Phytochemical Investigation of Polyphenols from the Aerial Parts of Tanacetum balsamita Used in Transylvanian Ethnobotany and Parallel Artificial Membrane Permeability Assay
Abstract
:1. Introduction
2. Results
2.1. Ethnobotanical Data in Selected Areas in Transylvania
2.2. Qualitative Analysis of Phenolic Compounds with UHPLC-DAD-ESI-MS/MS
2.2.1. Flavonoid Derivatives
2.2.2. Hydroxycinnamic Acid Derivatives
2.2.3. Other Constituents
2.3. Parallel Artificial Membrane Permeability Assay (PAMPA)
3. Discussion
4. Materials and Methods
4.1. Ethnobotanical Survey and Research Areas
4.2. Plant Material and Sample Preparation
4.3. Reagent and Chemicals
4.4. Phytochemical Analyses by Ultrahigh-Performance Liquid Chromatography (UHPLC) Coupled to Diode-Array Detector (DAD) and Mass Spectrometry (MS)
4.4.1. UHPLC Conditions
4.4.2. MS Conditions
4.5. Parallel Artificial Membrane Permeability Assay (PAMPA)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gurib-Fakim, A. Medicinal Plants: Traditions of Yesterday and Drugs of Tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Papp, N.; Birkás-Frendl, K.; Farkas, Á.; Pieroni, A. An Ethnobotanical Study on Home Gardens in a Transylvanian Hungarian Csángó Village (Romania). Genet. Resour. Crop Evol. 2013, 60, 1423–1432. [Google Scholar] [CrossRef]
- Papp, N.; Sali, N.; Csepregi, R.; Tóth, M.; Gyergyák, K.; Dénes, T.; Bartha, S.G.; Varga, E.; Kaszás, A.; Kőszegi, T. Antioxidant Potential of Some Plants Used in Folk Medicine in Romania. Farmacia 2019, 67, 323–330. [Google Scholar] [CrossRef]
- Papp, N.; Czégényi, D.; Tóth, M.; Dénes, T.; Bartha, S.G.; Csepregi, R.; Gyergyák, K.; Bukovics, P.; Stranczinger, S.; Varga, E.; et al. Ethnomedicine Survey on Folk Dermatology in Transylvania, Romania. Clin. Dermatol. 2022, 40, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Papp, N. “A Virágok …Mindegyik Orvosság”- Hagyományok és Népi Orvoslás Lövétén; Lövétei Közbirtokossága és Polgármesteri Hivatal: Lueta, Romania, 2018.
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, Antimicrobial, Antioxidant Properties and Effects on Cell Migration of Phenolic Compounds of Selected Transylvanian Medicinal Plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, V. Dictionary of Iranian Plant Names: Latin-English-Persian; Farhang Moaser: Tehran, Iran, 1998. [Google Scholar]
- Bagci, E.; Kocak, A. Essential Oil Composition of Two Endemic Tanacetum (T. nitens (Boiss.&Noe) Grierson and T. argenteum (Lam.) Willd. Subsp. argenteum) (Asteraceae) Taxa, Growing Wild in Turkey. Ind. Crops Prod. 2010, 31, 542–545. [Google Scholar] [CrossRef]
- Triana, J.; Eiroa, J.L.; Morales, M.; Pérez, F.J.; Brouard, I.; Marrero, M.T.; Estévez, S.; Quintana, J.; Estévez, F.; Castillo, Q.A.; et al. A Chemotaxonomic Study of Endemic Species of Genus Tanacetum from the Canary Islands. Phytochemistry 2013, 92, 87–104. [Google Scholar] [CrossRef]
- Zengin, G.; Sieniawska, E.; Senkardes, I.; Picot-Allain, M.C.N.; Ibrahime Sinan, K.; Fawzi Mahomoodally, M. Antioxidant Abilities, Key Enzyme Inhibitory Potential and Phytochemical Profile of Tanacetum poteriifolium Grierson. Ind. Crops Prod. 2019, 140, 111629. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Medicinal and Poisonous Plants. Common Names, Scientific Names, Eponyms, Synonyms, and Etymology (5 Volume Set); CRC Press: Boca Raton, FL, USA, 2012; Vol. V (R-Z). [Google Scholar]
- Dioscorides. De Materia Medica; Ibidis Press: Johannesburg, South Africa, 2000; ISBN 0-620-23435-0. [Google Scholar]
- Matthioli, P.A. Senensis, Medici, Compendium (Facsimile); In officina Valgrisiana: Venetiis, Italy, 1562. [Google Scholar]
- Matthioli, P.A. Herbář Neboli Bylinář. Commentarrii in Sex Libros Pedacii Dioscoridis (Facsimile); Melantrichius ab Aventino ad Inst. Vinc. Valgrisii: Pragae, Czech Republic, 1562. [Google Scholar]
- Rapaics, R. A Magyarság Virágai. A Virágkultusz Története; Királyi Magyar Természettudományi Társulat: Budapest, Hungary, 1932. [Google Scholar]
- Stirling, J. A késő középkori kertművészet szakrális szimbólumai a növénynevek tükrében. In “Lippay János” Tudományos Ülésszak Előadásai és Poszterei; Kertészeti és Élelmiszeripari Egyetem kiadványai: Budapest, Hungary, 1992; pp. 114–117. [Google Scholar]
- Berrár, J.; Károly, S. Régi Magyar Glosszárium. Szótárak, Szójegyzékek és Glosszák Egyesített Szótára; Akadémiai Kiadó: Budapest, Hungary, 1984. [Google Scholar]
- Clusius, C.; Beythe, I. Stirpium Nomenclator Pannonicus; Ex Officina Christophori Plantini: Németújvár, Austria, 1583. [Google Scholar]
- Schram, F. Népi növénynevek a XVIII. századból. Magy. Nyelvőr 1961, 1–4, 209–214. [Google Scholar]
- Priszter, S. Növényneveink. A Magyar és a Tudományos Növénynevek Szótára; Mezőgazda Kiadó: Budapest, Hungary, 1998. [Google Scholar]
- Koltay, E. Boldogasszony virágai. In Boldogasszony: Szűz Mária tisztelete Magyarországon és Közép-Európában; Szegedi Vallási Néprajzi Könyvtár; Néprajzi Tanszék: Szeged, Hungary, 2001; pp. 318–332. ISBN 963-482-319-X. [Google Scholar]
- Vörös, É. A Magyar Gyógynövények Neveinek Történeti-Etimológiai Szótára; A Debreceni Egyetem Magyar Nyelvtudományi Intézetének Kiadványai; Készült a Debreceni Egyetem Egyetemi és Nemzeti Könyvtárának Sokszorosító Üzemében: Debrecen, Hungary, 2008; ISBN 978-963-473-084-2. [Google Scholar]
- Rácz, J. Növénynevek Enciklopédiája. Az Elnevezések Eredete, a Növények Kultúrtörténete és Élettani Hatása; Tinta Kiadó: Budapest, Hungary, 2010; ISBN 978-963-9902-40-4. [Google Scholar]
- Melius Juhász, P. Herbarium; Heltai Gaspárné: Colosvárat, Romania, 1578. [Google Scholar]
- Varjas, B. XVI. Századi Magyar Orvosi Könyv; Erdélyi Tudományos Intézet: Kolozsvár, Romania, 1943. [Google Scholar]
- Lippay, J. Posoni Kert. II. Veteményes Kert; Cosmerovius Máté: Vienna, Austria, 1664. [Google Scholar]
- Fekete, A. Lugaskert, filegória, halastó. Erdélyi reneszánsz kertemlékeinkből. Korunk 2008, III, 46–51. [Google Scholar]
- Mollay, K. Hans Seyfridt Házipatikája és Eceteskönyvecskéje (1609–1633). Sopron Város Történeti Forrásai; B Sorozat; Győr-Moson-Sopron Megye Soproni Levéltára és a Soproni Múzeum: Sopron, Hungary, 1995; Volume 2. [Google Scholar]
- Hoffmann, G. Próbált Orvosságok. Medicusi és Borbélyi Mesterség. Régi Magyar Ember- és Állatorvosló Könyvek Radvánszky Béla Gyűjtéséből. (Adattár a XVI–XVIII. Század Szellemi Mozgalmainak Történetéhez, 9); MTA Irodalomtudományi Intézet: Budapest, Hungary, 1989. [Google Scholar]
- Mátyus, I. Ó és új Diaetetica, 4.; Landerer: Pozsony, Slovakia, 1789. [Google Scholar]
- Szent-Mihályi, M. Házi Orvosságok; Ambró: Vác, Hungary, 1791. [Google Scholar]
- Cyprian monk. Cypriánov herbár (Facsimile); monastery Červený kláštor: Červený Kláštor, Slovakia, 1766/1771. [Google Scholar]
- Veszelszki, A. A ‘Növevény-Plánták’ Országából Való Erdei, És Mezei Gyűjtemény, Vagy-Is Fa- És Fűszeres Könyv, Mellyben Azoknak Deák, Magyar, Német, Frantz, Tseh, És Oláh Neveik, Külső, Belső, És Köz Hasznaikkal Egyetemben Máthiolusból “s Más Több Fa-, És Fűvész-Írókból a” Köz-Rendű Hazafiak’ Kedvekért Szálanként Egybe-Szedettek; Veszelszki Antal által. Trattner-Kiss: Pesth, Hungary, 1798. [Google Scholar]
- Berde, K. A Magyar Nép Dermatológiája; A Magyar Orvosi Könyvkiadó Társulat Könyvtára: Budapest, Hungary, 1940. [Google Scholar]
- Márton, J. Növénynevek a Nép Nyelvében. Ethnographia 1892, 3, 55–60. [Google Scholar]
- Borbás, V. Balzsammenta. Pallas Nagy Lexikona 1893, II, 558. [Google Scholar]
- Szabó, T.E.A.; Péntek, J. Népi növényismereti gyűjtés (Tájékoztató és szemelvények). Művelődés 1974, 27, 51–57. [Google Scholar]
- Péntek, J.; Szabó, T.A. Egy háromszéki falu népi növényismerete. Ethnographia 1976, 87, 203–225. [Google Scholar]
- Sándor, M. Egy bihari parasztasszony hiedelmei. In Folklór Archívum 4; MTA Néprajzi Kutatócsoport: Budapest, Hungary, 1976; pp. 187–282. [Google Scholar]
- Péntek, J. Virágnyelv–Virágének. TETT 1979, 3, 15–20. [Google Scholar]
- Pálfalvi, P. Bűzlik-e a vénasszonyvirág? Hargita Népe 1990, 2, 2. [Google Scholar]
- Kovács, K. Kerti virágok és dísznövények Csíkszépvízen. Művelődés 1993, 42, 53. [Google Scholar]
- Gub, J. Borogatók, kenőcsök, sebtapaszok a Sóvidéken. Hazanéző 2000, 11, 27–29. [Google Scholar]
- Halmai, J. Adatok a “Herbárium” orvos-botanikai értékeléséhez. Commun. ex Bibl. Hist. Medicae Hung. 1962, 23, 281–334. [Google Scholar]
- Butura, V. Enciclopedie de Etnobotanică Românească; Editura Știinţifică și Enciclopedică: Bucharest, Romania, 1979. [Google Scholar]
- Péntek, J.; Szabó, T.A. Emberés Növényvilág. Kalotaszeg Növényzete és Népi Növényismerete; Kriterion: Bucuresti, Romania, 1985. [Google Scholar]
- Rab, J. Újabb népgyógyászati adatok Gyimesből. Gyógyszerészet 1982, 26, 325–333. [Google Scholar]
- Jaimand, K.; Rezaee, M.B. Chemical Constituents of Essential Oils from Tanacetum balsamita L. ssp. Balsamitoides (Schultz-Bip.) Grierson. from Iran. J. Essent. Oil Res. 2005, 17, 565–566. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.; Tabatabaie, S.J.; Nazemiyeh, H.; Aflatuni, A. N and K Nutrition Levels Affect Growth and Essential Oil Content of Costmary (Tanacetum balsamita L.). J. Food Agric. Environ. 2008, 6, 145–149. [Google Scholar]
- Hassanpouraghdam, M.B.; Tabatabaei, S.J.; Nazemiyeh, H.; Vojodi, L.; Aazami, M.-A.; Shoja, A. Chrysanthemum balsamita (L.) BAILL.: A Forgotten Medicinal Plant. Med. Biol. 2008, 15, 119–124. [Google Scholar]
- Hassanpouraghdam, M.B.; Tabatabaie, S.J.; Nazemiyeh, H.; Aflatuni, A. Effects of different concentrations of nutrient solution on vegetative growth and essential oil of costmary (Tanacetum balsamita L.). J. Agric. Sci. 2008, 18, 27–38. [Google Scholar]
- Hassanpouraghdam, M.B. Flowerhead Volatile Oil Composition of Soilless Culture-Grown Chrysanthemum balsamita L. Nat. Prod. Res. 2009, 23, 672–677. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Vojodi Mehrabani, L.; Kheiri, M.; Chrysargyris, A.; Tzortzakis, N. Physiological and Biochemical Responses of Tanacetum balsamita L. to the Foliar Application of Dobogen Biostimulant, Glucose and KNO3 under Salinity Stress. Sci. Rep. 2022, 12, 9320. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.A.; Jafari, A.A.; Sayedian, S.E. Evaluation of Aerial Biomass Yield and Essential Oil Content of Seven Species of Tanacetum. J. Hortic. Res. 2017, 25, 19–25. [Google Scholar] [CrossRef]
- Dalar, A. Plant Taxa Used in the Treatment of Diabetes in Van Province, Turkey. Int. J. Sec. Metab. 2018, 5, 171–185. [Google Scholar] [CrossRef]
- Güneş, F.; Özhatay, N. An Ethnobotanical Study from Kars Eastern Turkey. Biyolojik Çeşitlilik Ve Koruma 2011, 4, 30–41. [Google Scholar]
- Guarrera, P.M.; Forti, G.; Marignoli, S. Ethnobotanical and Ethnomedicinal Uses of Plants in the District of Acquapendente (Latium, Central Italy). J. Ethnopharmacol. 2005, 96, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Ghirardini, M.P.; Carli, M.; del Vecchio, N.; Rovati, A.; Cova, O.; Valigi, F.; Agnetti, G.; Macconi, M.; Adamo, D.; Traina, M.; et al. The Importance of a Taste. A Comparative Study on Wild Food Plant Consumption in Twenty-One Local Communities in Italy. J. Ethnobiol. Ethnomed. 2007, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Guarino, C.; De Simone, L.; Santoro, S. Ethnobotanical Study of the Sannio Area, Campania, Southern Italy. Ethnobot. Res. Appl. 2008, 6, 255–317. [Google Scholar] [CrossRef]
- Vitalini, S.; Puricelli, C.; Mikerezi, I.; Iriti, M. Plants, People and Traditions: Ethnobotanical Survey in the Lombard Stelvio National Park and Neighbouring Areas (Central Alps, Italy). J. Ethnopharmacol. 2015, 173, 435–458. [Google Scholar] [CrossRef] [PubMed]
- Sõukand, R.; Pieroni, A. The Importance of a Border: Medical, Veterinary, and Wild Food Ethnobotany of the Hutsuls Living on the Romanian and Ukrainian Sides of Bukovina. J. Ethnopharmacol. 2016, 185, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Cornara, L.; La Rocca, A.; Terrizzano, L.; Dente, F.; Mariotti, M.G. Ethnobotanical and Phytomedical Knowledge in the North-Western Ligurian Alps. J. Ethnopharmacol. 2014, 155, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Attighi Lorestani, F.; Khashaveh, A.; Attighi Lorestani, R. Fumigant Toxicity of Essential Oil from Tanacetum balsamita L. (Compositae) against Adults and Eggs of Callosobruchus maculatus F. (Coleoptera: Bruchidae). Arch. Phytopathol. Plant Prot. 2013, 46, 2080–2086. [Google Scholar] [CrossRef]
- Voigt, R.F.; Rogers, C.H.; Fischer, E.B. A Pharmacognostic Study of Chrysanthemum balsamita L., var. Tanacetoides Boiss., Together with a Study of Its Volatile Oil. J. Am. Pharm. Assoc. (1912) 1938, 27, 643–654. [Google Scholar] [CrossRef]
- Hoppe, H.A. Drogenkunde; De Gruyter & Co.: Hamburg, Germany, 1942. [Google Scholar]
- Linnaeus, C. Species Plantarum; Laurentius Salvius: Stockholm (Holmiae), Sweden, 1753. [Google Scholar]
- Csapó, J. Új Füves És Virágos Magyar Kert; Landerer Mihály: Pozsony, Slovakia, 1775. [Google Scholar]
- WFO World Flora Online. Available online: https://www.worldfloraonline.org/ (accessed on 18 May 2024).
- Mozaffarian, V. Flora of Iran, Asteraceae (Compositae): Tribes Anthemideae and Echinopeae, 1st ed.; Institute of Forests and Rangelands Press: Tehran, Iran, 2008. (In Persian) [Google Scholar]
- Király, G. Új Magyar Füvészkönyv. Magyarország Hajtásos Növényei. Határozókulcsok; Aggteleki Nemzeti Park Igazgatósága: Jósvafő, Hungary, 2009.
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 2010; Volume 4, pp. 171–172. [Google Scholar]
- Monfared, A.; Davarani, S.S.H.; Rustaiyan, A.; Masoudi, S. Composition of the Essential Oil of Tanacetum balsamita L. ssp. Balsamitoides (Schultz Bip.) Grierson from Iran. J. Essent. Oil Res. 2002, 14, 1–2. [Google Scholar] [CrossRef]
- Muresan, M.L.; Oniga, I.; Georgescu, C.; Paltinean, R.; Gligor, F.; Craciunas, M.T.; Oprean, R. Botanical and Phytochemical Studies on Tanacetum vulgare L. from Transylvania. Acta Med. Transilv. 2014, 2, 300–302. [Google Scholar]
- Todorova, M.N.; Ognyanov, I.V. Sesquiterpene Lactones in a Population of Balsamita major Cultivated in Bulgaria. Phytochemistry 1989, 28, 1115–1117. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.J.; Velasco-Negueruela, A.; Burzaco, A. Tanacetum balsamita L.: A Medicinal Plant from Guadalajara (Spain). Acta Hortic. 1992, 306, 188–193. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C.; Schwarz, H. Naturally Occurring Terpene Derivatives. XLVII. New Sesquiterpene from Tanacetum balsamita Subspecies Balsamitoides. Chem. Berichte 1975, 108, 1369–1372. [Google Scholar] [CrossRef]
- Bylaitė, E.; Venskutonis, R.; Roozen, J.P.; Posthumus, M.A. Composition of Essential Oil of Costmary [Balsamita major (L.) Desf.] at Different Growth Phases. J. Agric. Food Chem. 2000, 48, 2409–2414. [Google Scholar] [CrossRef] [PubMed]
- Gallori, S.; Flamini, G.; Bilia, A.R.; Morelli, I.; Landini, A.; Vincieri, F.F. Chemical Composition of Some Traditional Herbal Drug Preparations: Essential Oil and Aromatic Water of Costmary (Balsamita Suaveolens Pers.). J. Agric. Food Chem. 2001, 49, 5907–5910. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadi, M.; Ebrahimi, S.N.; Sonboli, A.; Miraghasi, F.; Ghiasi, S.; Arman, M.; Mosaffa, N. Cytotoxicity, antimicrobial activity and composition of essential oil from Tanacetum balsamita L. subsp. balsamita. Nat. Prod. Commun. 2009, 4, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Venskutonis, P.R. Costmary (Chrysanthemum balsamita) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 365–375. ISBN 978-0-12-416641-7. [Google Scholar]
- Kazemzadeh, M.; Yaghmaei, P.; Mohammadi, S. Analgesic and Anti-Inflammatory Effects of Tanacetum balsamita Essential Oil and One of Its Major Constituents (Quercetin) in Male Rats. Clin. Neurol. Neurosci. 2017, 1, 60–66. [Google Scholar] [CrossRef]
- Khodayari, M.; Basti, A.A.; Khanjari, A.; Misaghi, A.; Kamkar, A.; Shotorbani, P.M.; Hamedi, H. Effect of Poly(Lactic Acid) Films Incorporated with Different Concentrations of Tanacetum balsamita Essential Oil, Propolis Ethanolic Extract and Cellulose Nanocrystals on Shelf Life Extension of Vacuum-Packed Cooked Sausages. Food Packag. Shelf Life 2019, 19, 200–209. [Google Scholar] [CrossRef]
- Vukic, M.D.; Vukovic, N.L.; Obradovic, A.D.; Galovičová, L.; Čmiková, N.; Kačániová, M.; Matic, M.M. Chemical Composition and Biological Activity of Tanacetum balsamita Essential Oils Obtained from Different Plant Organs. Plants 2022, 11, 3474. [Google Scholar] [CrossRef]
- Servi, H.; Goren, N.; Sen, A.; Servi, E.Y. A New Eudesmanolide from Tanacetum balsamita L. and Biological Activities of Extracts. Nat. Prod. Res. 2023, 37, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Marculescu, A.; Sand, C.; Barbu, C.H.; Bobit, D.; Hanganu, D. Possibilities of Influencing the Biosynthesis and Accumulation of the Active Principles in Chrysanthemum balsamita L. Species. Rom. Biotechnol. Lett. 2001, 7, 577–584. [Google Scholar]
- Kubo, I.; Jamalamadaka, V.; Kamikawa, T.; Takahashi, K.; Tabata, K.; Kusumi, T. Absolute Stereochemistry of Tanabalin, an Insect Antifeedant Clerodane from Tanacetum balsamita. Chem. Lett. 1996, 25, 441–442. [Google Scholar] [CrossRef]
- Nickavar, B.; Amin, G.; Mehregan, N. Quercetine, a Major Flavonol Aglycon from Tanacetum balsamita L. Iran. J. Pharm. Res. 2003, 2, 249–250. [Google Scholar]
- Pukalskas, A.; Venskutonis, P.R.; Dijkgraaf, I.; van Beek, T.A. Isolation, Identification and Activity of Natural Antioxidants from Costmary (Chrysanthemum balsamita) Cultivated in Lithuania. Food Chem. 2010, 122, 804–811. [Google Scholar] [CrossRef]
- Ivashchenko, I.V. Chromatographic Analysis of Phenolic Compounds of Tanacetum balsamita L. (Asteraceae) under the Conditions of Introduction in Zhytomir Pollissya. Plant Physiol. Genet. 2016, 48, 178–183. [Google Scholar] [CrossRef]
- Ivashchenko, I.V. Antimicrobial Properties of Tanacetum balsamita L. (Asteraceae) Introduced in Ukrainian Polissya. Ukr. J. Ecol. 2017, 7, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Ahmadnejad-Asl-Gavgani, M.; Maham, M.; Dalair-Naghadeh, B. In Vitro Effects of Essential Oils of Tanacetum balsamita and Carvone on the Contractility of Bovine Ileum Smooth Muscles. Vet. Res. Forum. 2022, 13, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gevrenova, R.; Zengin, G.; Sinan, K.I.; Zheleva-Dimitrova, D.; Balabanova, V.; Kolmayer, M.; Voynikov, Y.; Joubert, O. An In-Depth Study of Metabolite Profile and Biological Potential of Tanacetum balsamita L. (Costmary). Plants 2023, 12, 22. [Google Scholar] [CrossRef]
- Gevrenova, R.; Balabanova, V.; Zheleva-Dimitrova, D.; Momekov, G. The Most Promising Southeastern European Tanacetum Species: A Review of Chemical Composition and Biological Studies. Pharmacia 2023, 70, 1067–1081. [Google Scholar] [CrossRef]
- Khatib, S.; Sobeh, M.; Faraloni, C.; Bouissane, L. Tanacetum Species: Bridging Empirical Knowledge, Phytochemistry, Nutritional Value, Health Benefits and Clinical Evidence. Front. Pharmacol. 2023, 14, 1169629. [Google Scholar] [CrossRef] [PubMed]
- Benedec, D.; Filip, L.; Vlase, L.; Bele, C.; Sevastre, B.; Raita, O.; Olah, N.-K.; Hanganu, D. In Vitro Study of Antioxidant Activity and Phenolic Content of Chrysanthemum balsamita Varieties. Pak. J. Pharm. Sci. 2016, 29, 1359–1364. [Google Scholar] [PubMed]
- Derakhshani, Z.; Hassani, A.; Sadaghiani, M.H.R.; Hassanpouraghdam, M.B.; Khalifani, B.H.; Dalkani, M. Effect of Zinc Application on Growth and Some Biochemical Characteristics of Costmary (Chrysanthemum balsamita L.). Commun. Soil Sci. Plant Anal. 2011, 42, 2493–2503. [Google Scholar] [CrossRef]
- Bączek, K.B.; Kosakowska, O.; Przybył, J.L.; Pióro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Węglarz, Z. Antibacterial and Antioxidant Activity of Essential Oils and Extracts from Costmary (Tanacetum balsamita L.) and Tansy (Tanacetum vulgare L.). Ind. Crops Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Kubo, A.; Kubo, I. Antimicrobial Agents from Tanacetum balsamita. J. Nat. Prod. 1995, 58, 1565–1569. [Google Scholar] [CrossRef]
- Bagci, E.; Kursat, M.; Kocak, A.; Gur, S. Composition and Antimicrobial Activity of the Essential Oils of Tanacetum balsamita L. subsp. balsamita and T. chiliophyllum (Fisch. et Mey.) Schultz Bip. var. chiliophyllum (Asteraceae) from Turkey. J. Essent. Oil Bear. Pl. 2008, 11, 476–484. [Google Scholar] [CrossRef]
- Bestmann, H.J.; Claßen, B.; Kobold, U.; Vostrowsky, O. Pflanzliche Insektizide, III [1], Pyrethrin I Im Etherischen Öl von Chrysanthemum balsamita L./Herbal Insecticides III [1]. Pyrethrin I in the Essential Oil of Chrysanthemum balsamita L. Z. für Naturforschung C 1986, 41, 725–728. [Google Scholar] [CrossRef]
- Sanz, A.; Silvan, A.M.; Abad, M.J.; Bermejo, P.; Villar, A.M. Anti-Inflammatory Activity of the Hexane Extract from Tanacetum balsamita. Methods Find. Exp. Clin. Pharmacol. 1997, 19, 177. [Google Scholar]
- Karaca, M.; Ozbek, H.; Akkan, H.; Tutuncu, M.; Özgökçe, F.; Him, A.; Bakir, B. Anti-Inflammatory Activities of Diethyl-Ether Extracts of Helichrysum Plicatum DC. and Tanacetum balsamita L. in Rats. Asian J. Anim. Vet. Adv. 2009, 4, 320–325. [Google Scholar] [CrossRef]
- Yousefi, M.; Adineh, H.; Sedaghat, Z.; Yilmaz, S.; Elgabry, S.E. Effects of Dietary Costmary (Tanacetum balsamita) Essential Oil on Growth Performance, Digestive Enzymes’ Activity, Immune Responses and Subjected to Ambient Ammonia of Common Carp Cyprinus Carpio. Aquaculture 2023, 569, 739347. [Google Scholar] [CrossRef]
- Alberti, Á.; Béni, S.; Lackó, E.; Riba, P.; Al-Khrasani, M.; Kéry, Á. Characterization of Phenolic Compounds and Antinociceptive Activity of Sempervivum tectorum L. Leaf Juice. J. Pharm. Biomed. Anal. 2012, 70, 143–150. [Google Scholar] [CrossRef]
- Bylund, D.; Norström, S.H.; Essén, S.A.; Lundström, U.S. Analysis of Low Molecular Mass Organic Acids in Natural Waters by Ion Exclusion Chromatography Tandem Mass Spectrometry. J. Chromatogr. A 2007, 1176, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Felegyi-Tóth, C.A.; Garádi, Z.; Darcsi, A.; Csernák, O.; Boldizsár, I.; Béni, S.; Alberti, Á. Isolation and Quantification of Diarylheptanoids from European Hornbeam (Carpinus betulus L.) and HPLC-ESI-MS/MS Characterization of Its Antioxidative Phenolics. J. Pharm. Biomed. Anal. 2022, 210, 114554. [Google Scholar] [CrossRef] [PubMed]
- Yur, S.; Tekin, M.; Göger, F.; Başer, K.H.C.; Özek, T.; Özek, G. Composition and Potential of Tanacetum Haussknechtii Bornm. Grierson as Antioxidant and Inhibitor of Acetylcholinesterase, Tyrosinase, and α-Amylase Enzymes. Int. J. Food Prop. 2017, 20, S2359–S2378. [Google Scholar] [CrossRef]
- Ouyang, H.; Fan, Y.; Wei, S.; Chang, Y.; He, J. Study on the Chemical Profile of Chrysanthemum (Chrysanthemum morifolium) and the Evaluation of the Similarities and Differences between Different Cultivars. Chem. Biodivers. 2022, 19, e202200252. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and Characterization of Chlorogenic Acids, Chlorogenic Acid Glycosides and Flavonoids from Lonicera henryi L. (Caprifoliaceae) Leaves by LC–MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Beelders, T.; De Beer, D.; Stander, M.A.; Joubert, E. Comprehensive Phenolic Profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and -MS/MS Reveals Novel Xanthone and Benzophenone Constituents. Molecules 2014, 19, 11760–11790. [Google Scholar] [CrossRef] [PubMed]
- Jakabfi-Csepregi, R.; Alberti, Á.; Felegyi-Tóth, C.A.; Kőszegi, T.; Czigle, S.; Papp, N. A Comprehensive Study on Lathyrus tuberosus L.: Insights into Phytochemical Composition, Antimicrobial Activity, Antioxidant Capacity, Cytotoxic, and Cell Migration Effects. Plants 2024, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Abaza, L.; Iswaldi, I.; Fernández-Gutiérrez, A.; Zarrouk, M.; Segura-Carretero, A. LC-MS-Based Metabolite Profiling of Methanolic Extracts from the Medicinal and Aromatic Species Mentha Pulegium and Origanum Majorana. Phytochem. Anal. 2015, 26, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Kerebba, N.; Oyedeji, A.O.; Byamukama, R.; Kuria, S.K.; Oyedeji, O.O. UHPLC-ESI-QTOF-MS/MS Characterisation of Phenolic Compounds from Tithonia diversifolia (Hemsl.) A. Gray and Antioxidant Activity. ChemistrySelect 2022, 7, e202104406. [Google Scholar] [CrossRef]
- Martucci, M.E.P.; Vos, R.C.H.D.; Carollo, C.A.; Gobbo-Neto, L. Metabolomics as a Potential Chemotaxonomical Tool: Application in the Genus Vernonia Schreb. PLoS ONE 2014, 9, e93149. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P.C. HPLC-ESI-MSn Characterization of Phenolic Compounds, Terpenoid Saponins, and Other Minor Compounds in Bituminaria bituminosa. Ind. Crops Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Ezzat, M.I.; El Gendy, S.N.; Saad, A.S.; Abdo, W.S.; EL Sayed, A.M.; Elmotayam, A.K. Secondary Metabolites from Lantana Camara L. Flowers Extract Exhibit in vivo Anti-Urolithiatic Activity in Adult Wistar Albino Rats. Nat. Prod. Res. 2020, 36, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Fan, C.; Jiang, Z.; Ye, W.; Yang, Y. Quality Assessment and Origin Tracing of Guangdong Liangcha Granules Using Direct Mass Spectrometry Fingerprinting. Anal. Methods 2012, 4, 3638–3645. [Google Scholar] [CrossRef]
- Tóth, G.; Alberti, Á.; Sólyomváry, A.; Barabás, C.; Boldizsár, I.; Noszál, B. Phenolic Profiling of Various Olive Bark-Types and Leaves: HPLC–ESI/MS Study. Ind. Crops Prod. 2015, 67, 432–438. [Google Scholar] [CrossRef]
- Kabbash, E.M.; Abdel-Shakour, Z.T.; El-Ahmady, S.H.; Wink, M.; Ayoub, I.M. Comparative Metabolic Profiling of Olive Leaf Extracts from Twelve Different Cultivars Collected in Both Fruiting and Flowering Seasons. Sci. Rep. 2023, 13, 612. [Google Scholar] [CrossRef]
- Cao, J.; Yin, C.; Qin, Y.; Cheng, Z.; Chen, D. Approach to the Study of Flavone Di-C-Glycosides by High Performance Liquid Chromatography-Tandem Ion Trap Mass Spectrometry and Its Application to Characterization of Flavonoid Composition in Viola Yedoensis. J. Mass Spectrom. 2014, 49, 1010–1024. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, R.; Song, W.; Miao, W.; Liu, J.; Chen, H.; Guo, D.; Ye, M. A Targeted Strategy to Analyze Untargeted Mass Spectral Data: Rapid Chemical Profiling of Scutellaria Baicalensis Using Ultra-High Performance Liquid Chromatography Coupled with Hybrid Quadrupole Orbitrap Mass Spectrometry and Key Ion Filtering. J. Chromatogr. A 2016, 1441, 83–95. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of Phenolics by LC–UV/Vis, LC–MS/MS and Sugars by GC in Melicoccus Bijugatus Jacq. ‘Montgomery’ Fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef]
- Gevrenova, R.; Zheleva-Dimitrova, D.; Balabanova, V.; Voynikov, Y.; Sinan, K.I.; Mahomoodally, M.F.; Zengin, G. Integrated Phytochemistry, Bio-Functional Potential and Multivariate Analysis of Tanacetum macrophyllum (Waldst. & Kit.) Sch.Bip. and Telekia Speciosa (Schreb.) Baumg. (Asteraceae). Ind. Crops Prod. 2020, 155, 112817. [Google Scholar] [CrossRef]
- Venditti, A.; Frezza, C.; Sciubba, F.; Serafini, M.; Bianco, A.; Cianfaglione, K.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Maggi, F. Volatile Components, Polar Constituents and Biological Activity of Tansy Daisy (Tanacetum macrophyllum (Waldst. et Kit.) Schultz Bip.). Ind. Crops Prod. 2018, 118, 225–235. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical Scheme for LC-MSn Identification of Chlorogenic Acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Takahara, H.; Shiraiwa, M. Purification and Characterization of Three Neutral Extracellular Isoperoxidases from Rye Leaves. Phytochemistry 2007, 68, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.M.; El Kashak, W.A.; Wink, M.; El Raey, M.A. New Isorhamnetin Derivatives from Salsola imbricata Forssk. Leaves with Distinct Anti-Inflammatory Activity. Pharmacogn. Mag. 2016, 12, S47–S51. [Google Scholar] [CrossRef] [PubMed]
- Marczak, Ł.; Znajdek-Awiżeń, P.; Bylka, W. The Use of Mass Spectrometric Techniques to Differentiate Isobaric and Isomeric Flavonoid Conjugates from Axyris amaranthoides. Molecules 2016, 21, 1229. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.-Y.; Ting, Y.; Wang, J.; Sun, J.; Guo, X.-F. Characterization and Identification of the Major Flavonoids in Phyllostachys Edulis Leaf Extract by UPLC–QTOF–MS/MS. Acta Chromatogr. 2020, 32, 228–237. [Google Scholar] [CrossRef]
- Waridel, P.; Wolfender, J.-L.; Ndjoko, K.; Hobby, K.R.; Major, H.J.; Hostettmann, K. Evaluation of Quadrupole Time-of-Flight Tandem Mass Spectrometry and Ion-Trap Multiple-Stage Mass Spectrometry for the Differentiation of C-Glycosidic Flavonoid Isomers. J. Chromatogr. A 2001, 926, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Wu, W.; Kirkpatrick, J.; Kuhnert, N. Profiling the Chlorogenic Acids and Other Caffeic Acid Derivatives of Herbal Chrysanthemum by LC−MSn. J. Agric. Food Chem. 2007, 55, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Alberti, Á.; Riethmüller, E.; Béni, S.; Kéry, Á. Evaluation of Radical Scavenging Activity of Sempervivum tectorum and Corylus avellana Extracts with Different Phenolic Composition. Nat. Prod. Commun. 2016, 11, 1934578X1601100412. [Google Scholar] [CrossRef]
- Ak, G.; Gevrenova, R.; Sinan, K.I.; Zengin, G.; Zheleva, D.; Mahomoodally, M.F.; Senkardes, I.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Tanacetum vulgare L. (Tansy) as an Effective Bioresource with Promising Pharmacological Effects from Natural Arsenal. Food Chem. Toxicol. 2021, 153, 112268. [Google Scholar] [CrossRef]
- Zengin, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamocilja, J.; Sokovic, M.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; et al. Phytochemical Characterization and Bioactivities of Five Apiaceae Species: Natural Sources for Novel Ingredients. Ind. Crops Prod. 2019, 135, 107–121. [Google Scholar] [CrossRef]
- Marczak, Ł.; Stobiecki, M.; Jasiński, M.; Oleszek, W.; Kachlicki, P. Fragmentation Pathways of Acylated Flavonoid Diglucuronides from Leaves of Medicago truncatula. Phytochem. Anal. 2010, 21, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Luo, Y.; Gao, B.; Sun, J.; Lu, W.; Liu, J.; Chen, P.; Zhang, Y.; Yu, L. (Lucy) Chemical Compositions of Chrysanthemum Teas and Their Anti-Inflammatory and Antioxidant Properties. Food Chem. 2019, 286, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Harnly, J.M. Identification of the Phenolic Components of Chrysanthemum Flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Végh, K.; Riethmüller, E.; Hosszú, L.; Darcsi, A.; Müller, J.; Alberti, Á.; Tóth, A.; Béni, S.; Könczöl, Á.; Balogh, G.T.; et al. Three Newly Identified Lipophilic Flavonoids in Tanacetum parthenium Supercritical Fluid Extract Penetrating the Blood-Brain Barrier. J. Pharm. Biomed. Anal. 2018, 149, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the Six Isomers of Dicaffeoylquinic Acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, F.; Wang, X.; Wu, Y.; Dong, M.; He, G.; Galyean, R.D.; He, L.; Huang, G. Identification of Antioxidant Phenolic Compounds in Feverfew (Tanacetum parthenium) by HPLC-ESI-MS/MS and NMR. Phytochem. Anal. 2007, 18, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Cuyckens, F.; Claeys, M. Mass Spectrometry in the Structural Analysis of Flavonoids. J. Mass Spectrom. 2004, 39, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; El-shabrawy, M.; Abdel-Hameed, E.-S.S.; Kawashty, S.A. Comparative Study of Mentha Species Growing Wild in Egypt: LC-ESI-MS Analysis and Chemosystematic Significance. J. Appl. Pharm. Sci. 2018, 8, 116–122. [Google Scholar] [CrossRef]
- Achour, M.; Mateos, R.; Ben Fredj, M.; Mtiraoui, A.; Bravo, L.; Saguem, S. A Comprehensive Characterisation of Rosemary Tea Obtained from Rosmarinus officinalis L. Collected in a Sub-Humid Area of Tunisia. Phytochem. Anal. 2018, 29, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Baranauskienė, R.; Kazernavičiūtė, R.; Pukalskienė, M.; Maždžierienė, R.; Venskutonis, P.R. Agrorefinery of Tanacetum vulgare L. into Valuable Products and Evaluation of Their Antioxidant Properties and Phytochemical Composition. Ind. Crops Prod. 2014, 60, 113–122. [Google Scholar] [CrossRef]
- Lai, J.-P.; Lim, Y.H.; Su, J.; Shen, H.-M.; Ong, C.N. Identification and Characterization of Major Flavonoids and Caffeoylquinic Acids in Three Compositae Plants by LC/DAD-APCI/MS. J. Chromatogr. B 2007, 848, 215–225. [Google Scholar] [CrossRef]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and Characterization of the Chlorogenic Acids in Green Robusta Coffee Beans by LC-MSn: Identification of Seven New Classes of Compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kuhnert, N. Hierarchical Scheme for Liquid Chromatography/Multi-Stage Spectrometric Identification of 3,4,5-Triacyl Chlorogenic Acids in Green Robusta Coffee Beans. Rapid Commun. Mass Spectrom. 2010, 24, 2283–2294. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Lamuela-Raventós, R.M.; Viladomat, F.; Bastida, J.; Codina, C. Qualitative Analysis of Phenolic Compounds in Apple Pomace Using Liquid Chromatography Coupled to Mass Spectrometry in Tandem Mode. Rapid Commun. Mass Spectrom. 2004, 18, 553–563. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid Chromatographic/Electrospray Ionization Tandem Mass Spectrometric Study of the Phenolic Composition of Cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural Characterization of Flavonoid Glycoconjugates and Their Derivatives with Mass Spectrometric Techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef]
- Könczöl, Á.; Müller, J.; Földes, E.; Béni, Z.; Végh, K.; Kéry, Á.; Balogh, G.T. Applicability of a Blood–Brain Barrier Specific Artificial Membrane Permeability Assay at the Early Stage of Natural Product-Based CNS Drug Discovery. J. Nat. Prod. 2013, 76, 655–663. [Google Scholar] [CrossRef]
- Borza, A. Dicţionar Etnobotanic; Editura Academiei Republicii Socialiste Romania: Bucureşti, Romania, 1968. [Google Scholar]
- Williams, C.A.; Harborne, J.B.; Eagles, J. Variations in Lipophilic and Polar Flavonoids in the Genus Tanacetum. Phytochemistry 1999, 52, 1301–1306. [Google Scholar] [CrossRef]
- Rusu, M.A.; Tamas, M.; Puica, C.; Roman, I.; Sabadas, M. The Hepatoprotective Action of Ten Herbal Extracts in CCl4 Intoxicated Liver. Phytother. Res. 2005, 19, 744–749. [Google Scholar] [CrossRef]
- Jarić, S.; Mačukanović-Jocić, M.; Djurdjević, L.; Mitrović, M.; Kostić, O.; Karadžić, B.; Pavlović, P. An Ethnobotanical Survey of Traditionally Used Plants on Suva Planina Mountain (South-Eastern Serbia). J. Ethnopharmacol. 2015, 175, 93–108. [Google Scholar] [CrossRef]
- Varga, E.; Orbán, K.; Finta, A.; Kursinszki, L.; Domokos, E. Phytochemical Analysis of Chrysanthemum balsamita Var. Tanacetoides. Acta Pharm. Hung. 2018, 88, 244–248. [Google Scholar]
- Devrnja, N.; Anđelković, B.; Aranđelović, S.; Radulović, S.; Soković, M.; Krstić-Milošević, D.; Ristić, M.; Ćalić, D. Comparative Studies on the Antimicrobial and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Methanol Extracts. S. Afr. J. Bot. 2017, 111, 212–221. [Google Scholar] [CrossRef]
- Ivanescu, B.; Tuchiluș, C.; Corciovă, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.-M.; Vlase, L. Antioxidant, Antimicrobial and Cytotoxic Activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum Extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Avdeef, A. Permeability-PAMPA. In Absorption and Drug Development; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 319–498. ISBN 978-1-118-28606-7. [Google Scholar]


| No. | tR (min) a | [M−H]− (m/z) | Fragment Ions (m/z) | Tentative Characterization | Presence of Compounds | Reference | |
|---|---|---|---|---|---|---|---|
| TbE b | TbW b | ||||||
| 1 | 0.75 | 133 | 115, 89, 73, 71 | malic acid d. | + | + | [106,107] |
| 2 | 0.96 | 341 | 377 [M+Cl]−, 179, 135 | caffeoyl-O-hexoside | + | + | [108] |
| 3 | 0.96 | 315 | 153, 152, 109, 108 | dihydroxybenzoyl-O-hexoside | + | + | [94,109] |
| 4 | 1.00 | 353 | 191, 179, 135 | 3-O-caffeoylquinic acid | + | + | [94,110,111] |
| 5 | 1.02 | 417 | 405, 285, 223, 152 | dihydroxybenzoyl-di-O-pentoside d | + | + | [112,113] |
| 6 | 1.15 | 357 | 195 | caffeoyl-O-pentahydroxyhexanoic acid | - | + | [94] |
| 7 | 1.23 | 305 | 225, 167, 147, 135 | epigallocatechin/gallocatechin isomer d | + | + | [114] |
| 8 | 1.25 | 563 | 387, 327, 297, 223, 207, 205 | dihydrosinapoyl-O-hexuronosyl-hexoside d | + | + | [113] |
| 9 | 1.29 | 353 | 707 [2M−H]−, 191 | trans-5-O-caffeoylquinic acid | + | + | [94,110,111] |
| 10 | 1.30 | 307 | 227, 189, 167, 148 | catechin/epicatechin hydrated d | + | - | [114,115] |
| 11 | 1.35 | 353 | 191, 179, 173 | 4-O-caffeoylquinic acid | - | + | [94,110,111] |
| 12 | 1.41 | 325 | 651 [2M−H]−, 163, 119 | p-coumaroyl-O-hexoside isomer | - | + | [108] |
| 13 | 1.55 | 593 | 1187 [2M−H]−, 503, 473, 383, 353 | apigenin-6,8-di-C-hexoside | + | + | [94,110,116,117] |
| 14 | 1.70 | 353 | 191 | cis-5-O-caffeoylquinic acid | - | + | [94,110,111] |
| 15 | 1.74 | 637 | 351, 285 | luteolin-di-O-hexuronoside d | + | + | [118] |
| 16 | 1.78 | 387 | 775 [2M−H]−, 207, 163, 119 | dihydrosinapoyl-O-hexoside d | + | + | [113,119] |
| 17 | 1.86 | 339 | 179, 161, 135 | caffeoyl-O-dimethyl-dihydroxybutanedioic acid d | + | + | [113] |
| 18 | 1.97 | 535 | 517, 373, 341, 323, 281, 251, 209, 193, 179, 161, 149, 135 | hydroxypinoresinol-O-hexoside d | + | + | [113] |
| 19 | 2.01 | 389 | 345, 227, 209, 183, 165 | oleoside d/secologanside d | + | + | [120,121] |
| 20 | 2.23 | 371 | 249, 121 | unknown | + | + | - |
| 21 | 2.28 | 563 | 503, 473, 443, 413, 383, 353 | apigenin-8-C-hexosyl-6-C-pentoside d | + | + | [117,122,123] |
| 22 | 2.30 | 367 | 191 | 5-O-feruloylquinic acid | + | + | [108,111] |
| 23 | 2.34 | 623 | 1247 [2M−H]−, 605, 561, 447, 327, 285 | luteolin-O-hexuronosyl-hexoside | + | + | [94,113] |
| 24 | 2.58 | 325 | 163, 119 | p-coumaroyl-O-hexoside isomer | + | + | [108,124] |
| 25 | 2.60 | 343 | 167, 113 | vanillyl-O-hexuronoside d | - | + | [125,126] |
| 26 | 2.63 | 367 | 193, 179, 134 | 3-O-feruloyl-quinic acid | + | + | [111,127] |
| 27 | 2.72 | 429 | 475 [M+HCOO]−, 411, 257, 227 | unknown | + | + | - |
| 28 | 2.84 | 799 | 619, 513, 351, 285, 193, 179, 161, 135 | luteolin-O-hexuronosyl-caffeoylhexuronoside d | - | + | [128] |
| 29 | 2.90 | 393 | 378, 355, 319, 295, 283 | unknown | + | + | - |
| 30 | 3.05 | 829 | 414 [M−2H]2−, 649, 513, 351, 315, 300, 179, 161, 135 | tetrahydroxy-methoxyflavone-O-hexuronosyl-caffeoylhexuronoside d | - | + | [113,129] |
| 31 | 3.15 | 651 | 299 | trihydroxy-methoxyflavone-O-hexuronosyl-hexuronoside d | + | + | [130] |
| 32 | 3.20 | 681 | 329 | trihydroxy-dimethoxyflavone-O-hexuronosyl-hexuronoside d | + | + | [130] |
| 33 | 3.54 | 431 | 341, 311, 283, 269 | apigenin-8-C-hexoside d | + | + | [110,131,132] |
| 34 | 3.70 | 677 | 515, 353, 341, 323, 191, 179, 161 | 3,5-O-dicaffeoylquinic acid-O-hexoside | - | + | [94,111,133] |
| 35 | 3.85 | 609 | 300, 301, 271, 255 | rutin c | + | + | [12,94,134,135] |
| 36 | 3.90 | 607 | 269 | apigenin-O-hexuronosyl-hexoside d | + | + | [136,137] |
| 37 | 4.07 | 461 | 923 [2M−H]−, 405, 285 | luteolin-O-hexuronoside | + | + | [94,135,138] |
| 38 | 4.17 | 593 | 417, 285 | luteolin-O-hexuronosyl-pentoside d | - | + | [12,94,138,139] |
| 39 | 4.23 | 491 | 983 [2M−H]−, 315, 287 | tetrahydroxy-methoxyflavone-O-hexuronoside | + | + | [94,135] |
| 40 | 4.30 | 637 | 1275 [2M−H]−, 299, 284 | trihydroxy-methoxyflavone-O-hexuronosyl-hexoside d | + | + | [137] |
| 41 | 4.31 | 667 | 1335 [2M−H]−, 491, 329, 314, 300 | trihydroxy-dimethoxyflavone-O-hexuronosyl-hexoside d | + | + | [130,137] |
| 42 | 4.39 | 463 | 301, 300 | quercetin-3-O-hexoside | - | + | [12,94,134,139] |
| 43 | 4.46 | 785 | 623, 461, 285, 179, 161 | luteolin-O-hexuronosyl-caffeoylhexoside d | - | + | [94,113,135] |
| 44 | 4.57 | 665 | 285 | luteolin-O-hexuronosyl-acetylhexoside d | + | + | [113] |
| 45 | 4.82 | 477 | 315, 300 | tetrahydroxy-methoxyflavone-O-hexoside | - | + | [94,135] |
| 46 | 4.94 | 813 | 329, 314, 299, 163, 145 | trihydroxy-dimethoxyflavone-O-hexuronosyl-coumaroylhexoside d | - | + | [113,130] |
| 47 | 5.14 | 445 | 313 | dihydroxy-dimethoxyflavone-O-pentoside d | + | + | [140] |
| 48 | 5.28 | 843 | 865 [M−2H+Na]−, 681, 351, 329, 314, 299, 193, 179, 161, 135 | trihydroxy-dimethoxyflavone-O-feruloyl-caffeoylhexuronoside d | + | + | [113,130] |
| 49 | 5.48 | 515 | 353, 335, 191, 179, 173, 161, 135 | 3,4-O-dicaffeoylquinic acid | - | + | [94,109,133,135,141,142] |
| 50 | 5.95 | 515 | 353, 191, 179, 135 | 3,5-O-dicaffeoylquinic acid | - | + | [94,109,133,135,141,142] |
| 51 | 6.45 | 515 | 353, 335, 191, 179, 135 | 1,3-O-dicaffeoylquinic acid d | - | + | [109,133,141] |
| 52 | 6.48 | 445 | 269 | apigenin-O-hexuronoside | + | + | [94] |
| 53 | 6.55 | 799 | 315, 300, 163, 145 | tetrahydroxy-methoxyflavone-O-hexuronosyl-coumaroylhexoside d | - | + | [113,137] |
| 54 | 6.60 | 829 | 329, 314, 299, 179, 161, 135 | trihydroxy-dimethoxyflavone-O-hexuronosyl-caffeoylhexoside d | - | + | [113,137] |
| 55 | 6.80 | 471 | 429, 411, 399, 267, 152 | unknown | + | - | [113] |
| 56 | 6.85 | 507 | 345, 330, 315, 287 | tetrahydroxy-dimethoxyflavone-O-hexoside d | - | + | [140] |
| 57 | 7.01 | 769 | 635, 623, 593, 327, 285, 163, 145 | luteolin-O-hexuronosyl-coumaroylhexoside d | + | + | [113] |
| 58 | 7.25 | 505 | 329, 314, 299, 271 | trihydroxy-dimethoxyflavone-O-hexuronoside | + | + | [94,130,135] |
| 59 | 7.43 | 503 | 445, 285, 175 | luteolin-O-acetylhexuronoside d | + | - | [143] |
| 60 | 7.50 | 507 | 329, 314, 299, 285, 271 | tetrahydroxy-dimethoxyflavone-O-hexoside d | - | + | [140] |
| 61 | 7.51 | 503 | 285, 175 | luteolin-O-acetylhexuronoside d | - | + | [143] |
| 62 | 7.65 | 561 | 369, 351, 191 | caffeoylquinic acid derivative d | + | - | [109] |
| 63 | 7.86 | 515 | 353, 317, 191, 179, 173, 135 | 4,5-O-dicaffeoylquinic acid | - | + | [94,109,133,135,141,142] |
| 64 | 8.05 | 679 | 379, 299, 284 | trihydroxy-methoxyflavone-O-hexuronosyl-acetylhexoside d | + | + | [140] |
| 65 | 8.25 | 501 | 367, 227, 193, 191, 179, 173, 161, 134 | 3-O-feruloylquinic acid derivative d | - | + | [111,127] |
| 66 | 8.73 | 521 | 399, 152 | trihydroxydihydrochalcone-di-C-pentoside d | + | + | [113] |
| 67 | 8.88 | 417 | 285 | luteolin-O-pentoside d | - | + | [143] |
| 68 | 8.94 | 501 | 537 [M+Cl]−, 399, 351, 152, 137 | trihydroxydihydrochalcone-C-glycoside derivative d | + | + | [113] |
| 69 | 9.24 | 445 | 491 [M+HCOO]−, 313 | dihydroxy-dimethoxyflavone-O-pentoside d | + | + | [94,109,135,140] |
| 70 | 9.69 | 813 | 667, 329, 314, 299, 285, 163, 145 | trihydroxy-dimethoxyflavone-O-hexuronosyl-coumaroylhexoside d | + | + | [140] |
| 71 | 9.70 | 461 | 323, 299, 179, 161, 137 | salicylic acid caffeoyl-O-hexoside d | - | + | [135] |
| 72 | 10.18 | 487 | 383, 353, 323, 285, 179, 163, 161, 145, 119 | coumaroyl-caffeoyl-O-hexoside d | + | + | [135] |
| 73 | 10.34 | 549 | 387, 207, 179, 161 | medioresinol-O-hexoside d/eucommin A d/dihydrosinapoyl-caffeoyl-O-hexoside d | + | + | [109,113,144,145] |
| 74 | 10.48 | 753 | 607, 269, 163, 145 | apigenin-O-hexuronosyl-coumaroylhexoside d | + | + | [113] |
| 75 | 10.58 | 783 | 637, 299, 284, 163, 145 | trihydroxy-methoxyflavone-O-hexuronosyl coumaroyhexoside d | + | + | [113] |
| 76 | 10.94 | 913 | 935 [M-2H+Na]−, 811, 769, 327, 285, 205, 175, 163, 145, 119 | luteolin-O-coumaroylglycoside derivative d | + | + | [143] |
| 77 | 11.14 | 547 | 583 [M+Cl]−, 507, 487, 329, 314, 299 | trihydroxy-dimethoxyflavone derivative d | + | + | [94,135,140] |
| 78 | 11.73 | 445 | 481 [M+Cl]−, 313 | dihydroxy-dimethoxyflavone-O-pentoside d | + | + | [94,135,140] |
| 79 | 12.46 | 419 | 153, 152, 121, 109, 108 | dihydroxybenzoyl-benzoyl-O-hexoside d | + | + | [143] |
| 80 | 12.76 | 783 | 367, 337, 299, 284, 269, 205, 163, 145 | trihydroxy-methoxyflavone-O-coumaroyl-sinapoylhexoside d | + | + | [143] |
| 81 | 12.83 | 285 | 257 | luteolin c | + | + | [12,94,109,135,138,139,140,146,147] |
| 82 | 13.01 | 315 | 300 | dihydroxy-methoxyflavone | + | + | [94,140] |
| 83 | 13.12 | 345 | 330, 315, 300, 287, 271 | tetrahydroxy-dimethoxyflavone | + | + | [94,140] |
| 84 | 13.36 | 677 | 515, 353, 191, 179, 173 | 3,4,5-tri-O-caffeoylquinic acid | + | + | [94,111,133,148,149] |
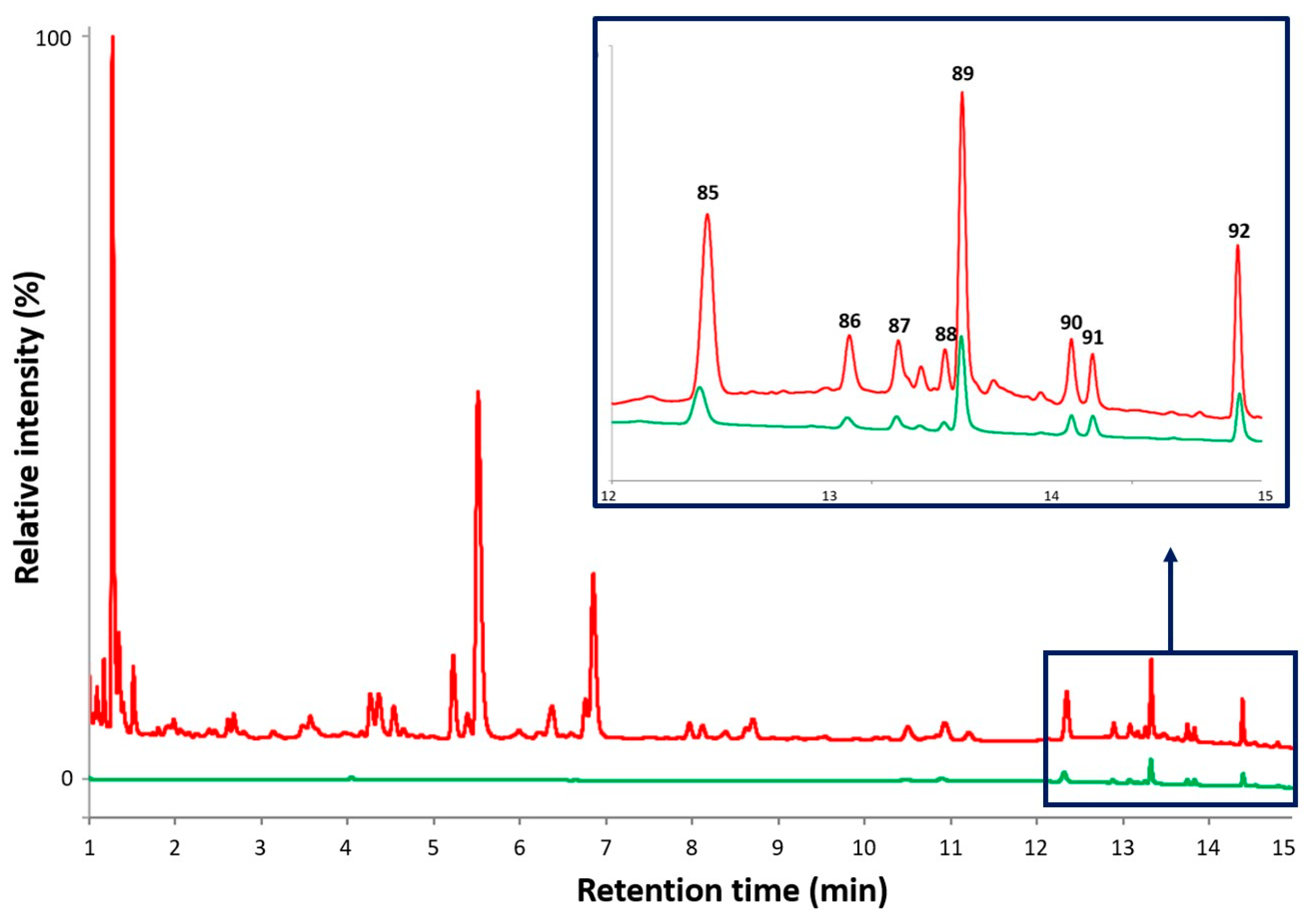
| 85 | 13.62 | 345 | 330, 315, 300, 287, 271 | tetrahydroxy-dimethoxyflavone | - | + | [94,140] |
| 86 | 13.74 | 269 | 227 | apigenin c | + | + | [94,140] |
| 87 | 13.98 | 299 | - | trihydroxy-methoxyflavone | + | + | [94,140] |
| 88 | 14.04 | 359 | 344, 329, 327, 314, 299, 284 | trihydroxy-trimethoxyflavone | + | + | [94,140] |
| 89 | 14.07 | 329 | 314, 299, 284, 271 | trihydroxy-dimethoxyflavone | + | + | [94,140] |
| 90 | 14.34 | 359 | 344, 329, 314, 299, 285, 271 | trihydroxy-trimethoxyflavone d | + | + | [94,140] |
| 91 | 14.42 | 359 | 344, 329, 314, 301, 286 | trihydroxy-trimethoxyflavone | + | + | [94,140] |
| 92 | 14.87 | 343 | 328, 313, 298 | dihydroxy-trimethoxyflavone | + | + | [94,140] |
| Compound | logPe PAMPA-BBB (n = 9) | logPe PAMPA-GI (n = 9) |
|---|---|---|
| Tetrahydroxy-dimethoxyflavone (85) | −4.78 ± 0.15 | −4.54 ± 0.13 |
| Apigenin (86) | −4.46 ± 0.11 | −4.75 ± 0.26 |
| Trihydroxy-methoxyflavone (87) | −4.56 ± 0.17 | −4.56 ± 0.19 |
| Trihydroxy-trimethoxyflavone (88) | −4.88 ± 0.13 | −4.75 ± 0.18 |
| Trihydroxy-dimethoxyflavone (89) | −4.46 ± 0.14 | −4.53 ± 0.13 |
| Trihydroxy-trimethoxyflavone (90) | −4.54 ± 0.08 | −4.74 ± 0.13 |
| Trihydroxy-trimethoxyflavone (91) | −4.43 ± 0.21 | −4.74 ± 0.14 |
| Dihydroxy-trimethoxyflavone (92) | −4.30 ± 0.13 | −4.93 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, Á.; Riethmüller, E.; Felegyi-Tóth, C.A.; Czigle, S.; Czégényi, D.; Filep, R.; Papp, N. Phytochemical Investigation of Polyphenols from the Aerial Parts of Tanacetum balsamita Used in Transylvanian Ethnobotany and Parallel Artificial Membrane Permeability Assay. Plants 2024, 13, 1652. https://doi.org/10.3390/plants13121652
Alberti Á, Riethmüller E, Felegyi-Tóth CA, Czigle S, Czégényi D, Filep R, Papp N. Phytochemical Investigation of Polyphenols from the Aerial Parts of Tanacetum balsamita Used in Transylvanian Ethnobotany and Parallel Artificial Membrane Permeability Assay. Plants. 2024; 13(12):1652. https://doi.org/10.3390/plants13121652
Chicago/Turabian StyleAlberti, Ágnes, Eszter Riethmüller, Csenge Anna Felegyi-Tóth, Szilvia Czigle, Dóra Czégényi, Rita Filep, and Nóra Papp. 2024. "Phytochemical Investigation of Polyphenols from the Aerial Parts of Tanacetum balsamita Used in Transylvanian Ethnobotany and Parallel Artificial Membrane Permeability Assay" Plants 13, no. 12: 1652. https://doi.org/10.3390/plants13121652
APA StyleAlberti, Á., Riethmüller, E., Felegyi-Tóth, C. A., Czigle, S., Czégényi, D., Filep, R., & Papp, N. (2024). Phytochemical Investigation of Polyphenols from the Aerial Parts of Tanacetum balsamita Used in Transylvanian Ethnobotany and Parallel Artificial Membrane Permeability Assay. Plants, 13(12), 1652. https://doi.org/10.3390/plants13121652






