Exploring the Neuroprotective Potential of Desmodium Species: Insights into Radical Scavenging Capacity and Mechanisms against 6-OHDA-Induced Neurotoxicity
Abstract
:1. Introduction
2. Results
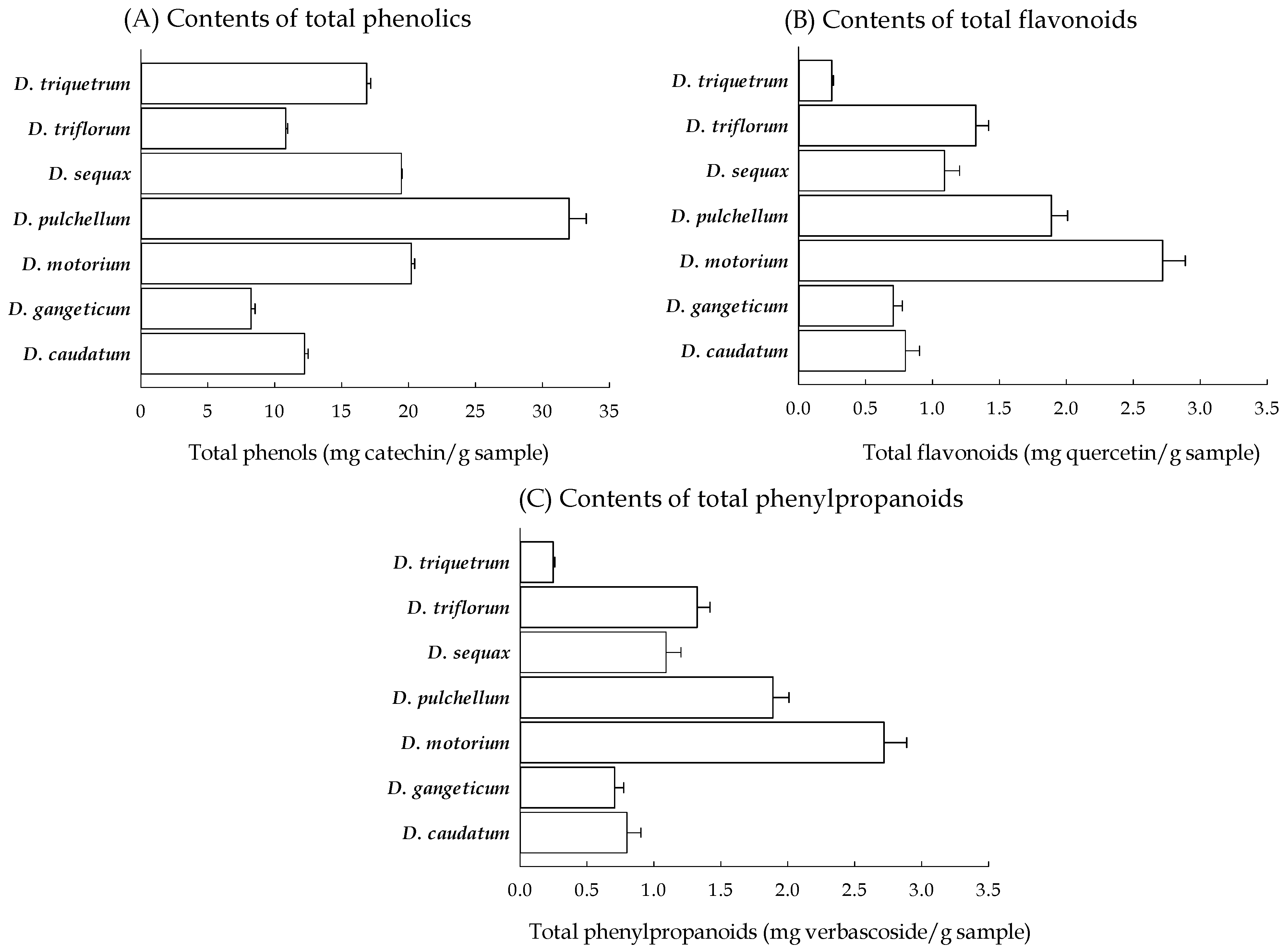
2.1. Antioxidant Phytoconstituent Contents of Seven Desmodium Plants
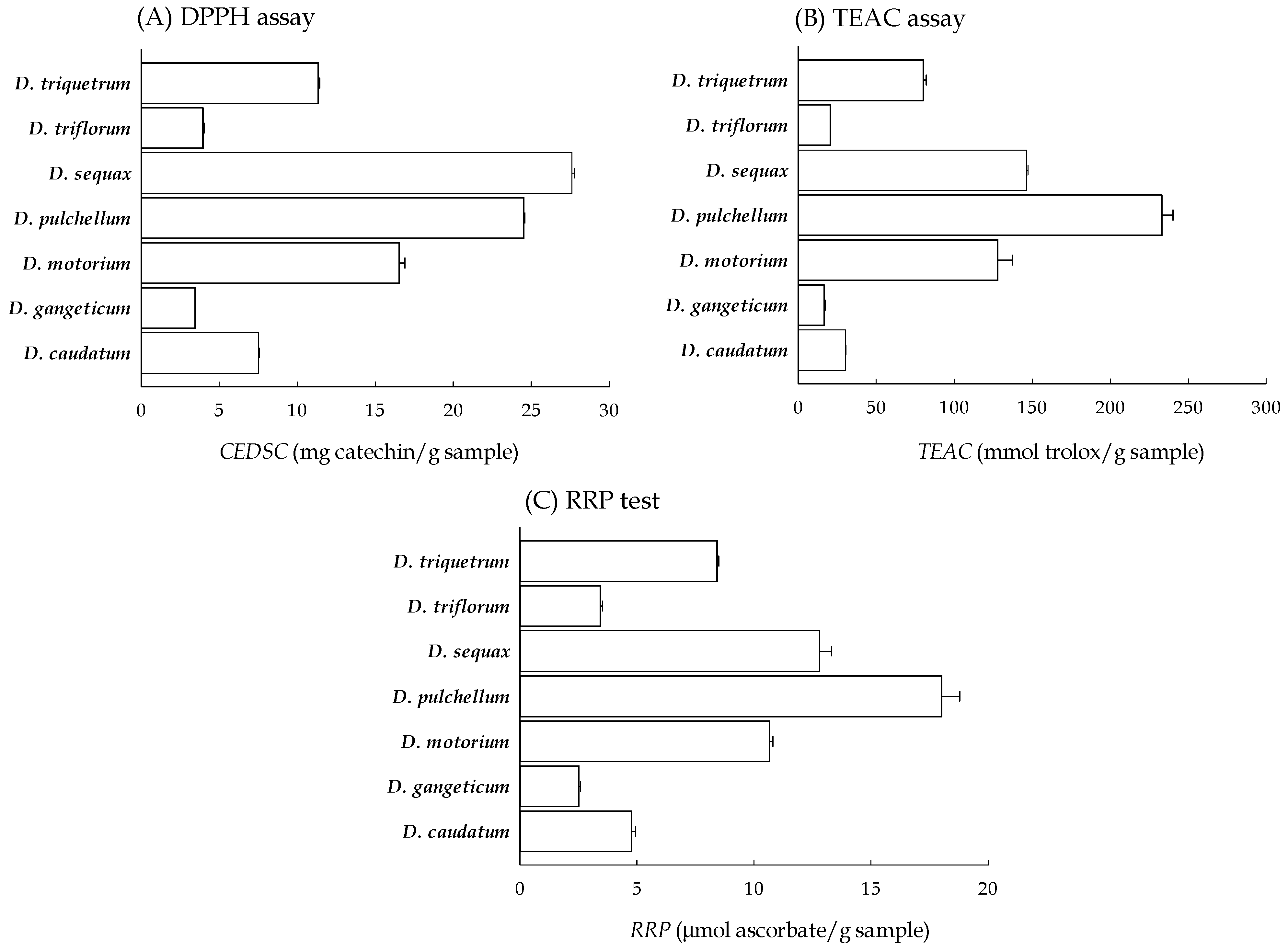
2.2. Radical Scavenging Capacities of Seven Desmodium Plants
2.3. MAO Inhibitory Activities of Seven Desmodium Plants
2.4. 6-OHDA Auto-Oxidation Inhibition of Three Desmodium Plants
2.5. Protective Effects against 6-OHDA-Induced or Rotenone-Induced Neurotoxicity in SH-SY5Y Cells
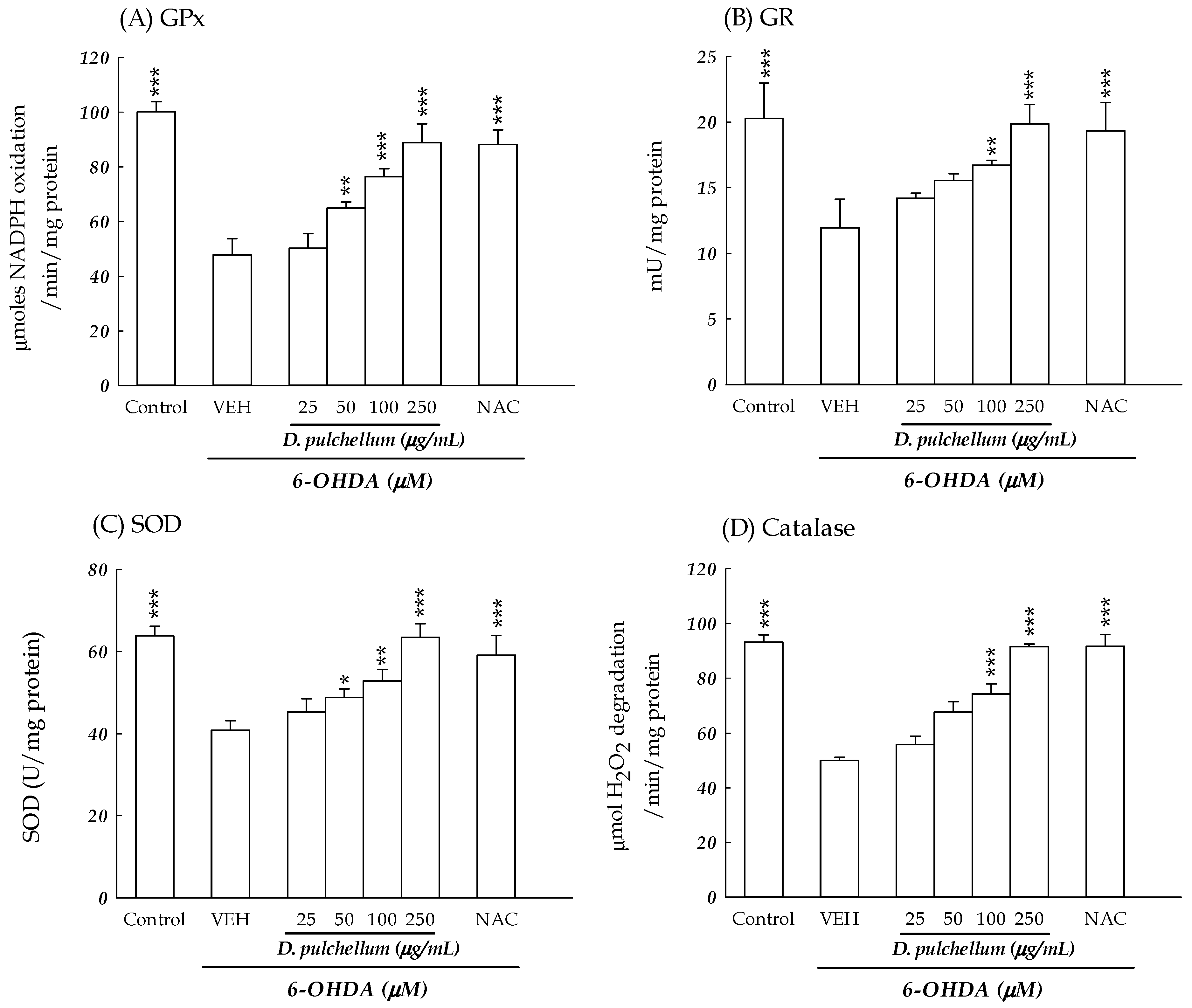
2.6. Intracellular ROS Levels and Antioxidant Enzymes Activities in 6-OHDA-Treated SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Plant Collection and Filtrate Preparation
4.2. Antioxidant Phytoconstituent Contents
4.2.1. Total Phenolic Contents
4.2.2. Total Flavonoid Contents
4.2.3. Total Phenylpropanoid Contents
4.3. Radical Scavenging Capacities
4.3.1. DPPH Radical Scavenging Capacities
4.3.2. ABTS Radical Scavenging Capacities
4.3.3. RRP Assay
4.3.4. Superoxide Scavenging Capacities
4.3.5. Hydrogen Peroxide Scavenging Capacities
4.3.6. Hydroxyl Radical Scavenging Capacities
4.4. MAO Inhibitory Activities
4.5. 6-OHDA Auto-Oxidation Inhibition
4.6. Protection against 6-OHDA-Induced Neurotoxicity in SH-SY5Y Cells
4.6.1. Cell Culture
4.6.2. Estimation of Cell Viability
4.6.3. Estimation of Intracellular ROS Levels
4.6.4. Estimation of Intracellular Antioxidant Makers
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Regensburger, M.; Ip, C.W.; Kohl, Z.; Schrader, C.; Urban, P.P.; Kassubek, J.; Jost, W.H. Clinical benefit of MAO-B and COMT inhibition in Parkinson’s disease: Practical considerations. J. Neural. Transm. 2023, 130, 847–861. [Google Scholar] [CrossRef] [PubMed]
- Bhawna; Kumar, A.; Bhatia, M.; Kapoor, A.; Kumar, P.; Kumar, S. Monoamine oxidase inhibitors: A concise review with special emphasis on structure activity relationship studies. Eur. J. Med. Chem. 2022, 242, 114655. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson’s Disease: Past, Present, and Future. J. Parkinsons Dis. 2022, 12, 477–493. [Google Scholar] [CrossRef]
- Mannan, A.; Singh, T.G.; Singh, V.; Garg, N.; Kaur, A.; Singh, M. Insights into the Mechanism of the Therapeutic Potential of Herbal Monoamine Oxidase Inhibitors in Neurological Diseases. Curr. Drug Targets 2022, 23, 286–310. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.; Teo, W.P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef]
- Deus, C.M.; Teixeira, J.; Raimundo, N.; Tucci, P.; Borges, F.; Saso, L.; Oliveira, P.J. Modulation of cellular redox environment as a novel therapeutic strategy for Parkinson’s disease. Eur. J. Clin. Investig. 2022, 52, e13820. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Gutierrez, M.T.; Serrano-Garcia, N.; Orozco-Ibarra, M. Rotenone-Induced Model of Parkinson’s Disease: Beyond Mitochondrial Complex I Inhibition. Mol. Neurobiol. 2023, 60, 1929–1948. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, C.; Hu, C.; Rahman, K.; Qin, L. The genus Desmodium (Fabaceae)-traditional uses in Chinese medicine, phytochemistry and pharmacology. J. Ethnopharmacol. 2011, 138, 314–332. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.P.; Lu, S.Y. DESMODIUM Desv. In Flora of Taiwan, 2nd ed.; Huang, T.C., Ed.; Editorial Committee of the Flora of Taiwan, Department of Botany, National Taiwan University: Taipei, Taiwan, 2000; Volume 33, pp. 251–270. [Google Scholar]
- Singh, V.; Singh, R.; Singh, M.P.; Katrolia, A. Therapeutic Role of Desmodium Species on its Isolated Flavonoids. Curr. Mol. Med. 2024, 24, 74–84. [Google Scholar] [CrossRef]
- Le Dang, Q.; Vu, H.D.; Nguyen, V.M.; Choi, G.J.; Hoa, L.T.P.; Dung, D.T.; Van Kiem, P.; Nhiem, N.X.; De Tran, Q.; Nguyen, Q.C.; et al. Desmodinosides A-E: New Flavonoid C-glycosides from Desmodium heterocarpon var. stigosum with hepatoprotective and antifungal activity. Fitoterapia 2023, 169, 105609. [Google Scholar] [CrossRef]
- Xu, Q.N.; Zhu, D.; Wang, G.H.; Lin, T.; Sun, C.L.; Ding, R.; Tian, W.J.; Chen, H.F. Phenolic glycosides and flavonoids with antioxidant and anticancer activities from Desmodium caudatum. Nat. Prod. Res. 2021, 35, 4534–4541. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Chae, D.; Hyun, J.W.; Kang, H.K.; Koh, Y.S.; Kim, Y.H. Anti-inflammatory and antioxidant activities of phenolic compounds from Desmodium caudatum leaves and stems. Arch. Pharm. Res. 2014, 37, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.X.; Sheng, W.B.; Liu, X.Y.; Cui, P.W.; Gong, L.M.; Xie, Q.L.; Wang, W.M.; Li, B.; Wang, W.; Zhou, X.D. The traditional ethnic herb Tadehagi triquetrum from China: A review of its phytochemistry and pharmacological activities. Pharm. Biol. 2022, 60, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.C.; Yue, S.J.; Guo, Z.L.; Xin, L.T.; Wang, C.Y.; Zhao, D.L.; Guan, H.S.; Wang, C.Y. Phytochemical Composition, Hepatoprotective, and Antioxidant Activities of Phyllodium pulchellum (L.) Desv. Molecules 2018, 23, 1361. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, C.; Huo, X.K.; Dong, P.P.; Zhang, B.J.; Zhang, H.L.; Huang, S.S.; Zhang, B.; Yu, S.M.; Zhong, M.; et al. Effect of Alkaloids Isolated from Phyllodium pulchellum on Monoamine Levels and Monoamine Oxidase Activity in Rat Brain. Evid. Based Complement. Altern. Med. 2016, 2016, 6826175. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhao, W.; Yang, Z.; Subbiah, V.; Suleria, H.A.R. Extraction and characterization of phenolic compounds and their potential antioxidant activities. Environ. Sci. Pollut. Res. Int. 2022, 29, 81112–81129. [Google Scholar] [CrossRef] [PubMed]
- Najera-Maldonado, J.M.; Salazar, R.; Alvarez-Fitz, P.; Acevedo-Quiroz, M.; Flores-Alfaro, E.; Hernandez-Sotelo, D.; Espinoza-Rojo, M.; Ramirez, M. Phenolic Compounds of Therapeutic Interest in Neuroprotection. J. Xenobiot. 2024, 14, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Dominguez-Lopez, I.; Lamuela-Raventos, R.M. The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of Aluminium Complexation Reaction for Flavonoid Content Assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Neelam; Khatkar, A.; Sharma, K.K. Phenylpropanoids and its derivatives: Biological activities and its role in food, pharmaceutical and cosmetic industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.W.; Chang, H.C.; Ching, H.; Lien, J.C.; Huang, H.C.; Wu, C.R. Antioxidant Effects and Phytochemical Properties of Seven Taiwanese Cirsium Species Extracts. Molecules 2021, 26, 3935. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Andres, C.M.C.; Perez de la Lastra, J.M.; Andres Juan, C.; Plou, F.J.; Perez-Lebena, E. Superoxide Anion Chemistry-Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Clement, M.V.; Long, L.H. Hydrogen peroxide in the human body. FEBS Lett. 2000, 486, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, G.; Demiryurek, A.T. Ferrous iron-induced luminol chemiluminescence: A method for hydroxyl radical study. J. Pharmacol. Toxicol. Methods 1998, 39, 179–184. [Google Scholar] [CrossRef]
- Wu, C.R.; Chang, H.C.; Cheng, Y.D.; Lan, W.C.; Yang, S.E.; Ching, H. Aqueous Extract of Davallia mariesii Attenuates 6-Hydroxydopamine-Induced Oxidative Damage and Apoptosis in B35 Cells Through Inhibition of Caspase Cascade and Activation of PI3K/AKT/GSK-3beta Pathway. Nutrients 2018, 10, 1449. [Google Scholar] [CrossRef]
- Ioghen, O.C.; Ceafalan, L.C.; Popescu, B.O. SH-SY5Y Cell Line In Vitro Models for Parkinson Disease Research-Old Practice for New Trends. J. Integr. Neurosci. 2023, 22, 20. [Google Scholar] [CrossRef]
- El-Habta, R.; Af Bjerken, S.; Virel, A. N-acetylcysteine increases dopamine release and prevents the deleterious effects of 6-OHDA on the expression of VMAT2, alpha-synuclein, and tyrosine hydroxylase. Neurol. Res. 2024, 46, 406–415. [Google Scholar] [CrossRef]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2’,7’-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Chen, T.H.; Wang, H.C.; Chang, C.J.; Lee, S.Y. Mitochondrial Glutathione in Cellular Redox Homeostasis and Disease Manifestation. Int. J. Mol. Sci. 2024, 25, 1314. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Lai, S.C.; Ho, Y.L.; Huang, S.C.; Huang, T.H.; Lai, Z.R.; Wu, C.R.; Lian, K.Y.; Chang, Y.S. Antioxidant and antiproliferative activities of Desmodium triflorum (L.) DC. Am. J. Chin. Med. 2010, 38, 329–342. [Google Scholar] [CrossRef]
- Chidambaram, U.; Pachamuthu, V.; Natarajan, S.; Elango, B.; Suriyanarayanan; Ramkumar, K.M. In vitro evaluation of free radical scavenging activity of Codariocalyx motorius root extract. Asian Pac. J. Trop. Med. 2013, 6, 188–194. [Google Scholar] [CrossRef]
- Tsai, C.J.; Huang, G.J.; Chiu, T.H.; Huang, S.S.; Huang, S.C.; Huang, T.H.; Lai, S.C.; Lee, C.Y. Antioxidant Activities of Phenolic Components from Various Plants of Desmodium Species. Afr. J. Pharm. Pharmacol. 2011, 5, 468–476. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Rawat, A.K. An ethnomedicinal, phytochemical and pharmacological profile of Desmodium gangeticum (L.) DC. and Desmodium adscendens (Sw.) DC. J. Ethnopharmacol. 2011, 136, 283–296. [Google Scholar] [CrossRef]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of In Vitro and In Vivo Antioxidant Activities of Six Flavonoids with Similar Structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef]
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J. Nutr. Sci. Vitaminol. 2001, 47, 357–362. [Google Scholar] [CrossRef]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial Effects of Epigallocatechin-3-O-Gallate, Chlorogenic Acid, Resveratrol, and Curcumin on Neurodegenerative Diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Pan, D.; Jiang, Y.; Zhang, W.; Yao, X.; Dai, Y.; Yu, Y.; Yao, X. Target discovery of chlorogenic acid derivatives from the flower buds of Lonicera macranthoides and their MAO B inhibitory mechanism. Fitoterapia 2019, 134, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Vasyliev, G.S.; Vorobyova, V.I.; Linyucheva, O.V. Evaluation of Reducing Ability and Antioxidant Activity of Fruit Pomace Extracts by Spectrophotometric and Electrochemical Methods. J. Anal. Methods Chem. 2020, 2020, 8869436. [Google Scholar] [CrossRef]
- Cheng, Z.; Yan, G.; Li, Y.; Chang, W. Determination of antioxidant activity of phenolic antioxidants in a Fenton-type reaction system by chemiluminescence assay. Anal. Bioanal. Chem. 2003, 375, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Tanaka, Y.; Kabbash, A.; Fujioka, T.; Ishizu, T.; Yagi, A. Monoamine oxidase inhibitors from Gentiana lutea. Phytochemistry 2004, 65, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.; Al-Ghafari, A.; Al Doghaither, H.; Hashish, S.; Salama, M.; Mudyanselage, A.W.; James, L.; Carter, W.G. Differential Effects of Paraquat, Rotenone, and MPTP on Cellular Bioenergetics of Undifferentiated and Differentiated Human Neuroblastoma Cells. Brain Sci. 2023, 13, 1717. [Google Scholar] [CrossRef]
- Chang, H.C.; Liu, K.F.; Teng, C.J.; Lai, S.C.; Yang, S.E.; Ching, H.; Wu, C.R. Sophora Tomentosa Extract Prevents MPTP-Induced Parkinsonism in C57BL/6 Mice Via the Inhibition of GSK-3beta Phosphorylation and Oxidative Stress. Nutrients 2019, 11, 252. [Google Scholar] [CrossRef]

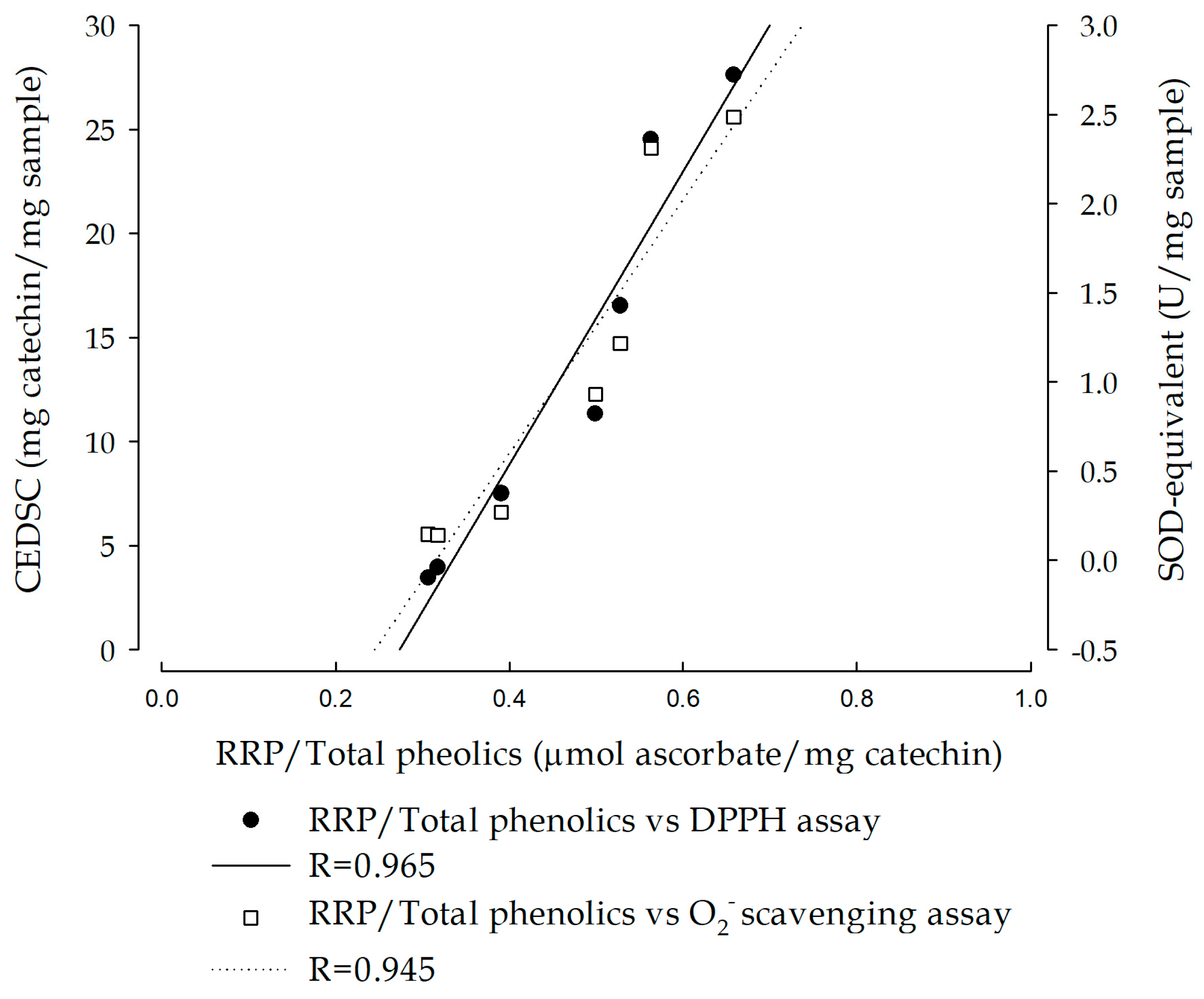




| DPPH | TEAC | O2− | H2O2 | OH− | RRP | TP | TF | |
|---|---|---|---|---|---|---|---|---|
| TEAC | 0.912 ** | |||||||
| O2− | 0.992 ** | 0.925 ** | ||||||
| H2O2 | 0.249 | 0.33 | 0.195 | |||||
| OH− | 0.033 | 0.068 | 0.064 | 0.252 | ||||
| RRP | 0.93 ** | 0.995 ** | 0.939 ** | 0.334 | 0.13 | |||
| TP | 0.835 * | 0.98 ** | 0.848 * | 0.362 | 0.139 | 0.978 ** | ||
| TF | 0.393 | 0.525 | 0.349 | 0.628 | −0.502 | 0.475 | 0.52 | |
| TPPs | 0.854 * | 0.903 * | 0.83 * | 0.537 | −0.169 | 0.892 * | 0.876 ** | 0.793 * |
| Samples | IC50 in MAO-A (mg/mL) | IC50 in MAO-B (mg/mL) | Ratio (MAO-B/MAO-A) |
|---|---|---|---|
| D. caudatum | 2.44 ± 0.25 | 4.41 ± 0.14 | 1.81 |
| D. gangeticum | 3.75 ± 0.10 | 5.45 ± 0.13 | 1.45 |
| D. motorium | 1.10 ± 0.10 | 2.31 ± 0.04 | 2.11 |
| D. pulchellum | 3.00 ± 0.08 | 6.23 ± 0.15 | 2.08 |
| D. sequax | 7.22 ± 0.67 | 1.13 ± 0.01 | 0.16 |
| D. triflorum | 4.29 ± 0.28 | 4.19 ± 0.11 | 0.98 |
| D. triquetrum | 2.94 ± 0.10 | 5.21 ± 0.08 | 1.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-C.; Lien, J.-C.; Hsueh, M.-C.; Wu, C.-R. Exploring the Neuroprotective Potential of Desmodium Species: Insights into Radical Scavenging Capacity and Mechanisms against 6-OHDA-Induced Neurotoxicity. Plants 2024, 13, 1742. https://doi.org/10.3390/plants13131742
Chang H-C, Lien J-C, Hsueh M-C, Wu C-R. Exploring the Neuroprotective Potential of Desmodium Species: Insights into Radical Scavenging Capacity and Mechanisms against 6-OHDA-Induced Neurotoxicity. Plants. 2024; 13(13):1742. https://doi.org/10.3390/plants13131742
Chicago/Turabian StyleChang, Hung-Chi, Jin-Cherng Lien, Min-Chung Hsueh, and Chi-Rei Wu. 2024. "Exploring the Neuroprotective Potential of Desmodium Species: Insights into Radical Scavenging Capacity and Mechanisms against 6-OHDA-Induced Neurotoxicity" Plants 13, no. 13: 1742. https://doi.org/10.3390/plants13131742
APA StyleChang, H.-C., Lien, J.-C., Hsueh, M.-C., & Wu, C.-R. (2024). Exploring the Neuroprotective Potential of Desmodium Species: Insights into Radical Scavenging Capacity and Mechanisms against 6-OHDA-Induced Neurotoxicity. Plants, 13(13), 1742. https://doi.org/10.3390/plants13131742







