Biochar Effectively Promoted Growth of Ardisia crenata by Affecting the Soil Physicochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Cultivation Medium
2.2. Experimental Treatments
2.3. Analysis of the Properties of Cultivation Medium
2.4. Measurement of Plant Growth Parameter
2.5. Measurement of Plant Physiological Parameters
2.6. Measurement of Plant Photosynthesis
2.7. Measurement of Root Parameters
2.8. Statistical Analysis
3. Results
3.1. Soil Physical and Chemical Properties and Nutrient Content
3.2. Growth Parameters of A. crenata
3.3. Root Parameters of A. crenata
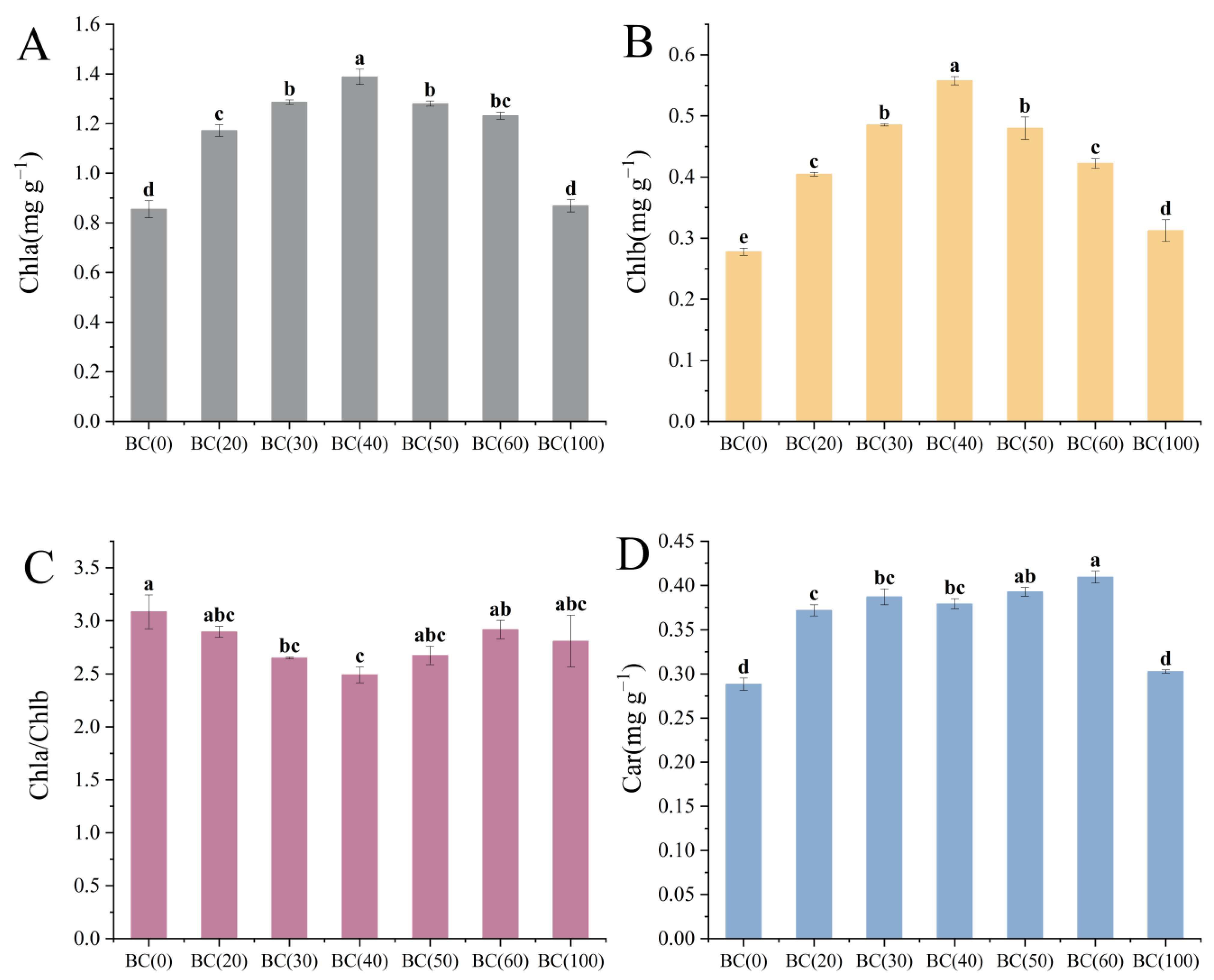
3.4. Plant Physiological Parameters
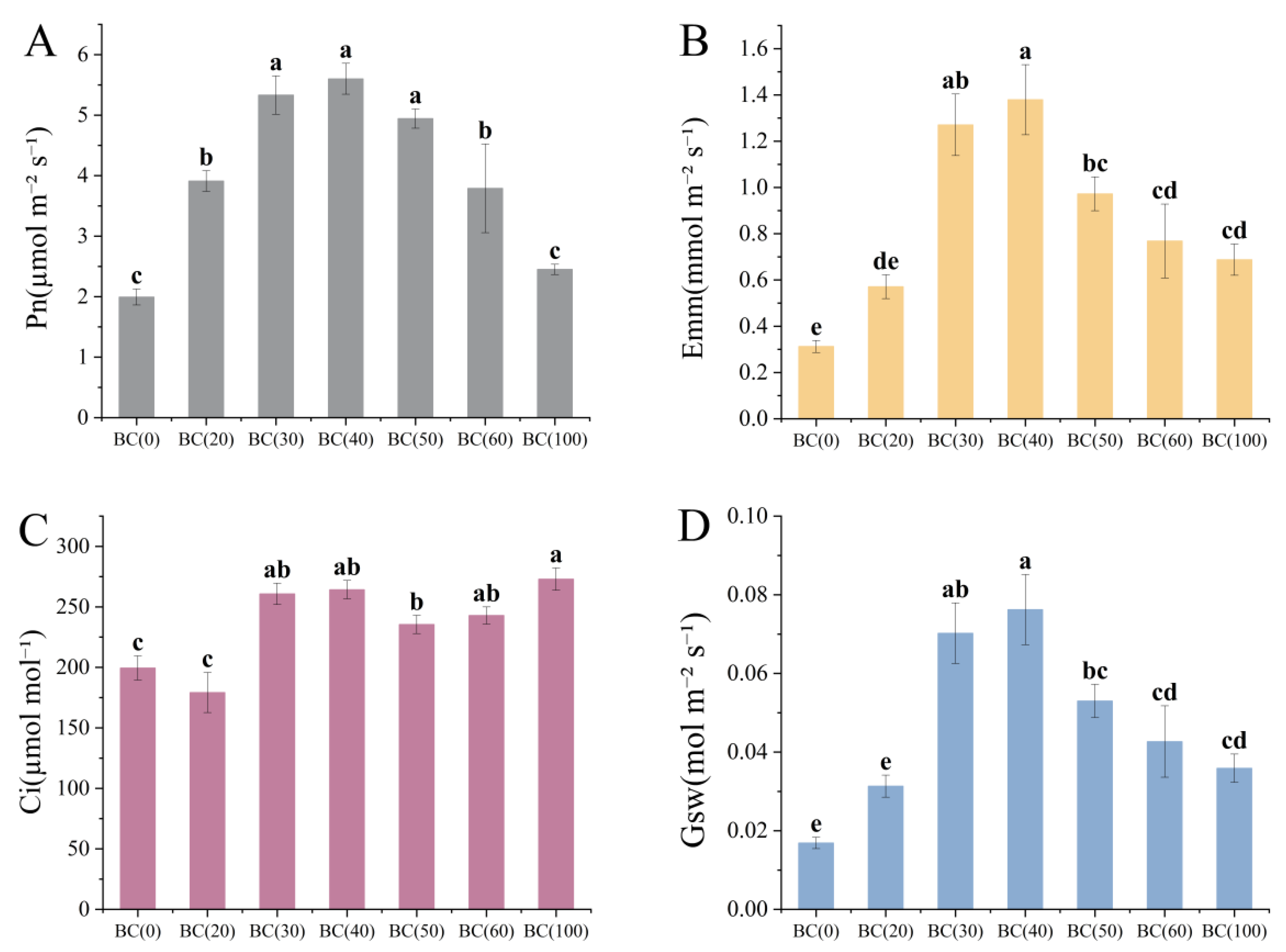
3.5. Plant Photosynthesis Parameters
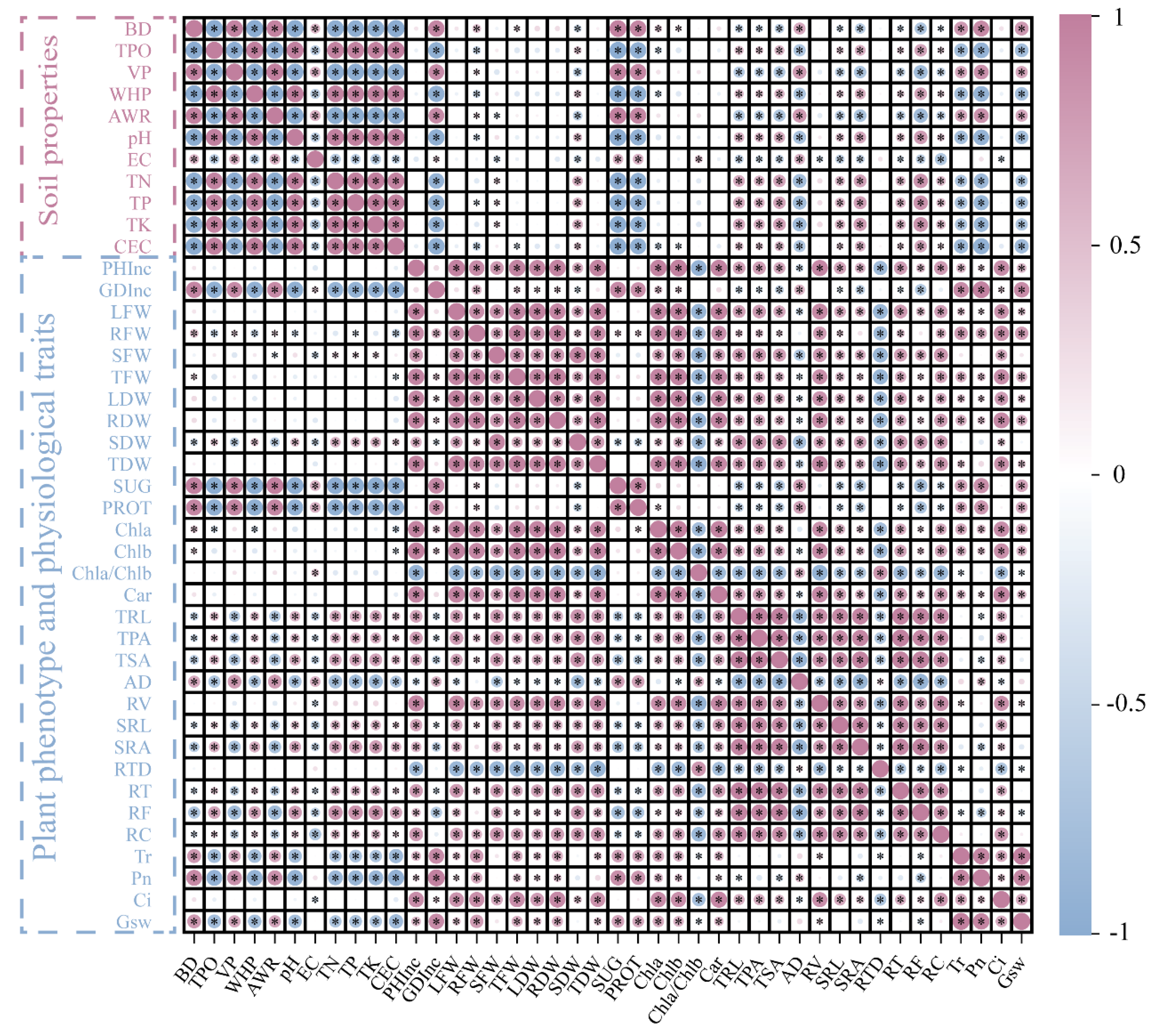
3.6. Correlation Analysis between Plant Traits and Soil Matrix Properties
3.7. Comprehensive Evaluation of Plant Growth Physiological Indexes
4. Discussions
4.1. The Plant Growth Promotion Effect of Biochar by Altering the Physiochemical Properties of Soil
4.2. Effects of Biochar Addition on Plant Roots
4.3. Effects of Biochar on Shoot Parts and Photosynthesis of Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abd Ghani, R.; Omar, S.; Jolánkai, M.; Tarnawa, Á.; Kende, Z.; Khalid, N.; Gyuricza, C.; Kassai, M.K. Soilless Culture Applications for Early Development of Soybean Crop (Glycine Max L. Merr). Agriculture 2023, 13, 1713. [Google Scholar] [CrossRef]
- Kamperidou, V.; Terzopoulou, P.; Barboutis, I. Marginal Lands Providing Tree-Crop Biomass as Feedstock for Solid Biofuels. Biofuels Bioprod. Biorefining 2021, 15, 1395–1405. [Google Scholar] [CrossRef]
- Barrett, G.E.; Alexander, P.D.; Robinson, J.S.; Bragg, N.C. Achieving Environmentally Sustainable Growing Media for Soilless Plant Cultivation Systems—A Review. Sci. Hortic. 2016, 212, 220–234. [Google Scholar] [CrossRef]
- Fussy, A.; Papenbrock, J. An Overview of Soil and Soilless Cultivation Techniques-Chances, Challenges and the Neglected Question of Sustainability. Plants 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing Water and Nutrient Losses from Soilless Cropping in Southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Insight into Biochar Properties and Its Cost Analysis. Biomass Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Aller, M.F. Biochar Properties: Transport, Fate, and Impact. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1183–1296. [Google Scholar] [CrossRef]
- Chen, W.; Meng, J.; Han, X.; Lan, Y.; Zhang, W. Past, Present, and Future of Biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Lehmann, J.; Cowie, A.; Masiello, C.A.; Kammann, C.; Woolf, D.; Amonette, J.E.; Cayuela, M.L.; Camps-Arbestain, M.; Whitman, T. Biochar in Climate Change Mitigation. Nat. Geosci. 2021, 14, 883–892. [Google Scholar] [CrossRef]
- Burrell, L.D.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long-Term Effects of Biochar on Soil Physical Properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of Biochar and Hydrochar Addition on Water Retention and Water Repellency of Sandy Soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Kang, S.-W.; Cheong, Y.H.; Yun, J.-J.; Park, J.-H.; Park, J.-H.; Seo, D.-C.; Cho, J.-S. Effect of Biochar Application on Nitrogen Use Efficiency for Sustainable and Productive Agriculture under Different Field Crops. J. Plant Nutr. 2021, 44, 2849–2862. [Google Scholar] [CrossRef]
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-Derived Biochar for Water Pollution Control and Sustainable Development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- Kazemi Shariat Panahi, H.; Dehhaghi, M.; Ok, Y.S.; Nizami, A.-S.; Khoshnevisan, B.; Mussatto, S.I.; Aghbashlo, M.; Tabatabaei, M.; Lam, S.S. A Comprehensive Review of Engineered Biochar: Production, Characteristics, and Environmental Applications. J. Clean. Prod. 2020, 270, 122462. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–Root Interactions Are Mediated by Biochar Nutrient Content and Impacts on Soil Nutrient Availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of Biochar, Compost and Biochar–Compost for Soil Quality, Maize Yield and Greenhouse Gas Emissions in a Tropical Agricultural Soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and Its Importance on Nutrient Dynamics in Soil and Plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical Cycling of Nitrogen and Phosphorus in Biochar-Amended Soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis Temperature Induced Changes in Characteristics and Chemical Composition of Biochar Produced from Conocarpus Wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar Characteristics and Application Rates Affecting Corn Growth and Properties of Soils Contrasting in Texture and Mineralogy. Geoderma 2015, 237–238, 105–116. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.-H.; Amirahmadi, E. How Do Different Feedstocks and Pyrolysis Conditions Effectively Change Biochar Modification Scenarios? A Critical Analysis of Engineered Biochars under H2O2 Oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Abu Zied Amin, A.E.-E. Impact of Corn Cob Biochar on Potassium Status and Wheat Growth in a Calcareous Sandy Soil. Commun. Soil Sci. Plant Anal. 2016, 47, 2026–2033. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, B.; Wu, S.; Feng, H.; Gao, M.; Zhang, B.; Liu, Y. After-Effects of Straw and Straw-Derived Biochar Application on Crop Growth, Yield, and Soil Properties in Wheat (Triticum Aestivum L.)-Maize (Zea Mays L.) Rotations: A Four-Year Field Experiment. Sci. Total Environ. 2021, 780, 146560. [Google Scholar] [CrossRef] [PubMed]
- Hagner, M.; Kemppainen, R.; Jauhiainen, L.; Tiilikkala, K.; Setälä, H. The Effects of Birch (Betula Spp.) Biochar and Pyrolysis Temperature on Soil Properties and Plant Growth. Soil Tillage Res. 2016, 163, 224–234. [Google Scholar] [CrossRef]
- Borges, B.M.M.N.; Strauss, M.; Camelo, P.A.; Sohi, S.P.; Franco, H.C.J. Re-Use of Sugarcane Residue as a Novel Biochar Fertiliser—Increased Phosphorus Use Efficiency and Plant Yield. J. Clean. Prod. 2020, 262, 121406. [Google Scholar] [CrossRef]
- Backer, R.G.M.; Saeed, W.; Seguin, P.; Smith, D.L. Root Traits and Nitrogen Fertilizer Recovery Efficiency of Corn Grown in Biochar-Amended Soil under Greenhouse Conditions. Plant Soil 2017, 415, 465–477. [Google Scholar] [CrossRef]
- Wan, H.; Liu, X.; Jiang, M.; Wei, Z.; Hossain, M.A.; Liu, F. Biochar Amendment Alters Root Morphology of Maize Plant: Its Implications in Enhancing Nutrient Uptake and Shoot Growth under Reduced Irrigation Regimes. Front. Plant Sci. 2023, 14, 1122742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Vivanco, J.M.; Manter, D.K. Nitrogen Fertilizer Rate Affects Root Exudation, the Rhizosphere Microbiome and Nitrogen-Use-Efficiency of Maize—ScienceDirect. Appl. Soil Ecol. 2016, 107, 324–333. [Google Scholar] [CrossRef]
- Xiang, Y.; Deng, Q.; Duan, H.; Guo, Y. Effects of Biochar Application on Root Traits: A Meta-Analysis. GCB Bioenergy 2017, 9, 1563–1572. [Google Scholar] [CrossRef]
- Kitajima, K.; Fox, A.M.; Sato, T.; Nagamatsu, D. Cultivar Selection Prior to Introduction May Increase Invasiveness: Evidence from Ardisia Crenata. Biol. Invasions 2006, 8, 1471–1482. [Google Scholar] [CrossRef]
- Kwon, K.-J.; Kwon, H.-J.; Oh, Y.-A.; Kim, S.-Y.; Park, B.-J. Particulate Matter Removal of Three Woody Plant Species, Ardisia Crenata, Ardisia Japonica, and Maesa Japonica. Sustainability 2021, 13, 11017. [Google Scholar] [CrossRef]
- Podolak, I.; Żuromska-Witek, B.; Grabowska, K.; Żebrowska, S.; Galanty, A.; Hubicka, U. Comparative Quantitative Study of Ardisiacrispin A in Extracts from Ardisia Crenata Sims Varieties and Their Cytotoxic Activities. Chem. Biodivers. 2021, 18, e2100335. [Google Scholar] [CrossRef]
- Tao, H.; Zhou, Y.; Yin, X.; Wei, X.; Zhou, Y. Two New Phenolic Glycosides with Lactone Structural Units from Leaves of Ardisia Crenata Sims with Antibacterial and Anti-Inflammatory Activities. Molecules 2022, 27, 4903. [Google Scholar] [CrossRef] [PubMed]
- Song, N.-N.; Yang, L.-M.; Zhang, M.-J.; An, R.-F.; Liu, W.; Huang, X.-F. Triterpenoid Saponins and Phenylpropanoid Glycoside from the Roots of Ardisia Crenata and Their Cytotoxic Activities. Chin. J. Nat. Med. 2021, 19, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Chen, H.; Lu, Y.; Liu, R.; Yuan, M.Y.; An, J.; Li, M.; Chua, L.S.; Kait, J.A.; Xin, L. Comparing the Efficacy and Safety of Atomization of Traditional Chinese Medicine Kai Hou Jian and Budesonide Suspension in Adult Acute Laryngitis: A Randomized Control Trial. Ann. Transl. Med. 2022, 10, 1019. [Google Scholar] [CrossRef]
- Gamliel, A.; van Bruggen, A.H.C. Maintaining Soil Health for Crop Production in Organic Greenhouses. Sci. Hortic. 2016, 208, 120–130. [Google Scholar] [CrossRef]
- Osman, N.; Barakbah, S.S. Parameters to Predict Slope Stability—Soil Water and Root Profiles. Ecol. Eng. 2006, 28, 90–95. [Google Scholar] [CrossRef]
- He, K.; He, G.; Wang, C.; Zhang, H.; Xu, Y.; Wang, S.; Kong, Y.; Zhou, G.; Hu, R. Biochar Amendment Ameliorates Soil Properties and Promotes Miscanthus Growth in a Coastal Saline-Alkali Soil. Appl. Soil Ecol. 2020, 155, 103674. [Google Scholar] [CrossRef]
- Zheng, K.; Cheng, J.; Xia, J.; Liu, G.; Xu, L. Effects of Soil Bulk Density and Moisture Content on the Physico-Mechanical Properties of Paddy Soil in Plough Layer. Water 2021, 13, 2290. [Google Scholar] [CrossRef]
- Azeez, J.O.; Van Averbeke, W. Dynamics of Soil pH and Electrical Conductivity with the Application of Three Animal Manures. Commun. Soil Sci. Plant Anal. 2012, 43, 865–874. [Google Scholar] [CrossRef]
- Bascomb, C.L. Rapid Method for the Determination of Cation-Exchange Capacity of Calcareous and Non-Calcareous Soils. J. Sci. Food Agric. 1964, 15, 821–823. [Google Scholar] [CrossRef]
- Begheyn, L.T. A Rapid Method to Determine Cation Exchange Capacity and Exchangeable Bases in Calcareous, Gypsiferous, Saline and Sodic Soils. Commun. Soil Sci. Plant Anal. 1987, 18, 911–932. [Google Scholar] [CrossRef]
- Yuen, S.H.; Pollard, A.G. Determination of Nitrogen in Soil and Plant Materials: Use of Boric Acid in the Micro-Kjeldahl Method. J. Sci. Food Agric. 1953, 4, 490–496. [Google Scholar] [CrossRef]
- Bremner, J.M. Determination of Nitrogen in Soil by the Kjeldahl Method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- David, D.J. The Determination of Exchangeable Sodium, Potassium, Calcium and Magnesium in Soils by Atomic-Absorption Spectrophotometry. Analyst 1960, 85, 495–503. [Google Scholar] [CrossRef]
- Ullah, R.; Abbas, Z.; Bilal, M.; Habib, F.; Iqbal, J.; Bashir, F.; Noor, S.; Qazi, M.A.; Niaz, A.; Baig, K.S.; et al. Method Development and Validation for the Determination of Potassium (K2O) in Fertilizer Samples by Flame Photometry Technique. J. King Saud Univ.—Sci. 2022, 34, 102070. [Google Scholar] [CrossRef]
- Levine, H.; Rowe, J.J.; Grimaldi, F.S. Molybdenum Blue Reaction and Determination of Phosphours in Waters Containing Arsenic, Silicon, and Germanium. Anal. Chem. 1995, 27, 258–262. [Google Scholar] [CrossRef]
- Tunçtürk, M.; Tunçtürk, R.; Yildirim, B.; Çiftçi, V. Changes of Micronutrients, Dry Weight and Plant Development in Canola (Brassica Napus L.) Cultivars under Salt Stress. Afr. J. Biotechnol. 2011, 10, 3726–3730. [Google Scholar]
- Huang, W.; Ratkowsky, D.A.; Hui, C.; Wang, P.; Su, J.; Shi, P. Leaf Fresh Weight Versus Dry Weight: Which Is Better for Describing the Scaling Relationship between Leaf Biomass and Leaf Area for Broad-Leaved Plants? Forests 2019, 10, 256. [Google Scholar] [CrossRef]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of Light Intensity on Physiological Changes, Carbon Allocation and Neutral Lipid Accumulation in Oleaginous Microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, J.; Zhang, N.; Yang, J.; Sa, R.; Wu, L. Effect of Alkali Stress on Soluble Sugar, Antioxidant Enzymes and Yield of Oat. J. Integr. Agric. 2013, 12, 1441–1449. [Google Scholar] [CrossRef]
- Walker, J.M. The Bicinchoninic Acid (BCA) Assay for Protein Quantitation. In The Protein Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 11–15. ISBN 978-1-59745-198-7. [Google Scholar]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. Chapter 2—A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2010; Volume 105, pp. 47–82. [Google Scholar]
- Ababsa, N.; Boudjabi, S.; Chenchouni, H. Biochar Amendments Changed Soil Properties and Improved Cereal Crop Growth Under Salt Stress. J. Soil Sci. Plant Nutr. 2023, 23, 4912–4925. [Google Scholar] [CrossRef]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Zhang, J.; Amonette, J.E.; Flury, M. Effect of Biochar and Biochar Particle Size on Plant-Available Water of Sand, Silt Loam, and Clay Soil. Soil Tillage Res. 2021, 212, 104992. [Google Scholar] [CrossRef]
- Pabin, J.; Lipiec, J.; Włodek, S.; Biskupski, A.; Kaus, A. Critical Soil Bulk Density and Strength for Pea Seedling Root Growth as Related to Other Soil Factors. Soil Tillage Res. 1998, 46, 203–208. [Google Scholar] [CrossRef]
- Yin, Y.; He, X.; Gao, R.; Ma, H.; Yang, Y. Effects of Rice Straw and Its Biochar Addition on Soil Labile Carbon and Soil Organic Carbon. J. Integr. Agric. 2014, 13, 491–498. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar Applications Influence Soil Physical and Chemical Properties, Microbial Diversity, and Crop Productivity: A Meta-Analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Abujabhah, I.S.; Bound, S.A.; Doyle, R.; Bowman, J.P. Effects of Biochar and Compost Amendments on Soil Physico-Chemical Properties and the Total Community within a Temperate Agricultural Soil. Appl. Soil Ecol. 2016, 98, 243–253. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M. Biochar and the Nitrogen Cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhu, L. Biochar Alters Microbial Community and Carbon Sequestration Potential across Different Soil pH. Sci. Total Environ. 2018, 622–623, 1391–1399. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Wallace, H.M.; Xu, C.-Y.; Van Zwieten, L.; Weng, Z.H.; Xu, Z.; Che, R.; Tahmasbian, I.; Hu, H.-W.; Bai, S.H. The Effects of Short Term, Long Term and Reapplication of Biochar on Soil Bacteria. Sci. Total Environ. 2018, 636, 142–151. [Google Scholar] [CrossRef]
- Zou, Z.; Fan, L.; Li, X.; Dong, C.; Zhang, L.; Zhang, L.; Fu, J.; Han, W.; Yan, P. Response of Plant Root Growth to Biochar Amendment: A Meta-Analysis. Agronomy 2021, 11, 2442. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.; Luo, Y. Biochar and Bacteria Inoculated Biochar Enhanced Cd and Cu Immobilization and Enzymatic Activity in a Polluted Soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of Biochar on Soil Available Inorganic Nitrogen: A Review and Meta-Analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of Biochar Amendment on Sorption and Leaching of Nitrate, Ammonium, and Phosphate in a Sandy Soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef]
- Wang, L.; Xue, C.; Nie, X.; Liu, Y.; Chen, F. Effects of Biochar Application on Soil Potassium Dynamics and Crop Uptake. J. Plant Nutr. Soil Sci. 2018, 181, 635–643. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Z.; Ma, Y.; Liu, J.; Liu, F. Effects of Biochar Amendment and Reduced Irrigation on Growth, Physiology, Water-Use Efficiency and Nutrients Uptake of Tobacco (Nicotiana tabacum L.) on Two Different Soil Types. Sci. Total Environ. 2021, 770, 144769. [Google Scholar] [CrossRef]
- Naisse, C.; Girardin, C.; Lefevre, R.; Pozzi, A.; Maas, R.; Stark, A.; Rumpel, C. Effect of Physical Weathering on the Carbon Sequestration Potential of Biochars and Hydrochars in Soil. GCB Bioenergy 2015, 7, 488–496. [Google Scholar] [CrossRef]
- Mia, S.; Dijkstra, F.A.; Singh, B. Chapter One—Long-Term Aging of Biochar: A Molecular Understanding with Agricultural and Environmental Implications. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 141, pp. 1–51. [Google Scholar]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-Mediated Changes in Soil Quality and Plant Growth in a Three Year Field Trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Ye, L.; Camps-Arbestain, M.; Shen, Q.; Lehmann, J.; Singh, B.; Sabir, M. Biochar Effects on Crop Yields with and without Fertilizer: A Meta-Analysis of Field Studies Using Separate Controls. Soil Use Manag. 2020, 36, 2–18. [Google Scholar] [CrossRef]
- Brennan, A.; Jiménez, E.M.; Puschenreiter, M.; Alburquerque, J.A.; Switzer, C. Effects of Biochar Amendment on Root Traits and Contaminant Availability of Maize Plants in a Copper and Arsenic Impacted Soil. Plant Soil 2014, 379, 351–360. [Google Scholar] [CrossRef]
- Olmo, M.; Villar, R. Changes in Root Traits Explain the Variability of Biochar Effects on Fruit Production in Eight Agronomic Species. Org. Agric. 2019, 9, 139–153. [Google Scholar] [CrossRef]
- Weemstra, M.; Mommer, L.; Visser, E.J.W.; van Ruijven, J.; Kuyper, T.W.; Mohren, G.M.J.; Sterck, F.J. Towards a Multidimensional Root Trait Framework: A Tree Root Review. New Phytol. 2016, 211, 1159–1169. [Google Scholar] [CrossRef]
- White, P.J.; George, T.S.; Dupuy, L.X.; Karley, A.J.; Valentine, T.A.; Wiesel, L.; Wishart, J. Root Traits for Infertile Soils. Front. Plant Sci. 2013, 4, 50349. [Google Scholar] [CrossRef]
- Minden, V.; Olde Venterink, H. Plant Traits and Species Interactions along Gradients of N, P and K Availabilities. Funct. Ecol. 2019, 33, 1611–1626. [Google Scholar] [CrossRef]
- Vanek, S.J.; Lehmann, J. Phosphorus Availability to Beans via Interactions between Mycorrhizas and Biochar. Plant Soil 2015, 395, 105–123. [Google Scholar] [CrossRef]
- Mohamed, I.; El-Meihy, R.; Ali, M.; Chen, F.; Raleve, D. Interactive Effects of Biochar and Micronutrients on Faba Bean Growth, Symbiotic Performance, and Soil Properties. J. Plant Nutr. Soil Sci. 2017, 180, 729–738. [Google Scholar] [CrossRef]
- Xiu, L.; Zhang, W.; Wu, D.; Sun, Y.; Zhang, H.; Gu, W.; Wang, Y.; Meng, J.; Chen, W. Biochar Can Improve Biological Nitrogen Fixation by Altering the Root Growth Strategy of Soybean in Albic Soil. Sci. Total Environ. 2021, 773, 144564. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, L.; Riaz, M.; Xia, H.; Jiang, C. Biochar Amendment Improved Fruit Quality and Soil Properties and Microbial Communities at Different Depths in Citrus Production. J. Clean. Prod. 2021, 292, 126062. [Google Scholar] [CrossRef]
- Domene, X.; Mattana, S.; Hanley, K.; Enders, A.; Lehmann, J. Medium-Term Effects of Corn Biochar Addition on Soil Biota Activities and Functions in a Temperate Soil Cropped to Corn. Soil Biol. Biochem. 2014, 72, 152–162. [Google Scholar] [CrossRef]
- Eberhard, S.; Finazzi, G.; Wollman, F.-A. The Dynamics of Photosynthesis. Annu. Rev. Genet. 2008, 42, 463–515. [Google Scholar] [CrossRef]
- Guo, L.; Bornø, M.L.; Niu, W.; Liu, F. Biochar Amendment Improves Shoot Biomass of Tomato Seedlings and Sustains Water Relations and Leaf Gas Exchange Rates under Different Irrigation and Nitrogen Regimes. Agric. Water Manag. 2021, 245, 106580. [Google Scholar] [CrossRef]
- Feng, W.; Yang, F.; Cen, R.; Liu, J.; Qu, Z.; Miao, Q.; Chen, H. Effects of Straw Biochar Application on Soil Temperature, Available Nitrogen and Growth of Corn. J. Environ. Manag. 2021, 277, 111331. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, J.; Wang, Y.; Yang, Q.; Chen, T.; Chen, Y.; Chi, D.; Xia, G.; Siddique, K.H.M.; Wang, T. Photosynthesis, Chlorophyll Fluorescence, and Yield of Peanut in Response to Biochar Application. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef]
- Guo, L.; Yu, H.; Kharbach, M.; Wang, J. The Response of Nutrient Uptake, Photosynthesis and Yield of Tomato to Biochar Addition under Reduced Nitrogen Application. Agronomy 2021, 11, 1598. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar Increases Soil Microbial Biomass with Changes in Extra- and Intracellular Enzyme Activities: A Global Meta-Analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Gao, Y.; Shao, G.; Yang, Z.; Zhang, K.; Lu, J.; Wang, Z.; Wu, S.; Xu, D. Influences of Soil and Biochar Properties and Amount of Biochar and Fertilizer on the Performance of Biochar in Improving Plant Photosynthetic Rate: A Meta-Analysis. Eur. J. Agron. 2021, 130, 126345. [Google Scholar] [CrossRef]




| Factor | pH | EC (ms cm−1) | TN (g kg−1) | TP (g kg−1) | TK (g kg−1) | CEC (cmol kg−1) |
|---|---|---|---|---|---|---|
| BC (0) | 5.31 ± 0.012 g | 2.04 ± 0.044 a | 2.998 ± 0.113 d | 0.941 ± 0.034 g | 4.547 ± 0.263 g | 16.869 ± 0.13 f |
| BC (20) | 5.523 ± 0.02 f | 1.98 ± 0.021 a | 3.303 ± 0.08 c | 2.86 ± 0.046 f | 5.964 ± 0.041 f | 16.879 ± 0.102 f |
| BC (30) | 5.933 ± 0.046 e | 1.99 ± 0.012 a | 3.379 ± 0.018 bc | 4.688 ± 0.013 e | 6.89 ± 0.015 e | 17.603 ± 0.132 e |
| BC (40) | 6.267 ± 0.02 d | 2.013 ± 0.023 a | 3.393 ± 0.011 bc | 6.71 ± 0.024 d | 7.495 ± 0.005 d | 18.551 ± 0.07 d |
| BC (50) | 6.707 ± 0.009 c | 2.01 ± 0.012 a | 3.508 ± 0.041 b | 8.023 ± 0.054 c | 8.005 ± 0.064 c | 19.701 ± 0.102 c |
| BC (60) | 7.077 ± 0.02 b | 2.03 ± 0.01 a | 3.51 ± 0.007 b | 8.966 ± 0.078 b | 8.767 ± 0.04 b | 21.179 ± 0.113 b |
| BC (100) | 8.667 ± 0.057 a | 1.97 ± 0.015 a | 4.015 ± 0.04 a | 15.079 ± 0.158 a | 11.72 ± 0.306 a | 27.163 ± 0.148 a |
| Factor | BD (g cm−3) | TPO (%) | VP (%) | WHP (%) | AWR (%) |
|---|---|---|---|---|---|
| BC (0) | 0.387 ± 0.003 a | 50.19% ± 0.13% e | 11.64% ± 0.39% a | 38.55% ± 0.52% e | 0.302 ± 0.014 a |
| BC (20) | 0.372 ± 0.002 b | 53.85% ± 0.72% d | 11.04% ± 0.57% ab | 42.81% ± 1.28% d | 0.259 ± 0.021 b |
| BC (30) | 0.368 ± 0.002 b | 54.53% ± 0.14% d | 10.88% ± 0.18% ab | 43.65% ± 0.21% d | 0.249 ± 0.005 b |
| BC (40) | 0.368 ± 0.003 b | 53.42% ± 0.53% d | 11.21% ± 0.34% ab | 42.21% ± 0.79% d | 0.266 ± 0.013 b |
| BC (50) | 0.363 ± 0.004 b | 58.71% ± 0.19% c | 10.20% ± 0.10% bc | 48.51% ± 0.29% c | 0.21 ± 0.003 c |
| BC (60) | 0.344 ± 0.003 c | 62.12% ± 0.12% b | 9.82% ± 0.27% cd | 52.30% ± 0.08% b | 0.188 ± 0.006 c |
| BC (100) | 0.285 ± 0.003 d | 74.86% ± 1.29% a | 9.00% ± 0.23% d | 65.86% ± 1.48% a | 0.137 ± 0.006 d |
| Factor | TRL (cm) | TPA (cm2) | TSA (cm2) | AD (mm) | RV (cm3) |
|---|---|---|---|---|---|
| BC (0) | 610.878 ± 7.8 d | 33.499 ± 0.808 d | 41.079 ± 0.809 d | 0.548 ± 0.011 | 3.951 ± 0.193 d |
| BC (20) | 762.032 ± 20.977 c | 41.527 ± 0.487 c | 49.819 ± 0.551 c | 0.544 ± 0.02 | 5.657 ± 0.252 bc |
| BC (30) | 838.686 ± 13.192 b | 45.936 ± 1.277 b | 58.015 ± 1.731 b | 0.522 ± 0.003 | 6.851 ± 0.495 a |
| BC (40) | 909.625 ± 10.536 a | 53.171 ± 1.372 a | 66.549 ± 1.48 a | 0.521 ± 0.003 | 7.34 ± 0.247 a |
| BC (50) | 936.336 ± 18.439 a | 54.085 ± 1.499 a | 64.537 ± 2.311 a | 0.529 ± 0.004 | 7.56 ± 0.435 a |
| BC (60) | 910.123 ± 6.33 a | 52.691 ± 1.325 a | 63.611 ± 0.856 a | 0.522 ± 0.01 | 6.555 ± 0.249 ab |
| BC (100) | 826.728 ± 5.672 b | 45.029 ± 0.538 b | 59.187 ± 0.502 b | 0.517 ± 0.009 | 5.358 ± 0.321 c |
| Factor | RT (k plant−1) | RF (k plant−1) | RC (k plant−1) | SRL (cm g−1) | SRA (cm2 g−1) | RTD (g cm−3) |
|---|---|---|---|---|---|---|
| BC (0) | 7.767 ± 0.096 d | 8.997 ± 0.057 e | 1.602 ± 0.01 c | 221.926 ± 2.553 b | 14.928 ± 0.326 c | 1.524 ± 0.042 |
| BC (20) | 8.684 ± 0.121 c | 10.315 ± 0.145 d | 1.945 ± 0.037 a | 250.92 ± 5.442 a | 16.452 ± 0.523 b | 1.448 ± 0.046 |
| BC (30) | 9.063 ± 0.053 c | 10.852 ± 0.184 c | 1.88 ± 0.009 b | 248.188 ± 4.792 a | 17.154 ± 0.441 ab | 1.458 ± 0.06 |
| BC (40) | 10.311 ± 0.179 a | 11.888 ± 0.144 b | 1.894 ± 0.007 ab | 250.165 ± 4.482 a | 18.295 ± 0.422 a | 1.435 ± 0.036 |
| BC (50) | 10.545 ± 0.328 a | 12.339 ± 0.173 a | 1.844 ± 0.031 b | 256.388 ± 11.276 a | 17.604 ± 0.688 ab | 1.431 ± 0.064 |
| BC (60) | 9.757 ± 0.097 b | 12.192 ± 0.061 ab | 1.881 ± 0.006 b | 254.969 ± 6.349 a | 17.798 ± 0.277 ab | 1.456 ± 0.033 |
| BC (100) | 9.093 ± 0.076 c | 11.803 ± 0.105 b | 1.863 ± 0.015 b | 244.138 ± 1.633 a | 17.485 ± 0.261 ab | 1.496 ± 0.05 |
| Index | Treatment | ||||||
|---|---|---|---|---|---|---|---|
| BC (0) | BC (20) | BC (30) | BC (40) | BC (50) | BC (60) | BC (100) | |
| PHInc | 0.131 | 0.606 | 0.749 | 0.990 | 0.701 | 0.569 | 0.374 |
| GDInc | 0.483 | 0.804 | 0.859 | 0.575 | 0.439 | 0.156 | 0.121 |
| LFW | 0.068 | 0.575 | 0.690 | 0.893 | 0.914 | 0.655 | 0.204 |
| RFW | 0.146 | 0.554 | 0.676 | 0.725 | 0.613 | 0.429 | 0.034 |
| SFW | 0.024 | 0.385 | 0.629 | 0.558 | 0.616 | 0.318 | 0.396 |
| TFW | 0.010 | 0.485 | 0.639 | 0.721 | 0.666 | 0.420 | 0.044 |
| LDW | 0.305 | 0.504 | 0.569 | 0.762 | 0.841 | 0.487 | 0.202 |
| RDW | 0.146 | 0.383 | 0.487 | 0.699 | 0.542 | 0.155 | 0.048 |
| SDW | 0.054 | 0.452 | 0.659 | 0.642 | 0.699 | 0.409 | 0.578 |
| TDW | 0.082 | 0.380 | 0.511 | 0.687 | 0.643 | 0.240 | 0.128 |
| SUG | 0.137 | 0.860 | 0.485 | 0.696 | 0.562 | 0.330 | 0.247 |
| PROT | 0.187 | 0.612 | 0.678 | 0.431 | 0.307 | 0.258 | 0.031 |
| Chla | 0.462 | 0.759 | 0.815 | 0.678 | 0.437 | 0.157 | 0.061 |
| Chlb | 0.086 | 0.758 | 0.787 | 0.550 | 0.611 | 0.859 | 0.253 |
| a/b | 0.186 | 0.606 | 0.667 | 0.419 | 0.308 | 0.237 | 0.032 |
| TRL | 0.035 | 0.441 | 0.647 | 0.837 | 0.909 | 0.839 | 0.614 |
| TPA | 0.033 | 0.377 | 0.565 | 0.875 | 0.914 | 0.855 | 0.527 |
| TSA | 0.076 | 0.364 | 0.633 | 0.913 | 0.847 | 0.817 | 0.671 |
| AD | 0.523 | 0.487 | 0.277 | 0.267 | 0.347 | 0.275 | 0.232 |
| RV | 0.064 | 0.187 | 0.564 | 0.754 | 0.771 | 0.743 | 0.650 |
| SRL | 0.040 | 0.285 | 0.566 | 0.782 | 0.820 | 0.728 | 0.567 |
| SPA | 0.011 | 0.526 | 0.477 | 0.512 | 0.623 | 0.598 | 0.405 |
| RTD | 0.114 | 0.412 | 0.549 | 0.773 | 0.637 | 0.675 | 0.614 |
| RT | 0.744 | 0.500 | 0.530 | 0.462 | 0.450 | 0.524 | 0.643 |
| RF | 0.051 | 0.286 | 0.383 | 0.702 | 0.762 | 0.560 | 0.390 |
| RC | 0.030 | 0.361 | 0.496 | 0.756 | 0.870 | 0.833 | 0.735 |
| Tr | 0.010 | 0.485 | 0.639 | 0.721 | 0.666 | 0.420 | 0.044 |
| Pn | 0.305 | 0.504 | 0.569 | 0.762 | 0.841 | 0.487 | 0.202 |
| Ci | 0.146 | 0.383 | 0.487 | 0.699 | 0.542 | 0.155 | 0.048 |
| Gsw | 0.054 | 0.452 | 0.659 | 0.642 | 0.699 | 0.409 | 0.578 |
| AS | 0.158 | 0.486 | 0.601 | 0.677 | 0.656 | 0.535 | 0.367 |
| Rank | 7 | 5 | 3 | 1 | 2 | 4 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, M.; Chen, X.; Pan, Y.; Wang, S.; Xue, L.; Duan, Y.; Ahmad, S.; Zhou, Y.; Zhao, K.; Peng, D. Biochar Effectively Promoted Growth of Ardisia crenata by Affecting the Soil Physicochemical Properties. Plants 2024, 13, 1736. https://doi.org/10.3390/plants13131736
Niu M, Chen X, Pan Y, Wang S, Xue L, Duan Y, Ahmad S, Zhou Y, Zhao K, Peng D. Biochar Effectively Promoted Growth of Ardisia crenata by Affecting the Soil Physicochemical Properties. Plants. 2024; 13(13):1736. https://doi.org/10.3390/plants13131736
Chicago/Turabian StyleNiu, Muqi, Xiuming Chen, Yun Pan, Shunshun Wang, Luyu Xue, Yanru Duan, Sagheer Ahmad, Yuzhen Zhou, Kai Zhao, and Donghui Peng. 2024. "Biochar Effectively Promoted Growth of Ardisia crenata by Affecting the Soil Physicochemical Properties" Plants 13, no. 13: 1736. https://doi.org/10.3390/plants13131736
APA StyleNiu, M., Chen, X., Pan, Y., Wang, S., Xue, L., Duan, Y., Ahmad, S., Zhou, Y., Zhao, K., & Peng, D. (2024). Biochar Effectively Promoted Growth of Ardisia crenata by Affecting the Soil Physicochemical Properties. Plants, 13(13), 1736. https://doi.org/10.3390/plants13131736







