Chemical Composition and Protective Possibilities of Juglans Nigra Leaves and Green Husks Extracts: DNA Binding and Micronucleus Assay in Human Lymphocytes
Abstract
:1. Introduction
2. Results and Discussions
| No | Compound Name | tR, min | Molecular Formula, [M–H]– | Calculated Mass, m/z | Exact Mass, m/z | Δ mDa | MS2 Fragments, (% Base Peak) | Husk | Leaf |
|---|---|---|---|---|---|---|---|---|---|
| Hydroxybenzoic acid derivatives | |||||||||
| 1 | HHDP-hexose | 0.54 | C20H17O14− | 481.06238 | 481.05966 | 2.72 | 249.03931 (4), 257.0087 (2), 275.01849 (67), 300.99759 (100), 481.05991 (12) | − | ✚ |
| 2 | Galloyl hexose | 0.59 | C13H15O10– | 331.06707 | 331.06551 | 1.56 | 125.02389 (9), 168.00566 (9), 169.01352 (71), 211.02385 (74), 241.03458 (7), 271.04474 (100) | ✚ | ✚ |
| 3 | Gallic acid | 0.61 | C7H5O5– | 169.01425 | 169.01339 | 0.86 | 125.02388 (100), 169.01350 (47) | ✚ | ✚ |
| 4 | Galloyl-HHDP-hexose | 1.26 | C27H21O18– | 633.07334 | 633.06992 | 3.41 | 169.0134 (9), 275.01837 (23), 300.99747 (100) | – | ✚ |
| 5 | p-Hydroxybenzoic acid | 2.33 | C7H5O3– | 137.02442 | 137.02384 | 0.58 | 93.03412 (100), 137.02374 (47) | ✚ | ✚ |
| 6 | Dihydroxybenzoyl hexose | 2.78 | C7H5O4– | 153.01933 | 153.01872 | 0.61 | 108.02118 (13), 109.02905 (100), 153.01872 (50) | ✚ | – |
| 7 | Digalloyl hexose | 5.13 | C20H19O14– | 483.07803 | 483.07526 | 2.77 | 125.02384 (18), 169.01346 (94), 271.04474 (100), 313.05499 (45), 331.06573 (24), 483.07538 (14) | ✚ | – |
| 8 | Di-gallate | 5.15 | C14H9O9– | 321.02521 | 321.02364 | 1.57 | 125.02381 (11), 158.03659 (12), 169.01343 (100) | ✚ | – |
| 9 | Methyl-galloyl hexose | 5.28 | C14H17O10– | 345.08272 | 345.08044 | 2.28 | 125.02382 (14), 151.00325 (10), 169.01347 (100) | ✚ | ✚ |
| 10 | Ethyl-galloyl hexose | 5.37 | C15H19O10– | 359.09837 | 359.09591 | 2.46 | 124.01598 (9), 151.00311 (16), 168.00560 (7), 169.01338 (26), 197.04462 (15), 359.09616 (100) | ✚ | ✚ |
| 11 | Dimethyl ellagic acid | 6.00 | C16H9O8– | 329.03029 | 329.02909 | 1.20 | 271.02356 (16), 299.01831 (24), 314.04178 (100), 329.02805 (33) | ✚ | ✚ |
| 12 | Urolithin M5 | 6.00 | C13H7O7– | 275.01973 | 275.01818 | 1.54 | 229.01335 (5), 231.02907 (4), 257.00781 (14), 275.01831 (100) | – | ✚ |
| 13 | Tri-galloyl-hexose | 6.03 | C27H23O18– | 635.08899 | 635.08556 | 3.43 | 169.01344 (100), 211.02388 (12), 271.04465 (10), 295.04465 (11), 313.05508 (88), 465.06509 (72) | ✚ | ✚ |
| 14 | Ethyl gallate | 6.10 | C9H9O5– | 197.04555 | 197.04444 | 1.11 | 124.01591 (30), 125.02377 (13), 168.00548 (11), 169.01334 (39), 197.04443 (100) | ✚ | ✚ |
| 15 | Ellagic acid pentoside | 6.11 | C19H13O12– | 433.04125 | 433.03858 | 2.67 | 299.98962 (61), 300.99738 (100), 433.03857 (23) | – | ✚ |
| 16 | Galloyl-coumaroyl hexose | 6.20 | C22H21O12– | 477.10385 | 477.10113 | 2.72 | 151.00302 (20), 163.03934 (50), 169.01352 (100), 301.03394 (44), 313.05511 (85), 477.10129 (26) | ✚ | – |
| 17 | Ellagic acid | 6.30 | C14H5O8– | 300.99899 | 300.99732 | 1.67 | 300.99753 (100) | ✚ | ✚ |
| 18 | Digalloy-feruloyl hexose I | 6.42 | C30H27O17– | 659.12537 | 659.12204 | 3.33 | 169.01331 (100), 211.02367 (30), 271.04453 (66), 313.0549 (22), 423.05460 (22), 483.07538 (51) | ✚ | ✚ |
| 19 | Ellagic acid galloyl pentose | 6.61 | C26H17O16– | 585.05221 | 585.04934 | 2.87 | 299.98926 (15), 300.99741 (100), 433.03864 (54) | – | ✚ |
| 20 | Digalloy-feruloyl hexose II | 6.91 | C30H27O17– | 659.12537 | 659.12192 | 3.46 | 169.01364 (4), 271.04468 (100), 331.06561 (8) | ✚ | ✚ |
| 21 | 4-O-Galloyl-chlorogenate | 7.47 | C23H21O13– | 505.09877 | 505.09584 | 2.92 | 135.04457 (16), 161.02388 (4), 173.04469 (43), 179.03410 (100), 191.05516 (100), 353.08551 (14) | ✚ | – |
| 22 | Galloyl-cinnamoyl hexose | 7.53 | C22H21O11– | 461.10894 | 461.10621 | 2.72 | 125.02386 (19), 147.04451 (27), 151.00304 (53), 161.06003 (22), 169.01344 (100), 211.02380 (20) | ✚ | ✚ |
| 23 | Urolithin C | 8.04 | C13H7O5– | 243.02990 | 243.02869 | 1.21 | 171.04427 (17), 199.03981 (16), 215.03362 (39), 243.02887 (100) | ✚ | ✚ |
| 24 | Galloyl deoxypentose | 8.43 | C12H13O8– | 285.06159 | 285.06014 | 1.46 | 124.01600 (6), 125.02383 (21), 168.00587 (3), 169.01340 (100), 285.06027 (15) | ✚ | ✚ |
| 25 | 5-O-Galloyl-chlorogenate | 8.88 | C23H21O13– | 505.09877 | 505.09598 | 2.79 | 135.04448 (11), 161.02402 (4), 173.04463 (6), 179.03413 (76), 191.05522 (100), 353.08551 (11) | ✚ | ✚ |
| 26 | Ethyl digallate | 8.89 | C16H13O9– | 349.05651 | 349.05419 | 2.32 | 169.01363 (2), 197.04449 (100) | ✚ | ✚ |
| 27 | p-Hydroxybenzyl-malonic acid | 9.01 | C10H9O5– | 209.04555 | 209.04442 | 1.13 | 92.02625 (15), 93.03411 (4), 136.01601 (6), 137.02373 (19), 165.05481 (100) | ✚ | ✚ |
| 28 | Methyl ellagic acid | 9.79 | C15H7O8– | 315.01464 | 315.01319 | 1.45 | 227.03426 (3), 241.05029 (4), 256.07297 (4), 299.98959 (100), 300.09918 (5), 315.01309 (29) | ✚ | ✚ |
| Hydroxycinnamic acid derivatives | |||||||||
| 29 | 1-O-Caffeoylquinic acid | 1.06 | C16H17O9– | 353.08781 | 353.08533 | 2.47 | 135.04459 (27), 179.03415 (73), 191.05525 (100) | ✚ | – |
| 30 | Caffeoyl hexose I | 4.95 | C15H17O9– | 341.08781 | 341.08561 | 2.19 | 135.04448 (24), 161.02371 (36), 177.05484 (21), 179.03410 (100), 221.04437 (45), 281.0654 (17) | ✚ | – |
| 31 | Esculetin | 5.06 | C9H5O4– | 177.01933 | 177.01843 | 0.91 | 115.05496 (4), 133.02888 (4), 159.04437 (35), 175.0392 (14), 177.01924 (100) | ✚ | ✚ |
| 32 | Caffeoylshikimic acid I | 5.68 | C16H15O8– | 335.07724 | 335.07569 | 1.55 | 135.04459 (45), 137.02382 (13), 155.03444 (9), 161.02371 (87), 173.04427 (4), 179.03410 (100) | ✚ | ✚ |
| 33 | 5-O-Caffeoylquinic acid | 5.70 | C16H17O9– | 353.08781 | 353.08540 | 2.40 | 135.04459 (27), 179.03415 (73), 191.05525 (100) | ✚ | – |
| 34 | 4-O-Feruloylquinic acid | 5.92 | C17H19O9– | 367.10346 | 367.10108 | 2.37 | 134.03656 (9), 137.02383 (5), 149.05981 (4), 155.03456 (6), 173.04471 (100), 193.04970 (26) | ✚ | – |
| 35 | p-Coumaric acid | 6.05 | C9H7O3– | 163.04007 | 163.03914 | 0.93 | 119.04967 (100), 163.03929 (14) | ✚ | ✚ |
| 36 | Caffeoyl hexose II | 7.10 | C15H17O9– | 341.08781 | 341.08548 | 2.32 | 135.04456 (22), 161.02373 (29), 179.03413 (100), 221.04456 (41), 251.05498 (10), 281.06537 (25) | ✚ | – |
| 37 | Ferulic acid | 7.16 | C10H9O4– | 193.05063 | 193.04962 | 1.01 | 93.03409 (11), 121.02881 (8), 121.06521 (4), 149.06004 (100), 175.03938 (12), 193.04961 (16) | – | ✚ |
| 38 | Hydroxy-methyl coumarin | 7.57 | C10H7O3– | 175.04007 | 175.03900 | 1.07 | 131.04953 (6), 175.03928 (100) | ✚ | ✚ |
| 39 | 4-O-Caffeoylquinic acid | 8.41 | C16H17O9– | 353.08781 | 353.08540 | 2.40 | 135.04459 (32), 173.04474 (100), 179.03416 (75), 191.05524 (53) | ✚ | – |
| 40 | Caffeoylshikimic acid II | 8.86 | C16H15O8– | 335.07724 | 335.07554 | 1.70 | 135.04456 (51), 137.02386 (8), 155.03407 (3), 161.02371 (48), 173.04446 (4), 179.03410 (100) | ✚ | ✚ |
| 41 | 5-O-Feruloylquinic acid | 8.88 | C17H19O9– | 367.10346 | 367.10104 | 2.41 | 134.03677 (27), 149.06021 (9), 155.03441 (4), 173.04466 (6), 193.04971 (100) | ✚ | – |
| 42 | Trimethoxycoumarin | 9.52 | C12H11O5– | 235.06120 | 235.05997 | 1.23 | 119.04974 (9), 163.03987 (9), 177.05469 (100), 191.03426 (10) | ✚ | ✚ |
| Flavonoid glycosides | |||||||||
| 43 | Myricetin 3-O-[2″-(ethyl-galloyl)]-rhamoside | 5.96 | C30H27O16– | 643.13046 | 643.12834 | 2.12 | 151.00316 (4), 169.01369 (11), 178.99771 (5), 197.04471 (10), 316.02106 (100), 317.02820 (24) | ✚ | – |
| 44 | Myricetin 3-O-hexoside-7-O-rhamnoside | 6.02 | C27H29O17– | 625.14102 | 625.13783 | 3.19 | 151.00325 (2), 178.99808 (5), 316.02103 (100), 317.02866 (59), 463.08588 (60) | – | ✚ |
| 45 | Myricetin 3-O-rhamnoside | 6.25 | C21H19O12– | 463.08820 | 463.08524 | 2.96 | 178.99792 (3), 316.02090 (100), 317.02863 (19) | ✚ | ✚ |
| 46 | Myricetin 3-O-pentoside | 6.29 | C20H17O12– | 449.07255 | 449.07236 | 0.19 | 135.02936 (10), 151.00291 (4), 199.03865 (6), 287.05582 (9), 316.02100 (100), 317.02841 (16) | ✚ | ✚ |
| 47 | Myricetin 3-O-rhamnoside-7-O-hexoside | 6.49 | C27H29O17– | 625.14102 | 625.13761 | 3.41 | 316.02066 (100), 317.02832 (97), 463.08521 (74), 464.09094 (2), 478.07269 (99), 479.08002 (50) | ✚ | ✚ |
| 48 | Taxifolin 3-O-pentoside | 6.49 | C20H19O11– | 435.09329 | 435.09070 | 2.58 | 125.0238 (23), 151.00302 (100), 178.99736 (15), 273.03864 (7), 285.03906 (64), 303.04251 (9) | ✚ | ✚ |
| 49 | Quercetin 3-O-pentoside | 6.52 | C20H17O11– | 433.07764 | 433.07470 | 2.94 | 300.02618 (100), 301.03384 (45) | ✚ | ✚ |
| 50 | Quercetin 3-O-rhamnoside | 6.63 | C21H19O11– | 447.09329 | 447.09046 | 2.82 | 151.00291 (4), 178.99786 (4), 255.02876 (3), 271.02267 (3), 300.02609 (100), 301.03384 (67) | ✚ | ✚ |
| 51 | Quercetin 3-O-hexoside-7-O-rhamnoside | 6.72 | C27H29O16– | 609.14611 | 609.14288 | 3.23 | 300.02609 (17), 301.03397 (100), 447.09103 (19), 462.07803 (89), 463.08493 (21) | ✚ | ✚ |
| 52 | Kaempferol 3-O-pentoside (Juglanin) | 6.83 | C20H17O10– | 417.08220 | 417.08063 | 1.57 | 101.02406 (6), 113.02426 (5), 255.02856 (10), 284.03116 (100), 285.03851 (28) | ✚ | ✚ |
| 53 | Myricetin 3-O-(2″-vanniloyl)-rhamnoside | 6.89 | C29H25O15– | 613.11989 | 613.11686 | 3.03 | 178.99765 (3), 316.02069 (100), 317.02817 (18), 433.12637 (12) | ✚ | ✚ |
| 54 | Myricetin 3-O-(2″-galloyl)-rhamnoside | 6.90 | C28H23O16– | 615.09916 | 615.09588 | 3.28 | 151.00298 (4), 178.99774 (10), 316.02118 (6), 317.02859 (100) | ✚ | ✚ |
| 55 | Myricetin 3-O-hexoside | 6.97 | C21H19O13– | 479.08311 | 479.08014 | 2.97 | 151.00307 (2), 316.02063 (100), 317.02805 (14) | ✚ | ✚ |
| 56 | Quercetin 3-O-(2″-vanniloyl)-rhamnoside | 7.15 | C29H25O14– | 597.12498 | 597.12146 | 3.52 | 151.00340 (5), 177.05467 (28), 178.99800 (7), 271.02271 (6), 300.02594 (100), 301.03381 (56) | ✚ | ✚ |
| 57 | Myricetin 3-O-(2″-p-hydroxybenzoyl)-rhamnoside | 7.36 | C28H23O14– | 583.10933 | 583.10683 | 2.50 | 195.06500 (12), 285.03873 (20), 316.02057 (100), 317.02817 (25) | ✚ | ✚ |
| 58 | Quercetin 3-O-(2″-sinapoyl)-rhamnoside | 7.42 | C32H29O15– | 653.15119 | 653.14845 | 2.74 | 151.00290 (7), 178.99768 (8), 223.06029 (7), 271.02347 (8), 300.02606 (100), 301.03381 (79) | ✚ | – |
| 59 | Taxifolin 3-O-(6″-galloyl)-hexoside | 7.50 | C28H25O16– | 617.11481 | 617.11282 | 1.99 | 125.02384 (6), 169.01346 (55), 273.03922 (31), 285.03906 (64), 303.049530 (100), 455.05960 (86) | ✚ | – |
| 60 | Isorhamnetin 3-O-rhamnoside | 7.52 | C22H21O11– | 461.10894 | 461.10644 | 2.49 | 145.02905 (45), 314.04196 (100), 315.05108 (41) | ✚ | ✚ |
| 61 | Quercetin 3-O-methyl-hexuronide | 7.53 | C22H19O13– | 491.08311 | 491.08084 | 2.27 | 175.03891 (8), 271.05014 (28), 300.02609 (100), 301.03381 (15), 447.09042 (24) | ✚ | ✚ |
| 62 | Myricetin 3-O-[2″-(methyl-galloyl)]-rhamoside | 7.65 | C29H25O16– | 629.11481 | 629.11208 | 2.73 | 169.01384 (13), 178.99789 (12), 183.02931 (7), 316.02115 (73), 317.02881 (100), 331.04623 (7) | – | ✚ |
| 63 | Myricetin 3-O-(2″-p-coumaroyl)-hexoside | 7.68 | C30H25O15– | 625.11989 | 625.11680 | 3.09 | 151.00291 (4), 178.99771 (11), 179.03271 (3), 316.02100 (46), 317.02866 (100), 463.08618 (7) | ✚ | ✚ |
| 64 | Quercetin 3-O-(2″-p-coumaroyl)-rhamnoside | 7.84 | C30H25O13– | 593.13007 | 593.12664 | 3.43 | 151.00284 (4), 178.99783 (4), 300.02600 (100), 301.03366 (56), 429.08151 (2), 447.09085 (5) | ✚ | ✚ |
| 65 | Kaempferol 3-O-(2″-p-coumaroyl)-rhamnoside | 8.06 | C30H25O12– | 577.13515 | 577.13245 | 2.70 | 119.04954 (4), 145.02881 (9), 163.03926 (7), 284.03134 (61), 285.03903 (100) | ✚ | ✚ |
| 66 | Myricetin 3-O-(2″-feruloyl)-rhamnoside | 8.45 | C31H27O15– | 639.13554 | 639.13233 | 3.22 | 178.99808 (3), 271.02359 (5), 316.02097 (100), 317.02841 (18) | ✚ | ✚ |
| 67 | Kaempferol 3-O-rhamnoside | 8.75 | C21H19O10– | 431.09837 | 431.09570 | 2.67 | 227.03384 (3), 255.02866 (9), 284.03122 (100), 285.03900 (95), 431.09540 (9) | ✚ | ✚ |
| 68 | Myricetin 3-O-(2″-p-coumaroyl)-rhamnoside | 8.76 | C30H25O14– | 609.12498 | 609.12193 | 3.05 | 178.99762 (5), 271.02374 (4), 287.01871 (4), 316.02087 (100), 317.02826 (23) | ✚ | ✚ |
| 69 | Quercetin 3-O-(2″-feruloyl)-rhamnoside | 8.78 | C31H27O14– | 623.14063 | 623.13806 | 2.57 | 151.00307 (8), 178.99770 (8), 271.0231 (8), 300.02597 (100), 301.03372 (91), 447.09412 (3) | ✚ | ✚ |
| 70 | Quercetin 3-O-hexuronide | 9.33 | C21H17O13– | 477.06746 | 477.06586 | 1.61 | 151.00308 (12), 178.99791 (9), 271.04382 (5), 301.03391 (100) | ✚ | – |
| 71 | Quercetin 3-O-(2″-galloyl)-rhamnoside | 9.95 | C28H23O15– | 599.10424 | 599.10137 | 2.87 | 151.00294 (8), 169.01347 (6), 178.99767 (8), 300.02606 (4), 301.03375 (100) | ✚ | ✚ |
| Flavonoid aglycones | |||||||||
| 72 | Santin | 6.05 | C18H15O7– | 343.08233 | 343.08017 | 2.16 | 285.03925 (16), 299.05457 (5), 313.03403 (100), 328.05753 (52), 343.08069 (15) | ✚ | – |
| 73 | Myricetin | 7.26 | C15H9O8– | 317.03029 | 317.02885 | 1.44 | 151.00304 (75), 178.99777 (93), 227.03392 (37), 245.04449 (39), 255.02879 (43), 317.02881 (100) | ✚ | ✚ |
| 74 | Dihydrokaempferol | 7.30 | C15H11O6– | 287.05611 | 287.05477 | 1.34 | 107.01334 (9), 125.02382 (5), 135.04445 (77), 151.00298 (100), 171.04417 (5), 199.03915 (9) | ✚ | ✚ |
| 75 | Quercetin | 7.37 | C15H9O7– | 301.03538 | 301.03392 | 1.46 | 121.02889 (16), 151.00302 (100), 178.99782 (55), 273.03931 (10), 301.03397 (83) | ✚ | ✚ |
| 76 | Quercetin 3-methyl ether | 7.51 | C16H11O7– | 315.05103 | 315.04923 | 1.80 | 151.00238 (3), 242.02019 (4), 271.02213 (5), 300.02603 (100), 315.04932 (52) | ✚ | ✚ |
| 77 | Naringin | 7.73 | C15H11O5– | 271.06120 | 271.05990 | 1.30 | 107.01345 (11), 119.04965 (37), 151.00294 (100), 177.01817 (13), 227.03337 (5), 271.02338 (26) | ✚ | ✚ |
| 78 | Luteolin | 7.81 | C15H9O6– | 285.04046 | 285.03903 | 1.43 | 169.01361 (8), 241.07059 (4), 285.03879 (100) | ✚ | ✚ |
| 79 | Isorhamnetin | 8.19 | C16H11O7– | 315.05103 | 315.04962 | 1.40 | 271.02371 (8), 300.02606 (100), 315.04953 (8) | ✚ | ✚ |
| 80 | Apigenin | 8.19 | C15H9O5– | 269.04555 | 269.04432 | 1.23 | 117.03402 (2), 149.02419 (2), 151.00276 (4), 225.0518 (2), 269.04428 (100) | ✚ | – |
| 81 | Luteolin 3′,4′-dimethyl ether | 8.67 | C17H13O6– | 313.07176 | 313.07034 | 1.42 | 269.04468 (33), 283.02335 (33), 297.03931 (25), 298.04672 (100), 313.07043 (57) | ✚ | – |
| 82 | Taxifolin | 8.67 | C15H11O7– | 303.05103 | 303.04973 | 1.30 | 185.02388 (10), 213.01805 (14), 231.06580 (16), 257.04465 (37), 285.03912 (100) | ✚ | – |
| 83 | Genistein | 8.92 | C15H9O5– | 269.04555 | 269.04422 | 1.33 | 137.02353 (2), 241.04845 (2), 269.04401 (100) | ✚ | ✚ |
| 84 | Isokaempferide | 9.02 | C16H11O6– | 299.05611 | 299.05468 | 1.43 | 255.02927 (9), 284.03119 (100), 299.05472 (37) | ✚ | – |
| 85 | Kaempferol | 9.20 | C15H9O6– | 285.04046 | 285.03914 | 1.32 | 169.01358 (8), 257.04599 (4), 285.03864 (100) | ✚ | ✚ |
| Quinones | |||||||||
| 86 | Juglanoside C gallate | 5.48 | C23H23O12– | 491.11950 | 491.11665 | 2.85 | 169.01335 (27), 211.02362 (25), 241.05040 (32), 271.04449 (100), 313.05499 (11), 473.10571 (10) | ✚ | ✚ |
| 87 | Isosclerone | 5.63 | C10H9O3– | 177.05572 | 177.05482 | 0.90 | 159.04437 (35), 177.05481 (100) | ✚ | ✚ |
| 88 | Juglanoside D | 5.80 | C16H19O9– | 355.10346 | 355.10111 | 2.35 | 175.03918 (100), 193.04956 (6), 235.05971 (3) | ✚ | ✚ |
| 89 | α-Hydrojuglone 4-hexoside | 5.96 | C16H17O8– | 337.09289 | 337.09117 | 1.73 | 175.03923 (100) | ✚ | ✚ |
| 90 | α-Hydrojuglone 4-hexoside gallate | 6.48 | C23H21O12– | 489.10385 | 489.10106 | 2.79 | 169.01343 (86), 174.03143 (76), 175.03922 (100), 211.02342 (28), 271.04471 (77), 313.05515 (75) | ✚ | ✚ |
| 91 | Jugnaphthalenoside A | 6.88 | C23H19O12– | 487.08820 | 487.08551 | 2.69 | 324.02600 (100), 325.03366 (51) | ✚ | ✚ |
| 92 | 1,4-Naphthalenedion hexoside | 7.17 | C16H15O8– | 335.07724 | 335.07559 | 1.65 | 135.04453 (22), 161.02367 (100), 335.07623 (11) | ✚ | – |
| 93 | 2-Methoxyjuglone | 7.51 | C11H7O4– | 203.03498 | 203.03395 | 1.04 | 174.03149 (31), 175.03922 (35), 203.03404 (100) | ✚ | ✚ |
| 94 | 2-Hydroxyjuglone | 7.51 | C10H5O4– | 189.01933 | 189.01839 | 0.94 | 161.02361 (14), 189.01833 (100) | ✚ | ✚ |
| 95 | 4,8-Dihydroxy-2-naphthalenecarboxylic acid hexoside | 9.16 | C17H17O10– | 381.08272 | 381.08012 | 2.60 | 174.03123 (9), 175.03949 (2), 218.02090 (100), 219.02734 (3) | ✚ | ✚ |
| Fatty acids | |||||||||
| 96 | 2-Hydroxy-9,12,15-octadecatrienoic acid | 9.88 | C18H29O3– | 293.21220 | 293.21027 | 1.93 | 121.10181 (32), 171.10199 (36), 183.13818 (90), 211.13284 (14), 235.16917 (45), 275.20035 (100) | ✚ | ✚ |
| 97 | Linoleic acid | 10.81 | C18H31O2– | 279.23295 | 279.23070 | 2.25 | 279.23141 (100) | ✚ | ✚ |
| 98 | cis-Octadecenoic acid | 11.36 | C18H33O2– | 281.24860 | 281.24706 | 1.54 | 281.24719 (100) | ✚ | – |
| 99 | Palmitic acid | 11.95 | C16H31O2– | 255.23295 | 255.23158 | 1.37 | 255.23174 (100) | ✚ | ✚ |
| Other metabolites | |||||||||
| 100 | Malic acid | 0.53 | C4H5O5– | 133.01420 | 133.01358 | 0.62 | 71.01352 (35), 72.99274 (9), 89.02402 (7), 115.00318 (100), 133.01364 (48) | ✚ | ✚ |
| 101 | Citric acid | 0.80 | C6H7O7– | 191.01973 | 191.01879 | 0.94 | 57.03428 (7), 85.02903 (35), 87.00828 (50), 111.00817 (100), 129.01872 (7), 191.01768 (7) | ✚ | ✚ |
| 102 | Dihydrophaseic acid | 5.80 | C15H21O5– | 281.13945 | 281.13789 | 1.55 | 123.08092 (74), 171.11708 (100), 189.12749 (36), 201.12729 (39), 207.13826 (25), 237.14822 (75) | ✚ | ✚ |
| 103 | Bergaptol | 8.54 | C11H5O4– | 201.01933 | 201.01831 | 1.02 | 173.02385 (4), 201.01852 (100) | ✚ | – |
| Characterization Parameters | Leaves | Green Husks |
|---|---|---|
| Total extracted substances (mg cm−3) | 56.3 ± 0.4 * | 33.5 ± 0.1 * |
| Total polyphenolic content (mg GA cm−3) | 10.66 ± 0.23 * | 6.24 ± 0.03 * |
| Total flavonoid content (mg R cm−3) | 7.95 ± 0.65 * | 2.70 ± 0.45 * |
| IC50 (µg cm−3) | 0.07 ± 0.01 | 0.11 ± 0.01 |
- Compared with control groups, statistically significant difference p < 0.01.
- Compared with amifostine—WR 2721, statistically significant difference p < 0.01.
- Compared with mitomycine—C, statistically significant difference p < 0.01.
3. Materials and Methods
3.1. Extract Preparation
3.2. The Electrochemical Measurements
3.3. Subjects
3.4. Characterization of Extracts
3.4.1. Total Extracted Substances
3.4.2. Total Polyphenolic Content Determination
3.4.3. Total Flavonoid Determination
3.4.4. DPPH Radical Scavenging Capacity
3.4.5. LC/MS Method for Metabolite Identification
3.5. In Vitro Cytokinesis-Block Micronucleus (MN) Assay
3.6. Statistics and Index Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheeseman, K.H.; Slater, T.F. An Introduction to Free Radical Biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Fenech, M. The Cytokinesis-Block Micronucleus Technique: A Detailed Description of the Method and Its Application to Genotoxicity Studies in Human Populations. Mutat. Res. Mol. Mech. Mutagen. 1993, 285, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. How to Characterize an Antioxidant: An Update. Biochem. Soc. Symp. 1995, 61, 73–101. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 Medicinal Plant Extracts for Antioxidant Capacity and Total Phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Lee, S.E.; Hwang, H.J.; Ha, J.-S.; Jeong, H.-S.; Kim, J.H. Screening of Medicinal Plant Extracts for Antioxidant Activity. Life Sci. 2003, 73, 167–179. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Karki, R. Phytochemical Profile and Biological Activity of Juglans Regia. J. Integr. Med. 2016, 14, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Vanden Heuvel, J.P.; Belda, B.J.; Hannon, D.B.; Kris-Etherton, P.M.; Grieger, J.A.; Zhang, J.; Thompson, J.T. Mechanistic Examination of Walnuts in Prevention of Breast Cancer. Nutr. Cancer 2012, 64, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Cheraghali, F.; Shojaee-Aliabadi, S.; Hosseini, S.M.; Mirmoghtadaie, L.; Mortazavian, A.M.; Ghanati, K.; Abedi, A.-S.; Moslemi, M. Characterization of Microcapsule Containing Walnut (Juglans Regia L.) Green Husk Extract as Preventive Antioxidant and Antimicrobial Agent. Int. J. Prev. Med. 2018, 9, 101. [Google Scholar] [CrossRef]
- Wenzel, J.; Storer Samaniego, C.; Wang, L.; Burrows, L.; Tucker, E.; Dwarshuis, N.; Ammerman, M.; Zand, A. Antioxidant Potential of Juglans Nigra, Black Walnut, Husks Extracted Using Supercritical Carbon Dioxide with an Ethanol Modifier. Food Sci. Nutr. 2017, 5, 223–232. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S310–S329. [Google Scholar] [CrossRef]
- Dawid-Pać, R. Medicinal Plants Used in Treatment of Inflammatory Skin Diseases. Postepy Dermatol. Alergol. 2013, 30, 170–177. [Google Scholar] [CrossRef]
- Ho, K.-V.; Schreiber, K.L.; Vu, D.C.; Rottinghaus, S.M.; Jackson, D.E.; Brown, C.R.; Lei, Z.; Sumner, L.W.; Coggeshall, M.V.; Lin, C.-H. Black Walnut (Juglans Nigra) Extracts Inhibit Proinflammatory Cytokine Production From Lipopolysaccharide-Stimulated Human Promonocytic Cell Line U-937. Front. Pharmacol. 2019, 10, 1059. [Google Scholar] [CrossRef]
- Fernández, P.; Vázquez, C.; Morales, L.; Bermejo, A.M. Analysis of Opiates, Cocaine and Metabolites in Urine by High-Performance Liquid Chromatography with Diode Array Detection (HPLC-DAD). J. Appl. Toxicol. 2005, 25, 200–204. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total Phenols, Antioxidant Potential and Antimicrobial Activity of Walnut (Juglans Regia L.) Green Husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef]
- Wang, J.; Tang, H.; Zhang, C.; Zhao, Y.; Derrien, M.; Rocher, E.; van-Hylckama Vlieg, J.E.T.; Strissel, K.; Zhao, L.; Obin, M.; et al. Modulation of Gut Microbiota during Probiotic-Mediated Attenuation of Metabolic Syndrome in High Fat Diet-Fed Mice. ISME J. 2015, 9, 1–15. [Google Scholar] [CrossRef]
- Aithal Juglone, a Naphthoquinone from Walnut, Exerts Cytotoxic and Genotoxic Effects against Cultured Melanoma Tumor Cells—Aithal—2009—Cell Biology International—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1016/j.cellbi.2009.06.018 (accessed on 5 December 2022).
- Rajković, K.M.; Vasić, M.; Drobac, M.; Mutić, J.; Jeremić, S.; Simić, V.; Stanković, J. Optimization of Extraction Yield and Chemical Characterization of Optimal Extract from Juglans Nigra L. Leaves. Chem. Eng. Res. Des. 2020, 157, 25–33. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Rabia, M.K.; Newair, E.F. Electrochemical Behaviour of Antioxidants: Part 2. Electrochemical Oxidation Mechanism of Quercetin at Glassy Carbon Electrode Modified with Multi-Wall Carbon Nanotubes. Arab. J. Chem. 2016, 9, 350–356. [Google Scholar] [CrossRef]
- Timbola, A.K.; de Souza, C.D.; Giacomelli, C.; Spinelli, A. Electrochemical Oxidation of Quercetin in Hydro-Alcoholic Solution. J. Braz. Chem. Soc. 2006, 17, 139–148. [Google Scholar] [CrossRef]
- Deepa, R.R.; Arulraj, A.A.D.; Vasantha, V.S.V. Evaluation of Antioxidant Property of Quinones and Calculation of Their Binding Constant Values with DNA by Electrochemical Technique. Der Pharma Chem 2018, 10, 69–78. [Google Scholar]
- Gavrilović, M.; Rajković, K.M.; Simić, V.; Jeremić, S.; Mirković, S.; Jevtić, A.S. Optimization of Ultrasound-Assisted Extraction of Total Polyphenolic Compounds from Juglans Nigra L. Leaves. J. Serbian Chem. Soc. 2018, 83, 1273–1284. [Google Scholar] [CrossRef]
- Grochová, D.; Šmardová, J. The Antimutagenic and Cytoprotective Effects of Amifostine: The Role of P53. J. Appl. Biomed. 2007, 5, 171–178. [Google Scholar] [CrossRef]
- Roncada, T.; Vicentini, V.E.P.; Mantovani, M.S. Possible Modulating Actions of Plant Extracts on the Chromosome Breaking Activity of MMC and Ara-C in Human Lymphocytes in Vitro. Toxicol. In Vitro 2004, 18, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Dragićević, M.; Stupar, A.; Uysal, A.; Şenkardes, I.; Sinan, K.I.; Picot-Allain, M.C.N.; Ak, G.; et al. UHPLC-LTQ OrbiTrap MS Analysis and Biological Properties of Origanum Vulgare Subsp. Viridulum Obtained by Different Extraction Methods. Ind. Crops Prod. 2020, 154, 112747. [Google Scholar] [CrossRef]
- Devidas, S.B.; Bhandari, P. Quantitative Determination of Specialised Metabolites in Different Parts of Juglans Regia Linn. and Carya Illinoinensis (Wangenh.) K. Koch by Using UHPLC-DAD-QTOF-MS/MS. Phytochem. Anal. 2024, 35, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Chen, M.; Zhou, F.; Cai, D.; Bai, H.; Wang, P.; Lei, H.; Ma, Q. Separation and Analysis of Flavonoid Chemical Constituents in Flowers of Juglans Regia L. by Ultra-High-Performance Liquid Chromatography-Hybrid Quadrupole Time-of-Flight Mass Spectrometry. J. Pharm. Biomed. Anal. 2019, 164, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Li, C.; Sun, K.; Li, W.; Liu, R. Quantitative Analysis of Bioactive Components in Walnut Leaves by UHPLC-Q-Orbitrap HRMS Combined with QAMS. Food Chem. 2020, 331, 127180. [Google Scholar] [CrossRef] [PubMed]
- Sheng, F.; Hu, B.; Jin, Q.; Wang, J.; Wu, C.; Luo, Z. The Analysis of Phenolic Compounds in Walnut Husk and Pellicle by UPLC-Q-Orbitrap HRMS and HPLC. Molecules 2021, 26, 3013. [Google Scholar] [CrossRef] [PubMed]
- Rapid Qualitative Profiling and Quantitative Analysis of Juglandis Mandshuricae Cortex and Seven Flavonoids by Ultra-high Performance Liquid Chromatography–Quadrupole/Orbitrap High-resolution Mass Spectrometry—Sun—2022—Journal of Separation Science—Wiley Online Library. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/jssc.202100658 (accessed on 3 May 2024).
- Ren, S.; Zhang, Q.; Jiang, M.; Chen, M.; Xu, X.; Wang, D.; Pan, Y.; Liu, X. Systematic Characterization of the Metabolites of Defatted Walnut Powder Extract in Vivo and Screening of the Mechanisms against NAFLD by UPLC-Q-Exactive Orbitrap MS Combined with Network Pharmacology. J. Ethnopharmacol. 2022, 285, 114870. [Google Scholar] [CrossRef]
- Wang, P.; Zhong, L.; Yang, H.; Zhang, J.; Hou, X.; Wu, C.; Zhang, R.; Cheng, Y. Comprehensive Comparative Analysis of Lipid Profile in Dried and Fresh Walnut Kernels by UHPLC-Q-Exactive Orbitrap/MS. Food Chem. 2022, 386, 132706. [Google Scholar] [CrossRef]
- Xu, X.; Song, Y.; Jiang, M.; Liu, M.; Zhang, X.; Wang, D.; Pan, Y.; Ren, S.; Liu, X. Screening of the Active Substances for the Assessment of Walnut Kernel in the Treatment of Scopolamine-Induced AD Animals. Mol. Nutr. Food Res. 2024, 68, 2200816. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.; Tešević, V.; Vajs, V.; Todorović, N.; Milosavljević, S.; Gođevac, D. Antioxidant Properties of Grape Seed Extract on Human Lymphocyte Oxidative Defence. Planta Med. 2008, 74, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Countryman, P.I.; Heddle, J.A. The Production of Micronuclei from Chromosome Aberrations in Irradiated Cultures of Human Lymphocytes. Mutat. Res. Mol. Mech. Mutagen. 1976, 41, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Surrallés, J.; Xamena, N.; Creus, A.; Catalán, J.; Norppa, H.; Marcos, R. Induction of Micronuclei by Five Pyrethroid Insecticides in Whole-Blood and Isolated Human Lymphocyte Cultures. Mutat. Res. Toxicol. 1995, 341, 169–184. [Google Scholar] [CrossRef]
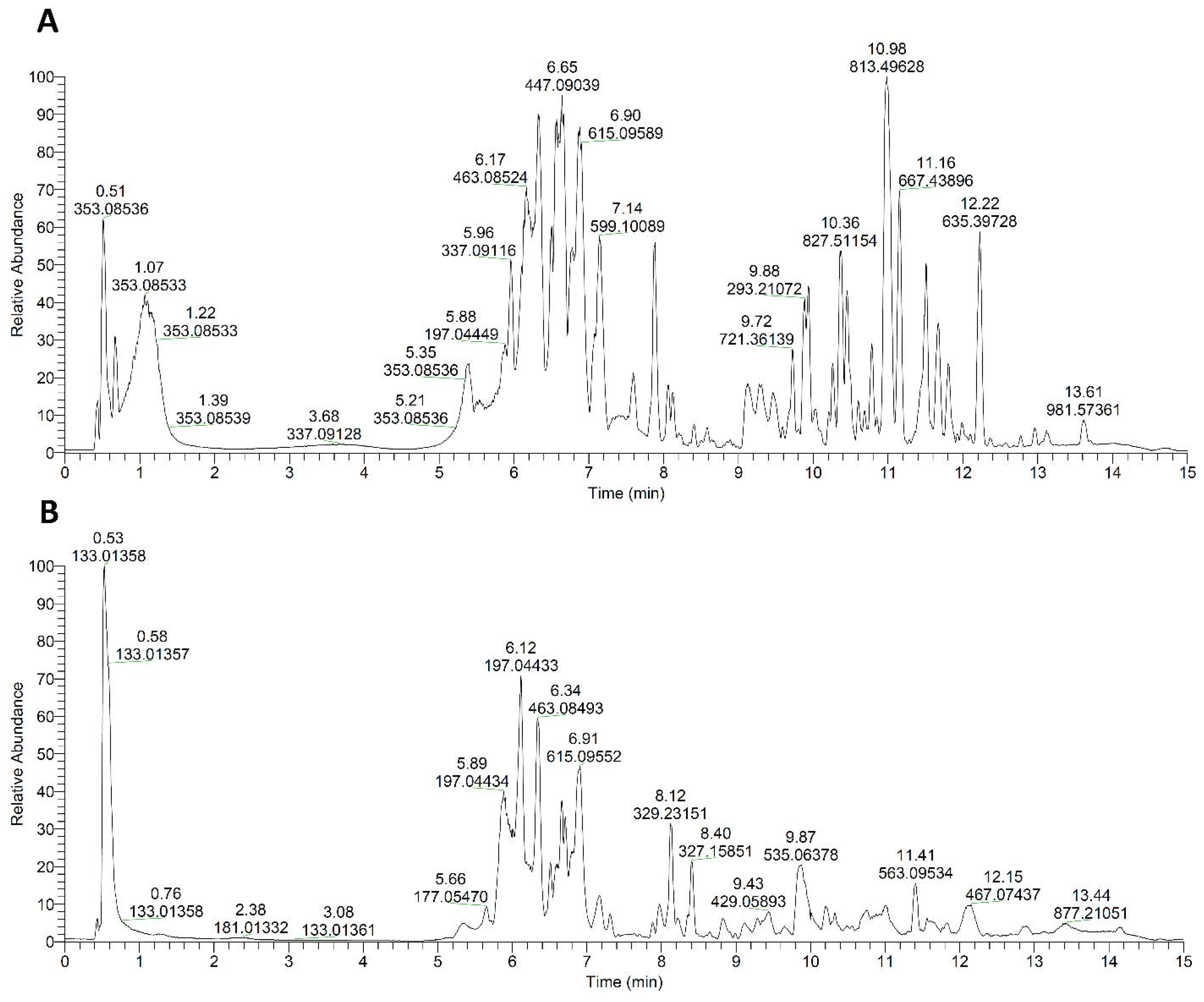
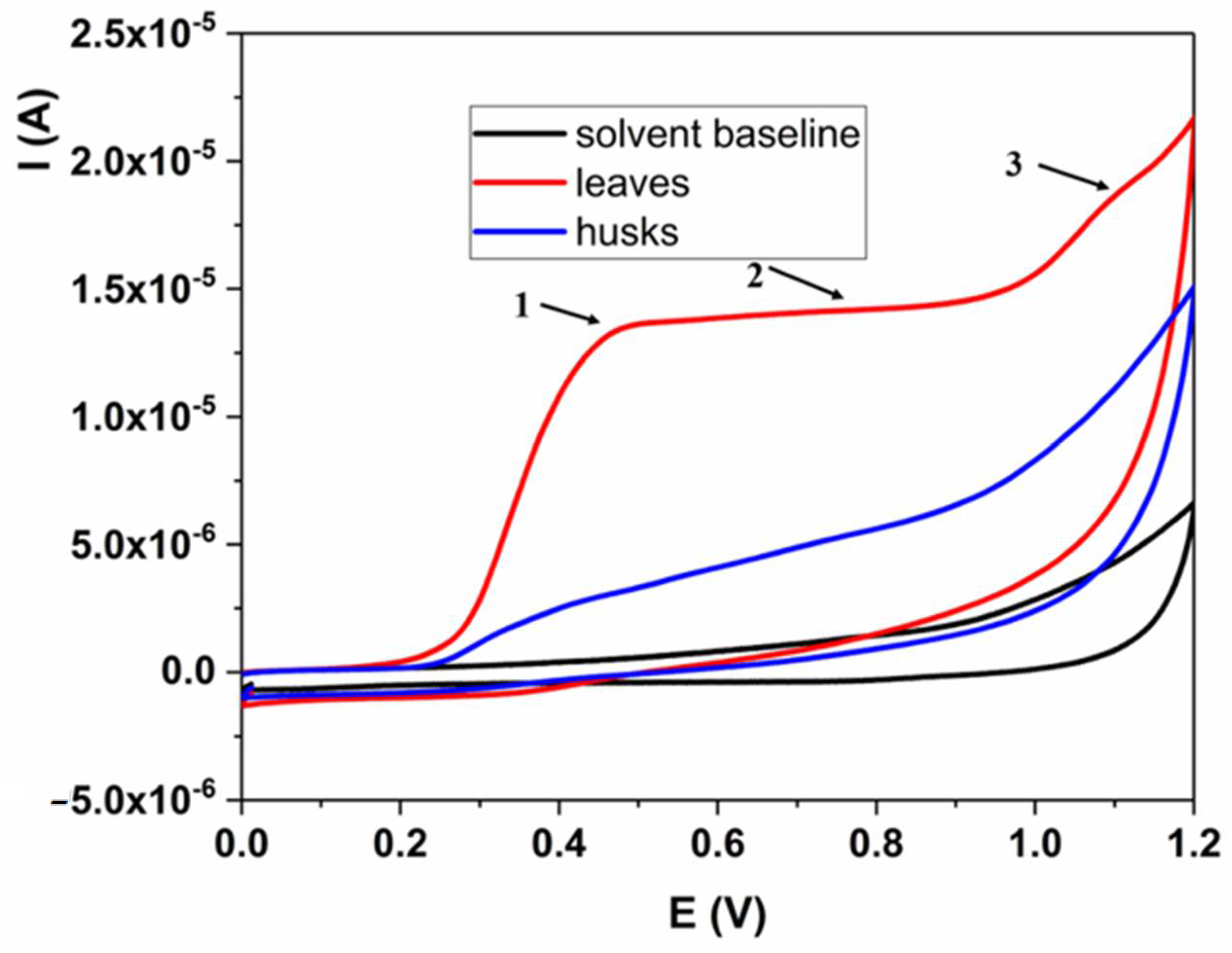
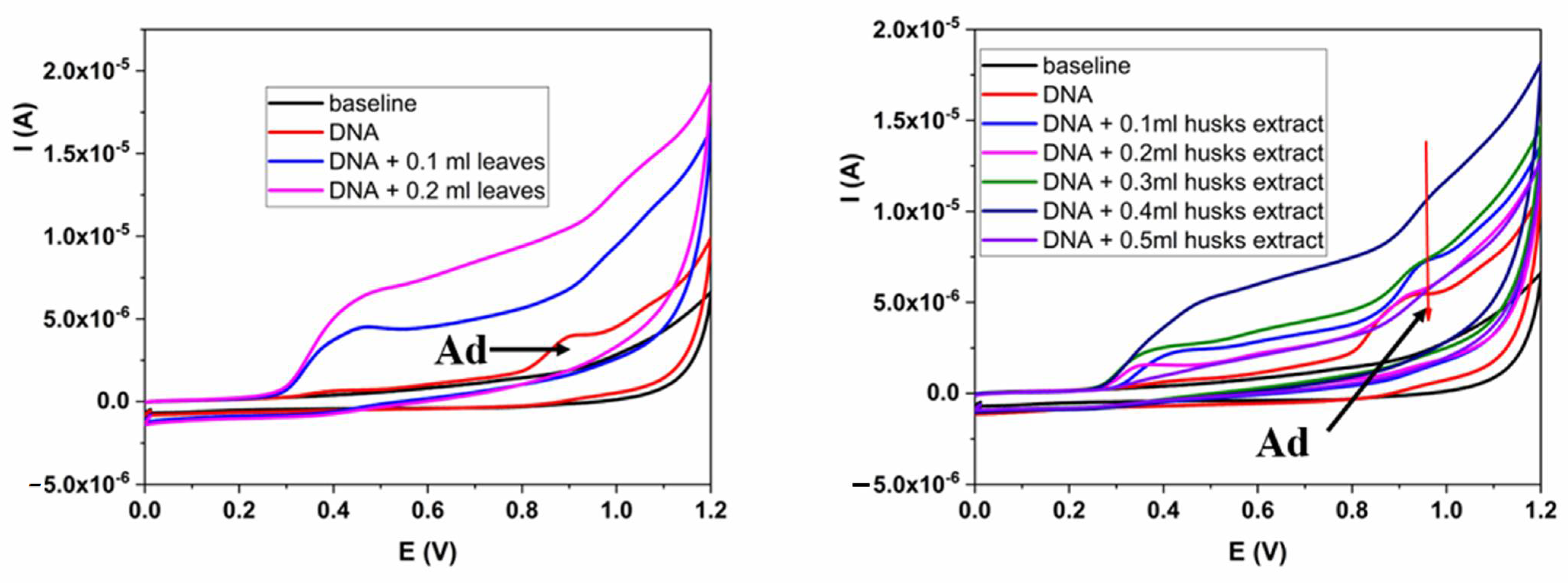
| Type of Extract | Binding Constant | Binding Site Size | Type of Interaction |
|---|---|---|---|
| Leaves | 3.56 × 104 M−1 | 0.14 | Electrostatic/intercalation |
| Green husks | 0.76 × 104 M−1 | 0.19 | Electrostatic/intercalation |
| Conc. | MN/1000 | % Bn Cell | MN/Bn | CBPI | Frequency |
|---|---|---|---|---|---|
| µg/mL | Bn cell | with MN | cell | of MN % | |
| Control | 26.85 ± 0.23 | 2.23 ± 0.08 | 1.19 ± 0.03 | 1.67 ± 0.03 | 100% |
| Amifos.—1.0 | 20.38 ± 0.58 a | 1.88 ± 0.04 | 1.21 ± 0.03 | 1.64 ± 0.02 | 75.9% (−24.1%) |
| MMC—0.2 | 34.82 ± 0.63 a,b | 3.36 ± 0.13 | 1.12 ± 0.03 | 1.71 ± 0.06 | 129.68% (+29.68%) |
| leaves—2.0 | 12.86 ± 0.43 a,b,c | 1.22 ± 0.02 | 1.10 ± 0.02 | 1.70 ± 0.08 | 47.89% (−52.11%) |
| leaves—4.0 | 11.23 ± 0.35 a,b,c | 1.27 ± 0.16 | 1.12 ± 0.07 | 1.61 ± 0.03 | 41.82% (−58.18%) |
| leaves—6.0 | 11.96 ± 0.38 a,b,c | 1.09 ± 0.01 | 1.21 ± 0.05 | 1.61 ± 0.03 | 44.54% (−55.46%) |
| green husks—2.0 | 10.99 ± 0.54 a,b,c | 1.15 ± 0.02 | 1.18± 0.03 | 1.66 ± 0.03 | 40.93% (−59.07%) |
| green husks—4.0 | 9.54 ± 0.32 a,b,c | 0.75 ± 0.07 | 1.17 ± 0.04 | 1.75 ± 0.06 | 35.53% (−64.47%) |
| green husks—6.0 | 10.39 ± 0.50 a,b,c | 0.86 ± 0.09 | 117 ± 0.05 | 1.60 ± 0.02 | 38.70% (−61.30%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajković, K.M.; Stanković, M.; Markićević, M.; Zavišić, G.; Vranješ-Đurić, S.; Janković, D.; Obradović, Z.; Stanković, D. Chemical Composition and Protective Possibilities of Juglans Nigra Leaves and Green Husks Extracts: DNA Binding and Micronucleus Assay in Human Lymphocytes. Plants 2024, 13, 1669. https://doi.org/10.3390/plants13121669
Rajković KM, Stanković M, Markićević M, Zavišić G, Vranješ-Đurić S, Janković D, Obradović Z, Stanković D. Chemical Composition and Protective Possibilities of Juglans Nigra Leaves and Green Husks Extracts: DNA Binding and Micronucleus Assay in Human Lymphocytes. Plants. 2024; 13(12):1669. https://doi.org/10.3390/plants13121669
Chicago/Turabian StyleRajković, Katarina M., Miroslava Stanković, Milan Markićević, Gordana Zavišić, Sanja Vranješ-Đurić, Drina Janković, Zorica Obradović, and Dalibor Stanković. 2024. "Chemical Composition and Protective Possibilities of Juglans Nigra Leaves and Green Husks Extracts: DNA Binding and Micronucleus Assay in Human Lymphocytes" Plants 13, no. 12: 1669. https://doi.org/10.3390/plants13121669





