Maritime Pine Rootstock Genotype Modulates Gene Expression Associated with Stress Tolerance in Grafted Stems
Abstract
1. Introduction
2. Results
2.1. Sequencing and Annotation of Stem Transcriptome
2.2. Variations among Graft Types: Differential Expression Analysis
2.3. Differences between Scion and Rootstock Stems from the Same Graft
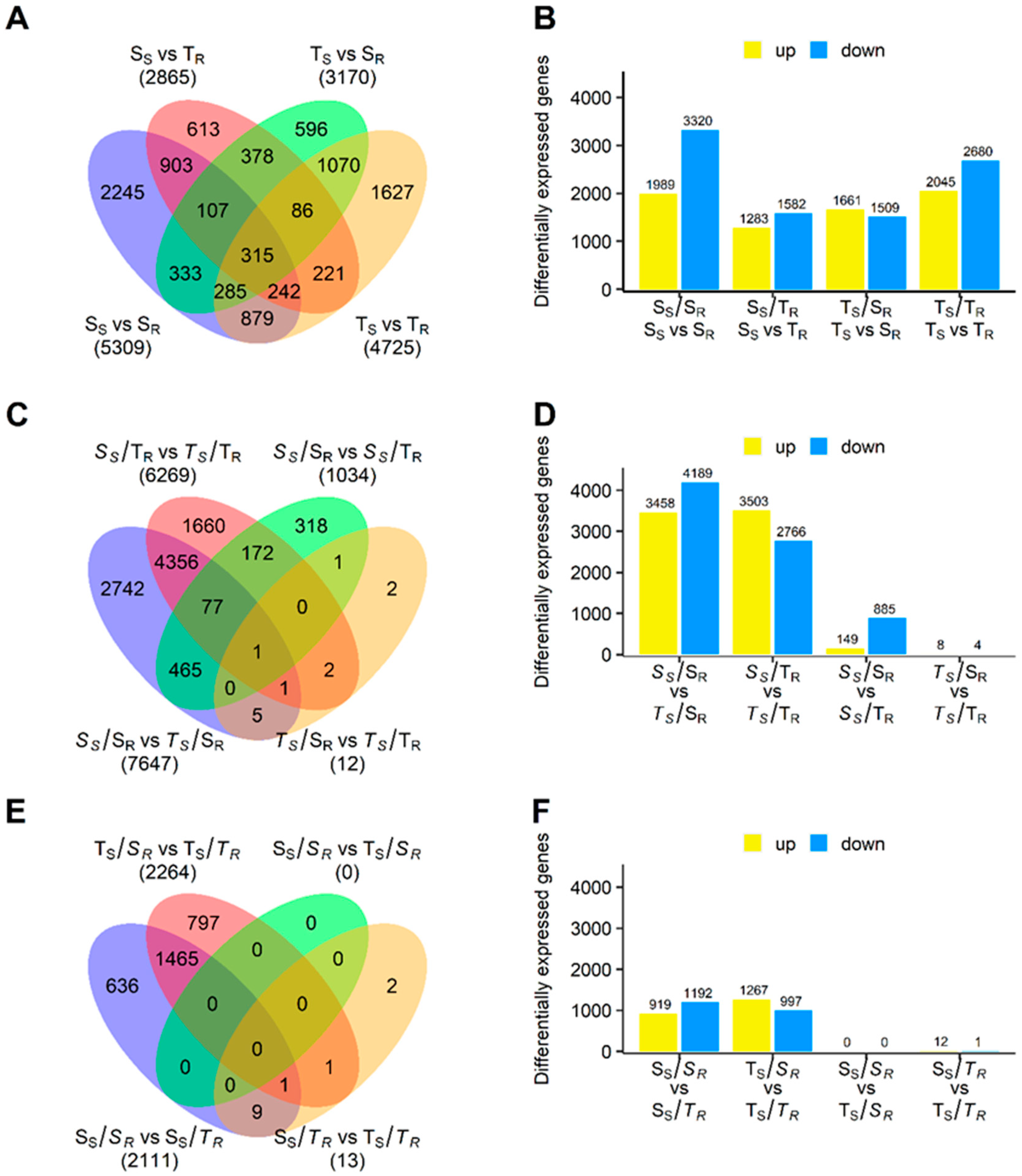
2.4. Identification of DEGs Associated with Differences between Drought-Sensitive and Drought-Tolerant Scion (SS vs. TS) and Rootstock (SR vs. TR) Stems
2.4.1. Comparisons among Scion Stems with Contrasting Drought Tolerance (SS vs. TS): SS/SR vs. TS/SR and SS/TR vs. TS/TR
2.4.2. Comparisons among Rootstock Stems with Contrasting Drought Tolerance (SR vs. TR): SS/SR vs. SS/TR and TS/SR vs. TS/TR
2.5. Analysis of Scion and Rootstock Genotype Interaction
2.5.1. Transcriptomic Responses of Scions: SS/SR vs. SS/TR and TS/SR vs. TS/TR
2.5.2. Transcriptomic Responses of Rootstocks: SS/SR vs. TS/SR or SS/TR vs. TS/TR
2.6. Gene Selection and Expression Analysis
2.6.1. Gene Selection of Drought-Sensitive Genotypes
2.6.2. Gene Selection of Drought-Tolerant Genotypes
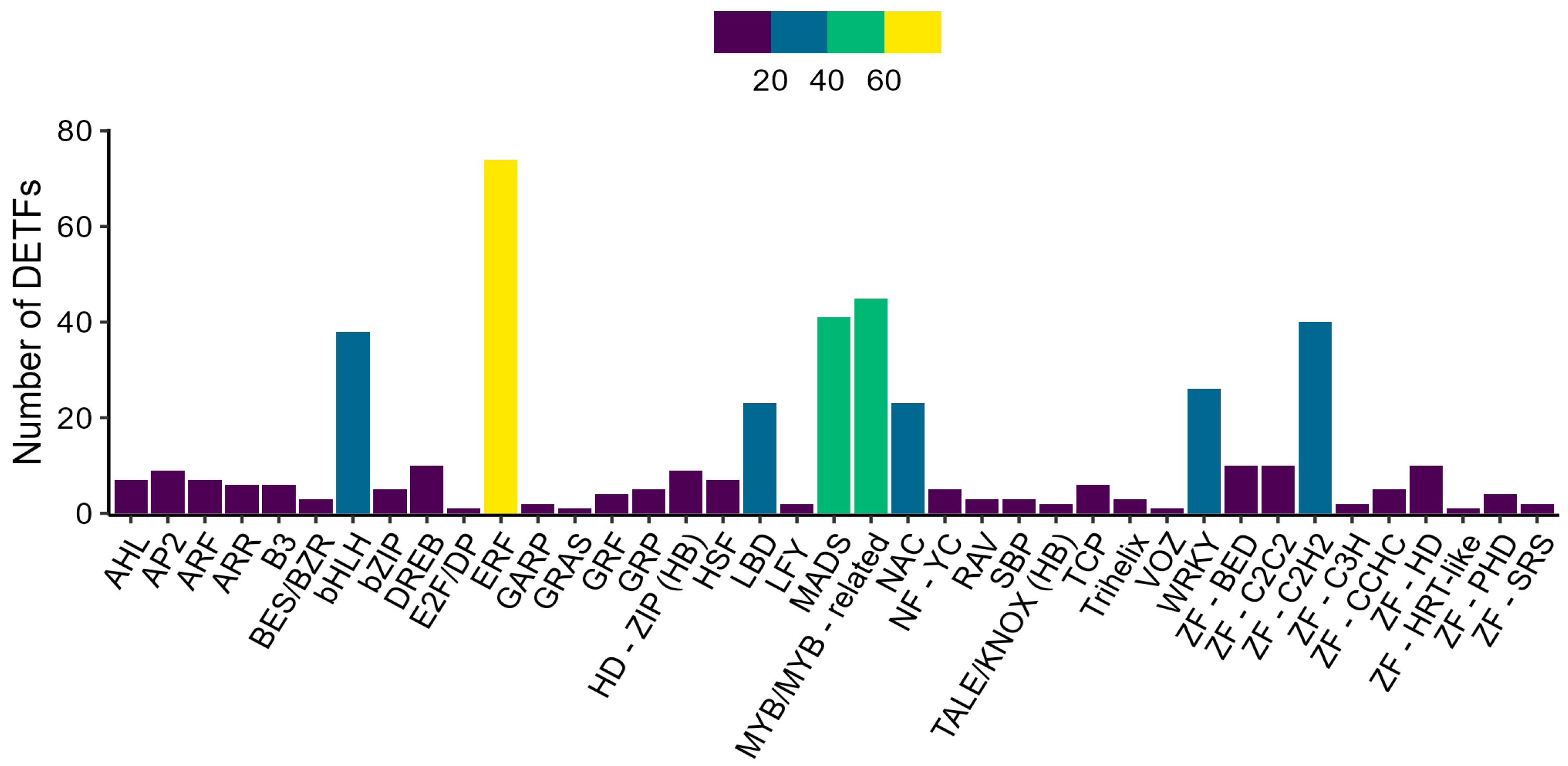
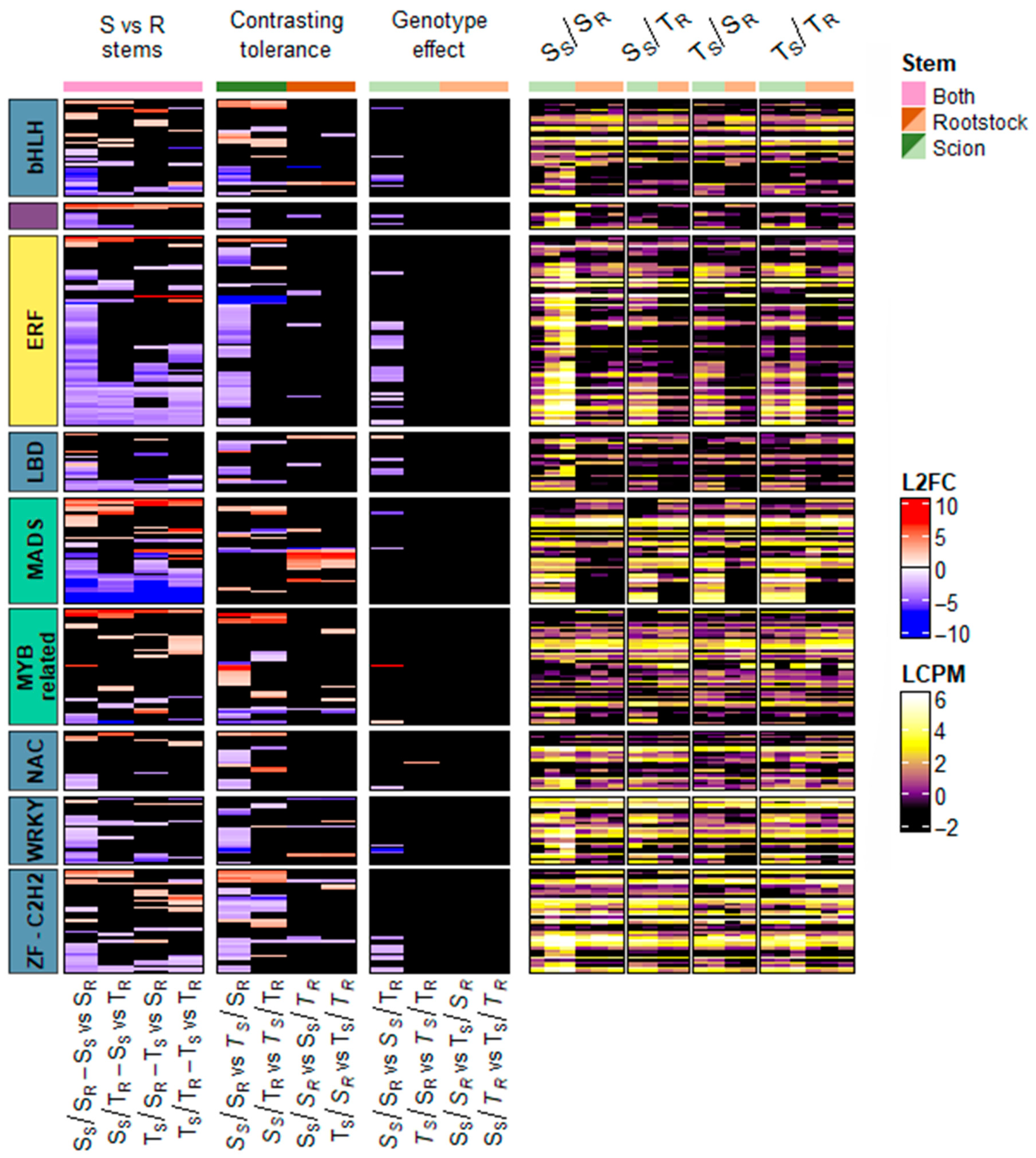
2.7. Analysis of Differentially Expressed Transcription Factors (DETFs)
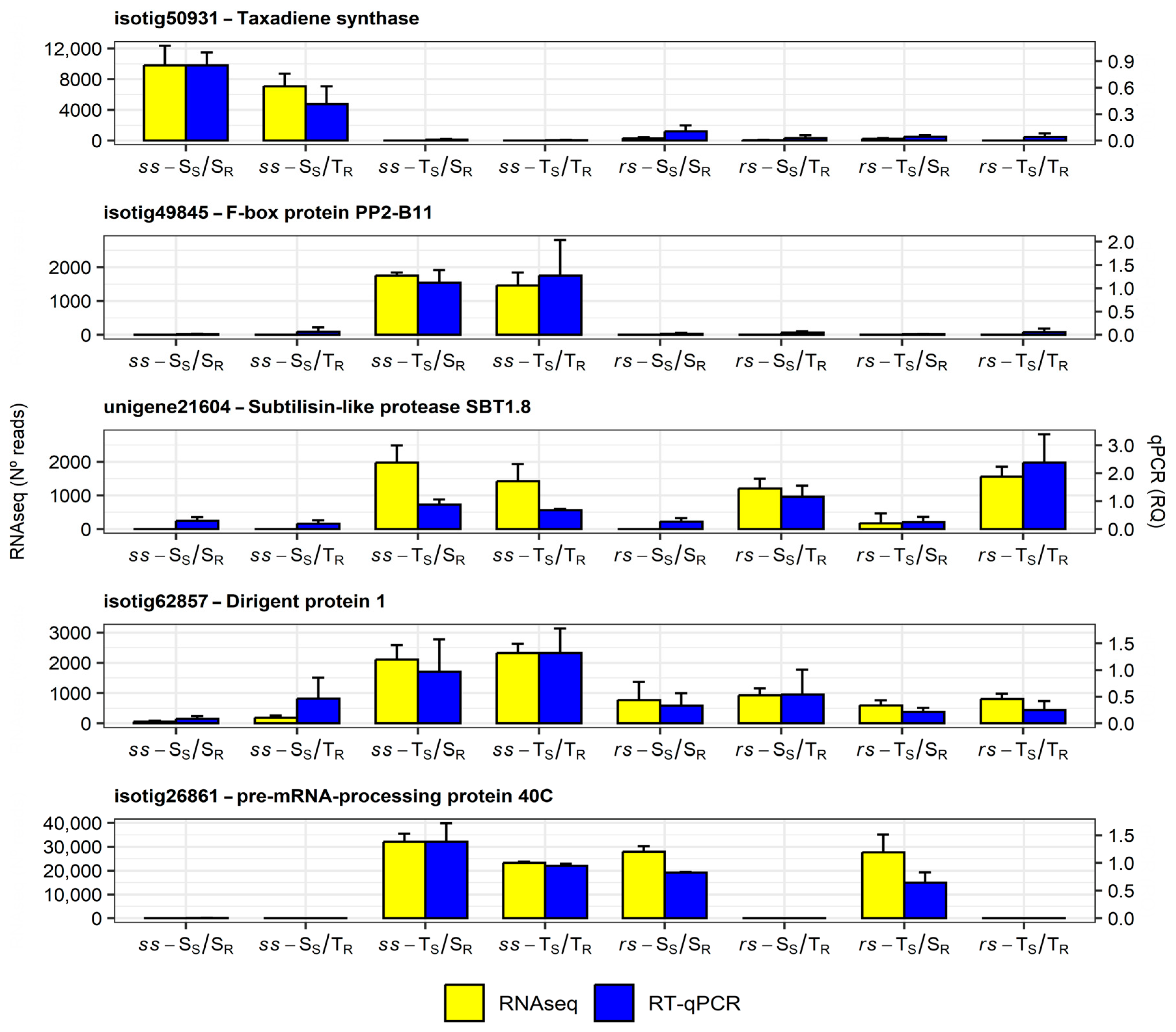
2.8. Gene Expression Analysis by Quantitative Real-Time PCR
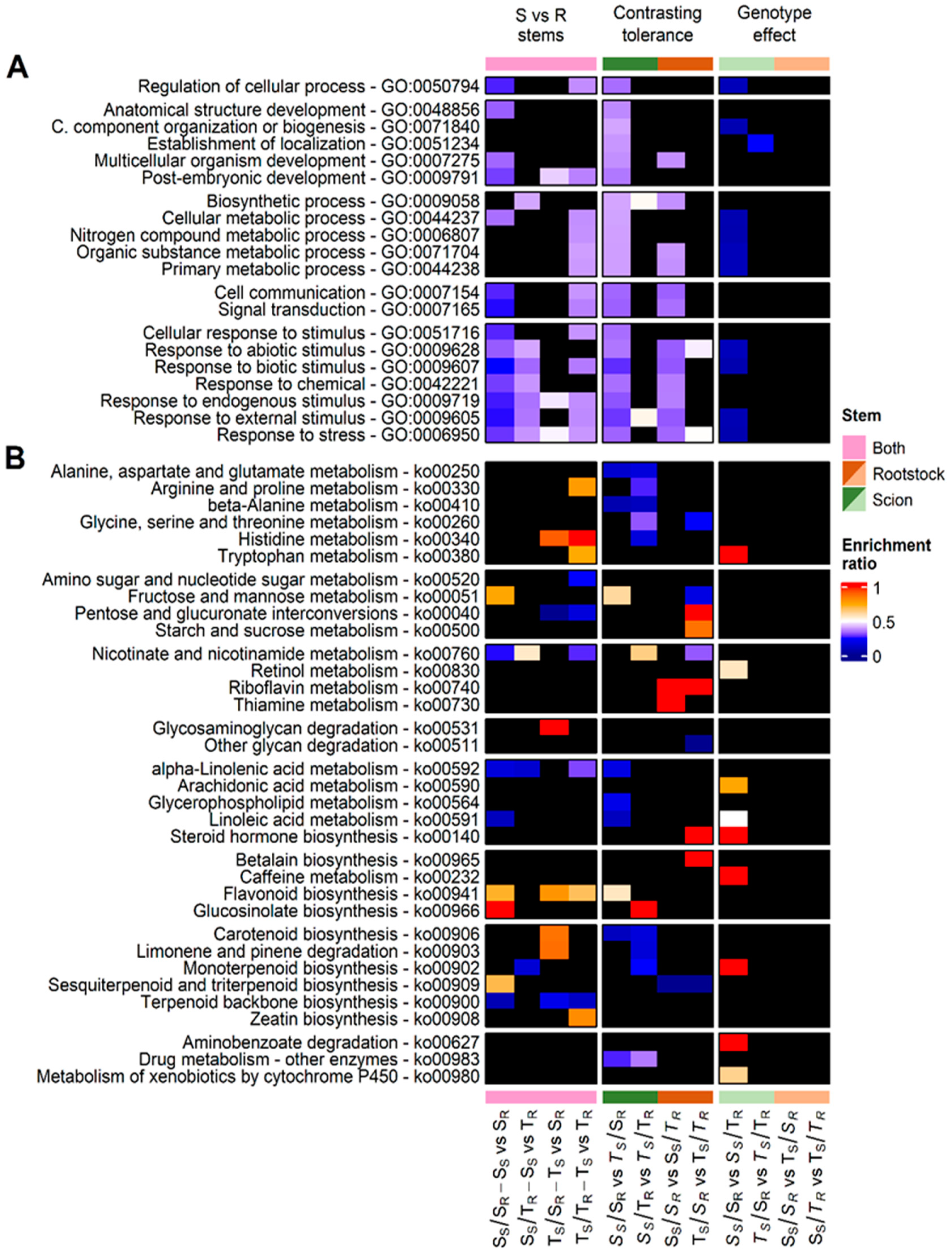
3. Discussion
3.1. Expression of Genes Involved in Pathogen Recognition in Pinus Pinaster Grafts Depended on Scion Provenance and Genotype Combination
3.2. Drought Susceptibility of P. Pinaster Grafts May Be Influenced by the Expression of Genes Involved in the Response to Biotic Stress
3.3. The Expression of Drought Tolerance Genes in P. pinaster Stems Could Predate the Drought Event in Tolerant Genotypes
3.4. Drought-Tolerant Rootstock (R18T) Controlled the Expression of Metabolism-Related Genes in Gal 1056 Stems
4. Material and Methods
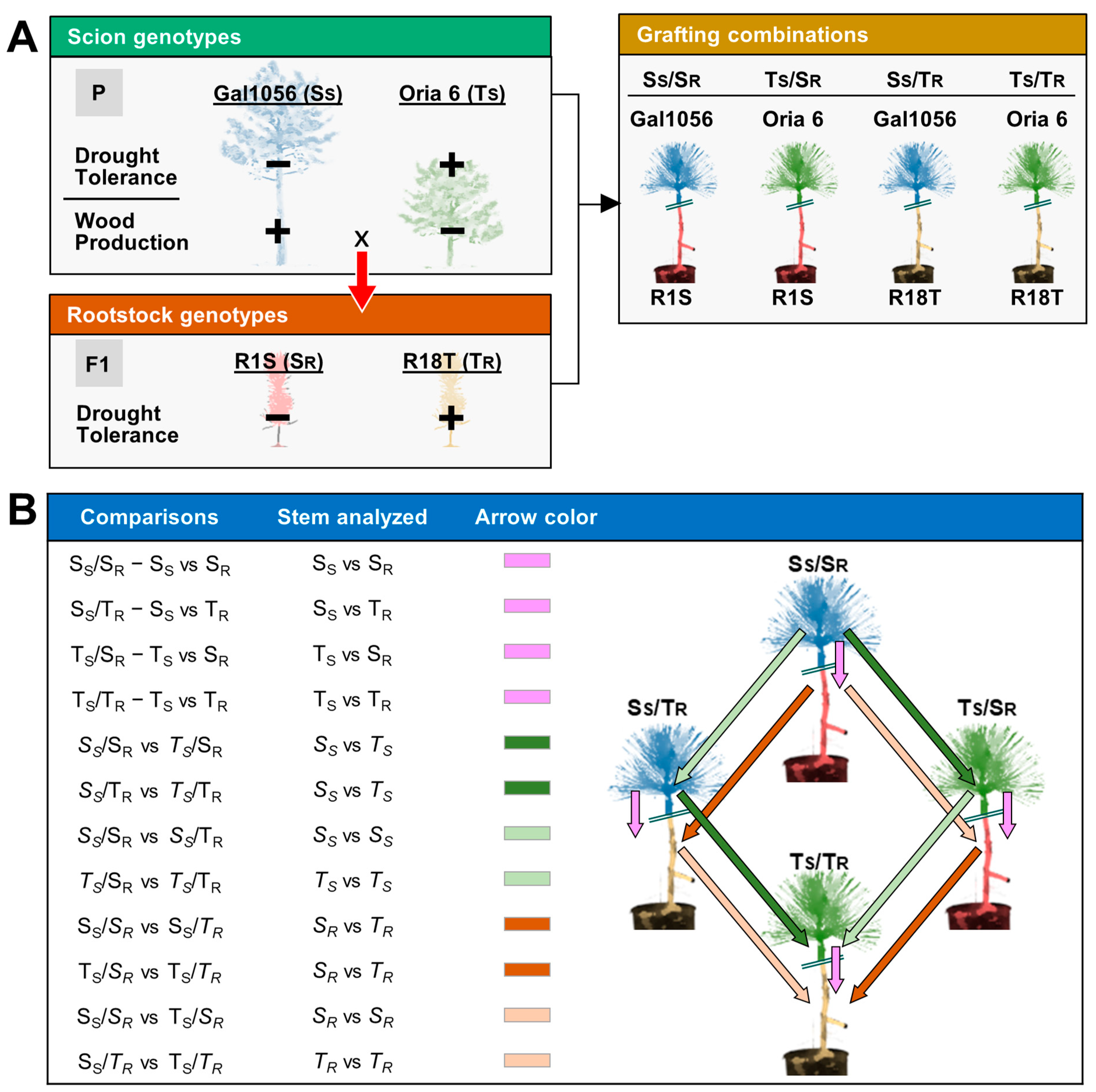
4.1. Plant Material and Experimental Design
4.2. RNA Extraction, RNA-Seq Library Preparation, and Sequencing
4.3. Sequence Analysis and Transcript Abundance Estimation
4.4. Differential Expression Analysis
- Analysis of the transcriptional differences between scion and rootstock stems of each P. pinaster graft combining genotypes with similar (SS/SR and TS/TR) or contrasting (SS/TR and TS/SR) responses to drought. The comparisons were SS/SR: Gal 1056 vs. R1S, TS/TR: Oria 6 vs. R18T, SS/TR: Gal 1056 vs. R18T, and TS/SR: Oria 6 vs. R1S (Scheme 1B).
- Analysis of the transcriptional variation between scion stems. Two types of comparisons were included: (1) identification of differentially expressed genes (DEGs) between drought-sensitive and drought-tolerant scion stems (SS vs. TS) grafted on the same rootstock genotype (SS/SR vs. TS/SR: Gal 1056/R1S vs. Oria 6/R1S and SS/TR vs. TS/TR: Gal 1056/R18T vs. Oria 6/R18T); and (2) identification of DEGs in drought-sensitive (SS) or drought-tolerant (TS) scion stems grafted on different rootstock genotypes, and therefore associated with rootstock interactions (SS/SR vs. SS/TR: Gal 1056/R1S vs. Gal 1056/R18T and TS/SR vs. TS/TR: Oria 6/R1S vs. Oria 6/R18T) (Scheme 1B).
- Analysis of the transcriptional variation between rootstock stems. Two types of comparisons were included: 1) identification of DEGs between rootstock genotypes (SR vs. TR) with the same grafted scion: SS/SR vs. SS/TR: Gal 1056/R1S vs. Gal 1056/R18T and TS/SR vs. TS/TR: Oria 6/R1S vs. Oria 6/R18T); and 2) identification of DEGs in drought-sensitive (SR) or tolerant (TR) rootstock stems with different grafted scions, associated with scion interactions (SS/SR vs. TS/SR: Gal 1056/R1S vs. Oria 6/R1S and SS/TR vs. TS/TR: Gal 1056/R18T vs. Oria 6/R18T) (Scheme 1B).
4.5. Pinus pinaster Transcriptome Annotation and Functional Enrichment Analysis
4.6. Gene Selection by Correlation Analysis and Validation by qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lecina-Diaz, J.; Martínez-Vilalta, J.; Alvarez, A.; Banqué, M.; Birkmann, J.; Feldmeyer, D.; Vayreda, J.; Retana, J. Characterizing Forest Vulnerability and Risk to Climate-change Hazards. Front. Ecol. Environ. 2021, 19, 126–133. [Google Scholar] [CrossRef]
- Avila, J.M.; Gallardo, A.; Gómez-Aparicio, L. Pathogen-Induced Tree Mortality Interacts with Predicted Climate Change to Alter Soil Respiration and Nutrient Availability in Mediterranean Systems. Biogeochemistry 2019, 142, 53–71. [Google Scholar] [CrossRef]
- Rubio-Cuadrado, Á.; López, R.; Rodríguez-Calcerrada, J.; Gil, L. Stress and Tree Mortality in Mediterranean Pine Forests: Anthropogenic Influences. In Pines and Their Mixed Forest Ecosystems in the Mediterranean Basin. Managing Forest Ecosystems; Ne’eman, G., Osem, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 38, pp. 141–181. ISBN 978-3-030-63625-8. [Google Scholar]
- Nunes, L.J.R.; Meireles, C.I.R.; Gomes, C.J.P.; Ribeiro, N.M.C.A. The Impact of Climate Change on Forest Development: A Sustainable Approach to Management Models Applied to Mediterranean-Type Climate Regions. Plants 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.; Cramer, W.; Carnicer, J.; Georgopoulou, E.; Hilmi, N.J.M.; Le Cozannet, G.; Lionello, P. Cross-Chapter Paper 4: Mediterranean Region. In Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK, 2022; pp. 2233–2272. ISBN 9781009325844. [Google Scholar]
- Thorpe, T.A.; Harry, I.S. Clonal Propagation of Conifers. In Plant Tissue Culture Manual; Lindsey, K., Ed.; Springer: Dordrecht, The Netherlands, 1991; pp. 487–502. ISBN 978-94-009-0103-2. [Google Scholar]
- Peng, L.; Searchinger, T.D.; Zionts, J.; Waite, R. The Carbon Costs of Global Wood Harvests. Nature 2023, 620, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Maître, D. Pines in Cultivation: A Global View; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Mariette, S.; Chagné, D.; Lézier, C.; Pastuszka, P.; Raffin, A.; Plomion, C.; Kremer, A. Genetic Diversity within and among Pinus pinaster Populations: Comparison between AFLP and Microsatellite Markers. Heredity 2001, 86, 469–479. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, M.; Rodríguez-Quilón, I.; Heuertz, M.; Hurel, A.; Grivet, D.; Jaramillo-Correa, J.P.; Vendramin, G.G.; Plomion, C.; Majada, J.; Alía, R.; et al. Polygenic Adaptation and Negative Selection across Traits, Years and Environments in a Long-lived Plant Species (Pinus pinaster Ait., Pinaceae). Mol. Ecol. 2022, 31, 2089–2105. [Google Scholar] [CrossRef]
- Correia, I.; Almeida, M.H.; Aguiar, A.; Alia, R.; David, T.S.; Pereira, J.S. Variations in Growth, Survival and Carbon Isotope Composition (13C) among Pinus pinaster Populations of Different Geographic Origins. Tree Physiol. 2008, 28, 1545–1552. [Google Scholar] [CrossRef]
- De La Mata, R.; Merlo, E.; Zas, R. Among-Population Variation and Plasticity to Drought of Atlantic, Mediterranean, and Interprovenance Hybrid Populations of Maritime Pine. Tree Genet. Genomes 2014, 10, 1191–1203. [Google Scholar] [CrossRef]
- González Martínez, S.C.; Gil, L.; Alia, R. Genetic Diversity Estimates of Pinus pinaster in the Iberian Peninsula: A Comparison of Allozymes and Quantitative Traits. For. Syst. 2005, 14, 3. [Google Scholar] [CrossRef]
- Alía, R.; Moro, J.; Denis, J.B.; Moro-Serrano, J. Performance of Pinus pinaster Provenances in Spain: Interpretation of the Genotype by Environment Interaction. Can. J. For. Res. 1997, 27, 1548–1559. [Google Scholar] [CrossRef]
- Meijón, M.; Feito, I.; Oravec, M.; Delatorre, C.; Weckwerth, W.; Majada, J.; Valledor, L. Exploring Natural Variation of Pinus pinaster Aiton Using Metabolomics: Is It Possible to Identify the Region of Origin of a Pine from Its Metabolites? Mol. Ecol. 2016, 25, 959–976. [Google Scholar] [CrossRef] [PubMed]
- Aranda, I.; Alía, R.; Ortega, U.; Dantas, Â.K.; Majada, J. Intra-Specific Variability in Biomass Partitioning and Carbon Isotopic Discrimination under Moderate Drought Stress in Seedlings from Four Pinus pinaster Populations. Tree Genet. Genomes 2010, 6, 169–178. [Google Scholar] [CrossRef]
- Gaspar, M.J.; Velasco, T.; Feito, I.; Alía, R.; Majada, J. Genetic Variation of Drought Tolerance in Pinus pinaster at Three Hierarchical Levels: A Comparison of Induced Osmotic Stress and Field Testing. PLoS ONE 2013, 8, e79094. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Recuenco, M.; Iturritxa, E.; Majada, J.; Alía, R.; Raposo, R. Adaptive Potential of Maritime Pine (Pinus pinaster) Populations to the Emerging Pitch Canker Pathogen, Fusarium Circinatum. PLoS ONE 2014, 9, e114971. [Google Scholar] [CrossRef] [PubMed]
- Hurel, A.; de Miguel, M.; Dutech, C.; Desprez-Loustau, M.; Plomion, C.; Rodríguez-Quilón, I.; Cyrille, A.; Guzman, T.; Alía, R.; González-Martínez, S.C.; et al. Genetic Basis of Growth, Spring Phenology, and Susceptibility to Biotic Stressors in Maritime Pine. Evol. Appl. 2021, 14, 2750–2772. [Google Scholar] [CrossRef] [PubMed]
- Bonga, J.M. A Comparative Evaluation of the Application of Somatic Embryogenesis, Rooting of Cuttings, and Organogenesis of Conifers. Can. J. For. Res. 2014, 45, 379–383. [Google Scholar] [CrossRef]
- Greenwood, M.S. 1. Rejuvenation of Forest Trees. Plant Growth Regul. 1987, 6, 1–12. [Google Scholar] [CrossRef]
- Mudge, K.; Janick, J.; Scofield, S.; Goldschmidt, E.E. A History of Grafting. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 437–493. [Google Scholar]
- Lindgren, D.; Prescher, F. Optimal Clone Number for Seed Orchards with Tested Clones. Silvae Genet. 2005, 54, 80–92. [Google Scholar] [CrossRef]
- Pérez-Luna, A.; Wehenkel, C.; Prieto-Ruíz, J.Á.; López-Upton, J.; Solís-González, S.; Chávez-Simental, J.A.; Hernández-Díaz, J.C. Grafting in Conifers: A Review. Pak. J. Bot. 2020, 52. [Google Scholar] [CrossRef]
- Melnyk, C.W.; Meyerowitz, E.M. Plant Grafting. Curr. Biol. 2015, 25, R183–R188. [Google Scholar] [CrossRef]
- Tsutsui, H.; Notaguchi, M. The Use of Grafting to Study Systemic Signaling in Plants. Plant Cell Physiol. 2017, 58, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.; Dridi, J.; Gutierrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, Biochemical and Molecular Responses in Four Prunus Rootstocks Submitted to Drought Stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, L.; Jittayasothorn, Y.; Kang, Y.; Jiao, C.; Fei, Z.; Zhong, G.-Y. Messenger RNA Exchange between Scions and Rootstocks in Grafted Grapevines. BMC Plant Biol. 2015, 15, 251. [Google Scholar] [CrossRef]
- Warschefsky, E.J.; Klein, L.L.; Frank, M.H.; Chitwood, D.H.; Londo, J.P.; von Wettberg, E.J.B.; Miller, A.J. Rootstocks: Diversity, Domestication, and Impacts on Shoot Phenotypes. Trends Plant Sci. 2016, 21, 418–437. [Google Scholar] [CrossRef]
- Thomas, H.R.; Frank, M.H. Connecting the Pieces: Uncovering the Molecular Basis for Long-distance Communication through Plant Grafting. New Phytol. 2019, 223, 582–589. [Google Scholar] [CrossRef]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT Protein Movement Contributes to Long-Distance Signaling in Floral Induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Small Peptide Modulates Stomatal Control via Abscisic Acid in Long-Distance Signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Notaguchi, M.; Higashiyama, T.; Suzuki, T. Identification of MRNAs That Move Over Long Distances Using an RNA-Seq Analysis of Arabidopsis/Nicotiana benthamiana Heterografts. Plant Cell Physiol. 2015, 56, 311–321. [Google Scholar] [CrossRef]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of Flowering and Storage Organ Formation in Potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef]
- González-Schain, N.D.; Díaz-Mendoza, M.; Żurczak, M.; Suárez-López, P. Potato CONSTANS Is Involved in Photoperiodic Tuberization in a Graft-transmissible Manner. Plant J. 2012, 70, 678–690. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Chatterjee, M.; Yu, Y.; Suh, S.-G.; Miller, W.A.; Hannapel, D.J. Dynamics of a Mobile RNA of Potato Involved in a Long-Distance Signaling Pathway. Plant Cell 2006, 18, 3443–3457. [Google Scholar] [CrossRef] [PubMed]
- Ghate, T.H.; Sharma, P.; Kondhare, K.R.; Hannapel, D.J.; Banerjee, A.K. The Mobile RNAs, StBEL11 and StBEL29, Suppress Growth of Tubers in Potato. Plant Mol. Biol. 2017, 93, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Bhogale, S.; Mahajan, A.S.; Natarajan, B.; Rajabhoj, M.; Thulasiram, H.V.; Banerjee, A.K. MicroRNA156: A Potential Graft-Transmissible MicroRNA That Modulates Plant Architecture and Tuberization in Solanum tuberosum ssp. Andigena. Plant Physiol. 2014, 164, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Adam, H.; Díaz-Mendoza, M.; Żurczak, M.; González-Schain, N.D.; Suárez-López, P. Graft-Transmissible Induction of Potato Tuberization by the MicroRNA MiR172. Development 2009, 136, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Zuntini, A.R.; Carruthers, T.; Maurin, O.; Bailey, P.C.; Leempoel, K.; Brewer, G.E.; Epitawalage, N.; Françoso, E.; Gallego-Paramo, B.; McGinnie, C.; et al. Phylogenomics and the Rise of the Angiosperms. Nature 2024, 629, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Sauquet, H.; Ramírez-Barahona, S.; Magallón, S. What Is the Age of Flowering Plants? J. Exp. Bot. 2022, 73, 3840–3853. [Google Scholar] [CrossRef]
- Sperry, J.S.; Hacke, U.G.; Pittermann, J. Size and Function in Conifer Tracheids and Angiosperm Vessels. Am. J. Bot. 2006, 93, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Simón, B.; Aranda, I.; López-Hinojosa, M.; Miguel, L.; Cervera, M.T. Scion-Rootstock Interaction and Drought Systemic Effect Modulate the Organ-Specific Terpene Profiles in Grafted Pinus pinaster Ait. Environ. Exp. Bot. 2021, 186, 104437. [Google Scholar] [CrossRef]
- López-Hinojosa, M.; de María, N.; Guevara, M.A.; Vélez, M.D.; Cabezas, J.A.; Díaz, L.M.; Mancha, J.A.; Pizarro, A.; Manjarrez, L.F.; Collada, C.; et al. Rootstock Effects on Scion Gene Expression in Maritime Pine. Sci. Rep. 2021, 11, 11582. [Google Scholar] [CrossRef]
- López-Goldar, X.; Zas, R.; Sampedro, L. Resource Availability Drives Microevolutionary Patterns of Plant Defences. Funct. Ecol. 2020, 34, 1640–1652. [Google Scholar] [CrossRef]
- Moreira, X.; Mooney, K.A.; Rasmann, S.; Petry, W.K.; Carrillo-Gavilán, A.; Zas, R.; Sampedro, L. Trade-Offs between Constitutive and Induced Defences Drive Geographical and Climatic Clines in Pine Chemical Defences. Ecol. Lett. 2014, 17, 537–546. [Google Scholar] [CrossRef]
- Moreira, X.; Zas, R.; Solla, A.; Sampedro, L. Differentiation of Persistent Anatomical Defensive Structures Is Costly and Determined by Nutrient Availability and Genetic Growth-Defence Constraints. Tree Physiol. 2015, 35, 112–123. [Google Scholar] [CrossRef]
- Andersen, E.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M. Disease Resistance Mechanisms in Plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Jones, J.D.G.; Ding, P. Plant Immune Networks. Trends Plant Sci. 2022, 27, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-Triggered Immunity: From Pathogen Perception to Robust Defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Van Ghelder, C.; Parent, G.J.; Rigault, P.; Prunier, J.; Giguère, I.; Caron, S.; Stival Sena, J.; Deslauriers, A.; Bousquet, J.; Esmenjaud, D.; et al. The Large Repertoire of Conifer NLR Resistance Genes Includes Drought Responsive and Highly Diversified RNLs. Sci. Rep. 2019, 9, 11614. [Google Scholar] [CrossRef]
- Liu, J.-J.; Ekramoddoullah, A.K.M. The CC-NBS-LRR Subfamily in Pinus monticola: Targeted Identification, Gene Expression, and Genetic Linkage with Resistance to Cronartium ribicola. Phytopathology 2007, 97, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Schoettle, A.W.; Sniezko, R.A.; Williams, H.; Zamany, A.; Rancourt, B. Fine Dissection of Limber Pine Resistance to Cronartium ribicola Using Targeted Sequencing of the NLR Family. BMC Genom. 2021, 22, 567. [Google Scholar] [CrossRef] [PubMed]
- Lauer, E.; Isik, F. Major QTL Confer Race-Nonspecific Resistance in the Co-Evolved Cronartium quercuum f. sp. Fusiforme–Pinus taeda Pathosystem. Heredity 2021, 127, 288–299. [Google Scholar] [CrossRef]
- Hernández-Escribano, L.; Visser, E.A.; Iturritxa, E.; Raposo, R.; Naidoo, S. The Transcriptome of Pinus pinaster under Fusarium circinatum Challenge. BMC Genom. 2020, 21, 28. [Google Scholar] [CrossRef]
- Modesto, I.; Sterck, L.; Arbona, V.; Gómez-Cadenas, A.; Carrasquinho, I.; Van de Peer, Y.; Miguel, C.M. Insights into the Mechanisms Implicated in Pinus pinaster Resistance to Pinewood Nematode. Front. Plant Sci. 2021, 12, 690857. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Gutiérrez, M.; Alonso, M.; Díaz, R. Assessing Genetic Variation in Resistance to Pinewood Nematode (Bursaphelenchus xylophilus) in Pinus radiata D. Don Half-Sib Families. Forests 2021, 12, 1474. [Google Scholar] [CrossRef]
- Cobo-Simón, I.; Maloof, J.N.; Li, R.; Amini, H.; Méndez-Cea, B.; García-García, I.; Gómez-Garrido, J.; Esteve-Codina, A.; Dabad, M.; Alioto, T.; et al. Contrasting Transcriptomic Patterns Reveal a Genomic Basis for Drought Resilience in the Relict Fir Abies Pinsapo Boiss. Tree Physiol. 2023, 43, 315–334. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Simón, I.; Gómez-Garrido, J.; Esteve-Codina, A.; Dabad, M.; Alioto, T.; Maloof, J.N.; Méndez-Cea, B.; Seco, J.I.; Linares, J.C.; Gallego, F.J. De Novo Transcriptome Sequencing and Gene Co-Expression Reveal a Genomic Basis for Drought Sensitivity and Evidence of a Rapid Local Adaptation on Atlas Cedar (Cedrus atlantica). Front. Plant Sci. 2023, 14, 1116863. [Google Scholar] [CrossRef] [PubMed]
- Blank, L.; Martín-García, J.; Bezos, D.; Vettraino, A.; Krasnov, H.; Lomba, J.; Fernández, M.; Diez, J. Factors Affecting the Distribution of Pine Pitch Canker in Northern Spain. Forests 2019, 10, 305. [Google Scholar] [CrossRef]
- Fariña-Flores, D.; Berbegal, M.; Iturritxa, E.; Hernandez-Escribano, L.; Aguín, O.; Raposo, R. Temporal and Spatial Variation in the Population Structure of Spanish Fusarium circinatum Infecting Pine Stands. J. Fungi 2023, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Stival Sena, J.; Giguère, I.; Rigault, P.; Bousquet, J.; Mackay, J. Expansion of the Dehydrin Gene Family in the Pinaceae Is Associated with Considerable Structural Diversity and Drought-Responsive Expression. Tree Physiol. 2018, 38, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ding, Y.; Jiang, Q.; Wang, F.; Sun, J.; Zhu, C. The Role of Receptor-like Protein Kinases (RLKs) in Abiotic Stress Response in Plants. Plant Cell Rep. 2017, 36, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Noutoshi, Y.; Ito, T.; Seki, M.; Nakashita, H.; Yoshida, S.; Marco, Y.; Shirasu, K.; Shinozaki, K. A Single Amino Acid Insertion in the WRKY Domain of the Arabidopsis TIR-NBS-LRR-WRKY-Type Disease Resistance Protein SLH1 (Sensitive to Low Humidity 1) Causes Activation of Defense Responses and Hypersensitive Cell Death. Plant J. 2005, 43, 873–888. [Google Scholar] [CrossRef]
- Sohn, K.H.; Segonzac, C.; Rallapalli, G.; Sarris, P.F.; Woo, J.Y.; Williams, S.J.; Newman, T.E.; Paek, K.H.; Kobe, B.; Jones, J.D.G. The Nuclear Immune Receptor RPS4 Is Required for RRS1SLH1-Dependent Constitutive Defense Activation in Arabidopsis thaliana. PLoS Genet. 2014, 10, e1004655. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Han, M.; Lee, S.-K.; Cho, J.-I.; Ryoo, N.; Heu, S.; Lee, Y.-H.; Bhoo, S.H.; Wang, G.-L.; Hahn, T.-R.; et al. A Comprehensive Expression Analysis of the WRKY Gene Superfamily in Rice Plants during Defense Response. Plant Cell Rep. 2006, 25, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hou, X.-L.; Xing, G.-M.; Liu, J.-X.; Duan, A.-Q.; Xu, Z.-S.; Li, M.-Y.; Zhuang, J.; Xiong, A.-S. Advances in AP2/ERF Super-Family Transcription Factors in Plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-J.; Liu, J.-Y. The Arabidopsis EAR-Motif-Containing Protein RAP2.1 Functions as an Active Transcriptional Repressor to Keep Stress Responses under Tight Control. BMC Plant Biol. 2010, 10, 47. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA Perception and Signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA Transport and Plant Water Stress Responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- MacGregor, D.R.; Zhang, N.; Iwasaki, M.; Chen, M.; Dave, A.; Lopez-Molina, L.; Penfield, S. ICE 1 and ZOU Determine the Depth of Primary Seed Dormancy in Arabidopsis Independently of Their Role in Endosperm Development. Plant J. 2019, 98, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hwang, H.; Hong, J.-W.; Lee, Y.-N.; Ahn, I.P.; Yoon, I.S.; Yoo, S.-D.; Lee, S.; Lee, S.C.; Kim, B.-G. A Rice Orthologue of the ABA Receptor, OsPYL/RCAR5, Is a Positive Regulator of the ABA Signal Transduction Pathway in Seed Germination and Early Seedling Growth. J. Exp. Bot. 2012, 63, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Chuong, N.N.; Nghia, D.H.T.; Le Thi, V.-A.; Tran, L.-S.P.; Hoang, X.L.T.; Thao, N.P. Type 2C Protein Phosphatases in Plant Signaling Pathways under Abiotic Stress. In Protein Phosphatases and Stress Management in Plants; Springer International Publishing: Cham, Switzerland, 2020; pp. 67–82. [Google Scholar]
- Rentsch, D.; Hirner, B.; Schmelzer, E.; Frommer, W.B. Salt Stress-Induced Proline Transporters and Salt Stress-Repressed Broad Specificity Amino Acid Permeases Identified by Suppression of a Yeast Amino Acid Permease-Targeting Mutant. Plant Cell 1996, 8, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Grallath, S.; Weimar, T.; Meyer, A.; Gumy, C.; Suter-Grotemeyer, M.; Neuhaus, J.-M.; Rentsch, D. The AtProT Family. Compatible Solute Transporters with Similar Substrate Specificity But Differential Expression Patterns. Plant Physiol. 2005, 137, 117–126. [Google Scholar] [CrossRef]
- Liu, M.; Wang, C.; Ji, Z.; Lu, J.; Zhang, L.; Li, C.; Huang, J.; Yang, G.; Yan, K.; Zhang, S.; et al. Regulation of Drought Tolerance in Arabidopsis Involves the PLATZ4-mediated Transcriptional Repression of Plasma Membrane Aquaporin PIP2;8. Plant J. 2023, 115, 434–451. [Google Scholar] [CrossRef]
- Vij, S.; Giri, J.; Dansana, P.K.; Kapoor, S.; Tyagi, A.K. The Receptor-Like Cytoplasmic Kinase (OsRLCK) Gene Family in Rice: Organization, Phylogenetic Relationship, and Expression during Development and Stress. Mol. Plant 2008, 1, 732–750. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-B.; Liu, C.; Tang, D.-Y.; Yan, L.; Wang, D.; Yang, Y.-Z.; Gui, J.-S.; Zhao, X.-Y.; Li, L.-G.; Tang, X.-D.; et al. The Receptor-Like Cytoplasmic Kinase STRK1 Phosphorylates and Activates CatC, Thereby Regulating H2O2 Homeostasis and Improving Salt Tolerance in Rice. Plant Cell 2018, 30, 1100–1118. [Google Scholar] [CrossRef] [PubMed]
- Fernández de Simón, B.; Sanz, M.; Cervera, M.T.; Pinto, E.; Aranda, I.; Cadahía, E. Leaf Metabolic Response to Water Deficit in Pinus pinaster Ait. Relies upon Ontogeny and Genotype. Environ. Exp. Bot. 2017, 140, 41–55. [Google Scholar] [CrossRef]
- de María, N.; Guevara, M.Á.; Perdiguero, P.; Vélez, M.D.; Cabezas, J.A.; López-Hinojosa, M.; Li, Z.; Díaz, L.M.; Pizarro, A.; Mancha, J.A.; et al. Molecular Study of Drought Response in the Mediterranean Conifer Pinus pinaster Ait.: Differential Transcriptomic Profiling Reveals Constitutive Water Deficit-independent Drought Tolerance Mechanisms. Ecol. Evol. 2020, 10, 9788–9807. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, Y.; Liu, S.; Xia, F.; Li, X.; Qi, B. Accumulation of Eicosapolyenoic Acids Enhances Sensitivity to Abscisic Acid and Mitigates the Effects of Drought in Transgenic Arabidopsis Thaliana. J. Exp. Bot. 2014, 65, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.; Chen, J.; Yin, Y. Cross-Talk of Brassinosteroid Signaling in Controlling Growth and Stress Responses. Biochem. J. 2017, 474, 2641–2661. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional Regulators of Plant Growth, Development, and Stress Responses. Plant Cell 2020, 32, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Shen, Y.; Pu, R.; Hou, M.; Wei, Q.; Zhang, X.; Li, G.; Ren, H.; Wu, G. Brassinosteroids Synthesised by CYP85A/A1 but Not CYP85A2 Function via a BRI1-like Receptor but Not via BRI1 in Picea abies. J. Exp. Bot. 2021, 72, 1748–1763. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, Z.; Qin, H.; Tan, J.; Ding, G. Exogenous Brassinosteroid Facilitates Xylem Development in Pinus massoniana Seedlings. Int. J. Mol. Sci. 2021, 22, 7615. [Google Scholar] [CrossRef]
- Seki, H.; Sawai, S.; Ohyama, K.; Mizutani, M.; Ohnishi, T.; Sudo, H.; Fukushima, E.O.; Akashi, T.; Aoki, T.; Saito, K.; et al. Triterpene Functional Genomics in Licorice for Identification of CYP72A154 Involved in the Biosynthesis of Glycyrrhizin. Plant Cell 2011, 23, 4112–4123. [Google Scholar] [CrossRef]
- Tanaka, K.; Asami, T.; Yoshida, S.; Nakamura, Y.; Matsuo, T.; Okamoto, S. Brassinosteroid Homeostasis in Arabidopsis Is Ensured by Feedback Expressions of Multiple Genes Involved in Its Metabolism. Plant Physiol. 2005, 138, 1117–1125. [Google Scholar] [CrossRef]
- Kleiber, A.; Duan, Q.; Jansen, K.; Verena Junker, L.; Kammerer, B.; Rennenberg, H.; Ensminger, I.; Gessler, A.; Kreuzwieser, J. Drought Effects on Root and Needle Terpenoid Content of a Coastal and an Interior Douglas Fir Provenance. Tree Physiol. 2017, 37, 1648–1658. [Google Scholar] [CrossRef] [PubMed]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The Variability of Terpenes in Conifers under Developmental and Environmental Stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Turtola, S.; Manninen, A.-M.; Rikala, R.; Kainulainen, P. Drought Stress Alters the Concentration of Wood Terpenoids in Scots Pine and Norway Spruce Seedlings. J. Chem. Ecol. 2003, 29, 1981–1995. [Google Scholar] [CrossRef]
- Paniagua, C.; Bilkova, A.; Jackson, P.; Dabravolski, S.; Riber, W.; Didi, V.; Houser, J.; Gigli-Bisceglia, N.; Wimmerova, M.; Budínská, E.; et al. Dirigent Proteins in Plants: Modulating Cell Wall Metabolism during Abiotic and Biotic Stress Exposure. J. Exp. Bot. 2017, 68, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Devasagayam, T.P.A.; Kamat, J.P.; Mohan, H.; Kesavan, P.C. Caffeine as an Antioxidant: Inhibition of Lipid Peroxidation Induced by Reactive Oxygen Species. Biochim. Biophys. Acta-Biomembr. 1996, 1282, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant Activity of Carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple Functional Roles of Flavonoids in Photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Lewis, D.R.; Ramirez, M.V.; Miller, N.D.; Vallabhaneni, P.; Ray, W.K.; Helm, R.F.; Winkel, B.S.J.; Muday, G.K. Auxin and Ethylene Induce Flavonol Accumulation through Distinct Transcriptional Networks. Plant Physiol. 2011, 156, 144–164. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, M.; Sanchez-Gomez, D.; Cervera, M.T.; Aranda, I. Functional and Genetic Characterization of Gas Exchange and Intrinsic Water Use Efficiency in a Full-Sib Family of Pinus pinaster Ait. in Response to Drought. Tree Physiol. 2012, 32, 94–103. [Google Scholar] [CrossRef] [PubMed]
- de Miguel, M.; Cabezas, J.-A.; de María, N.; Sánchez-Gómez, D.; Guevara, M.-Á.; Vélez, M.-D.; Sáez-Laguna, E.; Díaz, L.-M.; Mancha, J.-A.; Barbero, M.-C.; et al. Genetic Control of Functional Traits Related to Photosynthesis and Water Use Efficiency in Pinus pinaster Ait. Drought Response: Integration of Genome Annotation, Allele Association and QTL Detection for Candidate Gene Identification. BMC Genom. 2014, 15, 464. [Google Scholar] [CrossRef] [PubMed]
- Randomized Block Design. In The Concise Encyclopedia of Statistics; Springer: New York, NY, USA, 2008; pp. 447–448. ISBN 978-0-387-32833-1.
- BioBam Bioinformatics. OmicsBox—Bioinformatics Made Easy (version 2.0.36). 2019. Available online: https://www.biobam.com/ (accessed on 16 May 2024).
- Andrews, S. FastQC: A Quality Control Tool for High Thoughput Sequence Data. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 5 October 2020).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Kopylova, E.; Noé, L.; Touzet, H. SortMeRNA: Fast and Accurate Filtering of Ribosomal RNAs in Metatranscriptomic Data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talon, M.; Dopazo, J.; Conesa, A. High-Throughput Functional Annotation and Data Mining with the Blast2GO Suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Nagel, A.; Herter, T.; May, P.; Schroda, M.; Zrenner, R.; Tohge, T.; Fernie, A.R.; Stitt, M.; Usadel, B. Mercator: A Fast and Simple Web Server for Genome Scale Functional Annotation of Plant Sequence Data. Plant. Cell Environ. 2014, 37, 1250–1258. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manjarrez, L.F.; Guevara, M.Á.; de María, N.; Vélez, M.D.; Cobo-Simón, I.; López-Hinojosa, M.; Cabezas, J.A.; Mancha, J.A.; Pizarro, A.; Díaz-Sala, M.C.; et al. Maritime Pine Rootstock Genotype Modulates Gene Expression Associated with Stress Tolerance in Grafted Stems. Plants 2024, 13, 1644. https://doi.org/10.3390/plants13121644
Manjarrez LF, Guevara MÁ, de María N, Vélez MD, Cobo-Simón I, López-Hinojosa M, Cabezas JA, Mancha JA, Pizarro A, Díaz-Sala MC, et al. Maritime Pine Rootstock Genotype Modulates Gene Expression Associated with Stress Tolerance in Grafted Stems. Plants. 2024; 13(12):1644. https://doi.org/10.3390/plants13121644
Chicago/Turabian StyleManjarrez, Lorenzo Federico, María Ángeles Guevara, Nuria de María, María Dolores Vélez, Irene Cobo-Simón, Miriam López-Hinojosa, José Antonio Cabezas, José Antonio Mancha, Alberto Pizarro, María Carmen Díaz-Sala, and et al. 2024. "Maritime Pine Rootstock Genotype Modulates Gene Expression Associated with Stress Tolerance in Grafted Stems" Plants 13, no. 12: 1644. https://doi.org/10.3390/plants13121644
APA StyleManjarrez, L. F., Guevara, M. Á., de María, N., Vélez, M. D., Cobo-Simón, I., López-Hinojosa, M., Cabezas, J. A., Mancha, J. A., Pizarro, A., Díaz-Sala, M. C., & Cervera, M. T. (2024). Maritime Pine Rootstock Genotype Modulates Gene Expression Associated with Stress Tolerance in Grafted Stems. Plants, 13(12), 1644. https://doi.org/10.3390/plants13121644





