Bifenthrin Residues in Table Grapevine: Method Optimization, Dissipation and Removal of Residues in Grapes and Grape Leaves
Abstract
1. Introduction
2. Results
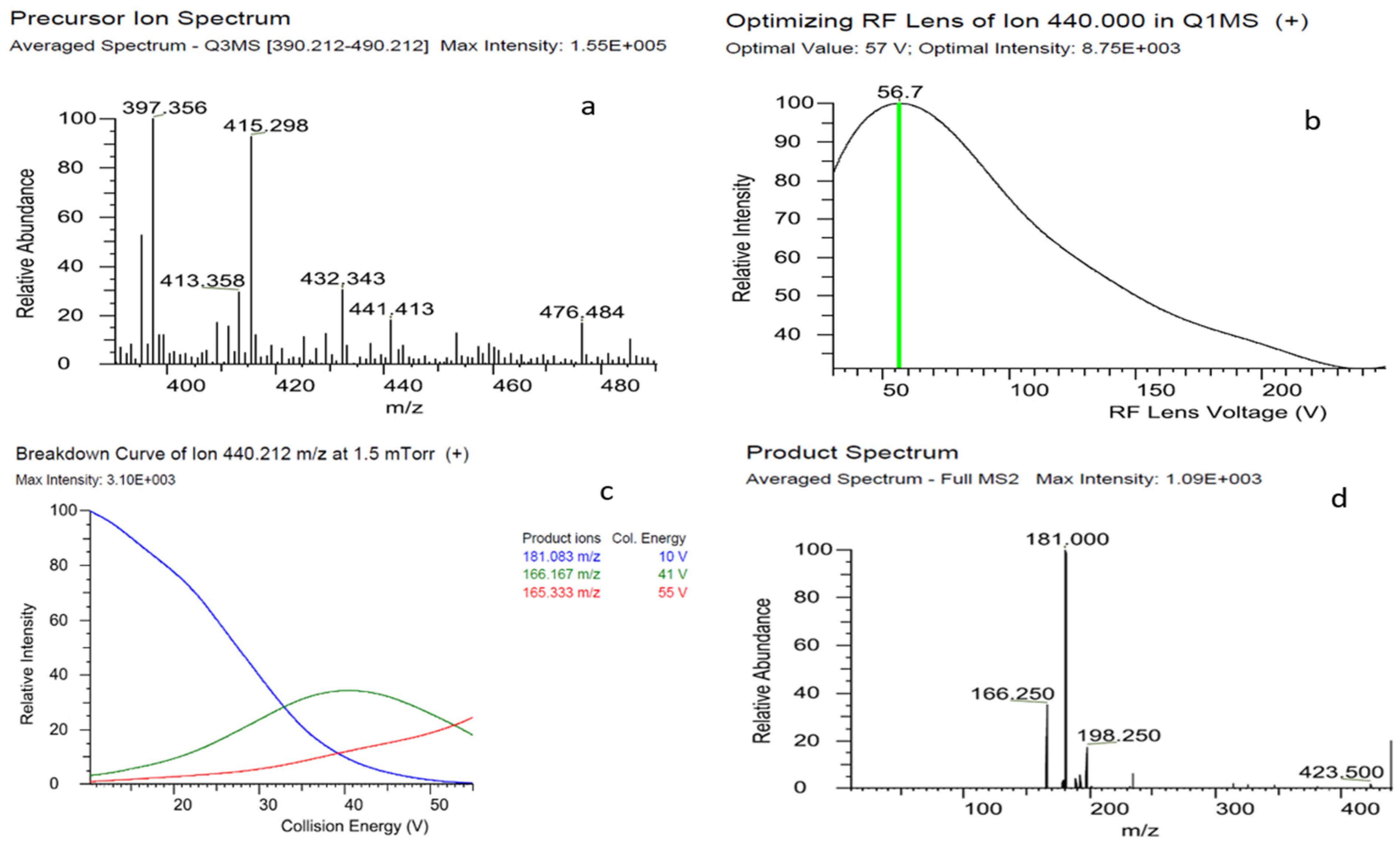
2.1. Optimization of LC-MS/MS
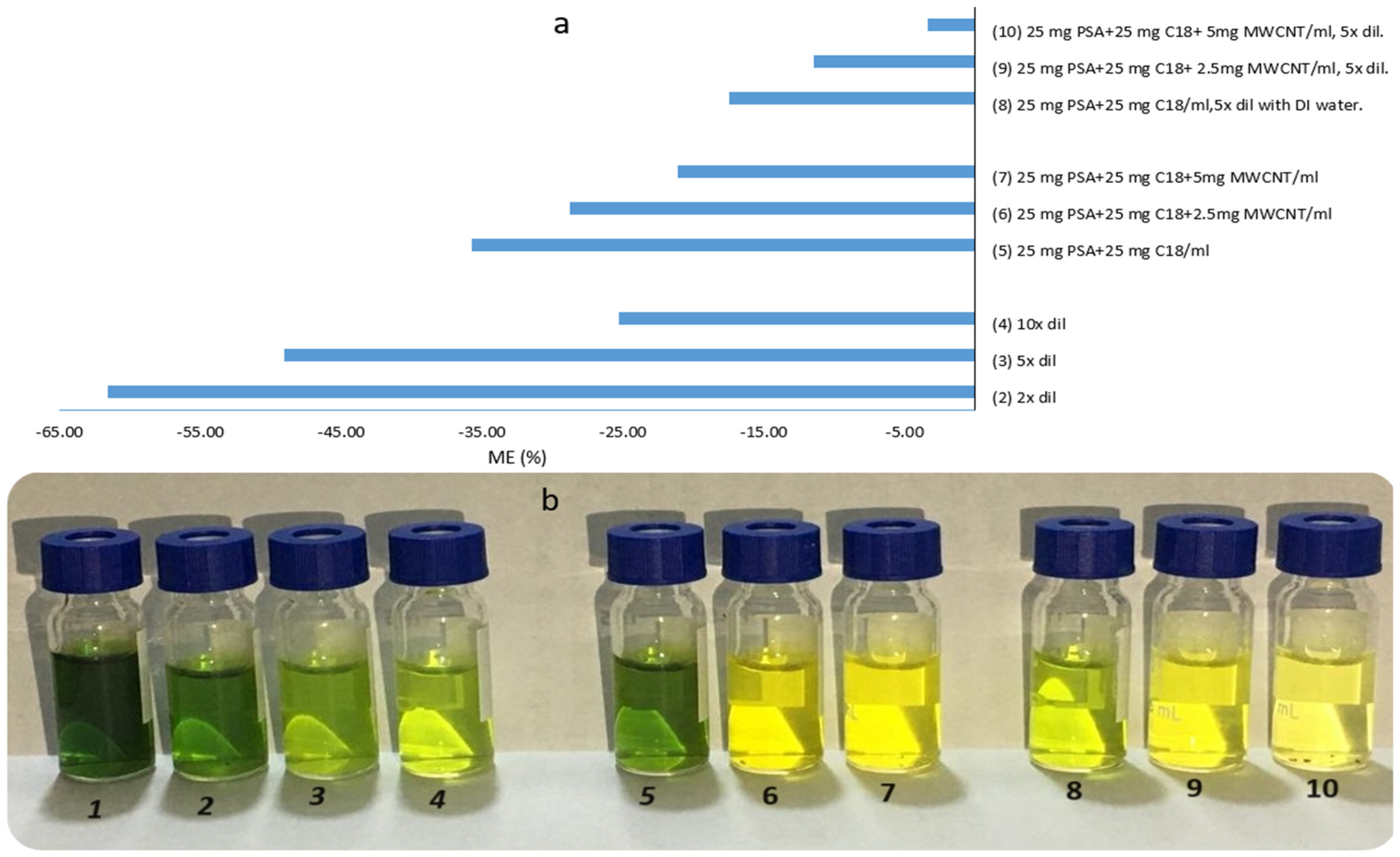
2.2. Optimization of Cleanup
2.3. Method Validation
2.3.1. Accuracy
2.3.2. Precision
2.3.3. LOD and LOQ
2.4. Dissipation Kinetics
2.5. Removal of Bifenthrin Residues by Washing
3. Materials and Methods
3.1. Chemicals
3.2. Standard Solutions
3.3. Sample Preparation, Extraction, and Cleanup
3.4. LC-MS/MS
3.5. Method Validation
3.6. Field Trial
3.7. Removal of Bifenthrin Residues by Washing
3.8. Statistical Analysis and Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2021. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 25 May 2023).
- Breckenridge, C.B.; Holden, L.; Sturgess, N.; Weiner, M.; Sheets, L.; Sargent, D.; Soderlund, D.M.; Choi, J.-S.; Symington, S.; Clark, J.M. Evidence for a Separate Mechanism of Toxicity for the Type I and the Type II Pyrethroid Insecticides. Neurotoxicology 2009, 30, S17–S31. [Google Scholar] [CrossRef]
- Beggel, S.; Werner, I.; Connon, R.E.; Geist, J.P. Sublethal Toxicity of Commercial Insecticide Formulations and Their Active Ingredients to Larval Fathead Minnow (Pimephales Promelas). Sci. Total Environ. 2010, 408, 3169–3175. [Google Scholar] [CrossRef]
- World Health Organization WHO. Global Insecticide Use for Vector-Borne Disease Control: A 10-Year Assessment (2010–2019), 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Liu, H.; Zhao, M.; Zhang, C.; Ma, Y.; Liu, W. Enantioselective Cytotoxicity of the Insecticide Bifenthrin on a Human Amnion Epithelial (FL) Cell Line. Toxicology 2008, 253, 89–96. [Google Scholar] [CrossRef]
- Gammon, D.W.; Liu, Z.; Chandrasekaran, A.; El-Naggar, S.F.; Kuryshev, Y.A.; Jackson, S. Pyrethroid Neurotoxicity Studies with Bifenthrin Indicate a Mixed Type I/II Mode of Action. Pest Manag. Sci. 2019, 75, 1190–1197. [Google Scholar] [CrossRef]
- Syed, F.; Awasthi, K.K.; Chandravanshi, L.P.; Verma, R.; Rajawat, N.K.; Khanna, V.K.; John, P.J.; Soni, I. Bifenthrin-Induced Neurotoxicity in Rats: Involvement of Oxidative Stress. Toxicol. Res. 2018, 7, 48–58. [Google Scholar] [CrossRef]
- Gargouri, B.; Bouchard, M.; Saliba, S.W.; Fetoui, H.; Fiebich, B.L. Repeated Bifenthrin Exposure Alters Hippocampal Nurr-1/AChE and Induces Depression-like Behavior in Adult Rats. Behav. Brain Res. 2019, 370, 111898. [Google Scholar] [CrossRef]
- Abdel Ghani, S.B.; Alhewairini, S.S.; Hrouzková, S. A Fast and Easy QuEChERS-DLLME Method Combined with GC-MS for Ethion and Bifenthrin Residues Determination and Study of Their Dissipation Dynamics in Palm Dates. Food Anal. Methods 2018, 11, 3542–3550. [Google Scholar] [CrossRef]
- Hanafi, A.; Hindy, M.; Ghani, S.A. Effect of Spray Application Techniques on Spray Deposits and Residues of Bifenthrin in Peas under Field Conditions. Pestic. Sci. 2016, 41, 49–54. [Google Scholar] [CrossRef]
- Bletsou, A.A.; Hanafi, A.H.; Dasenaki, M.E.; Thomaidis, N.S. Development of Specific LC-ESI-MS/MS Methods to Determine Bifenthrin, Lufenuron, and Iprodione Residue Levels in Green Beans, Peas, and Chili Peppers under Egyptian Field Conditions. Food Anal. Methods 2013, 6, 1099–1112. [Google Scholar] [CrossRef]
- Choi, J.-H.; Liu, X.; Kim, H.-K.; Shim, J.-H. Gas Chromatography Residue Analysis of Bifenthrin in Pears Treated with 2% Wettable Powder. Toxicol. Res. 2009, 25, 41–45. [Google Scholar] [CrossRef]
- Chen, L.; ShangGuan, L.; Wu, Y.; Xu, L.; Fu, F. Study on the Residue and Degradation of Fluorine-Containing Pesticides in Oolong Tea by Using Gas Chromatography–Mass Spectrometry. Food Control 2012, 25, 433–440. [Google Scholar] [CrossRef]
- Li, H.-P.; Lin, C.-H.; Jen, J.-F. Analysis of Aqueous Pyrethroid Residuals by One-Step Microwave-Assisted Headspace Solid-Phase Microextraction and Gas Chromatography with Electron Capture Detection. Talanta 2009, 79, 466–471. [Google Scholar] [CrossRef]
- Abdallah, O.I.; Alamer, S.S.; Alrasheed, A.M.; Almundarij, A.A. Residue Distribution, Safety Evaluation, Processing Factor and Dissipation Kinetics of Bifenthrin and Ethion in Date Fruits. Qual. Assur. Saf. Crops Foods. 2019, 11, 577–588. [Google Scholar] [CrossRef]
- Esteve-Turrillas, F.A.; Pastor, A.; de la Guardia, M. Determination of Pyrethroid Insecticide Residues in Vegetable Oils by Using Combined Solid-Phases Extraction and Tandem Mass Spectrometry Detection. Anal. Chim. Acta 2005, 553, 50–57. [Google Scholar] [CrossRef]
- Martínez, D.B.; Vázquez, P.P.; Galera, M.M.; García, M.D.G. Determination of Pyrethroid Insecticides in Vegetables with Liquid Chromatography Using Detection by Electrospray Mass Spectrometry. Chromatographia 2006, 63, 487–491. [Google Scholar] [CrossRef]
- EU. Commission Regulation (EC) No 178/2006 of 1 February 2006 Amending Regulation (EC) No 396/2005 of the European Parliament and of the Council to Establish Annex I Listing the Food and Feed Products to Which Maximum Levels for Pesticide Residues Apply. Off. J. 2006, 29, 3–25. [Google Scholar]
- Thurman, E.M.; Ferrer, I.; Barceló, D. Choosing between Atmospheric Pressure Chemical Ionization and Electrospray Ionization Interfaces for the HPLC/MS Analysis of Pesticides. Anal. Chem. 2001, 73, 5441–5449. [Google Scholar] [CrossRef]
- Chen, T.; Chen, G. Identification and Quantitation of Pyrethroid Pesticide Residues in Vegetables by Solid-phase Extraction and Liquid Chromatography/Electrospray Ionization Ion Trap Mass Spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1848–1854. [Google Scholar] [CrossRef]
- Lehotay, S. AOAC Official Method 2007.01 Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef]
- Pucci, V.; Di Palma, S.; Alfieri, A.; Bonelli, F.; Monteagudo, E. A Novel Strategy for Reducing Phospholipids-Based Matrix Effect in LC–ESI-MS Bioanalysis by Means of HybridSPE. J. Pharm. Biomed. Anal. 2009, 50, 867–871. [Google Scholar] [CrossRef]
- Ferrer, C.; Lozano, A.; Agüera, A.; Girón, A.J.; Fernández-Alba, A.R. Overcoming Matrix Effects Using the Dilution Approach in Multiresidue Methods for Fruits and Vegetables. J. Chromatogr. A 2011, 1218, 7634–7639. [Google Scholar] [CrossRef]
- Abdallah, O.; Abdel Ghani, S.; Hrouzková, S. Development of Validated LC-MS/MS Method for Imidacloprid and Acetamiprid in Parsley and Rocket and Evaluation of Their Dissipation Dynamics. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 392–399. [Google Scholar] [CrossRef]
- Pihlström, T.; Fernández-Alba, A.R.; Amate, C.F.; Poulsen, M.E.; Hardebusch, B.; Anastassiades, M.; Lippold, R.; Cabrera, L.C.; de Kok, A.; ORegan, F. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed SANTE 11312/2021. Sante 2021, 11312, v2. [Google Scholar]
- Li, Z.; Su, X.; Dong, C.; Zhou, J.; An, W.; Wang, C.; Jiao, B. Determination of Five Pesticides in Kumquat: Dissipation Behaviors, Residues and Their Health Risk Assessment under Field Conditions. Ecotoxicol Env. Saf. 2021, 228, 112958. [Google Scholar] [CrossRef]
- Abdel Ghani, S.B.; Abdallah, O.I. Method Validation and Dissipation Dynamics of Chlorfenapyr in Squash and Okra. Food Chem. 2016, 194, 516–521. [Google Scholar] [CrossRef]
- Tewary, D.K.; Kumar, V.; Ravindranath, S.D.; Shanker, A. Dissipation Behavior of Bifenthrin Residues in Tea and Its Brew. Food Control 2005, 16, 231–237. [Google Scholar] [CrossRef]
- Ghani, S.B.A.; Hanafi, A.; Nasr, I.N. Non-Toxic Washing Solutions for Decreasing Myclobutanil, Fenhexamid and Boscalid Residues in Sweet Pepper and Cherry Tomatoes. Aust. J. Basic Appl. Sci. 2010, 4, 3360–3365. [Google Scholar]
- Zhang, A.-A.; Sutar, P.P.; Bian, Q.; Fang, X.-M.; Ni, J.-B.; Xiao, H.-W. Pesticide Residue Elimination for Fruits and Vegetables: The Mechanisms, Applications, and Future Trends of Thermal and Non-Thermal Technologies. J. Future Foods 2022, 2, 223–240. [Google Scholar] [CrossRef]
- Naman, M.; Masoodi, F.A.; Wani, S.M.; Ahad, T. Changes in Concentration of Pesticide Residues in Fruits and Vegetables during Household Processing. Toxicol. Rep. 2022, 9, 1419–1425. [Google Scholar] [CrossRef]
- EU-MRL. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 10 April 2024).
- Kim, S.-W.; Abd El-Aty, A.M.; Rahman, M.M.; Choi, J.-H.; Lee, Y.-J.; Ko, A.-Y.; Choi, O.-J.; Jung, H.N.; Hacımüftüoğlu, A.; Shim, J.-H. The Effect of Household Processing on the Decline Pattern of Dimethomorph in Pepper Fruits and Leaves. Food Control 2015, 50, 118–124. [Google Scholar] [CrossRef]
- Malhat, F.; Abdallah, O. Residue Distribution and Risk Assessment of Two Macrocyclic Lactone Insecticides in Green Onion Using Micro-Liquid-Liquid Extraction (MLLE) Technique Coupled with Liquid Chromatography Tandem Mass Spectrometry. Environ. Monit. Assess. 2019, 191, 584. [Google Scholar] [CrossRef]
- European Commission. Commission Directive 2002/63/EC of 11 July 2002 Establishing Community Methods of Sampling for the Official Control of Pesticide Residues in and on Products of Plant and Animal Origin and Repealing Directive 79/700/EEC. EEC 2002, 2, 30–43. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Hoskins, W. Mathematical treatment of the rate of loss of pesticide residues. FAO Plant Prot. Bull. 1961, 9, 163–168. [Google Scholar]
- Hingmire, S.; Oulkar, D.P.; Utture, S.C.; Shabeer, T.A.; Banerjee, K. Residue analysis of fipronil and difenoconazole in okra by liquid chromatography tandem mass spectrometry and their food safety evaluation. Food Chem. 2015, 176, 145–151. [Google Scholar] [CrossRef]
- Abdallah, O.I.; Soliman, H.; El-Hefny, D.; Abd El-Hamid, R.; Malhat, F. Dissipation profile of sulfoxaflor on squash under Egyptian field conditions: A prelude to risk assessment. Int. J. Environ. Anal. Chem. 2021, 103, 3820–3834. [Google Scholar] [CrossRef]
| Grape | Grape Leaves | |
|---|---|---|
| Linearity range (µg/L) | 1–100 | 1–100 |
| R2 | 0.9997 | 0. 9964 |
| LOD (µg/L) | 0.48 | 0.42 |
| LOQ (µg/kg) | 2 | 20 |
| Intra-day repeatability (RSDr) (n = 6) | 5.51 a | 6.42 b |
| Inter-day repeatability (RSDR b) (n = 18) | 7.38 a | 6.72 b |
| Accuracy | 98.8 | 93.5 |
| Matrix effect (%) | −1.17 | −3.4 |
| Recommended Application Rate | Double Application Rate | |||
|---|---|---|---|---|
| Grapes | Grapes Leaves | Grapes | Grapes Leaves | |
| Intercept | −0.2542 | 2.9428 | 0.1707 | 3.433 |
| Slope | −0.1549 | −0.235 | −0.1672 | −0.208 |
| R2 a | 0.9873 | 0.9931 | 0.9886 | 0.9919 |
| K b | 0.1549 | 0.235 | 0.1672 | 0.208 |
| t½ | 4.08 | 1.885 | 3.54 | 2.14 |
| MRL c | 0.3 | 0.01 | 0.3 | 0.01 |
| PHI d | 6 | 32 | 8 | 36 |
| Grapes | Grapes Leaves | |
|---|---|---|
| Control, not treated | 0.6 ± 0.02 C | 10.4 ± 0.69 D |
| Running water, 1 min | 0.4 ± 0.04 B | 9.1 ± 0.36 C |
| Running water, 5 min | 0.3 ± 0.05 A | 7.9 ± 0.21 AB |
| Soaking, 1 min | 0.4 ± 0.03 B | 8.5 ± 0.40 BC |
| Soaking, 5 min | 0.3 ± 0.03 A | 6.9 ± 0.08 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhewairini, S.S.; Abd El-Hamid, R.M.; Ahmed, N.S.; Abdel Ghani, S.B.; Abdallah, O.I. Bifenthrin Residues in Table Grapevine: Method Optimization, Dissipation and Removal of Residues in Grapes and Grape Leaves. Plants 2024, 13, 1695. https://doi.org/10.3390/plants13121695
Alhewairini SS, Abd El-Hamid RM, Ahmed NS, Abdel Ghani SB, Abdallah OI. Bifenthrin Residues in Table Grapevine: Method Optimization, Dissipation and Removal of Residues in Grapes and Grape Leaves. Plants. 2024; 13(12):1695. https://doi.org/10.3390/plants13121695
Chicago/Turabian StyleAlhewairini, Saleh S., Rania M. Abd El-Hamid, Nevein S. Ahmed, Sherif B. Abdel Ghani, and Osama I. Abdallah. 2024. "Bifenthrin Residues in Table Grapevine: Method Optimization, Dissipation and Removal of Residues in Grapes and Grape Leaves" Plants 13, no. 12: 1695. https://doi.org/10.3390/plants13121695
APA StyleAlhewairini, S. S., Abd El-Hamid, R. M., Ahmed, N. S., Abdel Ghani, S. B., & Abdallah, O. I. (2024). Bifenthrin Residues in Table Grapevine: Method Optimization, Dissipation and Removal of Residues in Grapes and Grape Leaves. Plants, 13(12), 1695. https://doi.org/10.3390/plants13121695





