Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity
Abstract
:1. Introduction
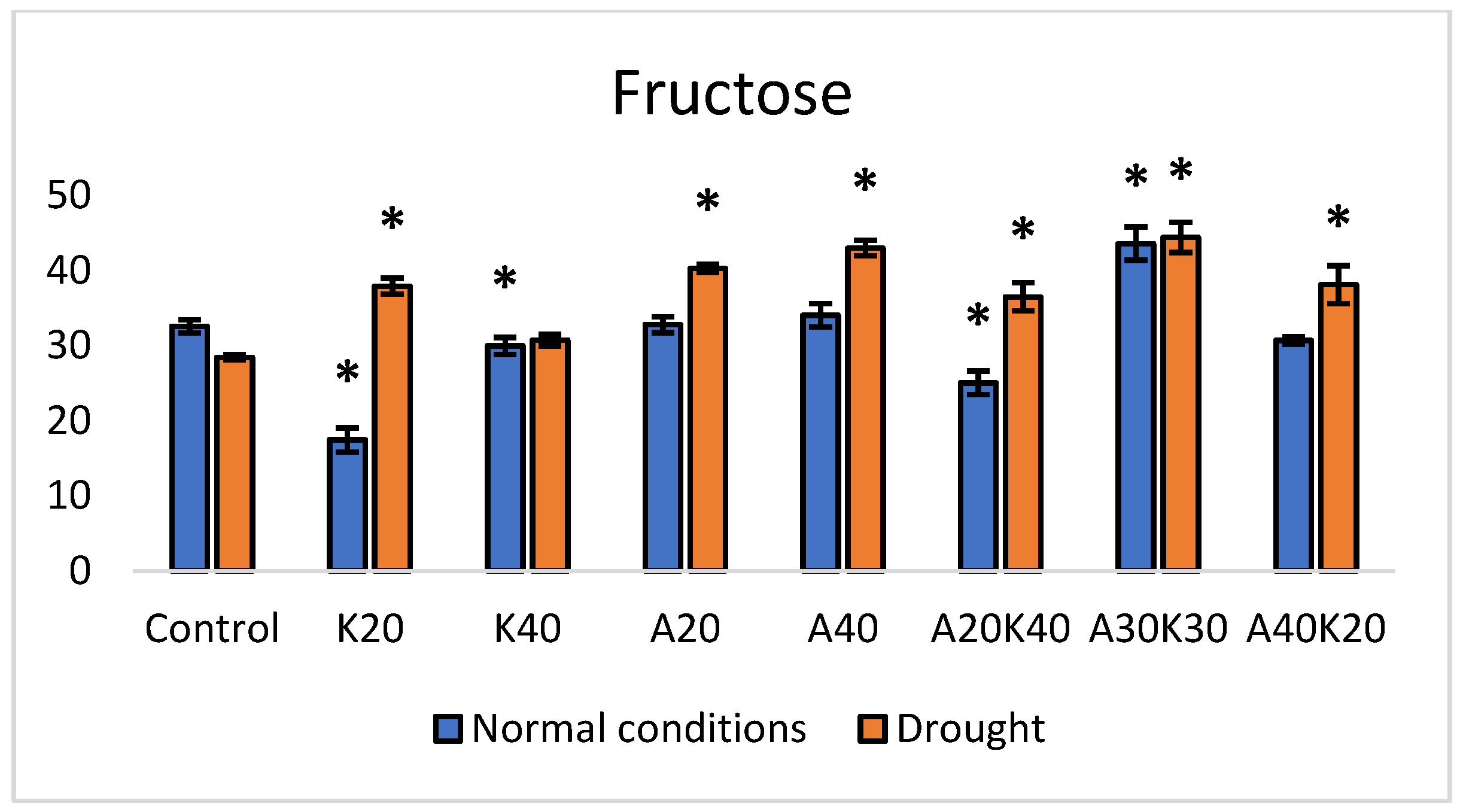
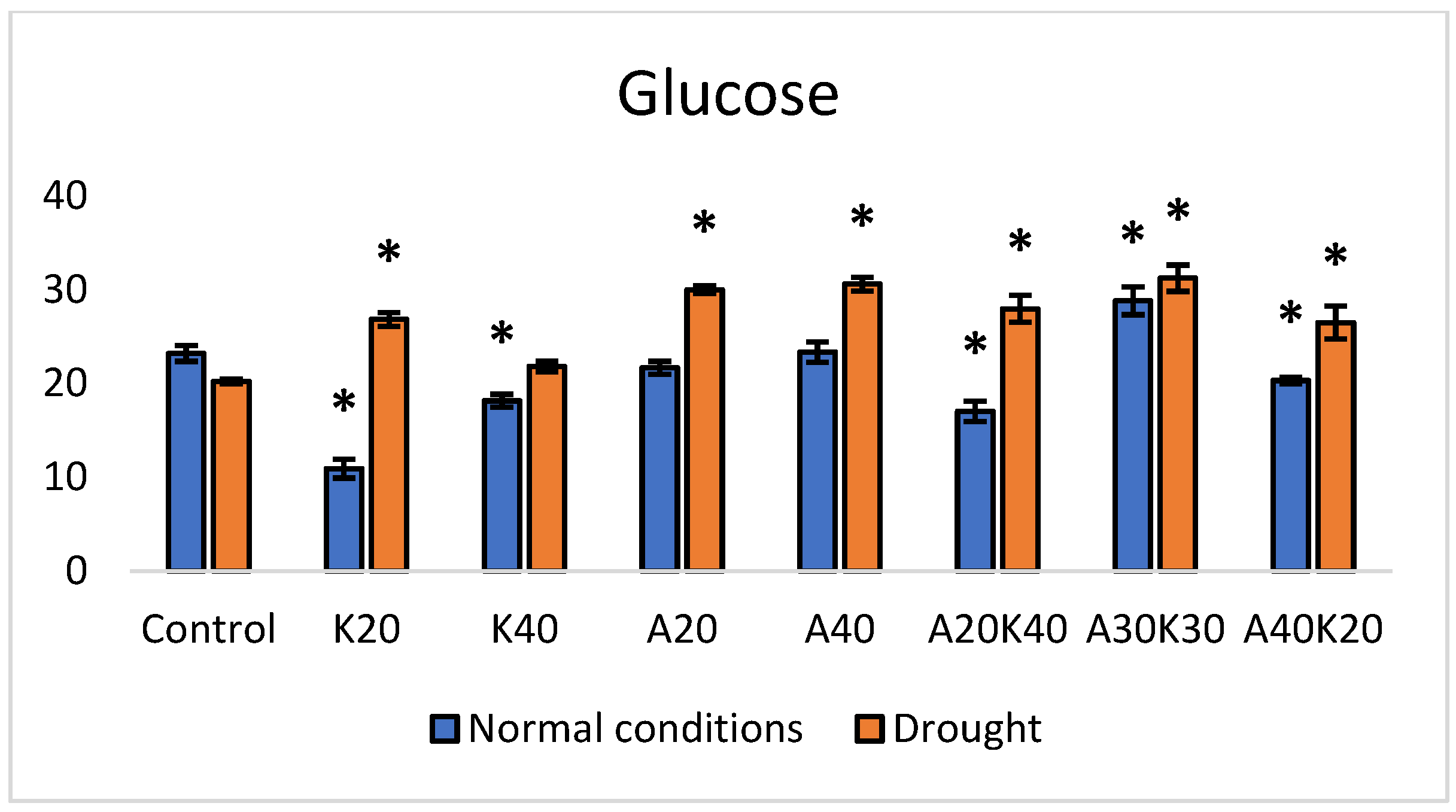
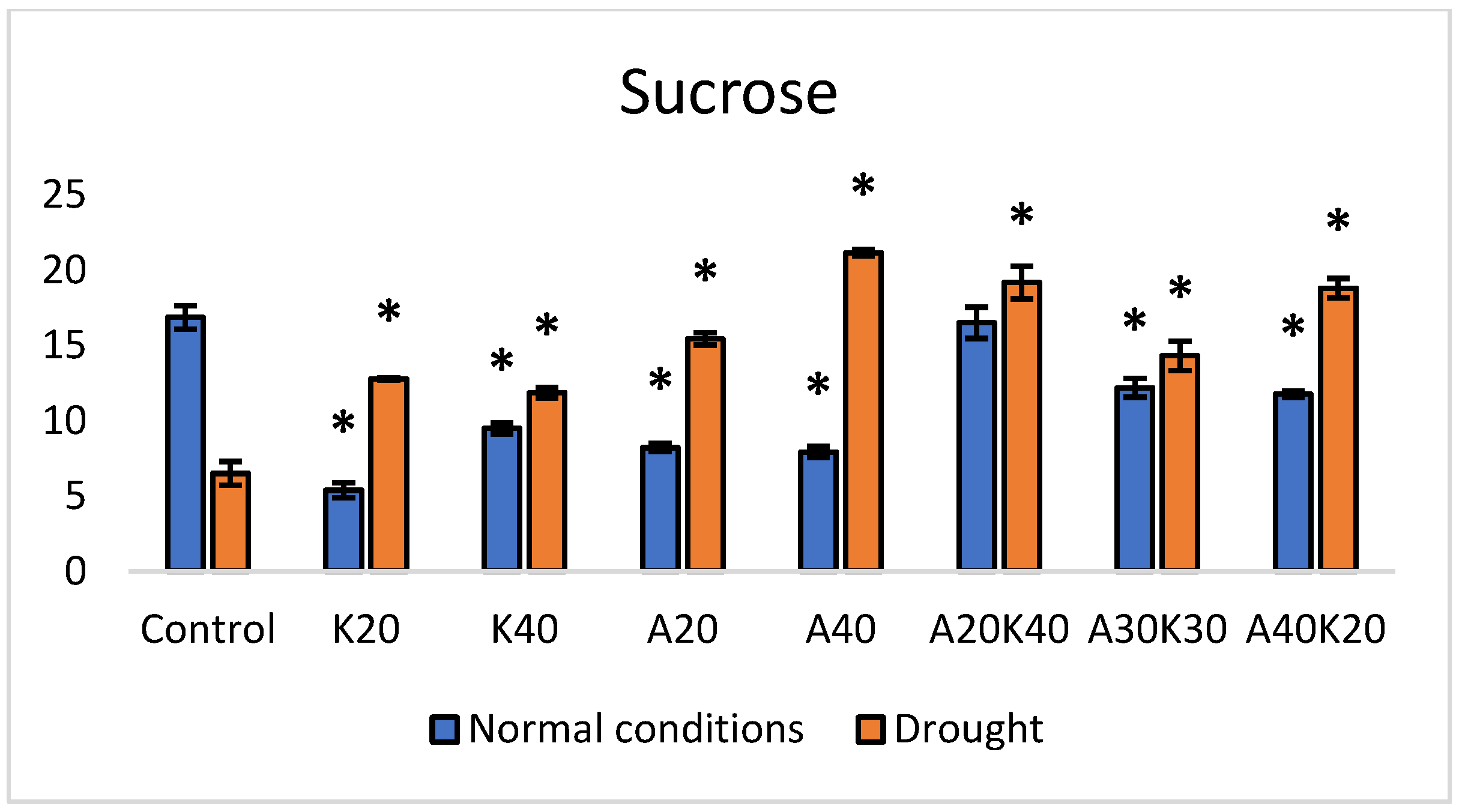
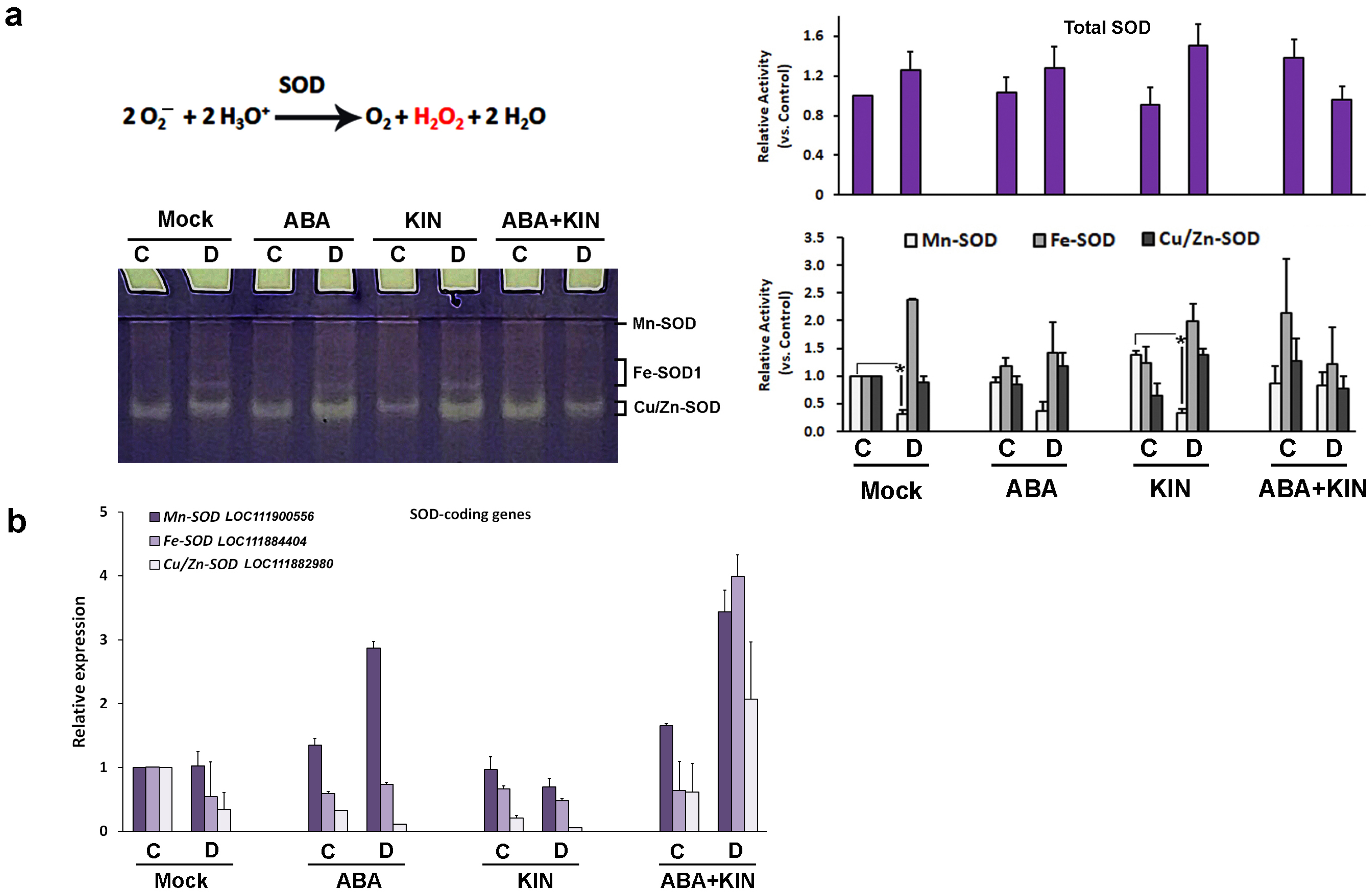
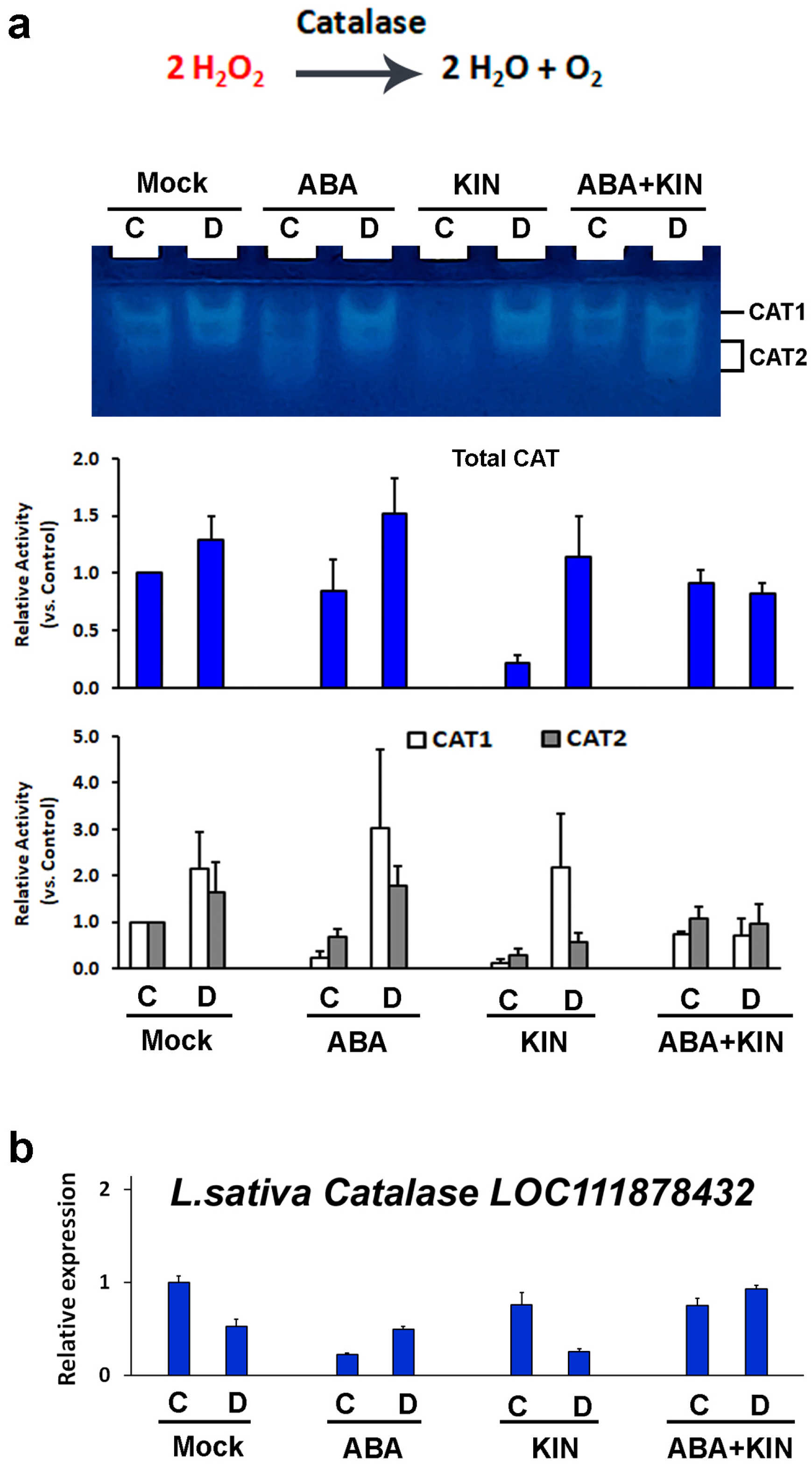
2. Results
3. Discussion
4. Materials and Methods
4.1. Growth Conditions
4.2. Sample Preparation for Determination of Sugars by HPLC
4.3. Determination of Sugars by HPLC
4.4. Non-Denaturing Electrophoresis and In-Gel Activity Staining of Antioxidant Enzymes
4.5. RT-PCR Analysis
4.6. Biometric Measurements
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosegrant, M.W.; Cline, S.A. Global Food Security: Challenges and Policies. Science 2003, 302, 1917–1919. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Perry, L.; Kisiala, A.; Olechowski, H.; Emery, R.J.N. Cytokinin Activity during Early Kernel Development Corresponds Positively with Yield Potential and Later Stage ABA Accumulation in Field-Grown Wheat (Triticum aestivum L.). Planta 2020, 252, 76. [Google Scholar] [CrossRef]
- Werner, T.; Nehnevajova, E.; Köllmer, I.; Novák, O.; Strnad, M.; Krämer, U.; Schmülling, T. Root-Specific Reduction of Cytokinin Causes Enhanced Root Growth, Drought Tolerance, and Leaf Mineral Enrichment in Arabidopsis and Tobacco. Plant Cell 2011, 22, 3905–3920. [Google Scholar] [CrossRef]
- Bielach, A.; Hrtyan, M.; Tognetti, V. Plants under Stress: Involvement of Auxin and Cytokinin. Int. J. Mol. Sci. 2017, 18, 1427. [Google Scholar] [CrossRef]
- Joshi, S.; Choukimath, A.; Isenegger, D.; Panozzo, J.; Spangenberg, G.; Kant, S. Improved Wheat Growth and Yield by Delayed Leaf Senescence Using Developmentally Regulated Expression of a Cytokinin Biosynthesis Gene. Front. Plant Sci. 2019, 10, 1285. [Google Scholar] [CrossRef]
- Ramireddy, E.; Hosseini, S.A.; Eggert, K.; Gillandt, S.; Gnad, H.; Von Wirén, N.; Schmülling, T. Root Engineering in Barley: Increasing Cytokinin Degradation Produces a Larger Root System, Mineral Enrichment in the Shoot and Improved Drought Tolerance. Plant Physiol. 2018, 177, 1078–1095. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, F1000 Faculty Rev-1554. [Google Scholar] [CrossRef]
- Cortleven, A.; Schmülling, T. Regulation of Chloroplast Development and Function by Cytokinin. J. Exp. Bot. 2015, 66, 4999–5013. [Google Scholar] [CrossRef]
- Rivero, R.M.; Gimeno, J.; Van Deynze, A.; Walia, H.; Blumwald, E. Enhanced Cytokinin Synthesis in Tobacco Plants Expressing PSARK::IPT Prevents the Degradation of Photosynthetic Protein Complexes During Drought. Plant Cell Physiol. 2010, 51, 1929–1941. [Google Scholar] [CrossRef]
- Piñol, R.; Simón, E. Effect of 24-Epibrassinolide on Chlorophyll Fluorescence and Photosynthetic CO2 Assimilation in Vicia faba Plants Treated with the Photosynthesis-Inhibiting Herbicide Terbutryn. J. Plant Growth Regul. 2009, 28, 97–105. [Google Scholar] [CrossRef]
- Hönig, M.; Plíhalová, L.; Husičková, A.; Nisler, J.; Doležal, K. Role of Cytokinins in Senescence, Antioxidant Defence and Photosynthesis. Int. J. Mol. Sci. 2018, 19, 4045. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Ozfidan, C.; Turkan, I.; Sekmen, A.H.; Seckin, B. Abscisic Acid-regulated Responses of aba2-1 under Osmotic Stress: The Abscisic Acid-inducible Antioxidant Defence System and Reactive Oxygen Species Production. Plant Biol. 2012, 14, 337–346. [Google Scholar] [CrossRef]
- Castro-Cegrí, A.; Sierra, S.; Hidalgo-Santiago, L.; Esteban-Muñoz, A.; Jamilena, M.; Garrido, D.; Palma, F. Postharvest Treatment with Abscisic Acid Alleviates Chilling Injury in Zucchini Fruit by Regulating Phenolic Metabolism and Non-Enzymatic Antioxidant System. Antioxidants 2023, 12, 211. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA Enhances the Antioxidant Defense System of Maize by Regulating the AsA-GSH Cycle under Drought Stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Mou, W.; Li, D.; Luo, Z.; Mao, L.; Ying, T. Transcriptomic Analysis Reveals Possible Influences of ABA on Secondary Metabolism of Pigments, Flavonoids and Antioxidants in Tomato Fruit during Ripening. PLoS ONE 2015, 10, e0129598. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Wilkinson, S.; Davies, W.J. Drought, Ozone, ABA and Ethylene: New Insights from Cell to Plant to Community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef]
- Zhang, K.; Gan, S.-S. An Abscisic Acid-AtNAP Transcription Factor-SAG113 Protein Phosphatase 2C Regulatory Chain for Controlling Dehydration in Senescing Arabidopsis Leaves. Plant Physiol. 2012, 158, 961–969. [Google Scholar] [CrossRef]
- Song, Y.; Xiang, F.; Zhang, G.; Miao, Y.; Miao, C.; Song, C.-P. Abscisic Acid as an Internal Integrator of Multiple Physiological Processes Modulates Leaf Senescence Onset in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 181. [Google Scholar] [CrossRef]
- Tahaei, A.; Soleymani, A.; Shams, M. Seed Germination of Medicinal Plant, Fennel (Foeniculum vulgare Mill), as Affected by Different Priming Techniques. Appl. Biochem. Biotechnol. 2016, 180, 26–40. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Zhao, S.; Liu, Z.; Feng, Y.; Wu, Y. Cytokinin Antagonizes ABA Suppression to Seed Germination of Arabidopsis by Downregulating ABI5 Expression. Plant J. 2011, 68, 249–261. [Google Scholar] [CrossRef]
- Davies, P.J. Plant Hormones: Biosythesis, Signal Transduction, Action! 3rd ed.; Davies, P.J., Ed.; Kluwer Academic: Dordrecht, The Netherlands; Boston, UK, 2004; ISBN 9781402026850. [Google Scholar]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-Dependent Photorespiration and the Protection of Photosynthesis during Water Deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef]
- Lalarukh, I.; Ashraf, M.A.; Azeem, M.; Hussain, M.; Akbar, M.; Ashraf, M.Y.; Javed, M.T.; Iqbal, N. Growth Stage-Based Response of Wheat (Triticum aestivum L.) to Kinetin under Water-Deficit Environment: Pigments and Gas Exchange Attributes. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2014, 64, 501–510. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of Exogenously Sourced Kinetin in Protecting Solanum lycopersicum from NaCl-Induced Oxidative Stress through up-Regulation of the Antioxidant System, Ascorbate-Glutathione Cycle and Glyoxalase System. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Singh, M.; Bashri, G.; Prasad, S.M.; Singh, V.P. Kinetin Alleviates UV-B-Induced Damage in Solanum lycopersicum: Implications of Phenolics and Antioxidants. J. Plant Growth Regul. 2019, 38, 831–841. [Google Scholar] [CrossRef]
- Acidri, R.; Sawai, Y.; Sugimoto, Y.; Handa, T.; Sasagawa, D.; Masunaga, T.; Yamamoto, S.; Nishihara, E. Exogenous Kinetin Promotes the Nonenzymatic Antioxidant System and Photosynthetic Activity of Coffee (Coffea arabica L.) Plants Under Cold Stress Conditions. Plants 2020, 9, 281. [Google Scholar] [CrossRef]
- Bozsó, Z.; Barna, B. Diverse Effect of Two Cytokinins, Kinetin and Benzyladenine, on Plant Development, Biotic Stress Tolerance, and Gene Expression. Life 2021, 11, 1404. [Google Scholar] [CrossRef]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Irshad, M.; Khan, A.L.; Waqas, M.; Shahzad, R.; Iqbal, A.; Ullah, N.; Rehman, G.; et al. Kinetin Modulates Physio-Hormonal Attributes and Isoflavone Contents of Soybean Grown under Salinity Stress. Front. Plant Sci. 2015, 6, 377. [Google Scholar] [CrossRef]
- Alscher, R.G. Role of Superoxide Dismutases (SODs) in Controlling Oxidative Stress in Plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Yang, P.; Guo, Y.; Qiu, L. Effects of Ozone-Treated Domestic Sludge on Hydroponic Lettuce Growth and Nutrition. J. Integr. Agric. 2018, 17, 593–602. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Reid, M.S.; Jiang, C.-Z. Controlling Plant Architecture by Manipulation of Gibberellic Acid Signalling in Petunia. Hortic. Res. 2014, 1, 14061. [Google Scholar] [CrossRef]
- Miyashima, S.; Roszak, P.; Sevilem, I.; Toyokura, K.; Blob, B.; Heo, J.; Mellor, N.; Help-Rinta-Rahko, H.; Otero, S.; Smet, W.; et al. Mobile PEAR Transcription Factors Integrate Positional Cues to Prime Cambial Growth. Nature 2019, 565, 490–494. [Google Scholar] [CrossRef]
- Dörffling, K.; Böttger, M. Steigerung der keimungshemmenden Wirkung von Abscisinsäure auf Lactuca-Früchte durch Saccharose und Glucose. Planta 1972, 103, 340–347. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, X.; Zhang, Y.; Dai, P.; Liang, B.; Li, J.; Sun, C.; Lin, X. Role of Sucrose in Modulating the Low-nitrogen-induced Accumulation of Phenolic Compounds in Lettuce (Lactuca sativa L.). J. Sci. Food Agric. 2020, 100, 5412–5421. [Google Scholar] [CrossRef]
- Ibrahim, H.A.; Abdellatif, Y.M.R. Effect of Maltose and Trehalose on Growth, Yield and Some Biochemical Components of Wheat Plant under Water Stress. Ann. Agric. Sci. 2016, 61, 267–274. [Google Scholar] [CrossRef]
- Zhang, W.; Du, T. Fresh/Brackish Watering at Growth Period Provided a Trade-off between Lettuce Growth and Resistance to NaCl-Induced Damage. Sci. Hortic. 2022, 304, 111283. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, G.; Wang, L.; Tang, Y. Effects of Spraying Abscisic Acid on Growth and Antioxidant Enzyme of Lettuce Seedlings under Salt Stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 199, 032015. [Google Scholar] [CrossRef]
- Vaseva, I.; Akiscan, Y.; Simova-Stoilova, L.; Kostadinova, A.; Nenkova, R.; Anders, I.; Feller, U.; Demirevska, K. Antioxidant Response to Drought in Red and White Clover. Acta Physiol. Plant 2012, 34, 1689–1699. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Arikan, B.; Alp, F.N.; Elbasan, F.; Zengin, G.; Cavusoglu, H.; Sakalak, H. The Hormetic Dose-Risks of Polymethyl Methacrylate Nanoplastics on Chlorophyll a Fluorescence Transient, Lipid Composition and Antioxidant System in Lactuca sativa. Environ. Pollut. 2022, 308, 119651. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- De Vylder, J.; Vandenbussche, F.; Hu, Y.; Philips, W.; Van Der Straeten, D. Rosette Tracker: An Open-Source Image Analysis Tool for Automatic Quantification of Genotype Effects. Plant Physiol. 2012, 160, 1149–1159. [Google Scholar] [CrossRef]


| Fresh Weight [g] | Dry Weight [g] | Leaf Area [mm2] | ||||
|---|---|---|---|---|---|---|
| Normal Conditions | Drought | Normal Conditions | Drought | Normal Conditions | Drought | |
| Control | 2.50 | 1.43 | 0.16 | 0.11 | 6237 | 2828 |
| K20 | 1.67 | 1.20 * | 0.10 * | 0.09 * | 4844 * | 3662 * |
| K40 | 1.54 * | 1.28 * | 0.09 * | 0.10 | 4885 * | 3301 * |
| A20 | 1.86 | 1.31 * | 0.11 | 0.10 | 5740 * | 2806 * |
| A40 | 2.40 | 1.42 | 0.13 | 0.10 | 5612 * | 3561 * |
| A20K40 | 1.90 | 1.27 * | 0.10 | 0.09 * | 5778 * | 3490 * |
| A30K30 | 1.96 | 1.49 * | 0.11 | 0.11 | 5002 * | 3558 * |
| A40K20 | 2.09 | 1.36 * | 0.11 | 0.10 | 6001 | 3600 * |
| Gene Name | Locus | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| Ls18S RNA | AH001680 | CGGGTGACGGAGAATTAGGG | TACCTCCCCGTGTCAGGATT |
| Ls Actin-7 | LOC111882438 | CTGGTGATGGTGTCTCCCAC | GGCGAGCTTCTCCTTCATGT |
| Ls Mn-SOD | LOC111900556 | CACCCCAGTATTTGGATGGCT | CTCCCTCCCCCTATGTGCTA |
| Ls Fe-SOD | LOC111884404 | GGGAATCCATGCAACCAGGA | AAAACAAGCCAAACCCAGCC |
| Ls Cu/Zn-SOD | LOC111882980 | CACTCTTACAGACGCTTTGCG | ATGGTGCCACTAACACCCTC |
| Ls Catalase | LOC111878432 | GCCATGCTGAACAGTACCCT | TCTCTCTCCTGGCTGCTTGA |
| Ls APX2 | LOC111882573 | GACATCGGCGATCTTCTGGT | TCTCGAAGCTTCCTCTTCGC |
| Ls POX N1 | LOC111896420 | CTATGGTTGATATTGGCGTCGT | ACAAAGTCGGCCATTGGAGAT |
| Ls POX5 | LOC111879417 | GGTCGCTAAAGCCTACTCCC | ACTTGGGTTGTTTGCTGGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbutis, M.; Vaseva, I.I.; Simova-Stoilova, L.; Todorova, D.; Pukalskas, A.; Samuolienė, G. Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity. Plants 2024, 13, 1641. https://doi.org/10.3390/plants13121641
Urbutis M, Vaseva II, Simova-Stoilova L, Todorova D, Pukalskas A, Samuolienė G. Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity. Plants. 2024; 13(12):1641. https://doi.org/10.3390/plants13121641
Chicago/Turabian StyleUrbutis, Martynas, Irina I. Vaseva, Lyudmila Simova-Stoilova, Dessislava Todorova, Audrius Pukalskas, and Giedrė Samuolienė. 2024. "Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity" Plants 13, no. 12: 1641. https://doi.org/10.3390/plants13121641
APA StyleUrbutis, M., Vaseva, I. I., Simova-Stoilova, L., Todorova, D., Pukalskas, A., & Samuolienė, G. (2024). Drought Protective Effects of Exogenous ABA and Kinetin on Lettuce: Sugar Content, Antioxidant Enzyme Activity, and Productivity. Plants, 13(12), 1641. https://doi.org/10.3390/plants13121641








