Arbuscular Mycorrhizal Fungi Selectively Promoted the Growth of Three Ecological Restoration Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Experimental Method
2.4. Data Analysis
3. Results
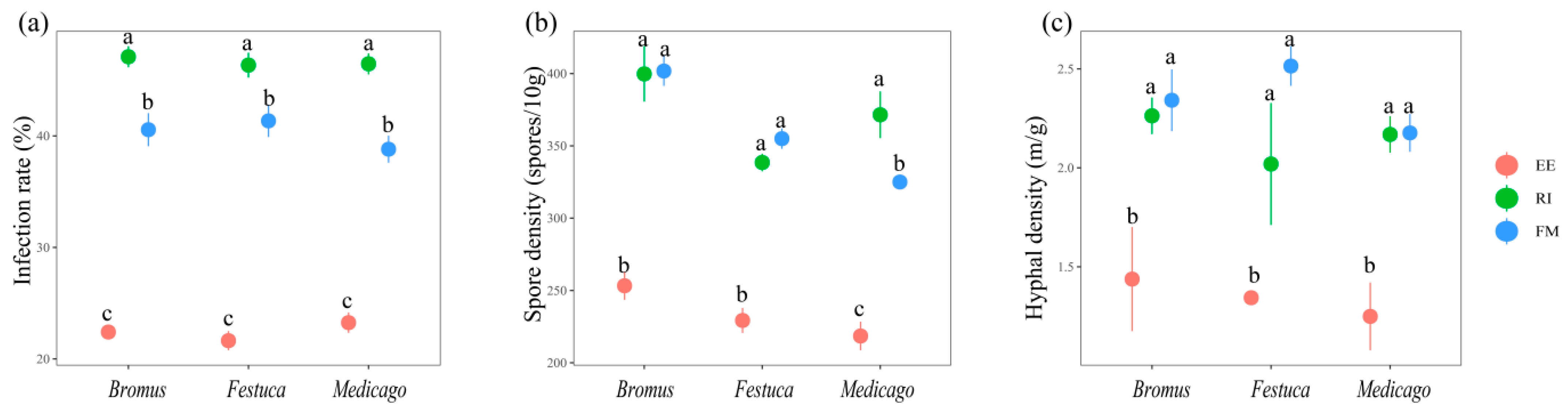
3.1. AMF Mycorrhizal Infection
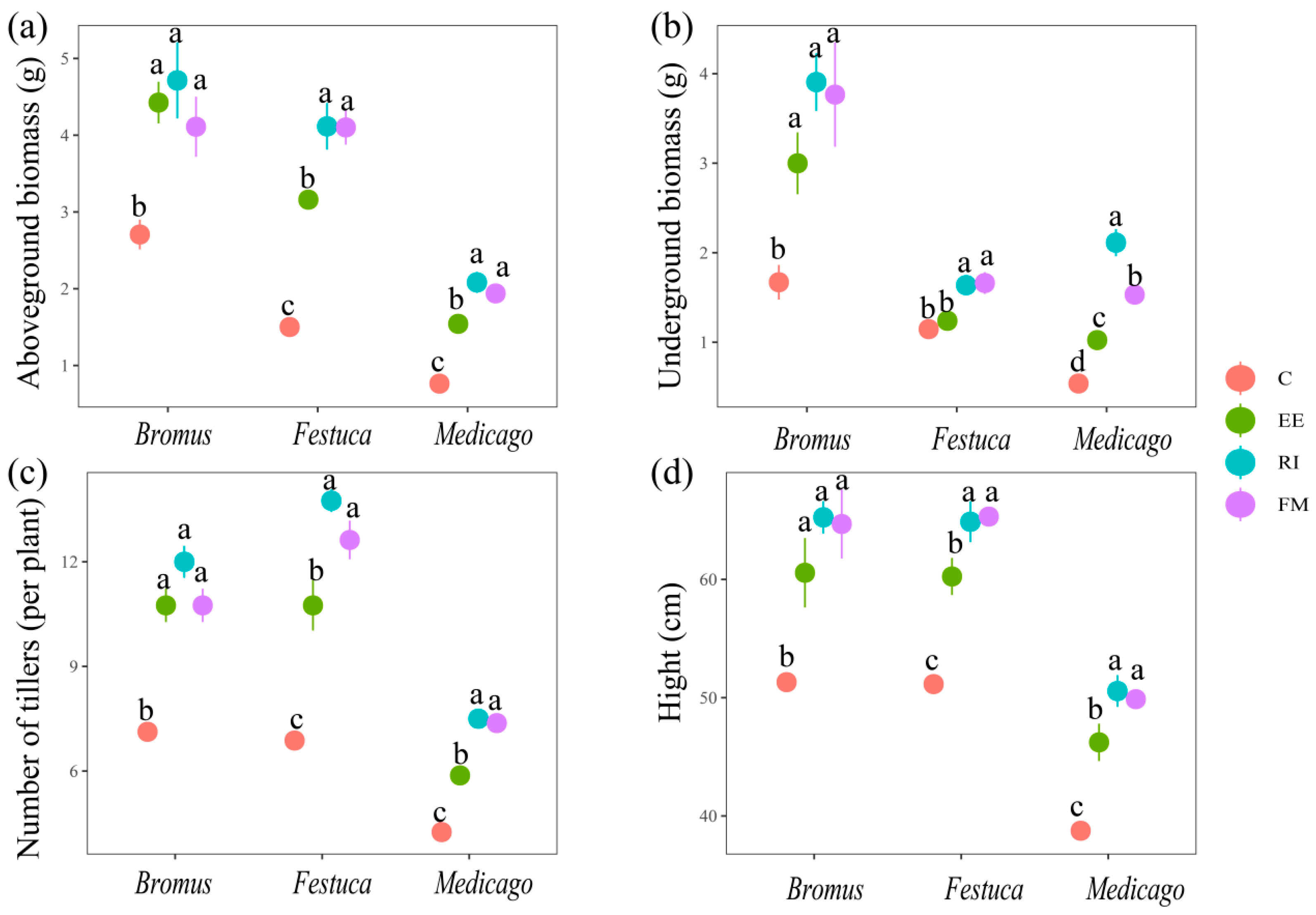
3.2. Plant Growth
3.3. N, P, and K Absorption and Stoichiometry
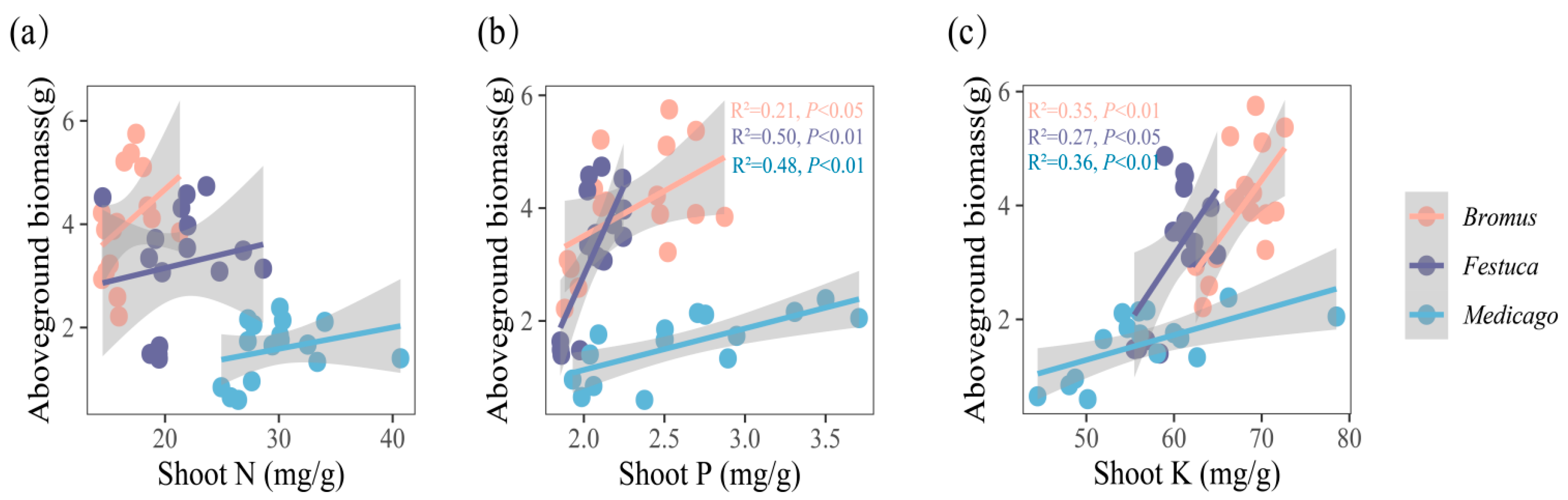
3.4. Relationship between Nutrient Uptake and Plant Growth
4. Discussion
4.1. Different AMF Have Different Symbiotic Relationships with Different Plants
4.2. Effect of AMF Inoculation on N, P, and K Uptake in Plants
4.3. Effects of AMF Inoculation on Plant Aboveground and Underground Biomass
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stevens, K.J.; Wall, C.B.; Janssen, J.A. Effects of arbuscular mycorrhizal fungi on seedling growth and development of two wetland plants, Bidens frondosa L., and Eclipta prostrata (L.) L., grown under three levels of water availability. Mycorrhiza 2011, 21, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Zubek, S.; Majewska, M.L.; Błaszkowski, J.; Stefanowicz, A.M.; Nobis, M.; Kapusta, P. Invasive plants affect arbuscular mycorrhizal fungi abundance and species richness as well as the performance of native plants grown in invaded soils. Biol. Fertil. Soils 2016, 52, 879–893. [Google Scholar] [CrossRef]
- Zhao, Y.; Cartabia, A.; Lalaymia, I.; Declerck, S. Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Bennett, A.E. Stressed out symbiotes: Hypotheses for the influence of abiotic stress on arbuscular mycorrhizal fungi. Oecologia 2016, 182, 625–641. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.-H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Nazari Deljou, M.; Marouf, A.; Jaberian Hamedan, H. Effect of inoculation with arbuscular mycorrhizal fungi (AMF) on Gerbera cut flower (Gerbera jamesonii) production in soilless cultivation. Acta Hortic. 2014, 1034, 417–422. [Google Scholar] [CrossRef]
- Asrar, A.; Abdel-Fattah, G.; Elhindi, K. Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 2012, 50, 305–316. [Google Scholar] [CrossRef]
- Majewska, M.L.; Rola, K.; Zubek, S. The growth and phosphorus acquisition of invasive plants Rudbeckia laciniata and Solidago gigantea are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 2017, 27, 83–94. [Google Scholar] [CrossRef]
- Selvaraj, T.; Chellappan, P. Arbuscular mycorrhizae: A diverse personality. J. Cent. Eur. Agric. 2006, 7, 349–358. [Google Scholar]
- Tian, H.; Drijber, R.; Zhang, J.; Li, X. Impact of long-term nitrogen fertilization and rotation with soybean on the diversity and phosphorus metabolism of indigenous arbuscular mycorrhizal fungi within the roots of maize (Zea mays L.). Agric. Ecosyst. Environ. 2013, 164, 53–61. [Google Scholar] [CrossRef]
- Selvakumar, G.; Shagol, C.C.; Kim, K.; Han, S.; Sa, T. Spore associated bacteria regulates maize root K+/Na+ ion homeostasis to promote salinity tolerance during arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2018, 18, 109. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Ohtomo, R. Mycorrhizal effects on growth, P uptake and Cd tolerance of the host plant vary among different AM fungal species. Soil Sci. Plant Nutr. 2015, 61, 359–368. [Google Scholar] [CrossRef]
- Alotaibi, M.O.; Saleh, A.M.; Sobrinho, R.L.; Sheteiwy, M.S.; El-Sawah, A.M.; Mohammed, A.E.; Elgawad, H.A. Arbuscular mycorrhizae mitigate aluminum toxicity and regulate proline metabolism in plants grown in acidic soil. J. Fungi 2021, 7, 531. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, A.K.; Suri, V.K. Agronomic bio-fortification and quality enhancement in okra–pea cropping system through arbuscular mycorrhizal fungi at varying phosphorus and irrigation regimes in Himalayan acid alfisol. J. Plant Nutr. 2017, 40, 1213–1229. [Google Scholar] [CrossRef]
- Zhen, L.; Yang, G.; Yang, H.; Chen, Y.; Liu, N.; Zhang, Y. Arbuscular mycorrhizal fungi affect seedling recruitment: A potential mechanism by which N deposition favors the dominance of grasses over forbs. Plant Soil 2014, 375, 127–136. [Google Scholar] [CrossRef]
- Rhouma, H.B.; Taski-Ajdukovic, K.; Zitouna, N.; Sdouga, D.; Milic, D.; Trifi-Farah, N. Assessment of the genetic variation in alfalfa genotypes using SRAP markers for breeding purposes. Chil. J. Agric. Res. 2017, 77, 332–339. [Google Scholar] [CrossRef]
- Tussipkan, D.; Manabayeva, S.A. Alfalfa (Medicago sativa L.): Genotypic Diversity and Transgenic Alfalfa for Phytoremediation. Front. Environ. Sci. 2022, 10, 828257. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, N.; Wei, Q.; Cao, Y.; Li, D.; Cui, G. Alfalfa modified the effects of degraded black soil cultivated land on the soil microbial community. Front. Plant Sci. 2022, 13, 938187. [Google Scholar] [CrossRef] [PubMed]
- DeKeyser, E.S.; Meehan, M.; Clambey, G.; Krabbenhoft, K. Cool season invasive grasses in northern Great Plains natural areas. Nat. Areas J. 2013, 33, 81–90. [Google Scholar] [CrossRef]
- Ellis-Felege, S.N.; Dixon, C.S.; Wilson, S.D. Impacts and management of invasive cool-season grasses in the Northern Great Plains: Challenges and opportunities for wildlife. Wildl. Soc. Bull. 2013, 37, 510–516. [Google Scholar] [CrossRef]
- Palit, R.; DeKeyser, E.S. Impacts and drivers of smooth brome (Bromus inermis Leyss.) invasion in native ecosystems. Plants 2022, 11, 1340. [Google Scholar] [CrossRef]
- Sato, H. Development and Future Application of Transgenic Tall Fescue (Festuca arundinacea Schreb.) with Improved Important Forage and Turf Traits. Jpn. Agric. Res. Q. 2022, 56, 1–6. [Google Scholar] [CrossRef]
- Malinowski, D.P.; Belesky, D.P. Tall fescue aluminum tolerance is affected by Neotyphodium coenophialum endophyte. J. Plant Nutr. 1999, 22, 1335–1349. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Koske, R.; Walker, C. Gigaspora erythropa, a new species forming arbuscular mycorrhizae. Mycologia 1984, 76, 250–255. [Google Scholar]
- Jakobsen, I.; Abbott, L.; Robson, A. External hyphae of vesicular-arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. New Phytol. 1992, 120, 371–380. [Google Scholar] [CrossRef]
- Bowman, R.A. A Rapid Method to Determine Total Phosphorus in Soils. Soil Sci. Soc. Am. J. 1988, 52, 1301–1304. [Google Scholar] [CrossRef]
- Li, X.N.; Zhang, W.W.; Zhao, C.Q.; Song, J.K.; Shi, R.S.; Xue, R.B.; Wang, C. Plant Diversity and Soil Physicochemical Properties in the wasteland of Yanqing District. Acta Agrestia Sin. 2019, 27, 695–701. [Google Scholar] [CrossRef]
- Liu, H.; Wu, M.; Chen, J.; Gao, Y.; Ren, A. Arbuscular mycorrhizal fungus identity modulates growth effects of endophyte-infected grasses on neighboring plants. Mycorrhiza 2020, 30, 663–670. [Google Scholar] [CrossRef]
- Putten, W.H.V.d. Climate Change, Aboveground-Belowground Interactions, and Species’ Range Shifts. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 365–383. [Google Scholar] [CrossRef]
- Croll, D.; Wille, L.; Gamper, H.A.; Mathimaran, N.; Lammers, P.J.; Corradi, N.; Sanders, I.R. Genetic diversity and host plant preferences revealed by simple sequence repeat and mitochondrial markers in a population of the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2008, 178, 672–687. [Google Scholar] [CrossRef]
- Ibijbijen, J.; Urquiaga, S.; Ismaili, M.; Alves, B.; Boddey, R. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition and nitrogen fixation of three varieties of common beans (Phaseolus vulgaris). New Phytol. 1996, 134, 353–360. [Google Scholar] [CrossRef]
- Richards, R. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, A.J.; Fagan, W.F.; Elser, J.J.; Enquist, B.J. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. Am. Nat. 2006, 168, E103–E122. [Google Scholar] [CrossRef] [PubMed]
- Ames, R.; Reid, C.; Porter, L.; Cambardella, C. Hyphal uptake and transport of nitrogen from two 15N-labelled sources by Glomus mosseae, a vesicular-arbuscular mycorrhizal fungus. New Phytol. 1983, 95, 381–396. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pfeffer, P.E.; Jin, H.; Abubaker, J.; Douds, D.D.; Allen, J.W.; Bücking, H.; Lammers, P.J.; Shachar-Hill, Y. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 2005, 435, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Tang, M.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 2011, 21, 423–430. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 297–299. [Google Scholar] [CrossRef]
- Atul-Nayyar, A.; Hamel, C.; Hanson, K.; Germida, J. The arbuscular mycorrhizal symbiosis links N mineralization to plant demand. Mycorrhiza 2009, 19, 239–246. [Google Scholar] [CrossRef]
- Pigna, M.; Caporale, A.; Cartes, P.; Cozzolino, V.; Mora, M.; Sommella, A.; Violante, A. Effects of arbuscular mycorrhizal inoculation and phosphorus fertilization on the growth of escarole (Cichorium endivia L.) in an arsenic polluted soil. J. Soil Sci. Plant Nutr. 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Liu, J.; Cui, M.; Huang, Y.; Tian, Y.; Chen, A.; Xu, G. The potassium transporter SlHAK10 is involved in mycorrhizal potassium uptake. Plant Physiol. 2019, 180, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Wassen, M.J.; Olde Venterink, H.G.; de Swart, E.O. Nutrient concentrations in mire vegetation as a measure of nutrient limitation in mire ecosystems. J. Veg. Sci. 1995, 6, 5–16. [Google Scholar] [CrossRef]
- Braakhekke, W.G.; Hooftman, D.A. The resource balance hypothesis of plant species diversity in grassland. J. Veg. Sci. 1999, 10, 187–200. [Google Scholar] [CrossRef]
- Masson-Boivin, C.; Sachs, J.L. Symbiotic nitrogen fixation by rhizobia—The roots of a success story. Curr. Opin. Plant Biol. 2018, 44, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Pfeffer, P.E.; Douds, D.D.; Piotrowski, E.; Lammers, P.J.; Shachar-Hill, Y. The uptake, metabolism, transport and transfer of nitrogen in an arbuscular mycorrhizal symbiosis. New Phytol. 2005, 168, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guo, F.; Zhang, J.-L.; Yang, S.; Meng, J.-J.; Geng, Y.; Wang, Q.; Li, X.-G.; Wan, S.-B. Arbuscular mycorrhizal fungi combined with exogenous calcium improves the growth of peanut (Arachis hypogaea L.) seedlings under continuous cropping. J. Integr. Agric. 2019, 18, 407–416. [Google Scholar] [CrossRef]
- Liu, S.; Guo, H.; Xu, J.; Song, Z.; Song, S.; Tang, J.; Chen, X. Arbuscular mycorrhizal fungi differ in affecting the flowering of a host plant under two soil phosphorus conditions. J. Plant Ecol. 2018, 11, 623–631. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Wei, S.; Xiao, X.; Su, C.; Jiang, P.; Song, Z.; Wang, T.; Yu, Z. Effects of arbuscular mycorrhizal fungi on the growth, nutrient uptake and glycyrrhizin production of licorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul. 2007, 52, 29–39. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Arbuscular mycorrhizal networks: Process and functions. A review. Agron. Sustain. Dev. 2010, 30, 581–599. [Google Scholar] [CrossRef]
- Enkhtuya, B.; Rydlová, J.; Vosátka, M. Effectiveness of indigenous and non-indigenous isolates of arbuscular mycorrhizal fungi in soils from degraded ecosystems and man-made habitats. Appl. Soil Ecol. 2000, 14, 201–211. [Google Scholar] [CrossRef]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Kasimatis, C.-N.; Kakabouki, I.; Leonidakis, D.; Danalatos, N.J.S.R. Assessment of plant growth promoting bacteria strains on growth, yield and quality of sweet corn. Sci. Rep. 2022, 12, 11598. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Shi, Y.; Chen, C.; Pang, Z.; Zhang, G.; Zhang, W.; Kan, H. Arbuscular Mycorrhizal Fungi Selectively Promoted the Growth of Three Ecological Restoration Plants. Plants 2024, 13, 1678. https://doi.org/10.3390/plants13121678
Xu H, Shi Y, Chen C, Pang Z, Zhang G, Zhang W, Kan H. Arbuscular Mycorrhizal Fungi Selectively Promoted the Growth of Three Ecological Restoration Plants. Plants. 2024; 13(12):1678. https://doi.org/10.3390/plants13121678
Chicago/Turabian StyleXu, Hengkang, Yuchuan Shi, Chao Chen, Zhuo Pang, Guofang Zhang, Weiwei Zhang, and Haiming Kan. 2024. "Arbuscular Mycorrhizal Fungi Selectively Promoted the Growth of Three Ecological Restoration Plants" Plants 13, no. 12: 1678. https://doi.org/10.3390/plants13121678
APA StyleXu, H., Shi, Y., Chen, C., Pang, Z., Zhang, G., Zhang, W., & Kan, H. (2024). Arbuscular Mycorrhizal Fungi Selectively Promoted the Growth of Three Ecological Restoration Plants. Plants, 13(12), 1678. https://doi.org/10.3390/plants13121678




