transparent, we removed the leaves and immersed them in distilled water for 5–10 min. Then, the leaves were removed and placed on a glass plate, and we gently tore the thin and transparent upper and lower epidermis with tweezers and placed them on a slide with a drop of distilled water to unfold, covered with a coverslip, gently pressed to squeeze out the excess water and air under the coverslip, and the excess water was carefully sucked off with absorbent paper to make a temporary slide. Finally, under 10 × 40 magnification, we respectively selected six fields of view with high clarity of the upper and lower epidermis, used a light microscope (Olympus-BX51) to observe the cells and stomata of the leaf epidermis, and photographed and preserved them. We randomly selected thirty stomata in the lower epidermis of each taxon, and due to the small number of stomata in the upper epidermis of some species, all stomata were selected. Then, we observed the cell shape, the type of stomatal apparatus of both the upper and lower epidermis, counted the number of cells, measured the size of the stomatal apparatus, and calculated the stomatal density and stomatal index. Stomatal density: number of stomata per unit area/unit area. Stomatal index: number of stomata per unit area/(number of stomata per unit area + number of epidermal cells in the same unit area) × 100%. The above measurement processes were carried out in MATO v4.2 software [36].
- (2)
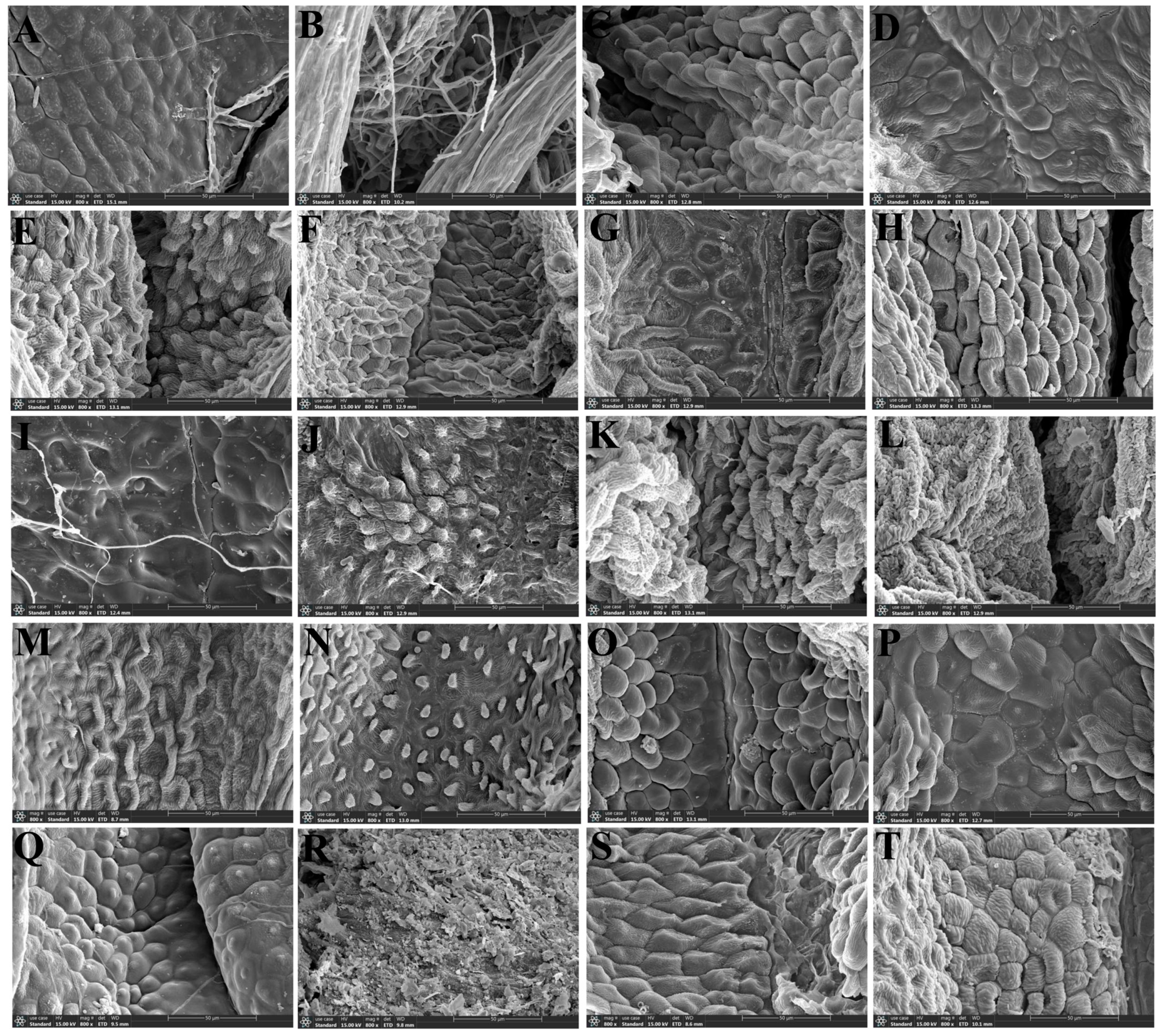
- Scanning electron microscope observation: The silica gel-dried basal leaves were washed with distilled water and then treated with different concentrations of ethanol for gradient dehydration (15%, 30%, 50%, 70%, 85%, 95% Ⅰ, 95% Ⅱ, 100% Ⅰ, 100% Ⅱ), with each concentration being processed for 1 min. Then, we took out the leaves and dried them naturally. Subsequently, the upper and lower epidermis of each species were cut out and were pasted on double-sided conductive adhesive and sprayed with gold coating, respectively. Finally, we observed and photographed the micromorphological features of the upper and lower epidermis using a JSM-7500F scanning electron microscope.
2.2. The Micromorphological and Anatomical Study on the Fruits of the Genus Sanicula L.
- (1)
- Observation of external micromorphological characteristics of fruits. We removed the fruits from the FAA fixation fluid and placed them on absorbent paper to allow them to dry naturally. Then, ten morphologically intact fruits of each species were randomly selected for observation under a stereomicroscope (SMZ25, Nikon Corp., Tokyo, Japan), and then the dorsal and commissure views of fruits of each species were observed, photographed and preserved. Finally, the length and width values of ten fruits of each species were measured using MATO v4.2 software [36], and we then calculated the mean values.
- (2)
- Observation of anatomical characteristics of fruits.
- a:
- Freehand slicing method. We observed and randomly selected ten morphologically intact fruits of each species using a stereomicroscope (SMZ25, Nikon Corp., Tokyo, Japan). Then, we used double-layer blades to cut the fruits in the vertical horizontal plane at the middle position of the fruits. Finally, we observed and photographed the transverse section of the fruits with a stereomicroscope (SMZ25, Nikon Corp., Tokyo, Japan).
- b:
- Paraffin sectioning method. We observed and randomly selected one or two morphologically intact fruits of each species and placed them in FAA fixation fluid for 2–24 h. After the fixation process was completed, we removed them and washed them with distilled water 2–3 times. Then, the fruits were subjected to gradient dehydration treatment with ethanol (30%, 50%, 70%, 80%, 90%, 100%) for 1 h at each concentration. After the gradient dehydration process was completed, we used xylene for deethanolization for 30 min. Finally, we permeabilized, embedded, sectioned, glued, dewaxed the treated fruit materials and stained them using toluidine blue solution (Toluidine Blue solution), and meanwhile, the slices were sealed with neutral gum to create permanent mounts. The anatomical features of the fruits (such as the vittae, endosperm, exocarp and mesocarp) were observed and photographed using a stereomicroscope (SMZ25, Nikon Corp., Tokyo, Japan).
- (3)
- Observation of micromorphological characteristics of fruits: We randomly selected one or two morphologically intact fruits of each species from the FAA fixation fluid. The remaining steps referred to the scanning electron microscopy method for leaf epidermis (Section 2.1 (2)).
2.3. The Morphological Study on the Pollen of the Genus Sanicula L.
- (1)
- Light microscope observation: The G. Erdtman acetic anhydride decomposition method [42] was used to treat pollen, and the specific steps were as follows:
- a:
- Take an appropriate amount of mature, dry and full pollen of each species in a 5 mL cryostat tube and submerge the pollen with 1–2 mL of glacial acetic acid for 24 h;
- b:
- Use a glass rod to thoroughly crush the pollen in the cryostat tube, filter impurities with a 100-mesh copper mesh, wash with glacial acetic acid and collect 5 mL of filtrate into a new cryostat tube;
- c:
- Put the new cryostat tube in a centrifuge at 5000 r/min for 10 min and then repeat again;
- d:
- Add a mixture of acetic anhydride and concentrate sulfuric acid (volume ratio = 9:1, currently used and prepared) into the cryostat tube containing discarded supernatant and add dropwise to 5 mL;
- e:
- Place the frozen tube in a water bath at 80 °C for 4 min, then remove and centrifuge at 5000 r/min for 10 min;
- f:
- Discard the supernatant from the frozen tube, add 4 mL of distilled water and centrifuge at 5000 r/min for 10 min;
- g:
- Discard the supernatant from the freezing tube, then add 2–3 drops of glycerol to the freezing tube and mix well;
- h:
- Pipette 1–2 drops of pollen suspension onto a glass slide and create a temporary slide.
- (2)
- Scanning electron microscope observation: Firstly, we observed and randomly selected ten dry, mature and full anthers of each species under a dissecting microscope. Then, the selected anthers were pasted on the double-sided conductive adhesive, and the pollen capsule was gently poked with a clean dissecting needle to release the pollen grains, so that they were evenly spread in all areas of the conductive adhesive, and the shell of the pollen was clamped away with tweezers. Finally, after gold spray coating, we used a JSM-7500F scanning electron microscope to observe and photograph the overall view of pollen, the polar view, the equatorial view, germ furrow and the exine ornamentation.
3. Results
3.1. The Morphological Characteristics of the Leaf Epidermis
3.1.1. Leaf Epidermal Characteristics by Light Microscope
3.1.2. Leaf Epidermal Characteristics by Scanning Electron Microscope
3.2. The Micromorphological and Anatomical Characteristics of the Fruits
3.3. The Micromorphological Characteristics of Pollen
4. Discussion
4.1. The Micromorphological Characteristics and Its Taxonomic Value of Leaf Epidermis in the Genus Sanicula L.
4.2. The Micromorphological Characteristics and Its Taxonomic Value of Fruit in the Genus Sanicula L.
4.3. The Micromorphological Characteristics and Its Taxonomic Value of Pollen in the Genus Sanicula L.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Wyk, B.E.; Tilney, P.M.; Magee, A.R. African Apiaceae: A Synopsis of the Apiaceae/Umbelliferae of Sub-Saharan Africa and Madagascar/Ben-Erik Van Wyk, Patricia M Tilney & Anthony R Magee; Briza Academic Books: Pretoria, South Africa, 2013. [Google Scholar]
- Li, H.M.; Wu, M.S.; Lai, Q.; Zhou, W.; Song, C.F. Complete chloroplast of four Sanicula taxa (Apiaceae) endemic to China: Lights into genome structure, comparative analysis, and phylogenetic relationships. BMC Plant Biol. 2023, 23, 444. [Google Scholar] [CrossRef] [PubMed]
- Song, B.N.; Liu, C.K.; Zhao, A.Q.; Tian, R.M.; Xie, D.F.; Xiao, Y.L.; Chen, H.; Zhou, S.D.; He, X.J. Phylogeny and diversification of genus Sanicula L. (Apiaceae): Novel insights from plastid phylogenomic analyses. BMC Plant Biol. 2024, 24, 70. [Google Scholar] [CrossRef] [PubMed]
- Pimenov, M.G.; Leonov, M.V. The Genera of the Umbelliferae: A Nomenclator; Royal Botanic Gardens: Kew, Richmond, UK, 1993. [Google Scholar] [CrossRef]
- Shan, R.H.; Constance, L. The Genus Sanicula (Umbelliferae) in the Old World and the New; University of California Press: California, UK, 1951; Volume 25, pp. 1–7. [Google Scholar]
- Sheh, M.L.; Phillippe, L.R. Sanicula L. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Beijing Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA; Science Press: Beijing, China, 2005; Volume 14, pp. 19–24. [Google Scholar]
- Xie, W.Y.; Ma, D.D.; Chen, F.; Wang, P.; Chen, J.F.; Chen, Z.H. Sanicula favovirens—A new species of the genus Sanicula (Umbelliferae) in Zhejiang. J. Hangzhou Norm. Univ. Nat. Sci. Ed. 2019, 18, 9–12. [Google Scholar]
- Liou, S.L. Sanicula L. In Flora Reipublicae Popularis Sinicae; Shan, R.H., Sheh, M.L., Eds.; Science Press: Beijing, China, 1979; Volume 55, pp. 35–63. [Google Scholar]
- Wolf, H. Umbelliferae-Saniculoideae. In Das Pfanzenreich; Engler, A., Ed.; Wilhelm Engelmann: Leipzig/Berlin, Germany, 1913; Volume IV (228), pp. 1–305. [Google Scholar]
- Pryer, K.M.; Phillippe, L.R. A synopsis of the genus Sanicula (Apiaceae) in eastern Canada. Can. J. Bot. 1989, 67, 694–707. [Google Scholar] [CrossRef]
- Calviño, C.I.; Downie, S.R. Circumscription and phylogeny of apiaceae subfamily saniculoideae based on chloroplast DNA sequences. Mol. Phylogenetics Evol. 2007, 44, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Behnke, H.D. The bases of angiosperm phylogeny: Ultrastructure. Ann. Mo. Bot. Gard. 1975, 62, 647–663. [Google Scholar] [CrossRef]
- Liang, X.L.; Shu, H.J.; Wang, T.; Cai, X.Z.; Cong, Y.Y.; Kuang, R.P. Research on karyotype analysis and leaf epidermal micromorphology of related species of Impatiens. Guihaia 2022, 42, 152–160. [Google Scholar]
- Yan, C.; Xiao, X.; Ran, Z.H.; Li, Z. Pollen morphology and leaf epidermal micromorphology of ten species (Camellia, sect. Tuberculata). Guihaia 2024, 1–14. [Google Scholar] [CrossRef]
- Wang, X.B.; Cao, Y.; Guo, W.; Ma, L.; Liu, X.L. Morphological characteristics of pollen from 44 species of Subgen. Yulania 2023, 50, 2417–2434. [Google Scholar] [CrossRef]
- Qin, X.Y.; Chang, Z.L.; Huang, X.M.; Hao, L.Z.; Fu, N.N.; Zhang, F.L. Study on the pollen and fruit morphology of Pugionium Gaertn. J. North. Agric. 2022, 50, 109–115. [Google Scholar]
- Yang, M.L.; Liao, C.Y. Palynological research on the Angelica species (Apiaceae) from North America. Acta Bot. Boreali-Occident. Sin. 2023, 43, 1236–1250. [Google Scholar]
- Zhou, X.T.; Yang, G.; Zhou, L.F.; Shao, A.J.; Wei, Y.; Chi, X.L.; Li, X.L. Study on the characteristics and microscopic identification of fruits of Bupleurum chinense and Bupleurum scorzonerifolium. Mod. Chin. Med. 2018, 20, 1251–1254. [Google Scholar] [CrossRef]
- Zhang, X.M.; He, X.J. Leaf epidermal characters of six species in Peucedanum and its taxonomic significance. Chin. Agric. Sci. Bull. 2009, 25, 177–180. [Google Scholar]
- Li, G.Q.; Hua, J.X. A comparison study on pollen morphology between Ferula and Heracleum of Umbeliferae. Chin. Agric. Sci. Bull. 2006, 22, 114–118. [Google Scholar]
- Liu, J. Systematics of Heracleum L. in Southwest China; Sichuan University: Chengdu, China, 2008. [Google Scholar]
- Li, L.B.; Yuan, C.Q.; Ting, C.; Cheo, T. A taxonomic study on the genus Cnidium in China. Acta Bot. Boreali-Occident. Sin. 1993, 13, 63–69+89–90. [Google Scholar]
- Ma, Y.H.; Zhang, X.M.; Zeng, C.T.; Zhao, H. Micro-morphology of leaf epidermis and its taxonomical significance of Sanicula L. Bull. Batanical Res. 2010, 30, 12–17. [Google Scholar]
- Yao, X.; Chen, Z.X.; Wang, Q.Z. Leaf epidermal micromorphological characters of 11 species of Sanicula L. Bull. Batanical Res. 2019, 39, 683–691. [Google Scholar]
- Guo, S.Q.; Wang, C.Q.; Liu, M.; Ru, J.; Wang, J.R.; Wang, X.W. Stomatal structures of some taxa in Apiaceae and their taxonomic values. Acta Bot. Boreali-Occident. Sin. 2016, 36, 1787–1793. [Google Scholar]
- Liu, Q.X.; Hui, H.; Li, B.Y.; Pan, Z.H. Comparative anatomical studies on the fruits of Saniculoideae in China (Umbelliferae) and its systematic significance. J. Plant Resour. Environ. 2002, 11, 1–8. [Google Scholar]
- Phillippe, L. A Biosystematic Study of Sanicula Section Sanicla; University of Tennessee: Knoxville, TN, USA, 1978. [Google Scholar]
- Ostroumova, T.A.; Kljuykov, E.V. Stomatal types in Chinese and Himalayan Umbelliferae. Feddes Repert. 2007, 118, 84–102. [Google Scholar] [CrossRef]
- Chen, Z.X.; Yao, X.Y.; Downie, S.R.; Wang, Q.Z. Fruit features of 15 species of Sanicula (Apiaceae) and their taxonomic significance. Plant Sci. J. 2019, 37, 1–9. [Google Scholar]
- Zhang, L.; Li, M.; Zhao, J.C. Fruit morphology of 23 species in Umbelliferae and its taxonomic significance. Acta Bot. Boreali-Occident. Sin. 2016, 36, 1787–1793. [Google Scholar]
- Chen, Z.X. Taxonomic Study on the Genus Sanicula (Apiaceae) from China. Master’s Thesis, Huaqiao University: Major of Biology, Quanzhou, China, 2019. [Google Scholar]
- Yang, C.; Chen, Z.X.; Yao, X.Y.; Wang, H.S.; Wang, Q.Z. Pollen morphology and systematic analysis of fifteen Sanicula species from China. Bull. Batanical Res. 2020, 40, 805–812. [Google Scholar]
- Baranova, M. Principles of comparative stomatographic studies of flowering plants. Bot. Rev. 1992, 58, 49–99. [Google Scholar] [CrossRef]
- Wilkinson, H.P. The Plant Surface (Mainly Leaf). Anatomy of the Dicotyledons, 2nd ed.; Clarendon Press: Oxford, UK, 1979; pp. 97–167. [Google Scholar] [CrossRef]
- Xu, X.R.; Zhou, S.D.; He, X.J. Pollen morphology and leaf epidermis micromorphological characters of genus Physospermopsis in China. Acta Bot. Boreali-Occident. Sin. 2021, 41, 766–774. [Google Scholar]
- Liu, L.J.; Wang, Q.; Zhang, Z.; Yu, Y. MATO: An updated tool for capturing and analyzing cytotaxonomic and morphological data. Innov. Life 2023, 1, 100010. [Google Scholar] [CrossRef]
- Kljuykov, E.V.; Liu, M.; Ostroumova, T.A.; Pimenov, M.G.; Tilney, P.M.; van Wyk, B.E.; van Stadenet, J. Towards a standardised terminology for taxonomically important morphological characters in the Umbelliferae. S. Afr. J. Bot. 2004, 70, 488–496. [Google Scholar] [CrossRef]
- Ostroumova, T.A. Fruit micromorphology of Siberian Apiaceae and its value for taxonomy of the family. Turczaninowia 2021, 24, 120–143. [Google Scholar] [CrossRef]
- Wang, K.F.; Wang, X.Z. Introduction to Pollen; Peking University Press: Beijing, China, 1983. [Google Scholar]
- Sheh, M.L.; Su, P. Pollen morphology of Hydrocotyloideae and Saniculoideae (Apiaceae) in China. J. Univ. Chin. Acad. Sci. 1992, 30, 126–136. [Google Scholar]
- Su, P.; Sheh, M.L. Pollen Atlas of Chinese Umbelliferae; Shanghai Science and Technology Press: Shanghai, China, 2001. [Google Scholar]
- Erdtman, G. Handbook of Spore Pollen. Spore Pollen Group of the Palaeobotanical Research Laboratory, Institute of Botany, Chinese Academy of Sciences, Translation; Science Press: Beijing, China, 1978. [Google Scholar]
- Stace, C.A. The significance of the leaf epidermis in the taxonomy of the Combretaceae: Conclusions. Bot. J. Linn. Soc. 1980, 81, 327–339. [Google Scholar] [CrossRef]
- Khan, R.; Abidin, S.Z.U.; Ahmad, M.; Zafar, M.; Liu, J.; Lubna; Jamshed, S.; Kiliç, Ö. Taxonomic importance of SEM and LM foliar epidermal micro-morphology: A tool for robust identification of gymnosperms. Flora 2019, 255, 42–68. [Google Scholar] [CrossRef]
- Zhang, H.; Zhuang, X.Y. Study on leaf epidermis of some plants of Theaceae. J. S. Chin. Agric. Univ. 2004, 25, 87–93. [Google Scholar]
- Ao, C.Q.; Chen, G.X.; Zhang, H.D. Leaf epidermis morphology of Camellia and its taxonomic significance. Acta Bot. Yunnan 2002, 24, 68–74. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, W.C.; Gong, X.; Liu, Z.W. Leaf epidermal morphology in Peucedanum L. (Umbelliferae) from China. Acta Bot. Gall. 2014, 161, 21–31. [Google Scholar] [CrossRef]
- Li, M.L.; Liao, C.Y.; Ye, C.; Zhang, J.Z.; Feng, T.Y.; Zhou, B. Analysis of leaf epidermal micromorphological characters of Ostericum Hoffm. (Apiaceae). Acta Bot. Boreali-Occident. Sin. 2017, 37, 1540–1549. [Google Scholar]
- Guo, X.L.; Yu, H.X.; Bai, J.; He, X.J. Morphological features of leaf epidermis of Chinese Chamaesium species (Apiaceae). Acta Bot. Boreali-Occident. Sin. 2017, 37, 486–494. [Google Scholar]
- Ren, H.Y.; Pang, Y.L.; He, X.J.; Deng, X.L.; Zhang, Y.C. Characters of leaf epidermis and systematic research in Pleurospermum from China. Acta Bot. Boreali-Occident. Sin. 2009, 29, 49–60. [Google Scholar]
- Zhou, J.; Liu, Z. Comparative morphology of the leaf epidermis in Ligusticum (Apiaceae) from China. Am. J. Plant Sci. 2018, 9, 1105–1123. [Google Scholar] [CrossRef]
- Li, H.M.; Song, C.F. Taxonomic studies on the genus Sanicula (Apiaceae) from China (I): The identity of S. orthacantha var. pumila and S. Pengshuiensis. Phytotaxa 2022, 532, 114–138. [Google Scholar] [CrossRef]
- Barthlott, W. Epidermal and seed surface characters of plants: Systematic applicability and some evolutionary aspects. Nord. J. Bot. 1981, 1, 345–355. [Google Scholar] [CrossRef]
- Ostroumova, T.A.; Pimenov, M.G. Carpological diversity of African Peucedanum s.l. (Umbelliferae). I. The species of southern Africa. Feddes Repert. 1997, 108, 299–318. [Google Scholar] [CrossRef]
- Chang, X.Q.; Liu, M.; Cheng, X.Y.; Wang, Y.T.; Wei, X.Y.; Wang, X.W. The phylogenetic significance of fruit structures in Bupleurum of the family Apiaceae. Acta Prataculturae Sin. 2015, 24, 108–119. [Google Scholar]
- Safina, L.K.; Pimenov, M.G. Carpology of the species of type subgenus of the genus Ferula and some problems of their systematics. Feddes Repert. 1990, 101, 135–151. [Google Scholar] [CrossRef]
- Cai, J.; Qin, H.H.; Lei, J.Q.; Liu, C.K.; He, X.J.; Zhou, S.D. The phylogeny of Seseli (Apiaceae, Apioideae): Insights from molecular and morphological data. BMC Plant Biol. 2022, 22, 534. [Google Scholar] [CrossRef]
- Liu, M.; Plunkett, G.M.; Lowry, P.P.; Van Wyk, B.E.; Tilney, P.M. The taxonomic value of fruit wing types in the order Apiales. Am. J. Bot. 2006, 93, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Kljuykov, E.V.; Zakharova, E.A.; Ostroumova, T.A.; Tilney, P.M. Most important carpological anatomical characters in the taxonomy of Apiaceae. Bot. J. Linn. Soc. 2021, 195, 532–544. [Google Scholar] [CrossRef]
- Tellería, M.C.; Daners, G. Pollen types in Southern New World Convolvulaceae and their taxonomic significance. Plant Syst. Evol. 2003, 243, 99–118. [Google Scholar] [CrossRef]
- Arora, A.; Modi, A. An acetolysis technique for pollen slide preparation. Indian J. Aerobiol. 2008, 21, 90–91. [Google Scholar]
- Qin, X.M.; Shen, G.M. Taxonomic study on the genus Ferula and its similar genera in Xinjiang. Arid Zone Res. 1990, 4, 23–33. [Google Scholar] [CrossRef]
- Meng, D.Y.; Zhou, S.D.; He, X.J. Pollen morphology of the genus Peucedanum from Sichuan and its systematic significance. Acta Bot. Boreali-Occident. Sin. 2004, 24, 2341–2345. [Google Scholar]
- Chen, W.W.; He, X.J. Zhang, X.M.; Pu, J.X. Pollen morphology of the genus Angelica from Southwest China and its systematic evolution analysis. Acta Bot. Boreali-Occident. Sin. 2007, 27, 1364–1372. [Google Scholar]
- Zhang, S.Y.; Liao, C.Y.; Li, M.L.; Chen, Y.; Zhou, B. Research on pollen morphologies of Ostericum Hoffm. (Apiaceae) of eight species from seventeen populations. Acta Bot. Boreali-Occident. Sin. 2018, 38, 2224–2235. [Google Scholar]
- Wang, Z.X.; Tan, J.B.; Peng, L.; He, X.J. Pollen morphology of sixteen Pimpinella species (Apiaceae) and the systematic analysis. Acta Bot. Boreali-Occident. Sin. 2012, 32, 1592–1598. [Google Scholar]
- Jardine, P.E.; Palazzesi, L.; Tellería, M.C.; Barreda, V.D. Why does pollen morphology vary? Evolutionary dynamics and morphospace occupation in the largest angiosperm order (Asterales). New Phytol. 2022, 234, 1075–1087. [Google Scholar] [CrossRef] [PubMed]






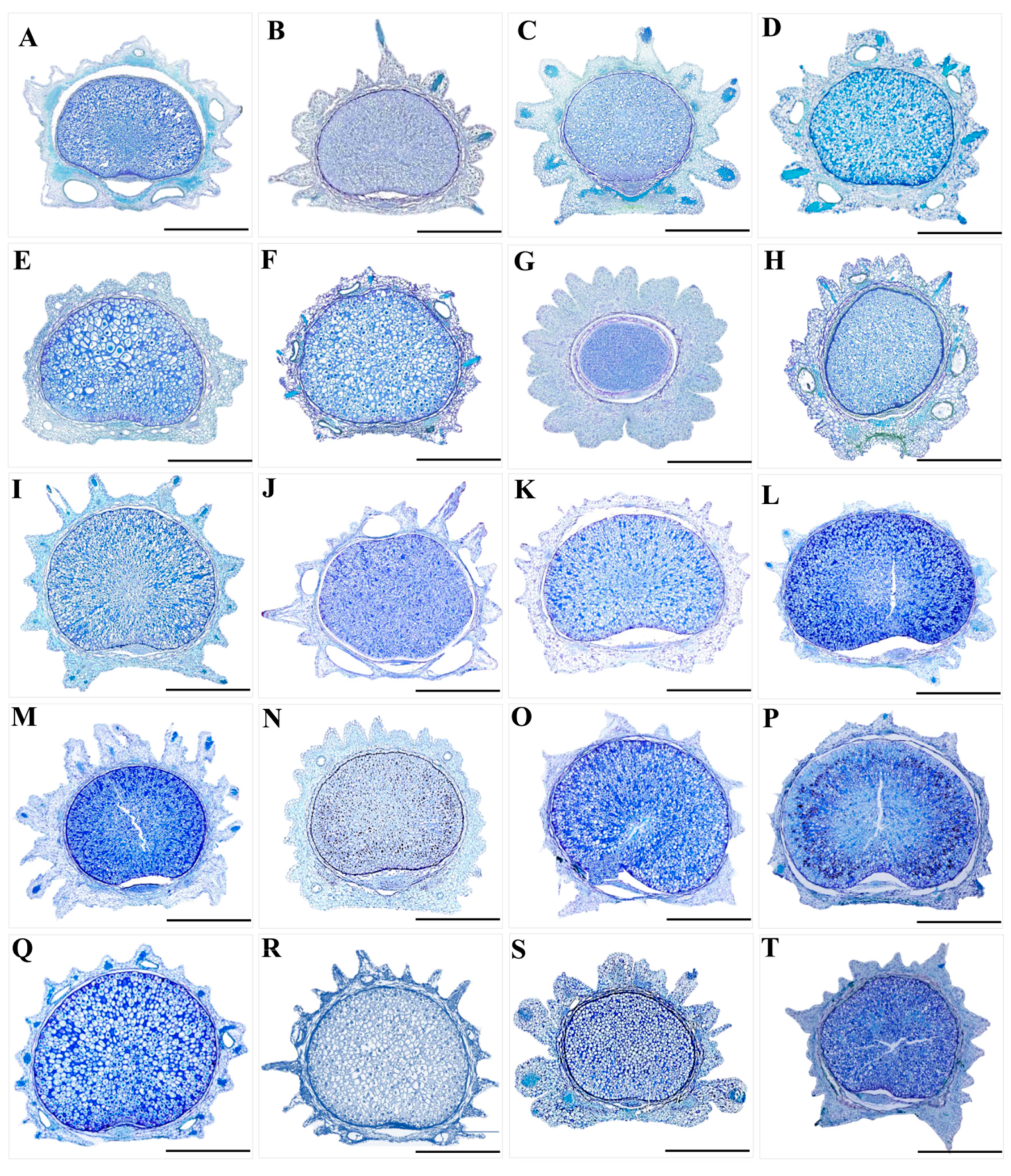
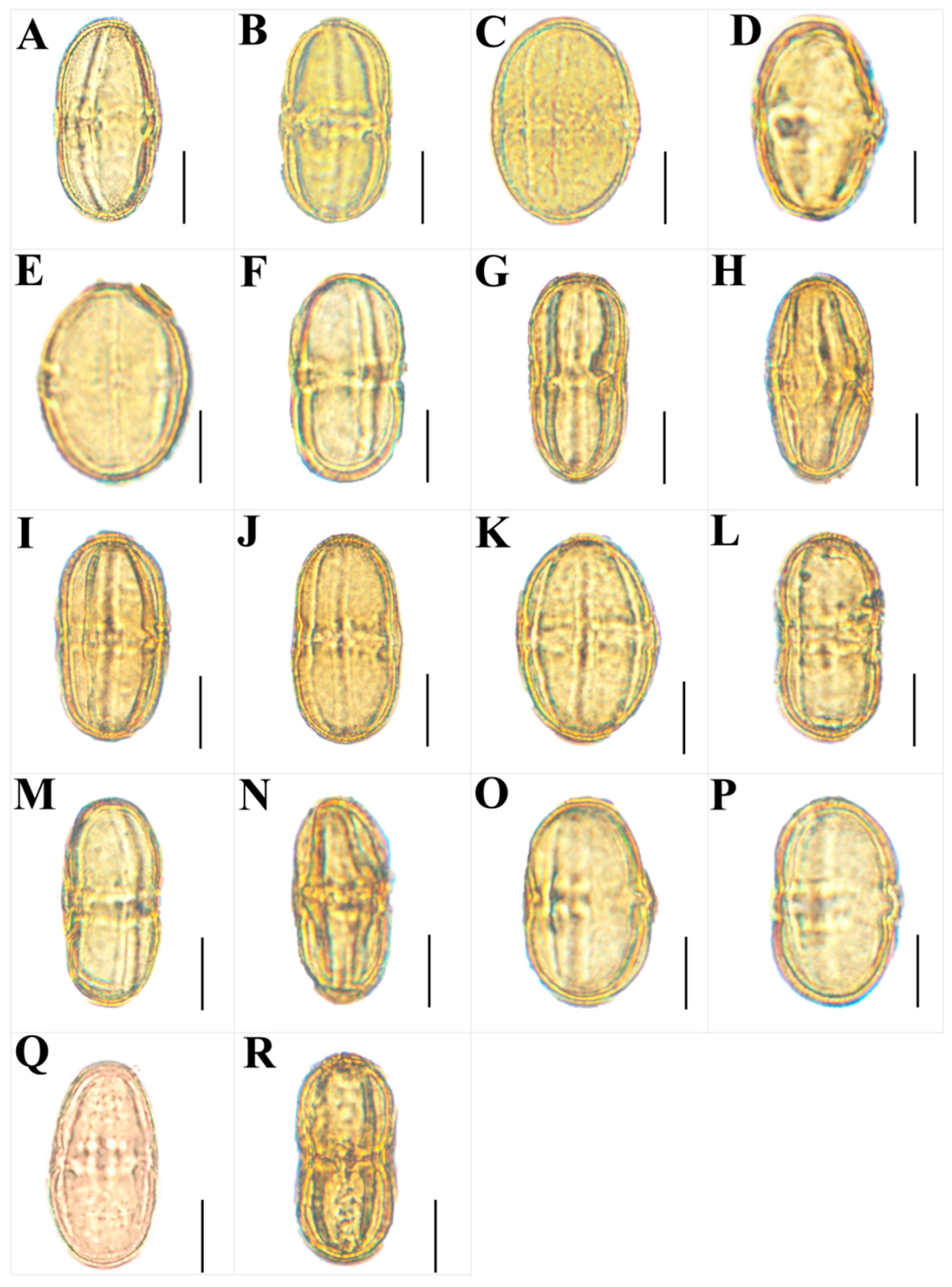
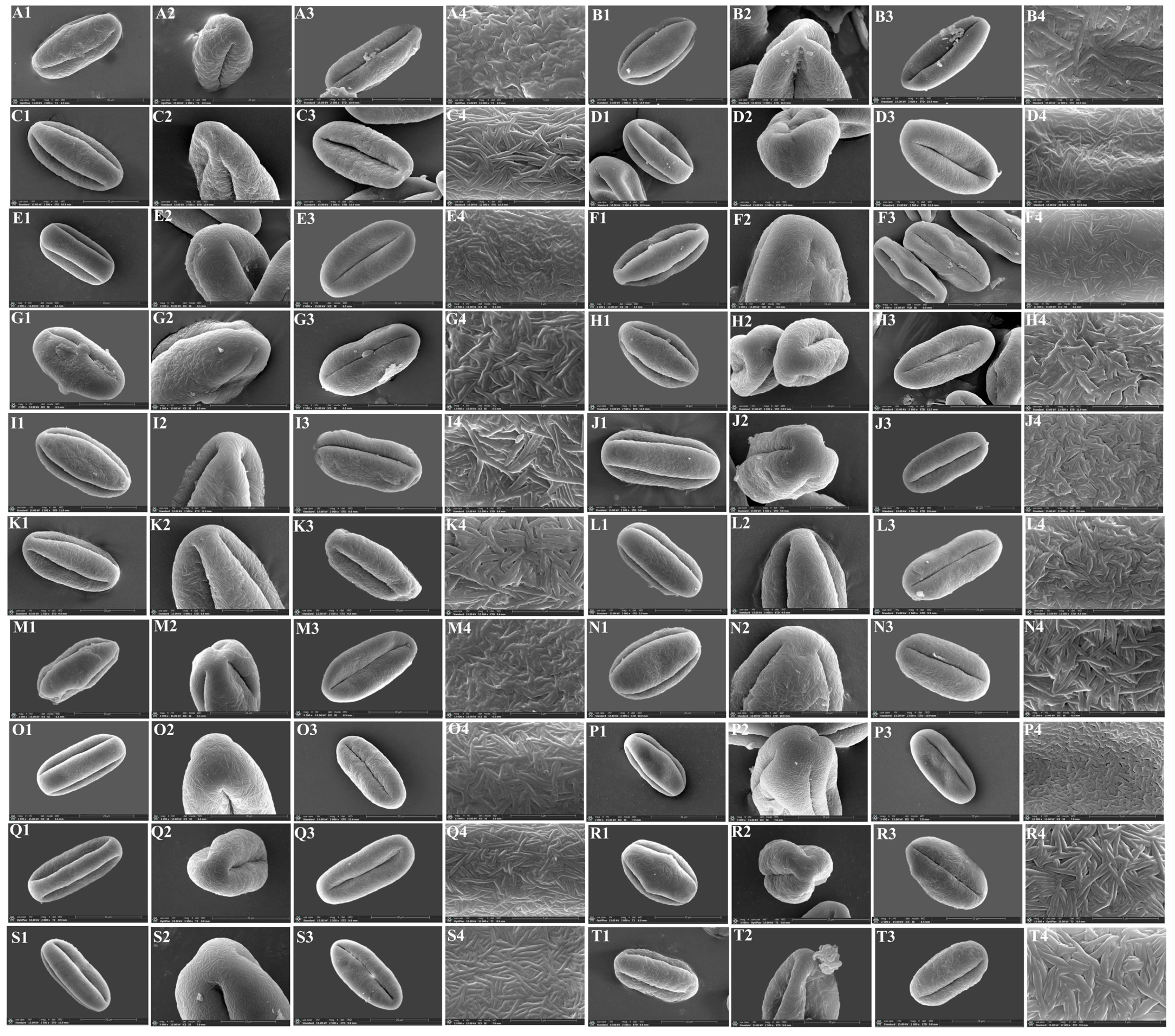
| Taxon | Shape | Size (mm) | Fruit Surface | Calyx Teeth | Endosperm on Commissural Side | Vittae |
|---|---|---|---|---|---|---|
| S. rubriflora | Ovoid or ovoid–globose | 6.97 × 2.68 | proximal part covered with scales, distal part covered with yellow uncinate bristles | ovate–lanceolate | slightly concave | 5 |
| S. astrantiifolia | Obovate or subglobose | 1.89 × 0.90 | proximal end with short bristles, distal end with uncinate bristles, bristles yellow or purple-red | linear–lanceolate | flat | Obscure |
| S. chinensis | Ovoid–globose | 3.28 × 1.37 | bristles uncinate above, dilated at base | linear | flat | 5 |
| S. giraldii | Narrowly ovoid | 3.86 × 1.74 | densely covered with developed yellow or purplish red uncinate bristles, long and hard | ovate and small, tip mucronate | flat | Obscure |
| S. caerulescens | Globose or ellipsoid | 2.35 × 0.68 | short and straight spinous-bristles usually fused at the base forming a thin tier | linear–lanceolate | slightly concave | 5 |
| S. serrata | Ovoid or ovoid–globose | 2.08 × 1.60 | proximal part covered with scales, distal part covered with slightly uncinate bristles, bristles pale yellow or purplish red | ovate | slightly concave | Obscure |
| S. elongata | Ovoid | 2.39 × 1.11 | densely covered with pale yellow scales | narrow–ovate | slightly concave | Obscure |
| S. brevispina | Oblong ovoid to ovoid | 1.95 × 1.25 | proximal end with degenerated to disappeared the prickles, nearly smooth, distal end with prickles and formed a thin layer; usually with erose-spinulose ribs and furrows smooth or barely spinulose | linear to lanceolate | slightly concave | Obscure |
| S. subgiraldii | Broadly ovate | 4.01 × 2.51 | rarely covered with purplish-red short bristles, proximal end with tubercles, obscure, distal end with uncinate bristles or straight | broadly ovate and large | slightly concave | Obscure |
| S. pengshuiensis | Ellipsoid | 2.38 × 0.77 | bristles in regular rows in furrows, ribs glabrous, stout and prominent | linear | flat | 5 |
| S. pauciflora | Long ellipsoid | 2.72 × 0.83 | densely covered with sharp prickles | long–lanceolate | flat | vittae 2 in commissural side |
| S. hacquetioides | Ovoid–globose | 2.81 × 2.08 | covered with scales and tubercles, but never spinulose | broadly ovate or obovate | flat | Obscure |
| S. orthacantha | Narrowly ovoid | 2.02 × 0.86 | short and straight spines | narrowly lanceolate | flat | Obscure |
| S. lamelligera | Long-ovoid | 1.97 × 0.72 | short and straight spines, never uncinate, fused at the base forming a thin tier | linear | slightly concave | 5 |
| S. oviformis | Ovoid | 2.13 × 1.44 | densely short and straight-spinulose; ribs prominent | linear–lanceolate | slightly concave | 5 |
| S. tienmuensis | Subglobose | 2.52 × 1.82 | covered with short and obtuse prickles, slight formed scales and tubercles | broadly ovate | slightly concave | Obscure |
| S. flavovirens | Ovoid–globose | 3.26 × 1.79 | proximal part covered with tubercles, distal part covered with yellow straight prickles | triangular–lanceolate, tip mucronate | flat | 5 |
| S. elata | Ovoid–globose | 2.27 × 1.35 | densely covered with yellow uncinate bristles | narrowly lanceolate | flat | 5 |
| S. rugulosa | Ellipsoid | 2.41 × 1.45 | densely covered with uncinate bristles | narrowly lanceolate | slightly concave | Obscure |
| S. nanchuanensis | Ellipsoid | 2.74 × 1.65 | proximal part covered with scales, rarely formed bristles | triangular–lanceolate | flat | Obscure |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Yong, F.; Liu, C.; Wang, Y.; Yang, L.; Chen, L.; Wang, Y.; Zhou, S.; He, X. The Micromorphology and Its Taxonomic Value of the Genus Sanicula L. in China (Apiaceae). Plants 2024, 13, 1635. https://doi.org/10.3390/plants13121635
Song B, Yong F, Liu C, Wang Y, Yang L, Chen L, Wang Y, Zhou S, He X. The Micromorphology and Its Taxonomic Value of the Genus Sanicula L. in China (Apiaceae). Plants. 2024; 13(12):1635. https://doi.org/10.3390/plants13121635
Chicago/Turabian StyleSong, Boni, Feng Yong, Changkun Liu, Yunyi Wang, Lei Yang, Lian Chen, Yuan Wang, Songdong Zhou, and Xingjin He. 2024. "The Micromorphology and Its Taxonomic Value of the Genus Sanicula L. in China (Apiaceae)" Plants 13, no. 12: 1635. https://doi.org/10.3390/plants13121635






