The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy
Abstract
1. Introduction
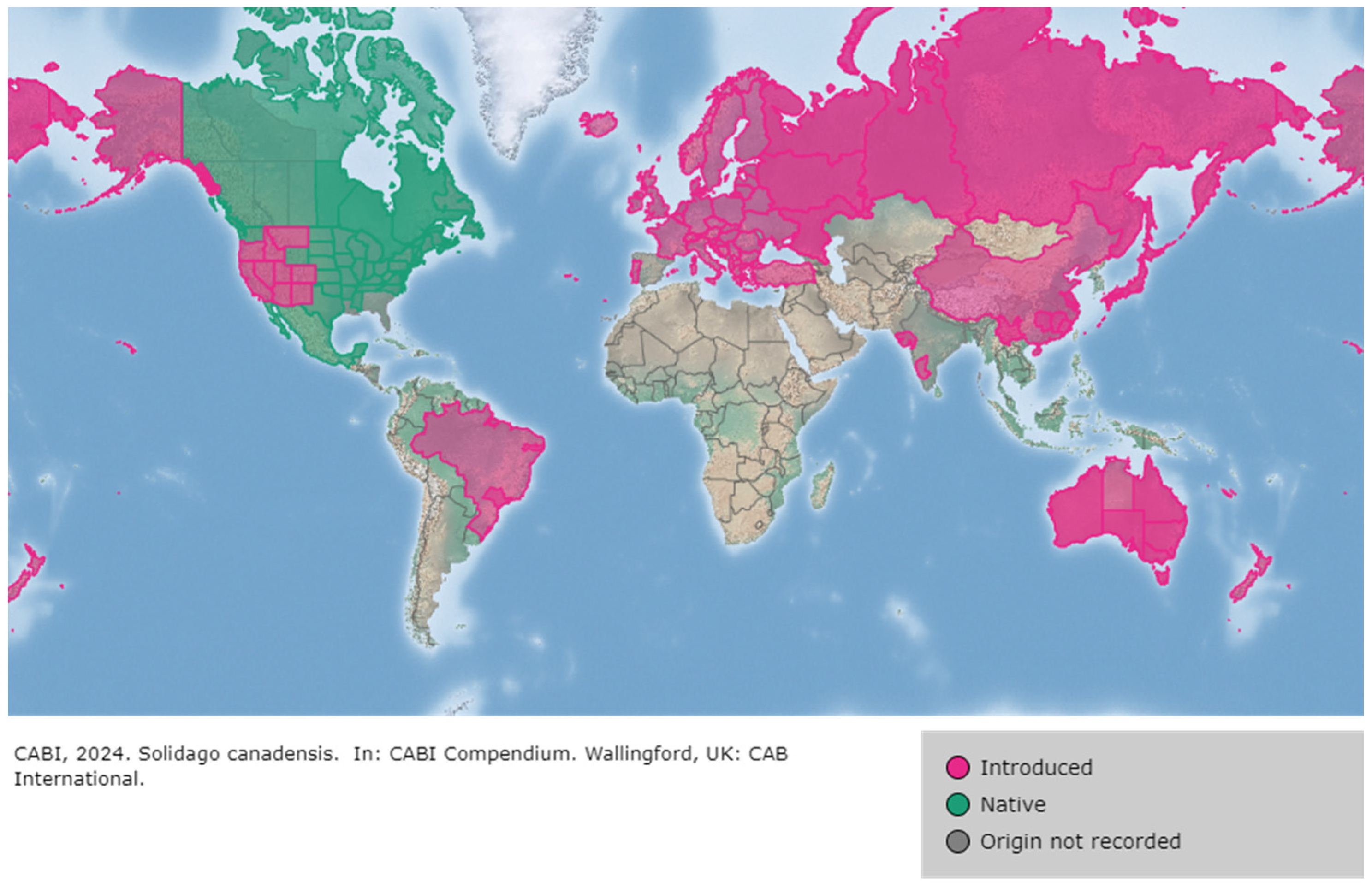
2. Solidago canadensis Origin and Distribution
3. Phytochemical Composition of Solidago canadensis
4. Behind the Invasiveness of S. canadensis
5. Ecosystem Services
5.1. Medicinal Ecosystem Services
5.2. Agriculture and Food Processing
5.3. Fuel
5.4. Others
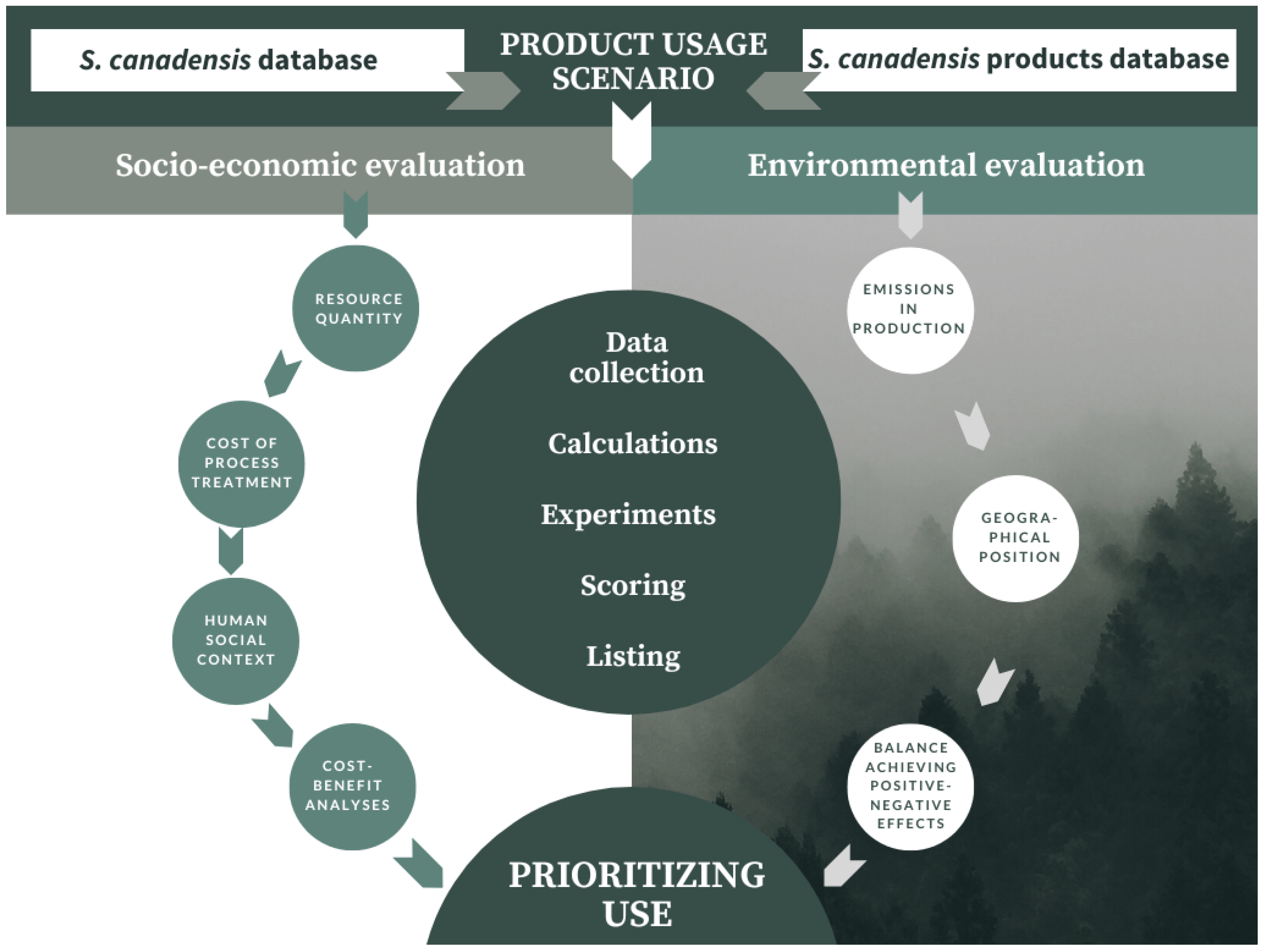
6. Solidago canadensis as a Resource—Validating Scenarios in Bioeconomy
7. Materials and Methods
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pejchar, L.; Mooney, H.A. Invasive Species, Ecosystem Services and Human Well-Being. Trends Ecol. Evol. 2009, 24, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Guido, A.; Pillar, V.D. Invasive Plant Removal: Assessing Community Impact and Recovery from Invasion. J. Appl. Ecol. 2017, 54, 1230–1237. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic Plant Invasions and the Enemy Release Hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Sladonja, B.; Poljuha, D.; Uzelac, M. Non-Native Invasive Species as Ecosystem Service Providers. In Ecosystem Services and Global Ecology; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Hegi, G. Illustrierte Flora von Mittel-Europa, Pteridophyta, Spermatophyta, Band III: Angiospermae, Dicotyledones 1, Teil 2, 1959–1979; Paul Parey: Berlin, Germany, 1979; ISBN 978-3-489-60020-6. [Google Scholar]
- Semple, J.C.; Beck, J.B. Revised Infrageneric Classification of Solidago (Asteraceae Astereae). Phytoneuron 2021, 10, 1–6. [Google Scholar]
- Kabuce, N.; Priede, N. NOBANIS—Invasive Alien Species Fact Sheet—Solidago canadensis. Available online: www.nobanis.org (accessed on 10 January 2024).
- Gallardo, B.; Bacher, S.; Bradley, B.; Comín, F.A.; Gallien, L.; Jeschke, J.M.; Sorte, C.J.B.; Vilà, M. InvasiBES: Understanding and Managing the Impacts of Invasive Alien Species on Biodiversity and Ecosystem Services. NeoBiota 2019, 50, 109–122. [Google Scholar] [CrossRef]
- EPPO Lists of Invasive Alien Plants. Available online: https://www.eppo.int/ACTIVITIES/invasive_alien_plants/iap_lists#iap (accessed on 12 January 2024).
- Popay, I.; Parker, C. Solidago canadensis (Canadian Goldenrod); CABI Compendium: Wallingford, UK, 2024. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, C.; Ma, L.; Qiang, S.; Silander, J.A.; Qi, L.L. Biotic Homogenization Caused by the Invasion of Solidago canadensis in China. J. Integr. Agric. 2013, 12, 835–845. [Google Scholar] [CrossRef]
- Dong, M.; Lu, J.Z.; Zhang, W.J.; Chen, J.K.; Li, B. Canada Goldenrod (Solidago canadensis): An Invasive Alien Weed Rapidly Spreading in China. J. Syst. Evol. 2006, 44, 72–85. [Google Scholar] [CrossRef]
- Denisow, B.; Wrzesien, M. The Importance of Field-Margin Location for Maintenance of Food Niches for Pollinators. J. Apic. Sci. 2015, 59, 27–37. [Google Scholar] [CrossRef]
- Mariychuk, R.; Gruľová, D.; Grishchenko, L.; Linnik, R.; Lisnyak, V. Green Synthesis Of Non-Spherical Gold Nanoparticles Using Solidago canadensis L. Extract. Appl. Nanosci. 2020, 10, 4817–4826. [Google Scholar] [CrossRef]
- Botha, T.L.; Elemike, E.E.; Horn, S.; Onwudiwe, D.C.; Giesy, J.P.; Wepener, V. Cytotoxicity of Ag, Au and Ag-Au Bimetallic Nanoparticles Prepared Using Golden Rod (Solidago canadensis) Plant Extract. Sci. Rep. 2019, 9, 4169. [Google Scholar] [CrossRef] [PubMed]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Invasive Solidago canadensis L. as a Resource of Valuable Biological Compounds. Potravin. Slovak J. Food Sci. 2019, 13, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Šutovská, M.; Capek, P.; Kocmálová, M.; Fraňová, S.; Pawlaczyk, I.; Gancarz, R. Characterization and Biological Activity of Solidago canadensis Complex. Int. J. Biol. Macromol. 2013, 52, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Leitner, P.; Fitz-Binder, C.; Mahmud-Ali, A.; Bechtold, T. Production of a Concentrated Natural Dye from Canadian Goldenrod (Solidago canadensis) Extracts. Dyes Pigments 2012, 93, 1416–1421. [Google Scholar] [CrossRef]
- Ye, J.-R.; Wang, N.; Wang, H.; Luo, H.; Ren, Y.; Shen, Q. Structure and Properties of Cellulose/Solidago canadensis L. Blend. Cellul. Chem. Technol. 2015, 49, 275–280. [Google Scholar]
- Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules 2019, 24, 1206. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Pan, S.H.; Zhu, X.W.; Tan, L.; Cao, Y.F. Anticancer Activity and Chemical Composition of Leaf Essential Oil from Solidago canadensis L. in China. Adv. Mater. Res. 2011, 347–353, 1584–1589. [Google Scholar] [CrossRef]
- Pimentel, D. Bioeconomics of Invasive Species: Integrating Ecology, Economics, Policy, and Management. BioScience 2009, 59, 1002–1003. [Google Scholar] [CrossRef]
- Zihare, L.; Blumberga, D. Insight into Bioeconomy. Solidago canadensis as a Valid Resource. Brief Review. Energy Procedia 2017, 128, 275–280. [Google Scholar] [CrossRef]
- Weber, E. The Dynamics of Plant Invasions: A Case Study of Three Exotic Goldenrod Species (Solidago L.) in Europe. J. Biogeogr. 1998, 25, 147–154. [Google Scholar] [CrossRef]
- EPPO Global Database. Available online: https://gd.eppo.int/taxon/SOOCA/distribution/CA (accessed on 12 February 2024).
- Moroń, D.; Lenda, M.; Skórka, P.; Szentgyörgyi, H.; Settele, J.; Woyciechowski, M. Wild Pollinator Communities Are Negatively Affected by Invasion of Alien Goldenrods in Grassland Landscapes. Biol. Conserv. 2009, 142, 1322–1332. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Shao, H.; Tang, S. Selected Aspects of Invasive Solidago canadensis with an Emphasis on Its Allelopathic Abilities: A Review. Chem. Biodivers. 2022, 19, e202200728. [Google Scholar] [CrossRef] [PubMed]
- Fursenco, C.; Calalb, T.; Uncu, L.; Dinu, M.; Ancuceanu, R. Solidago virgaurea L.: A Review of Its Ethnomedicinal Uses, Phytochemistry, and Pharmacological Activities. Biomolecules 2020, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of Two Invasive Plant Invaders in Europe (Solidago canadensis and Solidago gigantea) as Possible Sources of Botanical Insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Kasali, A.A.; Ekundayo, O.; Paul, C.; König, W.A. Epi-Cubebanes from Solidago canadensis. Phytochemistry 2002, 59, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Shelepova, O.; Vinogradova, Y.; Zaitchik, B.; Ruzhitsky, A.; Grygorieva, O.; Brindza, J. Constituents of the Essential Oil in Solidago canadensis L. from Eurasia. Potravin. Slovak J. Food Sci. 2018, 12, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.X.; He, W.; Wen, G.Y. The Constituents of the Essential Oil from Solidago canadensis L. Chin. Bull. Bot. 1999, 16, 178–181. [Google Scholar]
- Wang, K.J.; Li, N.; Chen, L.Z.; Yu, X.P. Chemical Constituents and Antifungal Activity of Essential Oil From Solidago canadensis. J. Plant Resour. Environ. 2006, 15, 34–36. [Google Scholar]
- Weyerstahl, P.; Marschall, H.; Christiansen, C.; Kalemba, D.; Góra, J. Constituents of the Essential Oil of Solidago canadensis (“Goldenrod”) from Poland—A Correction. Planta Med. 1993, 59, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Thiem, B. Constituents of the Essential Oils of Four Micropropagated Solidago Species. Flavour Fragr. J. 2004, 19, 40–43. [Google Scholar] [CrossRef]
- Nkuimi Wandjou, J.G.; Quassinti, L.; Gudžinskas, Z.; Nagy, D.U.; Cianfaglione, K.; Bramucci, M.; Maggi, F. Chemical Composition and Antiproliferative Effect of Essential Oils of Four Solidago Species (S. canadensis, S. gigantea, S. virgaurea and S.×niederederi). Chem. Biodivers. 2020, 17, e2000685. [Google Scholar] [CrossRef] [PubMed]
- Apati, P.; Szentmihályi, K.; Balázs, A.; Baumann, D.; Hamburger, M.; Kristó, T.S.; Szőke, É.; Kéry, Á. HPLC Analysis of the Flavonoids in Pharmaceutical Preparations from Canadian Goldenrod (Solidago canadensis). Chromatographia 2002, 56, S65–S68. [Google Scholar] [CrossRef]
- Woźniak, D.; Ślusarczyk, S.; Domaradzki, K.; Dryś, A.; Matkowski, A. Comparison of Polyphenol Profile and Antimutagenic and Antioxidant Activities in Two Species Used as Source of Solidaginis herba—Goldenrod. Chem. Biodiver. 2018, 15, e1800023. [Google Scholar] [CrossRef] [PubMed]
- Shelepova, O.; Vinogradova, Y.; Vergun, O.; Grygorieva, O.; Brindza, J. Assessment of Flavonoids and Phenolic Compound Accumulation in Invasive Solidago canadensis L. in Slovakia. Potravinarstvo Slovak. J. Food Sci. 2020, 14, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, M. The Chemical Relationship Between the Scent Features of Goldenrod (Solidago canadensis L.) Flower and Its Unifloral Honey. J. Food Compos. Anal. 2010, 23, 122–129. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Sonker, N.; Singh, P. Efficacy of Some Essential Oils Against Aspergillus flavus with Special Reference to Lippia alba Oil an Inhibitor of Fungal Proliferation and Aflatoxin B1 Production in Green Gram Seeds during Storage. J. Food Sci. 2016, 81, M928–M934. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Lybrand, D.; Xu, H.; Last, R.; Pichersky, E. How Plants Synthesize Pyrethrins: Safe and Biodegradable Insecticides. Trends Plant Sci. 2020, 25, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural Versatile Molecules With Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yadav, S.S. A Review On Health Benefits Of Phenolics Derived From Dietary Spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef] [PubMed]
- Zekič, J.; Vovk, I.; Glavnik, V. Extraction and Analyses of Flavonoids and Phenolic Acids from Canadian Goldenrod and Giant Goldenrod. Forests 2021, 12, 40. [Google Scholar] [CrossRef]
- Papp, I.; Apáti, P.; Andrasek, V.; Blázovics, A.; Balázs, A.; Kursinszki, L.; Kite, G.C.; Houghton, P.J.; Kéryl, Á. LC/MS Analysis of Antioxidant Plant Phenoloids. Chromatographia 2004, 60, 93–100. [Google Scholar] [CrossRef]
- Kelly, A.M.; de Oliveira, T.B.; Valverde, S.S. Determination of the Metabolic Profile of Solidago canadensis Using UFLC-PDA-ESI-TOF. Rodriguesia 2020, 71, e01062019. [Google Scholar] [CrossRef]
- Abdel Baki, P.M.; El-Sherei, M.M.; Khaleel, A.E.; Abdel Motaal, A.A.; Ibrahim Abdallah, H.M. Aquaretic Activity of Solidago canadensis L. Cultivated in Egypt and Determination of the Most Bioactive Fraction. Iran. J. Pharm. Res. 2019, 18, 922–937. [Google Scholar] [CrossRef] [PubMed]
- Radušienė, J.; Karpavičienė, B.; Vilkickytė, G.; Marksa, M.; Raudonė, L. Comparative Analysis of Root Phenolic Profiles and Antioxidant Activity of Five Native and Invasive Solidago L. Species. Plants 2024, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.J. Quarter-Century Explorations of Bioactive Polyphenols: Diverse Health Benefits. Front. Biosci. Landmark Ed. 2022, 27, 134. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of Dietary Polyphenols: The Role of Metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health Benefits of Polyphenols: A Concise Review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Avertseva, I.N.; Suleymanova, F.S.; Nesterova, O.V.; Reshetnyak, V.Y.; Matveenko, V.N.; Zhukov, P.A. Study of Polyphenolic Compounds in Extracts from Flowers and Leaves of Canadian Goldenrod and Dwarf Goldenrod (Solidago canadensis L. and Solidago nana Nitt.). Mosc. Univ. Chem. Bull. 2020, 75, 47–51. [Google Scholar] [CrossRef]
- Catford, J.A.; Jansson, R.; Nilsson, C. Reducing Redundancy in Invasion Ecology by Integrating Hypotheses into a Single Theoretical Framework. Divers. Distrib. 2009, 15, 22–40. [Google Scholar] [CrossRef]
- Weiher, E. On the Status of Restoration Science: Obstacles and Opportunities. Restor. Ecol. 2007, 15, 340–343. [Google Scholar] [CrossRef]
- Vilà, M.; Weiner, J. Are Invasive Plant Species Better Competitors Than Native Plant Species?—Evidence from Pair-Wise Experiments. Oikos 2004, 105, 229–238. [Google Scholar] [CrossRef]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do Allelopathic Compounds in Invasive Solidago canadensis s.l. Restrain the Native European Flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Schmid, B. No Evidence for an Evolutionary Increased Competitive Ability in an Invasive Plant. Ecology 2003, 84, 2816–2823. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, Y.; Zhang, S.; Hu, W. Simulated Warming Enhances Biological Invasion of Solidago canadensis and Bidens frondosa by Increasing Reproductive Investment and Altering Flowering Phenology Pattern. Sci. Rep. 2018, 8, 16073. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.Y.; Wang, Z.X.; He, Z.S.; He, W.M. Enhanced Precipitation Offsets Climate Warming Inhibition on Solidago canadensis Growth and Sustains Its High Tolerance. Glob. Ecol. Conserv. 2022, 34, e02023. [Google Scholar] [CrossRef]
- Zhou, X.H.; He, W.M. Climate Warming Facilitates Seed Germination in Native but Not Invasive Solidago canadensis Populations. Front. Ecol. Evol. 2020, 8, 595214. [Google Scholar] [CrossRef]
- Cappuccino, N.; Arnason, J.T. Novel Chemistry of Invasive Exotic Plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Qu, T.; Du, X.; Peng, Y.; Guo, W.; Zhao, C.; Losapio, G. Invasive Species Allelopathy Decreases Plant Growth and Soil Microbial Activity. PLoS ONE 2021, 16, e0246685. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Aschehoug, E.T. Invasive Plants Versus Their New and Old Neighbors: A Mechanism for Exotic Invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, B.; Zhang, S.; Tang, J.; Tu, C.; Hu, S.; Yong, J.W.H.; Chen, X. Enhanced Allelopathy and Competitive Ability of Invasive Plant Solidago canadensis In Its Introduced Range. J. Plant Ecol. 2013, 6, 253–263. [Google Scholar] [CrossRef]
- Likhanov, A.; Oliinyk, M.; Pashkevych, N.; Churilov, A.; Kozyr, M. The Role of Flavonoids in Invasion Strategy of Solidago canadensis L. Plants 2021, 10, 1748. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xiao, H.; Zhao, L.; Liu, J.; Wang, L.; Zhang, F.; Shi, Y.; Du, D. The Allelopathic Effects of Invasive Plant Solidago canadensis on Seed Germination and Growth of Lactuca sativa Enhanced by Different Types of Acid Deposition. Ecotoxicology 2016, 25, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Mei, L.X.; Tang, J.J.; Chen, X. Allelopathic Effects of Invasive Solidago canadensis L. on Germination and Growth of Native Chinese Plant Species. Allelopathy J. 2007, 19, 241–248. [Google Scholar]
- Judžentienė, A.; Būdienė, J.; Labanauskas, L.; Stancelytė, D.; Nedveckytė, I. Allelopathic Activity of Canadian Goldenrod (Solidago canadensis L.) Extracts on Seed Germination and Growth of Lettuce (Lactuca sativa L.) and Garden Pepper Cress (Lepidium sativum L.). Plants 2023, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.C.D.; Chmielowiec, C.; Szymura, T.H.; Szymura, M. Effects Of Extracts From Various Parts Of Invasive Solidago species On the Germination and Growth of Native Grassland Plant Species. PeerJ 2023, 11, e15676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tang, J.; Chen, X. Competitive Interaction Between the Invasive Solidago canadensis and Native Kummerowia striata in Lead Contaminated Soil. Bot. Stud. 2008, 49, 385–391. [Google Scholar]
- Wang, W.; Zhu, Q.; Dai, S.; Meng, L.; He, M.; Chen, S.; Zhao, C.; Dan, X.; Cai, Z.; Zhang, J.; et al. Effects of Solidago canadensis L. on Mineralization-Immobilization Turnover Enhance Its Nitrogen Competitiveness And Invasiveness. Sci. Total Environ. 2023, 882, 163641. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Yang, B.; Cui, M.; Dai, Z.; Xiang, J.; Zhang, H.; Li, G.; Li, J.; Javed, Q.; Du, D. Warming And Elevated Nitrogen Deposition Accelerate The Invasion Process of Solidago canadensis L. Ecol. Process. 2022, 11, 62. [Google Scholar] [CrossRef]
- Wan, L.Y.; Qi, S.S.; Dai, Z.C.; Zou, C.B.; Song, Y.G.; Hu, Z.Y.; Zhu, B.; Du, D.L. Growth Responses Of Canada Goldenrod (Solidago canadensis L.) to Increased Nitrogen Supply Correlate With Bioavailability of Insoluble Phosphorus Source. Ecol. Res. 2018, 33, 261–269. [Google Scholar] [CrossRef]
- Cui, M.; Yang, B.; Ren, G.; Stevanato, P.; Fan, X.; Huang, P.; Sun, J.; Du, D. Increased and Fluctuating Phosphorus Nutrient Availability Positively Affects the Growth of the Invasive Plant Solidago canadensis. Flora 2023, 309, 152422. [Google Scholar] [CrossRef]
- Walczyk, A.M.; Hersch-Green, E.I. Genome-Material Costs and Functional Trade-Offs in the Autopolyploid Solidago gigantea (Giant Goldenrod) Series. Am. J. Bot. 2023, 110, e16218. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Wang, Q.; Wang, L.; Liu, W.; Liu, X.; Huang, Y.; Christie, P. Response of Soil Enzymes and Microbial Communities to Root Extracts of the Alien Alternanthera philoxeroides. Arch. Agron. Soil Sci. 2018, 64, 708–717. [Google Scholar] [CrossRef]
- Liao, M.; Xie, X.M.; Peng, Y.; Ma, A.L. Changes of Soil Microbiological Characteristics After Solidago canadensis L. Invasion. Agric. Sci. China 2011, 10, 1064–1071. [Google Scholar] [CrossRef]
- Ye, X.Q.; Yan, Y.N.; Wu, M.; Yu, F.H. High Capacity of Nutrient Accumulation by Invasive Solidago canadensis in a Coastal Grassland. Front. Plant Sci. 2019, 10, 575. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.J.; Ma, L.N.; He, W.M. Arbuscular Mycorrhizal Fungi Help Explain Invasion Success of Solidago canadensis. Appl. Soil Ecol. 2020, 157, 103763. [Google Scholar] [CrossRef]
- Ye, X.Q.; Ma, R.X.; Lei, S.T.; Wu, M.; Yu, F.H. Maintained Nutrient Accumulation in Invasive Solidago canadensis in Response to Competition: Competition Tolerance And Nutrient Accumulation. Flora 2022, 295, 152136. [Google Scholar] [CrossRef]
- Yang, R.Y.; Tang, J.J.; Yang, Y.S.; Chen, X. Invasive and Non-Invasive Plants Differ in Response to Soil Heavy Metal Lead Contamination. Bot. Stud. 2007, 48, 453–458. [Google Scholar]
- Bielecka, A.; Królak, E. Solidago canadensis as a bioaccumulator and phytoremediator of Pb and Zn. Environ. Sci. Pollut. Res. 2019, 26, 36942–36951. [Google Scholar] [CrossRef] [PubMed]
- Mooney, H.A.; Hobbs, R.J. Invasive Species in a Changing World; Island Press: Washington, DC, USA, 2000. [Google Scholar]
- Charles, H.; Dukes, J.S. Impacts of Invasive Species on Ecosystem Services. In Biological Invasions; Nentwig, W., Ed.; Ecological Studies; Springer: Berlin/Heidelberg, Germany, 2007; pp. 217–237. [Google Scholar]
- National Research Council. Valuing Ecosystem Services: Toward Better Environmental Decision-Making; The National Academies Press: Washington, DC, USA, 2005. [CrossRef]
- Zihare, L.; Blumberga, D. Market Opportunities for Cellulose Products from Combined Renewable Resources. Environ. Clim. Technol. 2017, 19, 33–38. [Google Scholar] [CrossRef]
- European Medicines Agency, Committee on Herbal Medicinal Products (HMPC). Assessment Report For Herbal Substance(s), Herbal Preparations or Combinations Thereof With Traditional Use: Solidago virgaurea L., Herba. London, 4 September 2008. EMEA/HMPC/ 285759/2007. Available online: http://www.ema.europa.eu (accessed on 1 April 2024).
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, Antioxidant and Antimicrobial Activities of Leaf and Bark Extracts of Solidago canadensis L. Ind. Crops Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Kołodziej, B.; Kowalski, R.; Kedzia, B. Antibacterial and Antimutagenic Activity of Extracts Aboveground Parts of Three Solidago Species: Solidago virgaurea L., Solidago canadensis L. and Solidago gigantea Ait. J. Med. Plant Res. 2011, 5, 6770–6779. [Google Scholar] [CrossRef]
- Deepa, N.; Ravichandiran, V. Antimicrobial Activity of Extractives of Solidago canadensis L. Int. J. Res. Pharm. Sci. 2010, 1, 411–413. [Google Scholar]
- Mishra, D.; Joshi, S.; Bisht, G.; Pilkhwal, S. Chemical Composition and Antimicrobial Activity of Solidago canadensis Linn. Root Essential Oil. J. Basic Clin. Pharm. 2010, 1, 187–190. [Google Scholar] [PubMed]
- Chanotiya, C.S.; Yadav, A. Natural Variability In Enantiomeric Composition of Bioactive Chiral Terpenoids in the Essential Oil of Solidago canadensis L. from Uttarakhand, India. Nat. Prod. Commun. 2008, 3, 263–266. [Google Scholar] [CrossRef]
- Prosser, I.; Altug, I.G.; Phillips, A.L.; Konig, W.A.; Bouwmeester, H.J.; Beale, M.H. Enantiospecific (+)- and (-)-Germacrene-D synthases, Cloned from Goldenrod, Reveal a Functionally Active Variant of the Universal Isoprenoid-biosynthesis Aspartate-Rich Motif. Arch. Biochem. Biophys. 2004, 432, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Thiem, B.; Wesolowska, M.; Skrzypczak, L.; Budzianowski, J. Phenolic Compounds in Two Solidago, L. Species from In Vitro Culture. Acta Pol. Pharm. 2001, 58, 277–281. [Google Scholar] [PubMed]
- Bradette-Hébert, M.; Legault, J.; Lavoie, S.; Pichette, A. A New Labdane Diterpene from the Flowers of Solidago canadensis. Chem. Pharm. Bull. 2008, 56, 82–84. [Google Scholar] [CrossRef][Green Version]
- Urbanowicz, C.; Muñiz, P.A.; McArt, S.H. Honey Bees and Wild Pollinators Differ in Their Preference for and Use of Introduced Floral Resources. Ecol. Evol. 2020, 10, 6741–6751. [Google Scholar] [CrossRef] [PubMed]
- Štefanić, E.; Puškadija, Z.; Štefanić, I.; Bubalo, D. Goldenrod: A Valuable Plant for Beekeeping in North-Eastern Croatia. Bee World 2003, 84, 88–92. [Google Scholar] [CrossRef]
- Liu, S.; Shao, X.; Wei, Y.; Li, Y.; Xu, F.; Wang, H. Solidago canadensis L. Essential Oil Vapor Effectively Inhibits Botrytis cinerea Growth and Preserves Post-Harvest Quality of Strawberry as a Food Model System. Front. Microbiol. 2016, 7, 1179. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Bai, Y.; Wang, Y.; Kong, H. Allelopathic Effects of the Extracts from an Invasive Species Solidago canadensis L. on Microcystis aeruginosa. Lett. Appl. Microbiol. 2013, 57, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Weber, E. Biological Flora of Central Europe: Solidago altissima L. Flora 2000, 195, 123–134. [Google Scholar] [CrossRef]
- Łapczyńska-Kordon, B.; Ślipek, Z.; Słomka-Polonis, K.; Styks, J.; Hebda, T.; Francik, S. Physicochemical Properties of Biochar Produced from Goldenrod Plants. Materials 2022, 15, 2615. [Google Scholar] [CrossRef] [PubMed]
- Rodionova, M.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel Production: Challenges and Opportunities. Int. J. Hydrogen Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Yao, Y.; Sheng, H.; Luo, Y.; He, M.; Li, X.; Zhang, H.; He, W.; An, L. Optimization of Anaerobic Co-Digestion of Solidago canadensis L. Biomass and Cattle Slurry. Energy 2014, 78, 122–127. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Poluszyńska, J.; Rosik-Dulewska, C.; Sporek, M.; Lenkiewicz, M. Uses Of Weeds As An Economical Alternative To Processed Woodbiomass And Fossil Fuels. Ecol. Eng. 2016, 95, 485–491. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Skrzypczak, D.; Kocek, D.; Mironiuk, M.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Valorization of Bio-Based Post-Extraction Residues of Goldenrod and Alfalfa as Energy Pellets. Energy 2020, 194, 116898. [Google Scholar] [CrossRef]
- Ciesielczuk, T.; Poluszynska, J.; Sporek, M. Potential Uses For Solid Biofuels From NON-Food Crops. Proc. ECOpole 2014, 8, 23e6. [Google Scholar] [CrossRef]
- Wiatrowska, B.M.; Wawro, A.; Gieparda, W.; Waliszewska, B. Bioethanol Production Potential and Other Biomass Energy Properties of Invasive Reynoutria, Solidago,and Spiraea Plants. Forests 2022, 13, 1582. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef]
- Tang, J.W.; Cheng, J.H.; Wang, K.N.; Zhang, Q.Z. Characteristics and Mechanism of Cadmium Adsorption By Solidago canadensis-Derived Biochar. J. Agro-Environ. Sci. 2019, 38, 1339–1348. [Google Scholar]
- Bechtold, T.; Mahmud-Ali, A.; Mussak, R. Natural Dyes For Textile Dyeing: A Comparison Of Methods To Assess The Quality Of Canadian Golden Rod Plant Material. Dyes Pigm. 2007, 75, 287–293. [Google Scholar] [CrossRef]
- Mahmud-Ali, A.; Fitz-Binder, C.; Bechtold, T. Aluminium Based Dye Lakes From Plant Extracts For Textile Coloration. Dyes Pigm. 2012, 94, 533–540. [Google Scholar] [CrossRef]
- IPBES Summary for Policymakers of the Thematic Assessment Report on Invasive Alien Species and Their Control of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; Roy, H.E., Pauchard, A., Stoett, P., Renard Truong, T., Bacher, S., Galil, B.S., Hulme, P.E., Ikeda, T., Sankaran, K.V., McGeoch, M.A., et al., Eds.; IPBES Secretariat: Bonn, Germany, 2023. [Google Scholar]
- Epanchin-Niell, R.S. Economics of Invasive Species Policy and Management. Biol. Invasions 2017, 19, 3333–3354. [Google Scholar] [CrossRef]
- Blumberga, D.; Muizniece, I.; Blumberga, A.; Baranenko, D. Biotechonomy Framework for Bioenergy Use. Energy Procedia 2016, 95, 76–80. [Google Scholar] [CrossRef]
- Harris, H.E.; Patterson, W.F.; Ahrens, R.N.M.; Allen, M.S.; Chagaris, D.D.; Larkin, S.L. The Bioeconomic Paradox of Market-Based Invasive Species Harvest: A Case Study of the Commercial Lionfish Fishery. Biol. Invasions 2023, 25, 1595–1612. [Google Scholar] [CrossRef]

| Sample Type and Origin | Separation Method | Identified Compounds | Reference | |
|---|---|---|---|---|
| Phytochemical Group | No | |||
| Essential oils from leaves (Hungary) | GC/MS | Terpenoids | 66 | [30] |
| Essential oils from inflorescences (Hungary) | 71 | |||
| Essential oils from roots (Hungary) | 69 | |||
| Essential oils from the green parts (Poland) | GC-MS and NMR | Terpenoids | 16 | [31] |
| Essential oils from leaves at the vegetative stage (Russia, Moscow Main Botanical Garden) | GC-MS | 15 | ||
| Essential oils from leaves at the blooming stage (Russia, Moscow Main Botanical Garden) | 15 | |||
| Essential oils from inflorescences (Russia, Moscow Main Botanical Garden) | 11 | |||
| Essential oils from aerial parts (Moscow region, Krasnogorsk district) | 15 | |||
| Essential oils from aerial parts (Austria) | GC-MS | Terpenoids | 15 | [32] |
| Essential oils from aerial parts (Tver’ region) | 16 | |||
| Essential oils from aerial parts (Penza region) | 14 | |||
| Essential oils from aerial parts (Kazakhstan) | 15 | |||
| Essential oils from aerial parts (Altaj region) | 15 | |||
| Essential oils from aerial parts (Sakhalin region) | 14 | |||
| Essential oils from aerial parts (Ukraine) | 15 | |||
| Essential oils from aerial parts (Tula region) | 16 | |||
| Essential oils from aerial parts (Slovakia) | GC-MS | Terpenoids | 5 | [21] |
| Essential oils from leaves and inflorescences (China) | GC-MS | Terpenoids | 6 | [33] |
| Essential oils from the whole plant (China) | GC-MS | Terpenoids | 7 | [34] |
| Essential oils from aerial parts (Poland) | GC-MS | Terpenoids | 6 | [35] |
| Essential oils from aerial parts (Poland) | GC-MS | Terpenoids | 3 | [36] |
| Essential oils from aerial parts (Lithuania) | GC-MS | Terpenoids | 6 | [37] |
| Air-dried herbs (Hungary) | HPLC-MS | Flavonoids | 7 | [38] |
| Phenolic acids | 2 | |||
| Aerial parts (Poland) | UPLC/ESI-MS | Flavonoids | 9 | [39] |
| Phenolic acids | 9 | |||
| Roots with rhizomes (Poland) | Phenolic acids | 9 | ||
| Inflorescences and leaves (Slovakia) | LC-MS/MS | Flavonoids | 8 | [40] |
| Phenolic acids | 8 | |||
| Flowers (Hungary) | GC–MS | Terpenoids | 50 | [41] |
| Compounds with benzene ring | 2 | |||
| Open-chain alcohols, aldehydes, and ketones | 3 | |||
| Honey (Hungary) | GC–MS | Terpenoids | 42 | |
| Compounds with benzene ring | 13 | |||
| Open-chain alcohols, aldehydes and ketones | 7 | |||
| Lactones | 2 | |||
| Esters of open-chain acids | 2 | |||
| Fatty acids | 1 | |||
| Open-chain and ringed, saturated and unsaturated hydrocarbons | 15 | |||
| Polyphenolic Group | Identified Individual Compounds | Presence of Compounds in the Extraction Solvent | |||||
|---|---|---|---|---|---|---|---|
| Methanol | Ethanol | Acetone | Water | Ether: ethanol | |||
| Phenolic acids | Hydroxy- benzoic acids  | Gallic acid | [40] | [40] | |||
| Protocatechuic acid | [40] | [40] | [40] | [40] | |||
| Vanillic acid | [40] | [40] | |||||
| Syringic acid | [40] | [40] | |||||
| Hydroxy- cinnamic acids 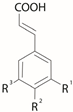 | |||||||
| 5-O-Feruloylquinic acid | [39] | ||||||
| Caffeoyl-di-feruloylquinic acid | [39] | ||||||
| 1-Caffeoylquinic acid | [39] | ||||||
| 4-Caffeoylquinic acid | [39] | ||||||
| 5-Caffeoylquinic acid | [39] | ||||||
| t-Ferulic acid | [40] | [40] | [40] | [40] | |||
| Chlorogenic acid | [38,39,40,48,49] | [40] | [40] | [40,50] | |||
| Caffeic acid | [38,40,51] | [40] | |||||
| 3,4-O-Dicaffeoylquinic acid | [39,52] | ||||||
| 3,5-O-Dicaffeoylquinic acid | [39,52] | ||||||
| Coumaric acid | [40] | [40] | [40] | [40] | |||
| Flavonoids | Flavonols  | Quercetin | [38,39,40,48,49,51] | [40] | [40] | [40] | |
| Quercitrin | [38,39,40,48,49] | [40] | [40] | [40,50] | |||
| Hyperoside | [38,48,49] | ||||||
| Isoquercitrin | [38,48,49] | [50] | |||||
| Rutin | [38,39,40,48,49,51] | [40] | [40] | [40,50] | |||
| Quercetin-O-hexoside | [39] | ||||||
| Quercetin-(acetyl)-hexoside | [48] | ||||||
| Quercetin-(rhamnosyl)- hexoside | [48] | ||||||
| Quercetin-3-O-(6′-O-acetyl)-β-D-glucopyranoside | [51] | ||||||
| Isorhamnetin 3-O-hexoside-7-O-deoxyhexoside | [39] | ||||||
| Isorhamnetin-(acetyl)-hexoside | [50] | ||||||
| Isorhamnetin-(rhamnosyl)-hexoside | [51] | ||||||
| Isorhamnetin-3-O-β-D-glucopyranoside | [51] | ||||||
| Kaempferol | [39,40,49,51] | [40] | [40] | [40,50] | |||
| Kaempferol-(rhamnosyl)-hexoside isomers | [48] | ||||||
| Nicotiflorin | [38] | [50] | |||||
| Hesperidin | [40] | [40] | [40] | [40] | |||
| Afzelin | [38,49] | ||||||
| Kaempferol-3-O-(6′-O-acetylyl)- β-D-glucopyranoside | [51] | ||||||
| Kaempferol-3-O-β-D-apiofuranoside | [51] | ||||||
| Kaempferol-O-hexoside-deoxyhexoside | [39] | ||||||
Flavanols  | Epicatechin | [40] | [40] | [40] | [40] | ||
| Catechin | [40] | [40] | |||||
Flavanones 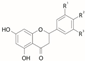 | Hydroxy flavanone | [40] | [40] | [40] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poljuha, D.; Sladonja, B.; Uzelac Božac, M.; Šola, I.; Damijanić, D.; Weber, T. The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants 2024, 13, 1745. https://doi.org/10.3390/plants13131745
Poljuha D, Sladonja B, Uzelac Božac M, Šola I, Damijanić D, Weber T. The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants. 2024; 13(13):1745. https://doi.org/10.3390/plants13131745
Chicago/Turabian StylePoljuha, Danijela, Barbara Sladonja, Mirela Uzelac Božac, Ivana Šola, Danijela Damijanić, and Tim Weber. 2024. "The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy" Plants 13, no. 13: 1745. https://doi.org/10.3390/plants13131745
APA StylePoljuha, D., Sladonja, B., Uzelac Božac, M., Šola, I., Damijanić, D., & Weber, T. (2024). The Invasive Alien Plant Solidago canadensis: Phytochemical Composition, Ecosystem Service Potential, and Application in Bioeconomy. Plants, 13(13), 1745. https://doi.org/10.3390/plants13131745











