Different Phenotypic, Photosynthetic, and Physiological Responses to Flooding between Q. nuttallii and Q. palustris
Abstract
:1. Introduction
2. Results
2.1. The Impact of Flooding on Plant Growth and Leaf Color
2.2. The Impact of Flooding on Gas Exchange and Chlorophyll Fluorescence Parameters
2.3. The Impact of Flooding on Biochemical Parameters in Both Oak Species
2.4. The Impact of Flooding on Endogenous Hormone Levels
2.5. Pearson Correlation Coefficients (PCCs) and Principal Component Analysis (PCA) of Physiological Parameters under Flooding Stress
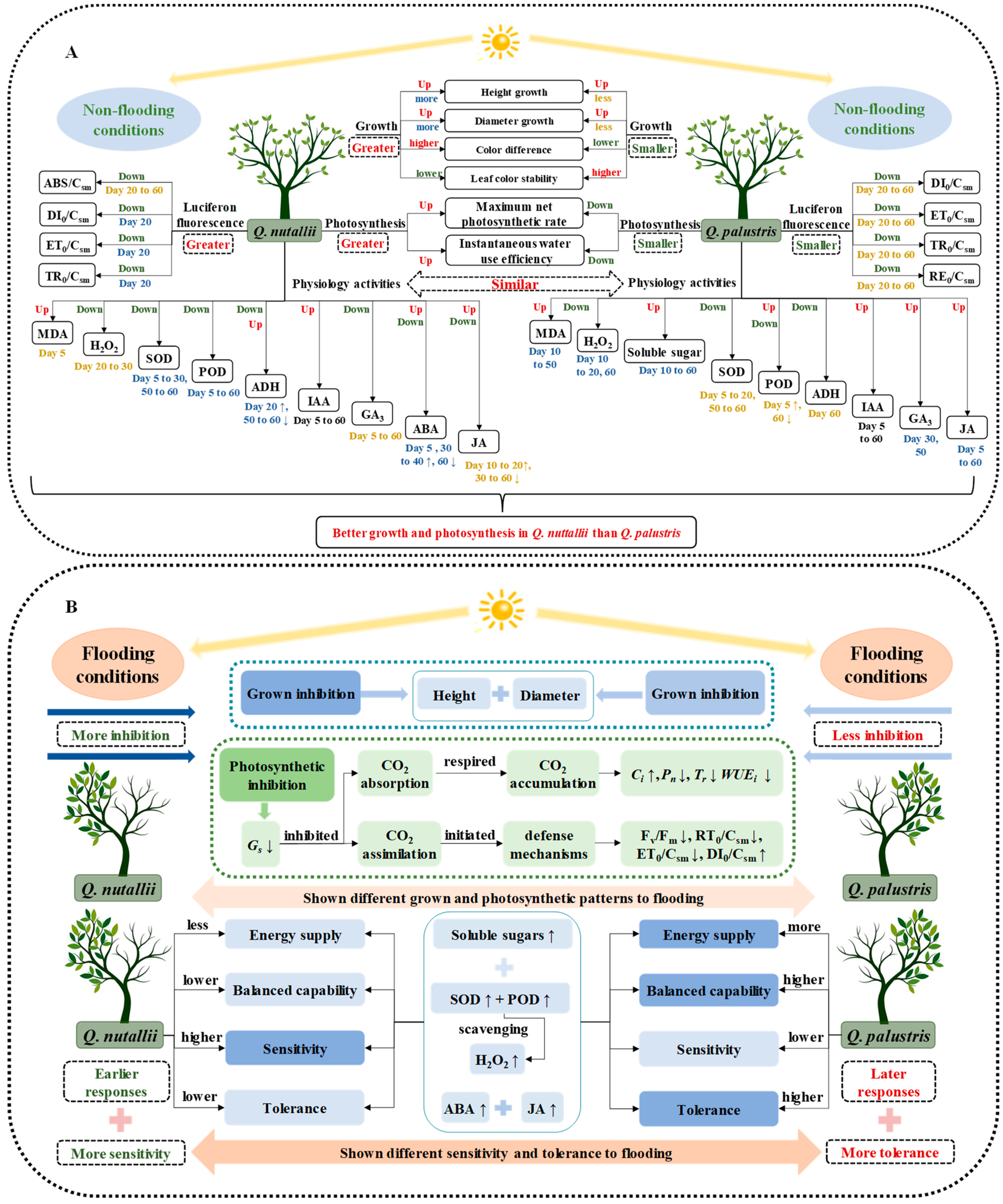
3. Discussion
3.1. The Two Species Had Contrasting Growth Responses
3.2. Higher Tolerance and Lower Sensitivity Were Observed in Q. palustris
4. Materials and Methods
4.1. Study Area
4.2. Plant Material and Experimental Design
4.3. Measurement of Gas Exchange and Chlorophyll Fluorescence Parameters
4.4. Measurements of Seedling Growth Traits and Leaf Color
4.5. Harvest and Measurements of MDA, H2O2, and Soluble Sugar
4.6. Analysis of Enzyme Activities and Endogenous Hormone Levels
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Luo, F.-L.; Matsubara, S.; Chen, Y.; Wei, G.-W.; Dong, B.-C.; Zhang, M.-X.; Yu, F.-H. Consecutive Submergence and De-Submergence Both Impede Growth of a Riparian Plant during Water Level Fluctuations with Different Frequencies. Environ. Exp. Bot. 2018, 155, 641–649. [Google Scholar] [CrossRef]
- Shen, C.; Yuan, J.; Qiao, H.; Wang, Z.; Liu, Y.; Ren, X.; Wang, F.; Liu, X.; Zhang, Y.; Chen, X.; et al. Transcriptomic and Anatomic Profiling Reveal the Germination Process of Different Wheat Varieties in Response to Waterlogging Stress. BMC Genet. 2020, 21, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, D.; Qi, Q.; Tong, S.; Wang, X.; An, Y.; Lu, X. Flooding Effects on Population and Growth Characteristics of Bolboschoenus planiculmis in Momoge Wetland, Northeast China. Ecol. Indic. 2022, 137, 108730. [Google Scholar] [CrossRef]
- Perata, P.; Armstrong, W.; Voesenek, L.A.C.J. Plants and Flooding Stress. New Phytol. 2011, 190, 269–273. [Google Scholar] [CrossRef]
- Striker, G.G. Flooding Stress on Plants: Anatomical, Morphological and Physiological Responses. In Botany; Books on Demand GmbH: Norderstedt, Germany, 2012; Volume 1, pp. 3–28. [Google Scholar] [CrossRef]
- Wegner, L.H. Oxygen Transport in Waterlogged Plants. In Waterlogging Signalling and Tolerance in Plants; Mancuso, S., Shabala, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–22. ISBN 978-3-642-10304-9. [Google Scholar]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding Tolerance: Suites of Plant Traits in Variable Environments. Funct. Plant Biol. 2009, 36, 665. [Google Scholar] [CrossRef]
- Iqbal, Z.; Sarkhosh, A.; Balal, R.M.; Gómez, C.; Zubair, M.; Ilyas, N.; Khan, N.; Shahid, M.A. Silicon Alleviate Hypoxia Stress by Improving Enzymatic and Non-Enzymatic Antioxidants and Regulating Nutrient Uptake in Muscadine Grape (Muscadinia rotundifolia Michx.). Front. Plant Sci. 2021, 11, 618873. [Google Scholar] [CrossRef]
- Su, Q.; Sun, Z.; Liu, Y.; Lei, J.; Zhu, W.; Nanyan, L. Physiological and Comparative Transcriptome Analysis of the Response and Adaptation Mechanism of the Photosynthetic Function of Mulberry (Morus alba L.) Leaves to Flooding Stress. Plant Signal. Behav. 2022, 17, 2094619. [Google Scholar] [CrossRef]
- Ferner, E.; Rennenberg, H.; Kreuzwieser, J. Effect of Flooding on C Metabolism of Flood-Tolerant (Quercus robur) and Non-Tolerant (Fagus sylvatica) Tree Species. Tree Physiol. 2012, 32, 135–145. [Google Scholar] [CrossRef]
- Maric, A.; Hartman, S. The Leaf Sheath Promotes Prolonged Flooding Protection by Giving Rise to Specialized Adventitious Roots. New Phytol. 2023, 238, 1337–1339. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding Stress: Acclimations and Genetic Diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A.C.J. The Role of Ethylene in Metabolic Acclimations to Low Oxygen. New Phytol. 2021, 229, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, Y.; Huang, D.; Wang, H.; Cao, Q.; Fan, P.; Yang, N.; Zheng, P.; Wang, R. The Effect of Climate Change on the Richness Distribution Pattern of Oaks (Quercus L.) in China. Sci. Total Environ. 2020, 744, 140786. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Z.; Chen, W.; Cannon, C.H.; Raes, N.; Slik, J.W.F. Major Declines of Woody Plant Species Ranges under Climate Change in Y Unnan, C Hina. Divers. Distrib. 2014, 20, 405–415. [Google Scholar] [CrossRef]
- Sloan, J.L.; Islam, M.A.; Jacobs, D.F. Reduced Translocation of Current Photosynthate Precedes Changes in Gas Exchange for Quercus Rubra Seedlings under Flooding Stress. Tree Physiol. 2016, 36, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Gao, Z.; Chen, H.; Chen, C.; Yu, F. Exponential Fertilization Regimes Improved Growth and Nutrient Status of Quercus Nuttallii Container Seedlings. Agronomy 2022, 12, 669. [Google Scholar] [CrossRef]
- Holladay, C.-A.; Kwit, C.; Collins, B. Woody Regeneration in and around Aging Southern Bottomland Hardwood Forest Gaps: Effects of Herbivory and Gap Size. For. Ecol. Manag. 2006, 223, 218–225. [Google Scholar] [CrossRef]
- Hayford, I.; Knapp, B.O.; Kabrick, J.M. Responses of Natural and Artificial Pin Oak (Quercus palustris) Reproduction to a Sequence of Silvicultural Release Treatments in Bottomland Hardwood Forests in Southern Missouri. For. Ecol. Manag. 2023, 529, 120699. [Google Scholar] [CrossRef]
- Gravatt, D.A.; Kirby, C.J. Patterns of Photosynthesis and Starch Allocation in Seedlings of Four Bottomland Hardwood Tree Species Subjected to Flooding. Tree Physiol. 1998, 18, 411–417. [Google Scholar] [CrossRef]
- Folzer, H.; Dat, J.F.; Capelli, N.; Rieffel, D.; Badot, P.-M. Response of Sessile Oak Seedlings (Quercus petraea) to Flooding: An Integrated Study. Tree Physiol. 2006, 26, 759–766. [Google Scholar] [CrossRef]
- Black, R.A. Water Relations of Quercus Palustris: Field Measurements on an Experimentally Flooded Stand. Oecologia 1984, 64, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sample, R.D.; Cook, J.; Babst, B.A. Resiliency of Nuttall Oak but Not Shumard Oak to Winter and Spring Flood: Dormancy Alone Does Not Confer Flood Tolerance. Trees 2023, 37, 1121–1136. [Google Scholar] [CrossRef]
- Jacques, R.; Stovall, J.; Comer, C.; Williams, H.; Symmank, M. Herbivory and Flooding Impacts on Planted Bottomland Hardwood Seedlings. For. Int. J. For. Res. 2022, 95, 215–228. [Google Scholar] [CrossRef]
- Glenz, C.; Schlaepfer, R.; Iorgulescu, I.; Kienast, F. Flooding Tolerance of Central European Tree and Shrub Species. For. Ecol. Manag. 2006, 235, 1–13. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Responses of Woody Plants to Flooding and Salinity. Tree Physiol. 1997, 17, 490. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Wang, J.; Deng, W.; Liao, L.; Li, M. Different Eco-Physiological Responses between Male and Female Populus Deltoides Clones to Waterlogging Stress. For. Ecol. Manag. 2011, 262, 1963–1971. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, W.; Hashiguchi, A.; Nishimura, M.; Tian, J.; Komatsu, S. Metabolic Profiles of Flooding-Tolerant Mechanism in Early-Stage Soybean Responding to Initial Stress. Plant Mol. Biol. 2017, 94, 669–685. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Duan, H. Effects of Drought and Flooding on Growth and Physiology of Cinnamomum Camphora Seedlings. Forests 2023, 14, 1343. [Google Scholar] [CrossRef]
- Shimamura, S.; Mochizuki, T.; Nada, Y.; Fukuyama, M. Formation and Function of Secondary Aerenchyma in Hypocotyl, Roots and Nodules of Soybean (Glycine max) under Flooded Conditions. Plant Soil 2003, 251, 351–359. [Google Scholar] [CrossRef]
- Henshaw, T.L.; Gilbert, R.A.; Scholberg, J.M.S.; Sinclair, T.R. Soya Bean (Glycine Max L. Merr.) Genotype Response to Early-season Flooding: II. Aboveground Growth and Biomass. J. Agron. Crop Sci. 2007, 193, 189–197. [Google Scholar] [CrossRef]
- Pires, J.L.F.; Soprano, E.; Cassol, B. Adaptações morfofisiológicas da soja em solo inundado. Pesq. Agropec. Bras. 2002, 37, 41–50. [Google Scholar] [CrossRef]
- Else, M.A.; Janowiak, F.; Atkinson, C.J.; Jackson, M.B. Root Signals and Stomatal Closure in Relation to Photosynthesis, Chlorophyll a Fluorescence and Adventitious Rooting of Flooded Tomato Plants. Ann. Bot. 2009, 103, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Hill, C.B.; Zhou, G.; Zhang, X.-Q.; Jia, Y.; Li, C. Opportunities for Improving Waterlogging Tolerance in Cereal Crops—Physiological Traits and Genetic Mechanisms. Plants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Gong, X.; Xu, Y.; Li, H.; Chen, X.; Song, Z. Antioxidant Activation, Cell Wall Reinforcement, and Reactive Oxygen Species Regulation Promote Resistance to Waterlogging Stress in Hot Pepper (Capsicum annuum L.). BMC Plant Biol. 2022, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; Cochard, H. Hydraulic Failure Defines the Recovery and Point of Death in Water-Stressed Conifers. Plant Physiol. 2009, 149, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Juzoń, K.; Czyczyło-Mysza, I.; Ostrowska, A.; Marcińska, I.; Skrzypek, E. Chlorophyll Fluorescence for Prediction of Yellow Lupin (Lupinus luteus L.) and Pea (Pisum sativum L.) Susceptibility to Drought. Photosynthetica 2019, 57, 950–959. [Google Scholar] [CrossRef]
- Bhatt, U.; Sharma, S.; Soni, V. Differential Photosynthetic Responses in Riccia Gangetica under Heat, Cold, Salinity, Submergence, and UV-B Stresses. J. Photochem. Photobiol. 2022, 12, 100146. [Google Scholar] [CrossRef]
- Li, M.; López, R.; Venturas, M.; Pita, P.; Gordaliza, G.G.; Gil, L.; Rodríguez-Calcerrada, J. Greater Resistance to Flooding of Seedlings of Ulmus Laevis than Ulmus Minor Is Related to the Maintenance of a More Positive Carbon Balance. Trees 2015, 29, 835–848. [Google Scholar] [CrossRef]
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and Pathogenic Plant-microbe Interactions during Flooding Stress. Plant Cell Environ. 2022, 45, 2875–2897. [Google Scholar] [CrossRef]
- Sinsawat, V.; Leipner, J.; Stamp, P.; Fracheboud, Y. Effect of Heat Stress on the Photosynthetic Apparatus in Maize (Zea mays L.) Grown at Control or High Temperature. Environ. Exp. Bot. 2004, 52, 123–129. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A. Investigating Deleterious Effects of Ultraviolet (UV) Radiations on Wheat by a Quick Method. Acta Physiol. Plant. 2015, 37, 121. [Google Scholar] [CrossRef]
- Hura, T.; Hura, K.; Ostrowska, A.; Dziurka, K. Rapid Plant Rehydration Initiates Permanent and Adverse Changes in the Photosynthetic Apparatus of Triticale. Plant Soil 2015, 397, 127–145. [Google Scholar] [CrossRef]
- Park, S.-U.; Lee, C.-J.; Kim, S.-E.; Lim, Y.-H.; Lee, H.-U.; Nam, S.-S.; Kim, H.S.; Kwak, S.-S. Selection of Flooding Stress Tolerant Sweetpotato Cultivars Based on Biochemical and Phenotypic Characterization. Plant Physiol. Biochem. 2020, 155, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Limami, A.M. Adaptations of Nitrogen Metabolism to Oxygen Deprivation in Plants. In Low-Oxygen Stress in Plants: Oxygen Sensing and Adaptive Responses to Hypoxia; van Dongen, J.T., Licausi, F., Eds.; Springer: Vienna, Austria, 2014; pp. 209–221. ISBN 978-3-7091-1254-0. [Google Scholar]
- Miao, L.-F.; Yang, F.; Han, C.-Y.; Pu, Y.-J.; Ding, Y.; Zhang, L.-J. Sex-Specific Responses to Winter Flooding, Spring Waterlogging and Post-Flooding Recovery in Populus Deltoides. Sci. Rep. 2017, 7, 2534. [Google Scholar] [CrossRef]
- Yin, D.; Chen, S.; Chen, F.; Guan, Z.; Fang, W. Morphological and Physiological Responses of Two Chrysanthemum Cultivars Differing in Their Tolerance to Waterlogging. Environ. Exp. Bot. 2009, 67, 87–93. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in Photosynthesis and Some Antioxidant Enzymatic Activities of Mungbean Subjected to Waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Ye, Y.; Tam, N.F.Y.; Wong, Y.S.; Lu, C.Y. Growth and Physiological Responses of Two Mangrove Species (Bruguiera gymnorrhiza and Kandelia candel) to Waterlogging. Environ. Exp. Bot. 2003, 49, 209–221. [Google Scholar] [CrossRef]
- Yiu, J.-C.; Juang, L.-D.; Fang, D.Y.-T.; Liu, C.-W.; Wu, S.-J. Exogenous Putrescine Reduces Flooding-Induced Oxidative Damage by Increasing the Antioxidant Properties of Welsh Onion. Sci. Hortic. 2009, 120, 306–314. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Miao, L.-F. Comparative Physiological and Proteomic Responses to Drought Stress in Two Poplar Species Originating from Different Altitudes. Physiol. Plant. 2010, 139, 388–400. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, S.; Xu, X.; Korpelainen, H.; Li, C. Changes in Antioxidant Enzyme Activities and Isozyme Profiles in Leaves of Male and Female Populus cathayana Infected with Melampsora larici-populina. Tree Physiol. 2010, 30, 116–128. [Google Scholar] [CrossRef]
- Blumstein, M.; Gersony, J.; Martínez-Vilalta, J.; Sala, A. Global Variation in Nonstructural Carbohydrate Stores in Response to Climate. Glob. Chang. Biol. 2023, 29, 1854–1869. [Google Scholar] [CrossRef]
- Herschbach, C.; Mult, S.; Kreuzwieser, J.; Kopriva, S. Influence of Anoxia on Whole Plant Sulphur Nutrition of Flooding-tolerant Poplar (Populus Tremula × P. Alba). Plant Cell Environ. 2005, 28, 167–175. [Google Scholar] [CrossRef]
- Jaeger, C.; Gessler, A.; Biller, S.; Rennenberg, H.; Kreuzwieser, J. Differences in C Metabolism of Ash Species and Provenances as a Consequence of Root Oxygen Deprivation by Waterlogging. J. Exp. Bot. 2009, 60, 4335–4345. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Jover, S.; Quiñones, A.; Forner-Giner, M.Á.; Rodríguez-Gamir, J.; Legaz, F.; Primo-Millo, E.; Iglesias, D.J. Flooding Affects Uptake and Distribution of Carbon and Nitrogen in Citrus Seedlings. J. Plant Physiol. 2012, 169, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Kreuzwieser, J.; Papadopoulou, E.; Rennenberg, H. Interaction of Flooding with Carbon Metabolism of Forest Trees. Plant Biol. 2004, 6, 299–306. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, X.; Ma, W.; Song, J.; Rahman, S.U.; Wang, J.; Zhang, Y. Morphological and Physiological Responses to Cyclic Drought in Two Contrasting Genotypes of Catalpa bungei. Environ. Exp. Bot. 2017, 138, 77–87. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, A.; Jiang, M. Involvement of Polyamine Oxidase in Abscisic Acid-induced Cytosolic Antioxidant Defense in Leaves of Maize. J. Integr. Plant Biol. 2009, 51, 225–234. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone Balance and Abiotic Stress Tolerance in Crop Plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Shi, W.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z. Exogenous Abscisic Acid Alleviates Zinc Uptake and Accumulation in P. Opulus × Canescens Exposed to Excess Zinc. Plant Cell Environ. 2015, 38, 207–223. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Chen, M.; Chen, J.; Pan, Y.; Chen, Y.; Rahman, S.U.; Fan, J.; Zhang, Y. Influence of Nitrogen Availability on Cd Accumulation and Acclimation Strategy of Populus Leaves under Cd Exposure. Ecotoxicol. Environ. Saf. 2019, 180, 439–448. [Google Scholar] [CrossRef]
- Song, J.; Finnegan, P.M.; Liu, W.; Li, X.; Yong, J.W.H.; Xu, J.; Zhang, Q.; Wen, Y.; Qin, K.; Guo, J.; et al. Mechanisms Underlying Enhanced Cd Translocation and Tolerance in Roots of Populus Euramericana in Response to Nitrogen Fertilization. Plant Sci. 2019, 287, 110206. [Google Scholar] [CrossRef]
- Zhu, L.; Wen, W.; Thorpe, M.R.; Hocart, C.H.; Song, X. Combining Heat Stress with Pre-Existing Drought Exacerbated the Effects on Chlorophyll Fluorescence Rise Kinetics in Four Contrasting Plant Species. IJMS 2021, 22, 10682. [Google Scholar] [CrossRef]
- Carreño, J.; Martínez, A.; Almela, L.; Fernández-López, J.A. Proposal of an Index for the Objective Evaluation of the Colour of Red Table Grapes. Food Res. Int. 1995, 28, 373–377. [Google Scholar] [CrossRef]
- Zhang, C.C.; Wang, K.L.; Ren, H.D.; Chang, J.; Yao, X.H. Variation in Pigments in Pecan Testa during Kernel Development and Storage. Food Chem. 2024, 438, 137989. [Google Scholar] [CrossRef]
- He, K.; Yang, J.; Huang, L. Physiological Responses of Seedlings of Two Oak Species to Flooding Stress. For. Stud. China 2008, 10, 259–264. [Google Scholar] [CrossRef]
- Alvarez, M.; Huygens, D.; Fernandez, C.; Gacitua, Y.; Olivares, E.; Saavedra, I.; Alberdi, M.; Valenzuela, E. Effect of Ectomycorrhizal Colonization and Drought on Reactive Oxygen Species Metabolism of Nothofagus dombeyi Roots. Tree Physiol. 2009, 29, 1047–1057. [Google Scholar] [CrossRef]
- Shi, H.; Ma, W.; Song, J.; Lu, M.; Rahman, S.U.; Bui, T.T.X.; Vu, D.D.; Zheng, H.; Wang, J.; Zhang, Y. Physiological and Transcriptional Responses of Catalpa bungei to Drought Stress under Sufficient- and Deficient-Nitrogen Conditions. Tree Physiol. 2017, 37, 1457–1468. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The Role of Superoxide Anion in the Autoxidation of Epinephrine and a Simple Assay for Superoxide Dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Tyler, D.D. A Protective Function of Superoxide Dismutase during Respiratory Chain Activity. Biochim. Biophys. Acta (BBA)—Bioenerg. 1975, 396, 335–346. [Google Scholar] [CrossRef]
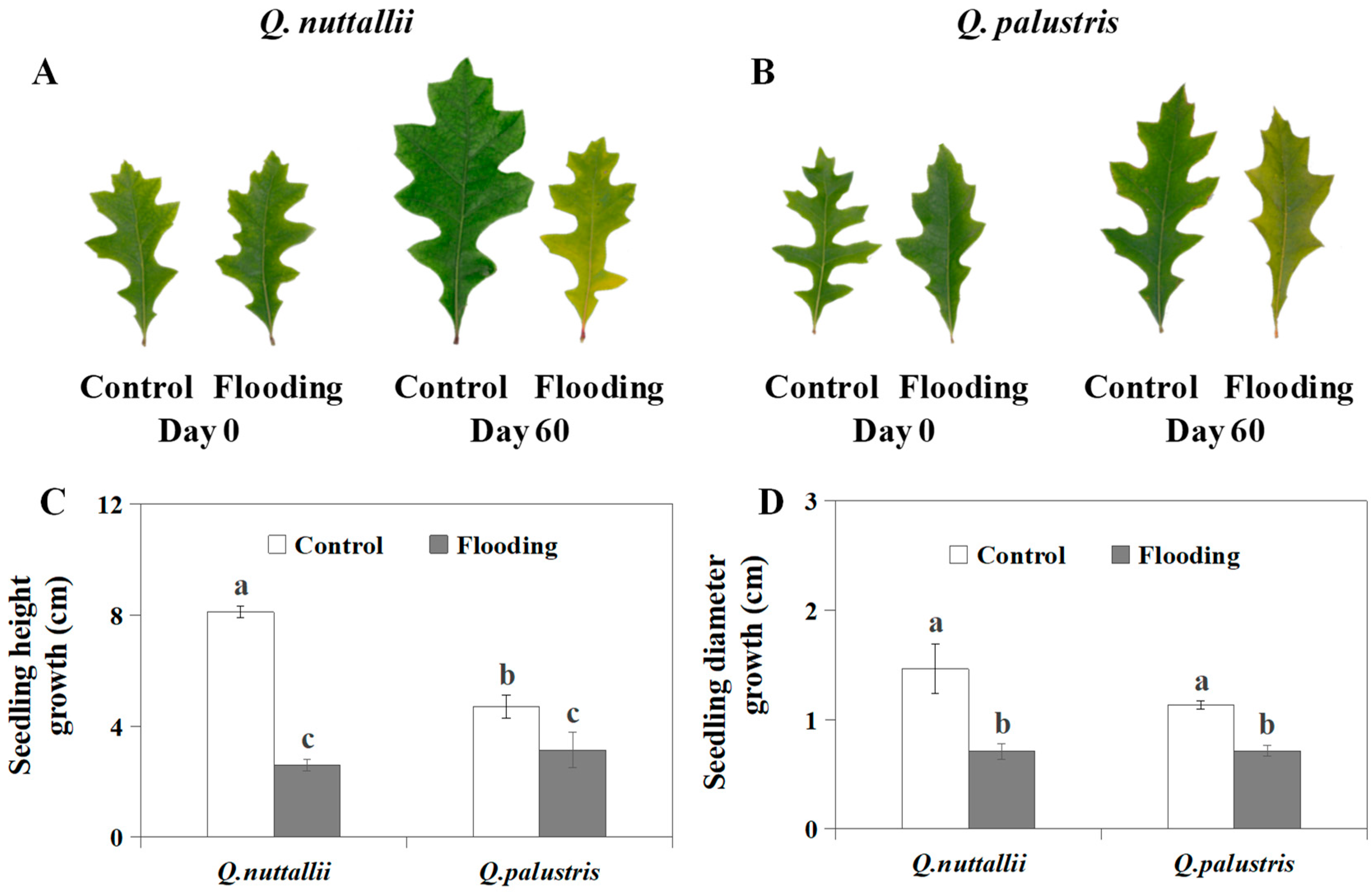
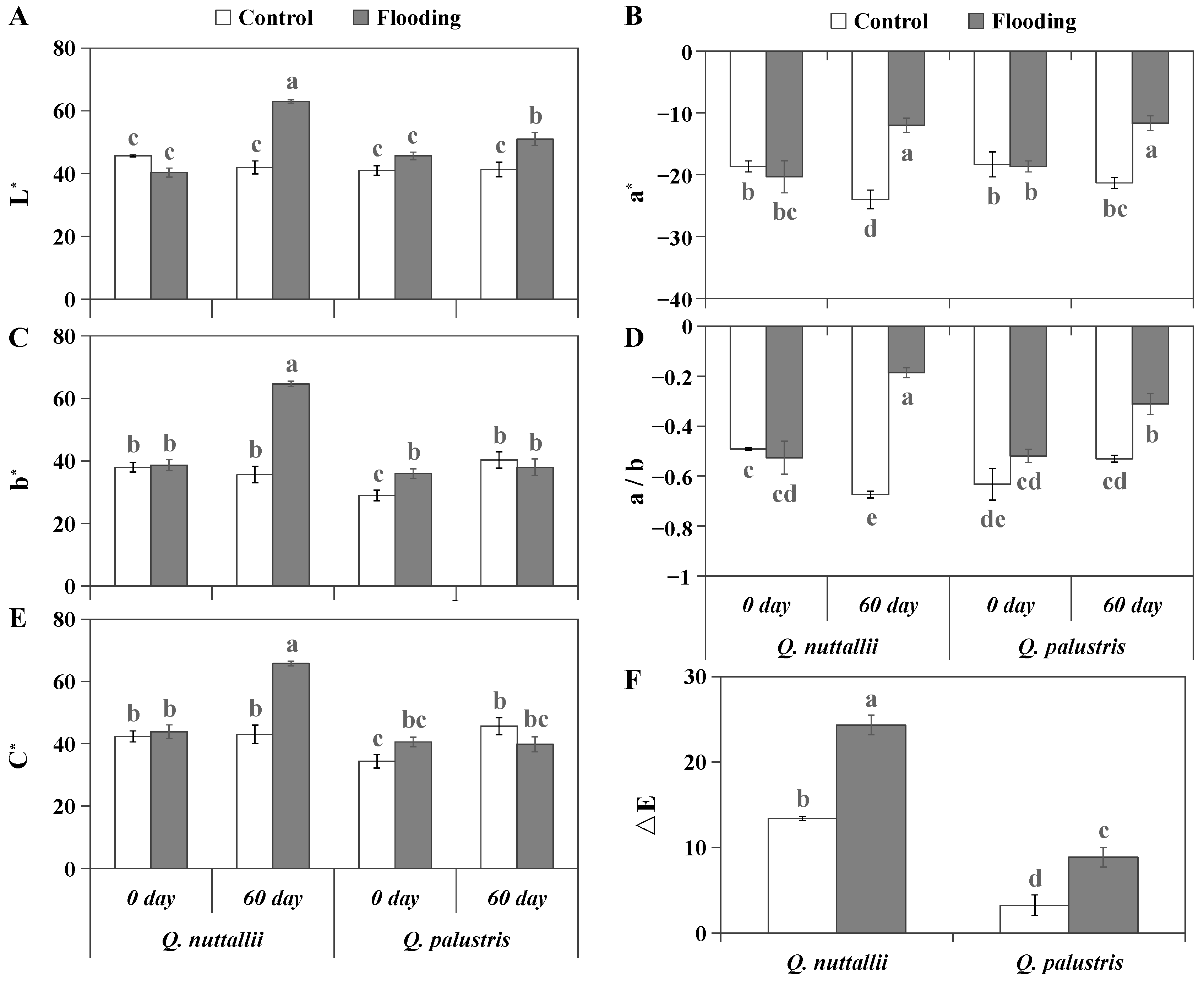
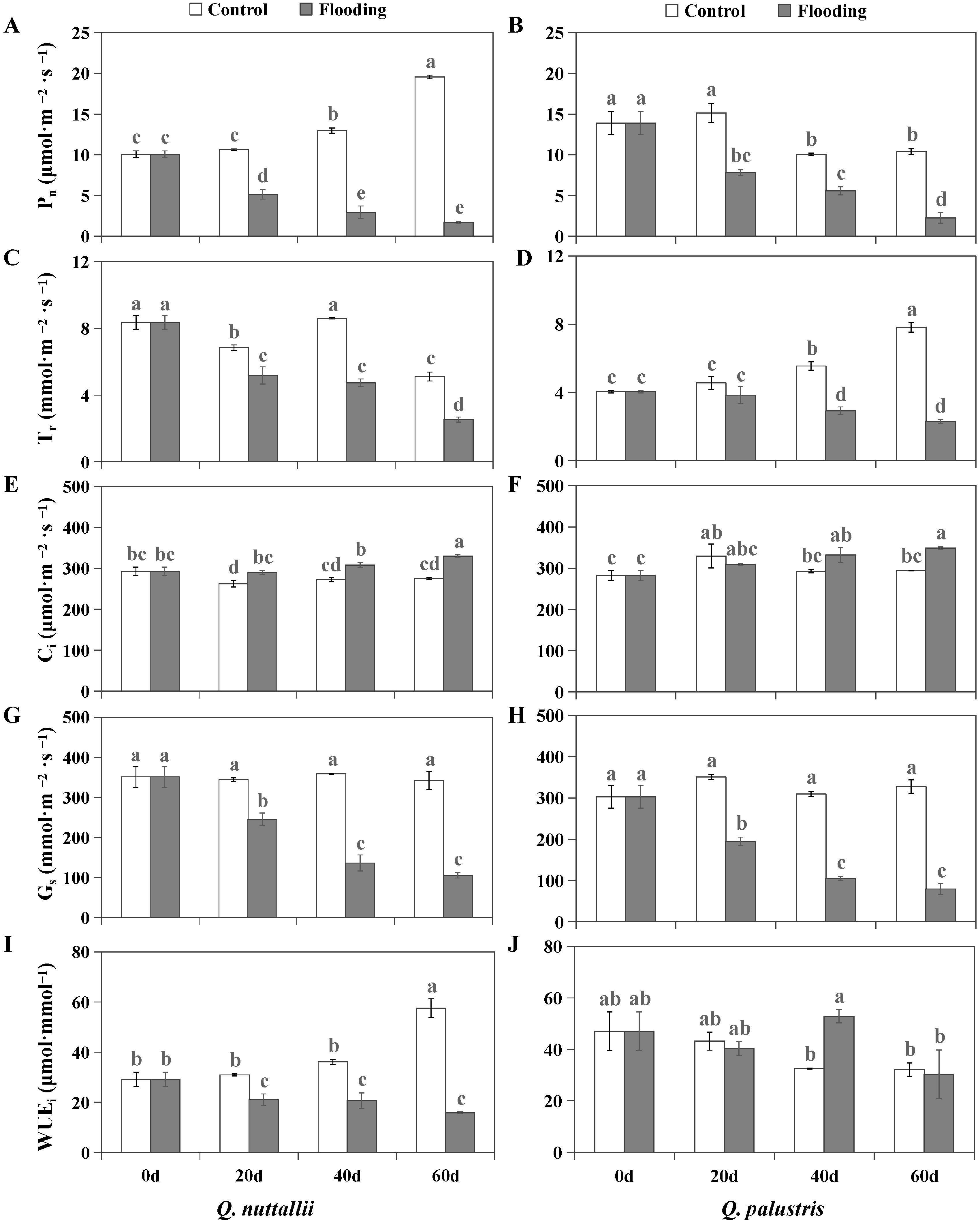

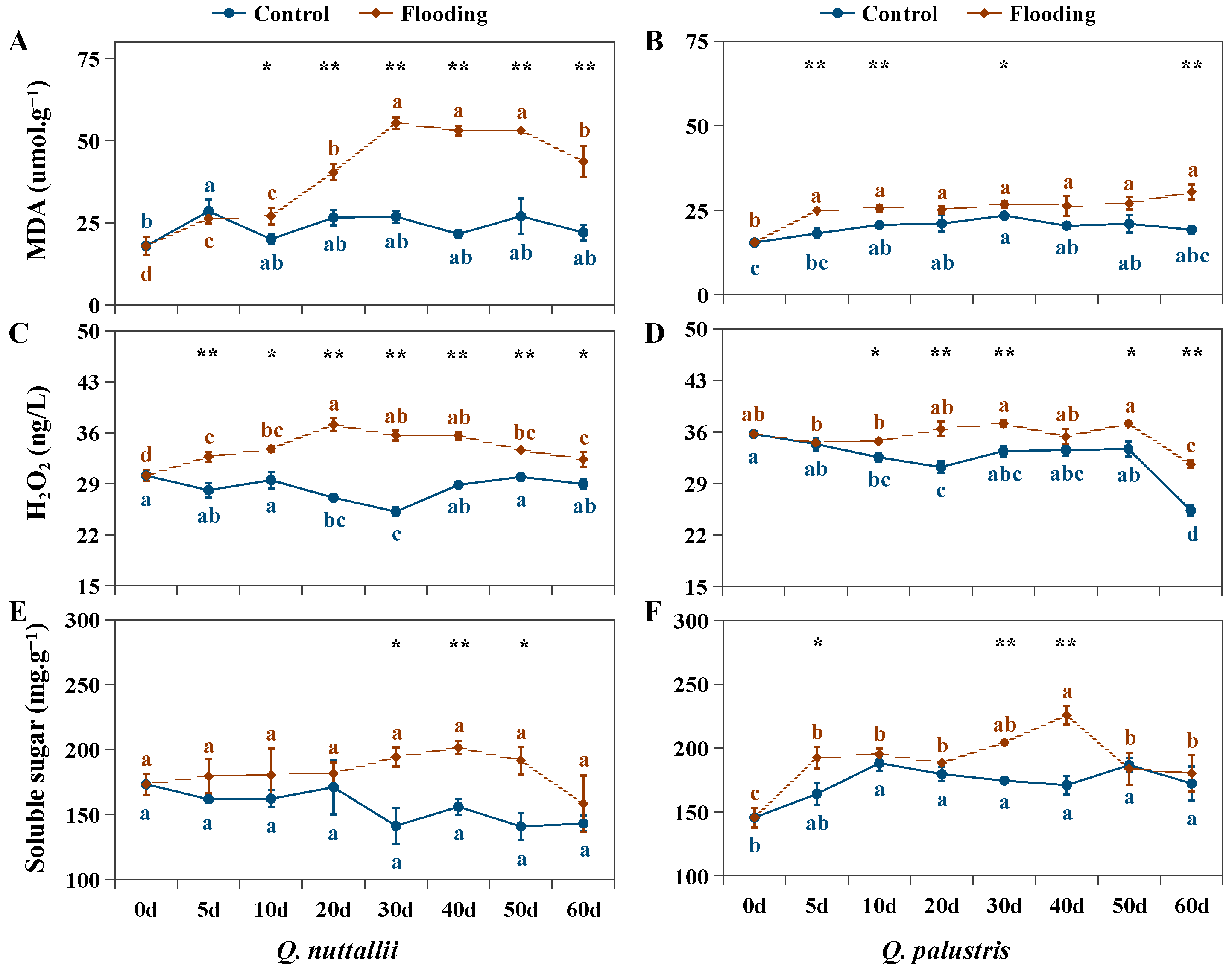
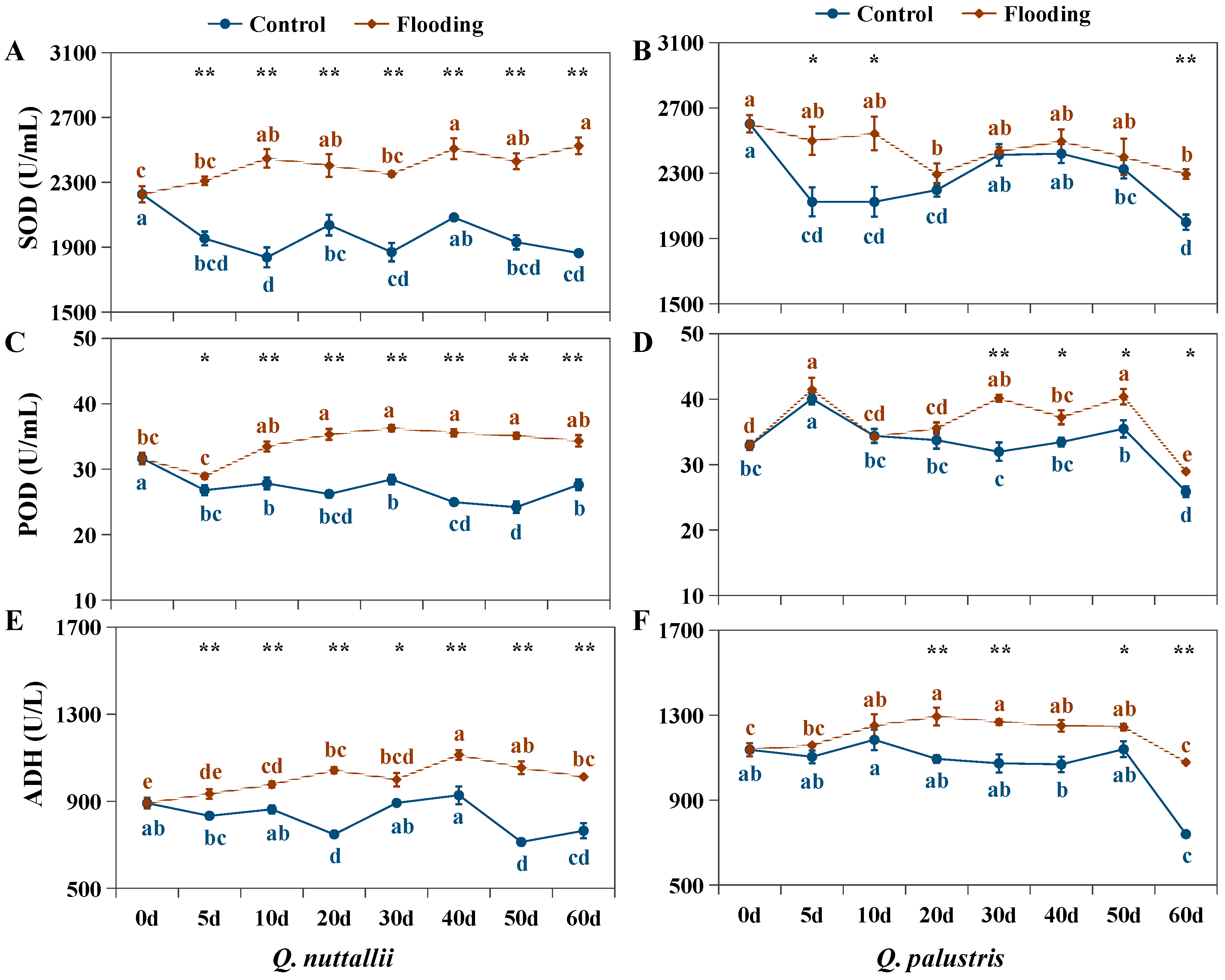
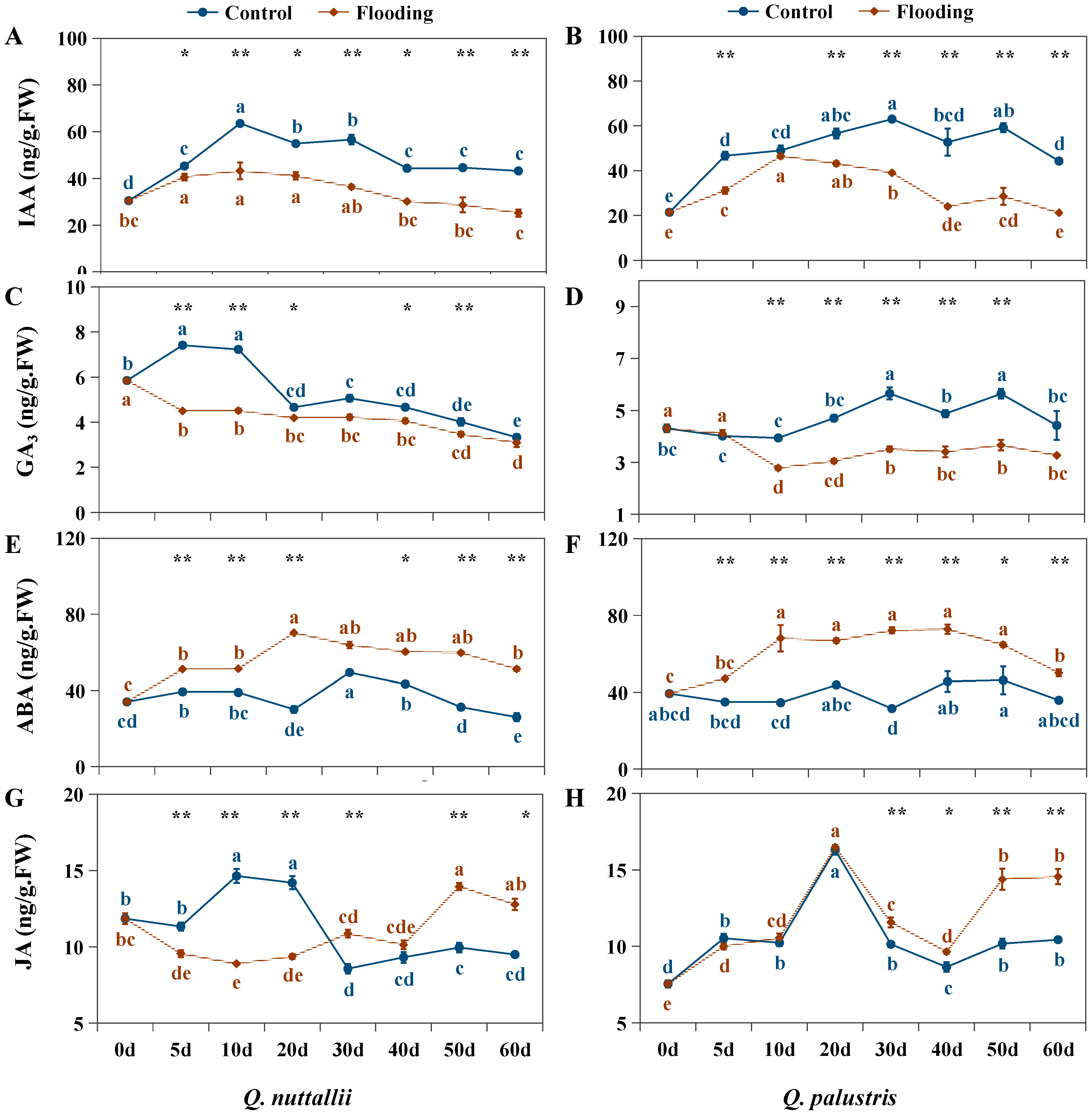
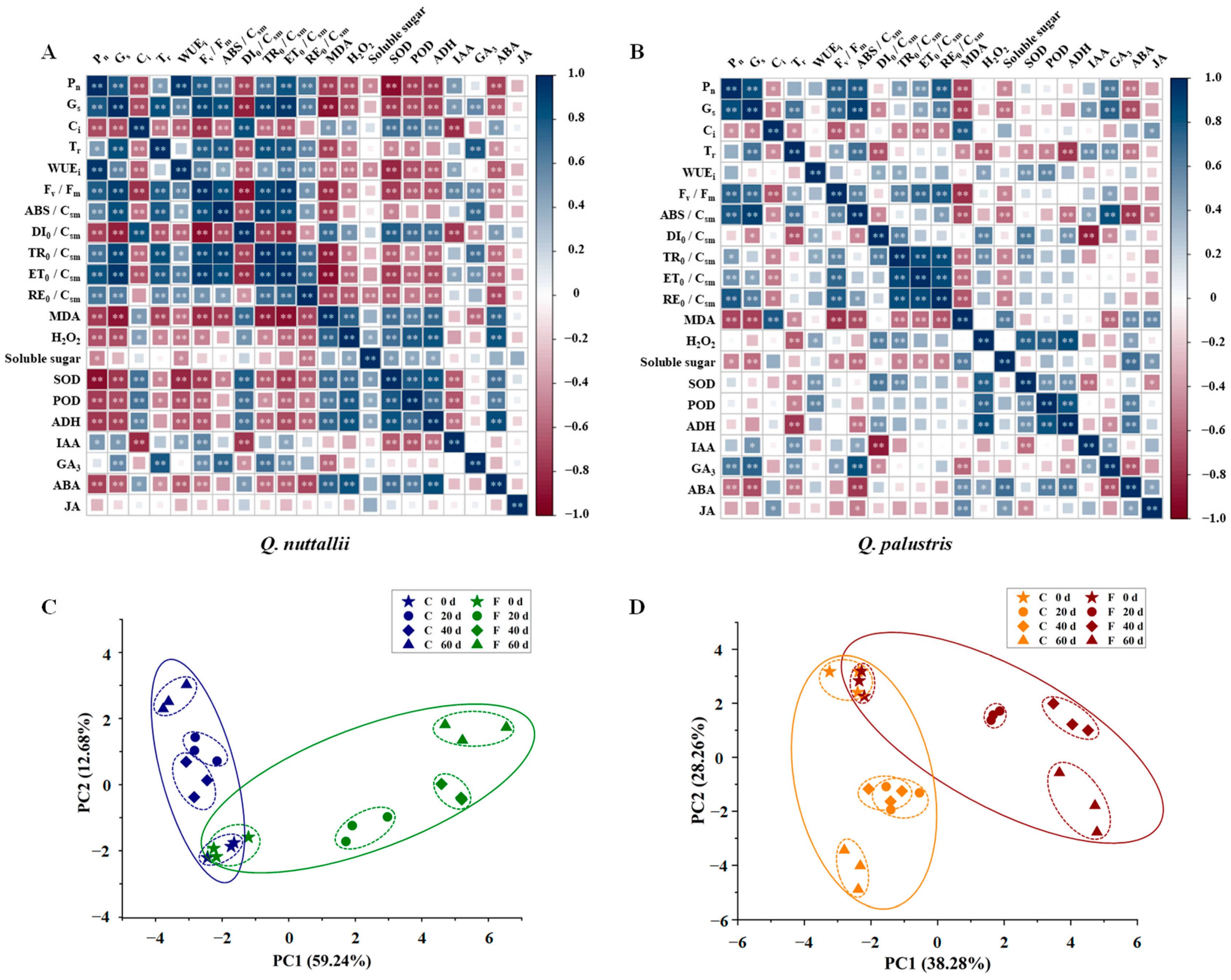
| Phenotypic Parameters | Q. nuttallii (Day 60) | Q. palustris (Day 60) |
|---|---|---|
| Height (growth amount) | 0.32 ↓ | 0.67 ↓ |
| Diameter (growth amount) | 0.48 ↓ | 0.63 ↓ |
| ΔE | 1.82 ↑ | 2.71 ↑ |
| L* | 1.50 ↑ | 1.23 ↑ |
| a* | 2.00 ↑ | 1.83 ↑ |
| b* | 1.81 ↑ | 0.94 ns |
| a/b | 3.62 ↑ | 1.70 ↑ |
| C* | 1.53 ↑ | 0.87 ns |
| Indexes | Species | Treatment | Time | Species × Treatment | Species × Time | Treatment × Time | Species × Treatment × Time | |
|---|---|---|---|---|---|---|---|---|
| 1 | Pn | * | ** | ** | ** | ** | ** | ** |
| 2 | Gs | ** | ** | ** | Ns | Ns | ** | Ns |
| 3 | Ci | ** | ** | * | Ns | * | ** | Ns |
| 4 | Tr | ** | ** | ** | Ns | ** | ** | ** |
| 5 | WUEi | ** | ** | Ns | ** | ** | ** | ** |
| 6 | Fv/Fm | ** | ** | ** | ** | ** | ** | ** |
| 7 | ABS/CSm | Ns | ** | ** | ** | ** | ** | ** |
| 8 | DI0/CSm | ** | ** | ** | ** | ** | ** | * |
| 9 | TR0/CSm | ** | ** | ** | ** | Ns | ** | ** |
| 10 | ET0/CSm | ** | ** | ** | ** | Ns | ** | ** |
| 11 | RE0/CSm | ** | ** | ** | Ns | ** | * | Ns |
| 12 | MDA | ** | ** | ** | ** | ** | ** | ** |
| 13 | H2O2 | ** | ** | ** | ** | ** | ** | ** |
| 14 | Soluble sugar | ** | ** | ** | Ns | * | * | Ns |
| 15 | SOD | ** | ** | ** | ** | ** | ** | * |
| 16 | POD | ** | ** | ** | ** | ** | ** | * |
| 17 | ADH | ** | ** | ** | Ns | ** | ** | ** |
| 18 | IAA | Ns | ** | ** | * | ** | ** | ** |
| 19 | GA3 | ** | ** | ** | Ns | ** | ** | ** |
| 20 | ABA | ** | ** | ** | Ns | ** | ** | ** |
| 21 | JA | Ns | ** | ** | ** | ** | ** | ** |
| Photosynthetic Indexes | Q. nuttallii | Q. palustris | ||||
|---|---|---|---|---|---|---|
| 20 d | 40 d | 60 d | 20 d | 40 d | 60 d | |
| Pn | 0.48 ↓ | 0.23 ↓ | 0.09 ↓ | 0.52 ↓ | 0.55 ↓ | 0.21 ↓ |
| Gs | 0.71 ↓ | 0.38 ↓ | 0.31 ↓ | 0.56 ↓ | 0.34 ↓ | 0.24 ↓ |
| Ci | 1.11 ↑ | 1.13 ↑ | 1.20 ↑ | 0.94 ns | 1.13 ns | 1.18 ↑ |
| Tr | 0.76 ↓ | 0.55 ↓ | 0.49 ↓ | 0.84 ns | 0.53 ↓ | 0.29 ↓ |
| WUEi | 0.64 ↓ | 0.40 ↓ | 0.17 ↓ | 0.62 ↓ | 1.05 ns | 0.71 ns |
| Fv/Fm | 0.95 ↓ | 0.73 ↓ | 0.66 ↓ | 1.00 ns | 0.94 ↓ | 0.88 ↓ |
| ABS/CSm | 1.07 ns | 0.80 ↓ | 0.64 ↓ | 0.66 ↓ | 0.58 ↓ | 0.59 ↓ |
| DI0/CSm | 1.22 ↑ | 1.47 ↑ | 1.63 ↑ | 1.20 ↑ | 1.42 ↑ | 1.38 ↑ |
| TR0/CSm | 0.93 ns | 0.46 ↓ | 0.44 ↓ | 1.26 ↑ | 1.09 ns | 0.93 ns |
| ET0/CSm | 0.87 ns | 0.31 ↓ | 0.30 ↓ | 1.39 ↑ | 0.91 ns | 0.67 ↓ |
| RE0/CSm | 0.71 ↓ | 0.74 ↓ | 0.73 ↓ | 1.08 ns | 0.82 ns | 0.58 ↓ |
| Physiological Indexes | 5 d | 10 d | 20 d | 30 d | 40 d | 50 d | 60 d |
|---|---|---|---|---|---|---|---|
| MDA | 0.92 ns | 1.35 ↑ | 1.52 ↑ | 2.06 ↑ | 2.47 ↑ | 1.97 ↑ | 1.99 ↑ |
| H2O2 | 1.16 ↑ | 1.15 ↑ | 1.37 ↑ | 1.42 ↑ | 1.23 ↑ | 1.12 ↑ | 1.12 ↑ |
| Soluble sugar | 1.11 ns | 1.11 ns | 1.06 ns | 1.38 ↑ | 1.29 ↑ | 1.36 ↑ | 1.11 ns |
| SOD | 1.18 ↑ | 1.33 ↑ | 1.18 ↑ | 1.26 ↑ | 1.20 ↑ | 1.26 ↑ | 1.35 ↑ |
| POD | 1.08 ↑ | 1.20 ↑ | 1.35 ↑ | 1.28 ↑ | 1.42 ↑ | 1.45 ↑ | 1.24 ↑ |
| ADH | 1.12 ↑ | 1.13 ↑ | 1.39 ↑ | 1.12 ↑ | 1.20 ↑ | 1.48 ↑ | 1.32 ↑ |
| IAA | 0.90 ↓ | 0.68 ↓ | 0.75 ↓ | 0.64 ↓ | 0.68 ↓ | 0.64 ↓ | 0.58 ↓ |
| GA3 | 0.61 ↓ | 0.62 ↓ | 0.90 ↓ | 0.83 ns | 0.87 ↓ | 0.86 ↓ | 0.93 ns |
| ABA | 1.30 ↑ | 1.32 ↑ | 2.34 ↑ | 1.29 ns | 1.39 ↑ | 1.91 ↑ | 1.97 ↑ |
| JA | 0.84 ↓ | 0.61 ↓ | 0.66 ↓ | 1.27 ↑ | 1.09 ns | 1.40 ↑ | 1.35 ↑ |
| Physiological Indexes | 5 d | 10 d | 20 d | 30 d | 40 d | 50 d | 60 d |
|---|---|---|---|---|---|---|---|
| MDA | 1.38 ↑ | 1.24 ↑ | 1.20 ns | 1.14 ↑ | 1.29 ns | 1.29 ns | 1.58 ↑ |
| H2O2 | 1.01 ns | 1.07 ↑ | 1.17 ↑ | 1.11 ↑ | 1.05 ns | 1.10 ↑ | 1.25 ↑ |
| Soluble sugar | 1.17 ↑ | 1.04 ns | 1.05 ns | 1.17 ↑ | 1.32 ↑ | 0.98 ns | 1.05 ns |
| SOD | 1.18 ↑ | 1.20 ↑ | 1.04 ns | 1.01 ns | 1.03 ns | 1.03 ns | 1.15 ↑ |
| POD | 1.04 ns | 1.00 ns | 1.05 ns | 1.26 ↑ | 1.11 ↑ | 1.14 ↑ | 1.12 ↑ |
| ADH | 1.05 ns | 1.06 ns | 1.18 ↑ | 1.18 ↑ | 1.17 ns | 1.09 ↑ | 1.46 ↑ |
| IAA | 0.67 ↓ | 0.95 ns | 0.76 ↓ | 0.62 ↓ | 0.46 ↓ | 0.48 ↓ | 0.48 ↓ |
| GA3 | 1.03 ns | 0.71 ↓ | 0.65 ↓ | 0.62 ↓ | 0.70 ↓ | 0.65 ↓ | 0.74 ns |
| ABA | 1.35 ↑ | 1.97 ↑ | 1.53 ↑ | 2.29 ↑ | 1.60 ↑ | 1.40 ↑ | 1.40 ↑ |
| JA | 0.95 ns | 1.03 ns | 1.01 ns | 1.14 ns | 1.12 ↑ | 1.41 ↑ | 1.40 ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, T.; Wang, M.; Li, X.; Chen, Y.; Zhang, W. Different Phenotypic, Photosynthetic, and Physiological Responses to Flooding between Q. nuttallii and Q. palustris. Plants 2024, 13, 1658. https://doi.org/10.3390/plants13121658
Sun T, Wang M, Li X, Chen Y, Zhang W. Different Phenotypic, Photosynthetic, and Physiological Responses to Flooding between Q. nuttallii and Q. palustris. Plants. 2024; 13(12):1658. https://doi.org/10.3390/plants13121658
Chicago/Turabian StyleSun, Tiantian, Mengzhu Wang, Xin Li, Yongxia Chen, and Wangxiang Zhang. 2024. "Different Phenotypic, Photosynthetic, and Physiological Responses to Flooding between Q. nuttallii and Q. palustris" Plants 13, no. 12: 1658. https://doi.org/10.3390/plants13121658
APA StyleSun, T., Wang, M., Li, X., Chen, Y., & Zhang, W. (2024). Different Phenotypic, Photosynthetic, and Physiological Responses to Flooding between Q. nuttallii and Q. palustris. Plants, 13(12), 1658. https://doi.org/10.3390/plants13121658





